Abstract
Purpose
The aims of this study were to determine outcomes for patients with inflammatory breast cancer (IBC) treated with multimodality therapy, to identify factors associated with locoregional recurrence, and to determine which patients may benefit from radiation dose escalation.
Methods and Materials
We retrospectively reviewed 256 consecutive patients with nonmetastatic IBC treated at our institution between 1977 and 2004.
Results
The 192 patients who were able to complete the planned course of chemotherapy, mastectomy, and postmastectomy radiation had significantly better outcomes than the 64 patients who did not. The respective 5-year outcome rates were: locoregional control (84% vs. 51%), distant metastasis–free survival (47% vs. 20%), and overall survival (51% vs. 24%) (p < 0.0001 for all comparisons). Univariate factors significantly associated with locoregional control in the patients who completed plan treatment were response to neoadjuvant chemotherapy, surgical margin status, number of involved lymph nodes, and use of taxanes. Increasing the total chest-wall dose of postmastectomy radiation from 60 Gy to 66 Gy significantly improved locoregional control for patients who experienced less than a partial response to chemotherapy, patients with positive, close, or unknown margins, and patients <45 years of age.
Conclusions
Patients with IBC who are able to complete treatment with chemotherapy, mastectomy, and postmastectomy radiation have a high probability of locoregional control. Escalation of postmastectomy radiation dose to 66 Gy appears to benefit patients with disease that responds poorly to chemotherapy, those with positive, close, or unknown margin status, and those <45 years of age.
Keywords: Inflammatory breast cancer, Radiation dose, Locoregional therapy
INTRODUCTION
Despite advances in medical, surgical, and radiation oncology, inflammatory breast cancer (IBC) remains a therapeutic challenge, with a high risk of rapid disease progression and early distant dissemination (1, 2). More than 70% of patients with IBC have clinically localized disease without distant metastases at initial presentation and thus are candidates for potentially curative combined-modality treatments (3). Chemotherapy has transformed what was once a uniformly fatal disease (1) into one with 5-year survival rates of 40% (4–7).
With improvements in systemic treatments, locoregional management became a critical component in curative treatment. Several studies have found an association between locoregional control (LRC) and overall survival (OS) in patients with IBC treated with chemotherapy (8–10). Locoregional recurrence in this disease is invariably associated with distant dissemination and death (11–13). Over the past three decades, the locoregional treatment strategy for patients with IBC has evolved. Initially radiation was used alone (14); but after systemic treatments became available, neoadjuvant chemotherapy followed by mastectomy and radiation became the standard (15).
To offset the rapid proliferative potential of IBC (16), researchers at our institution began investigating accelerated hyperfractionated radiotherapy. This strategy uses twice-daily treatments to shorten the treatment course and thereby minimize the risk of tumor repopulation during therapy. Another aspect of radiation therapy that has changed over time at our institution is the total dose delivered to the chest wall and draining lymphatics, which has increased from 60 Gy to 66 Gy in 1985.
In this article we retrospectively analyze our experience in treating IBC with multimodality therapy. One objective of this analysis was to define which subsets of patients with IBC benefit from a more aggressive radiation treatment schedule and which patients are at lower risk for locoregional recurrence with conventional radiation treatment schedules that carry a lower risk of normal tissue toxicity.
METHODS AND MATERIALS
Patients
For the purpose of this study, an institutional review board–approved retrospective chart review was performed on all patients with IBC treated at The University of Texas M. D. Anderson Cancer Center from 1977 through 2004. All patients who had nonmetastatic IBC treated with radiation therapy and a curative intent at our institution were included. In each case, the diagnosis of IBC was made by a multidisciplinary team after the patient presented with the clinical triad of ridging, peau d’orange, and diffuse skin erythema in the setting of a rapid clinical onset, typically <3 months. Patients who had a neglected, locally advanced breast cancer with secondary signs of erythema and edema were considered not to have inflammatory breast cancer, and these patients were not included in this study. All diagnoses of cancer were confirmed by biopsy before initiation of therapy.
Data from each patient’s medical record were used to stage the disease clinically according to the American Joint Committee on Cancer TNM classification (6th ed). Therefore patients with supraclavicular metastases without distant metastases were considered to have N3 disease and were included in the analysis. All patients had a complete history and physical examination, bilateral mammography, ultrasonography of regional nodes and breast tissue, chest X-ray, bone scan, and routine blood cell counts, and serum chemistry tests. A liver–spleen scan was performed routinely until computed tomography of the abdomen and pelvis became available. Some patients underwent bone marrow biopsy, positron emission tomography, and/or magnetic resonance imaging as part of their workup.
Treatment
All patients included in this analysis were treated with an initial intent to deliver curative treatment that included chemotherapy, mastectomy, and radiotherapy (and hormonal therapy if indicated). Most patients received sequential treatment with initial chemotherapy, mastectomy, adjuvant chemotherapy, and postmastectomy radiation. Patients whose disease was refractory to neoadjuvant chemotherapy were treated in an ad hoc manner that was customized to their disease (described in Results). A few patients in this series underwent initial surgical resection at an outside facility and were treated in our institution with adjuvant chemotherapy and radiation.
From 1977 to 1982, the standard neoadjuvant chemotherapy regimen used in our institution consisted of 5-fluorouracil, doxorubicin, and cyclophosphamide (FAC) for three or four cycles. Vincristine and prednisone were added to the regimen in 1982 but were discontinued in 1987 because the data suggested that they added no benefit. Patients whose cancer remained inoperable after initial FAC chemotherapy were given methotrexate, vinblastine, and folinic acid for two or three cycles. If the disease remained inoperable or progressed, preoperative radiation was given or, in select cases, radiotherapy alone. Most patients also received adjuvant chemotherapy. Taxanes were introduced as adjuvant therapy in 1994. Postmenopausal women with estrogen receptor–positive or progesterone receptor–positive disease were treated with tamoxifen. In more recent years, hormonal treatment also became standard for premenopausal patients with estrogen receptor–positive or progesterone receptor–positive disease, and aromatase inhibitors became available for postmenopausal patients. Trastuzumab was used in only a few patients who were treated in the later years of the study period for a HER2/neu-positive cancer.
Response to neoadjuvant chemotherapy was evaluated via findings on physical examination and radiographic studies. A clinical complete response (CR) was defined as the resolution of all clinical signs of disease within the primary site and lymph nodes. A clinical partial response (PR) was defined as a reduction of at least 50% in the clinical skin changes and the bidirectional measurements of tumor mass defined by clinical and radiographic means. All other responses were categorized as no response.
After neoadjuvant chemotherapy, most patients underwent a modified radical mastectomy. A resection margin of <2 mm was considered as close/positive, and a margin ≥2 mm as negative. Patients with close/positive margins most commonly began postmastectomy radiation 4 to 6 weeks after surgery, followed by adjuvant chemotherapy. Patients with negative margins most commonly received adjuvant chemotherapy followed by postoperative radiation therapy.
Radiotherapy dose and technique used at our institution for IBC during the years studied have been described elsewhere (14, 17). The target volume of treatment after mastectomy was the chest wall and draining lymphatics. The most common postmastectomy radiation treatment approach used paired photon tangent fields that targeted the lateral chest wall and were matched with an appositional electron field that targeted the medial chest wall and internal mammary nodal chain. The supraclavicular fossa and axillary apex were treated with a matched photon field. Tissue equivalent bolus (3- to 5-mm thick) was placed on the chest wall every treatment for the first 10 treatments, every other treatment for the next 10 treatments, and then as needed based on the clinical response of the skin overlying the chest wall. The goal of the bolus schedule was to cause brisk erythema with dry desquamation, which we had reported previously to correlate with the highest probability of LRC (15).
The dose and fractionation schema for the patients who were treated with postmastectomy radiation changed over time. From 1977 to 1981 the chest wall and draining lymphatics were treated daily to 50 Gy in 2-Gy fractions, followed by a 10-Gy boost to the chest wall. From 1982 to 1985, accelerated hyperfractionated radiotherapy was adopted as the standard technique, and most patients were treated to a dose of 45 Gy in 1.5-Gy fractions given twice daily followed by a 15-Gy boost, which was also delivered at 1.5 Gy twice daily. After 1985, the technique remained the same but the overall dose was increased by 10%, with most patients receiving 51 Gy in 1.5-Gy fractions followed by a 15-Gy boost also delivered twice daily.
The dose and fractionation schedules for the patients who required preoperative radiation because of a poor response to chemotherapy also changed over time. Between 1977 and 1985, preoperative irradiation comprised an initial dose of 50 Gy in 25 fractions given once daily or 45 Gy in 30 fractions with twice-daily fractionation. After 1985, most patients were treated to 51 Gy in 34 fractions with twice-daily fractionation. Surgery was typically performed 4 to 6 weeks after preoperative radiation. No boost fields were used except in cases of known infraclavicular or supraclavicular involvement that would not be dissected. These regions were given a boost of 6 to 9 Gy.
Statistical analysis
Differences in categorical variables were compared by Chi-square analysis. Differences in means were assessed by analysis of variance or t test where appropriate. The LRC, OS, and distant metastasis–free survival (DMFS) and the probability of developing late toxicity, graded according to the Radiation Therapy Oncology Group (RTOG)/European Organization for Research and Treatment of Cancer (EORTC) late radiation morbidity scoring schema (18), were estimated using the Kaplan-Meier method. All time points were calculated from the date of diagnosis. All locoregional recurrences and distant metastasese were counted as events for the endpoints of LRC and DMFS, independent of whether they occurred as first or secondary events. Multivariate Cox regression analysis was used to determine which disease and treatment variables were independent predictors of outcome.
RESULTS
The median follow-up for the surviving patients was 64 months (range, 7–240 months). For the 256 patients, there were 56 locoregional recurrences, 152 distant failures, and 159 deaths. Of the 256 patients, 192 (75%) successfully completed treatment as intended with a combination of doxorubicin-based chemotherapy, mastectomy, and postmastectomy radiation. A total of 64 patients (25%) were not able to complete the intended course of treatment because of poor disease response. Of these 64 patients, 21 underwent preoperative radiation followed by surgery because of poor response to preoperative chemotherapy; 21 underwent definitive radiation alone because of disease extents; and 22 experienced local disease recurrence after mastectomy but before postmastectomy radiation. These 64 patients more commonly had supraclavicular lymph node involvement at presentation as well as positive surgical margins after mastectomy than the group of 192 patients who were treated with adjuvant postmastectomy radiation as planned.
Locoregional control and survival
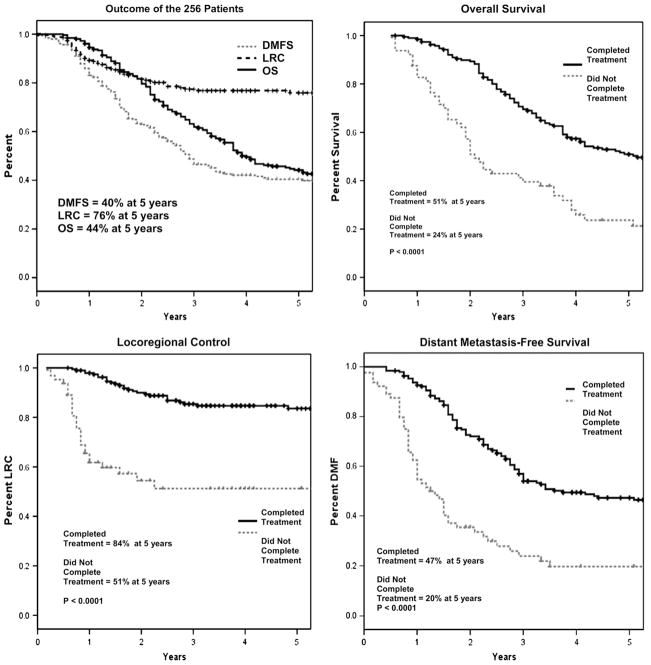
Figure 1 shows the actuarial rates of LRC, DMFS, and OS for the 256 patients as a whole and a comparison of the 192 patients who completed the planned course of treatment and the 64 who did not. The actuarial 5-year LRC, DMFS, and OS rates for the entire population were 76%, 40%, and 44%, respectively. Not surprisingly, patients who were able to complete multimodality therapy as intended had significantly better rates of 5-year LRC (84% vs. 51%), DMFS (47% vs. 20%), and OS (51% vs. 24%) than those who could not complete the treatment as planned (p <0.0001 for all comparisons). A multivariate analysis for the entire group confirmed that inability to complete treatment as plan was independently associated with a statistically inferior outcome for all three endpoints.
Fig. 1.
(Top left) Overall survival (OS), locoregional control (LRC), and distant metastasis–free survival (DMFS) in all patients in the series (n = 256). (Top right) OS in patients able to complete combined-modality therapy as intended (group 1, n = 192) vs. OS in patients who did not complete therapy as intended (group 2, n = 64). (Bottom left) LRC as a function of group. (Bottom right) DMFS as a function of group.
Patient, tumor, and treatment characteristics
Table 1 details the patient, tumor, and treatment characteristics for the entire study population and the 192 patients who were able to complete the planned course of treatment. In all, 95% of patients received neoadjuvant chemotherapy. Of the patients who received neoadjuvant chemotherapy, 21% achieved a clinical CR, 59% a clinical PR, and 20% less than a PR.
Table 1.
Patient, tumor, and treatment characteristics for entire patient population and for subgroup of patients who completed the planned course of treatment*
| Characteristic | All patients (n = 256) | Patients completing planned treatment (n = 192) |
|---|---|---|
| Patient | ||
| Follow-up of patients alive | ||
| Median (mo) | 64 | 78 |
| Range (mo) | 7–240 | 7–232 |
| Age (y) | ||
| Median | 49 | 49 |
| Range | 24–76 | 24–76 |
| Race/ethnicity | ||
| White | 184 (72%) | 141 (73%) |
| African American | 30 (12%) | 21 (11%) |
| Hispanic | 34 (13%) | 23 (12%) |
| Asian | 4 (2%) | 3 (2%) |
| Other | 4 (2%) | 4 (2%) |
| Menopausal status | ||
| Pre/peri | 97 (38%) | 77 (56%) |
| Post | 124 (48%) | 108 (40%) |
| Unknown | 35 (14%) | 7 (4%) |
| Tumor | ||
| N stage | ||
| N0 | 36 (14%) | 26 (14%) |
| N1 | 102 (40%) | 81 (42%) |
| N2 | 84 (33%) | 62 (32%) |
| N3 | 22 (9%) | 17 (9%) |
| Nx | 12 (5%) | 6 (3%) |
| Supraclavicular nodes | ||
| Positive | 35 (14%) | 17 (9%) |
| Negative | 187 (73%) | 144 (75%) |
| Unknown | 34 (13%) | 31 (16%) |
| ER status | ||
| Negative | 119 (46%) | 93 (48%) |
| Positive | 66 (26%) | 50 (26%) |
| Unknown | 77 (30%) | 49 (26%) |
| Her2neu status | ||
| Negative | 37 (14%) | 24 (13%) |
| Positive | 21 (8%) | 20 (10%) |
| Unknown | 198 (77%) | 148 (77%) |
| Treatment | ||
| Neoadjuvant | 244 (95%) | 183 (95%) |
| chemotherapy | ||
| Taxane used | 80 (31%) | 77 (40%) |
| Response to chemotherapy† | ||
| CR | 43 (21%) | 42 (23%) |
| PR | 118 (59%) | 111 (61%) |
| NR | 40 (20%) | 30 (16%) |
| Surgical margin status | ||
| Negative | 133 (52%) | 133 (70%) |
| Close/positive | 26 (10%) | 16 (8%) |
| Unknown | 97 (38%) | 43 (22%) |
| No. of nodes removed | ||
| Median | 13 | 13 |
| Range | 0–43 | 0–43 |
| No. of nodes pathologically positive | ||
| 0–3 | 90 (47%) | 90 (47%) |
| ≥4 | 102 (53%) | 102 (53%) |
| XRT fractionation | ||
| Once daily | 47 (20%) | 38 (20%) |
| Twice daily | 204 (80%) | 154 (80%) |
| XRT dose | ||
| Median 60 Gy | NA | 79 (41%) |
| Range (Gy) | NA | 43.5–60 |
| Median 66 Gy | NA | 113 (59%) |
| Range (Gy) | NA | 61–72 |
Abbreviations: CR = complete response; NR = less than a partial response; NA= not applicable (in that surgeries varied); PR = partial response; XRT = radiotherapy; ER = estrogen receptor.
Data are numbers of patients with percentages in parentheses, unless otherwise specified.
Excludes 9 patients who did not receive neoadjuvant chemotherapy.
For the 192 patients who went on to have a mastectomy and postmastectomy radiation, at the time of surgical resection, negative margins were achieved in 70% of patients, whereas in the remaining 30% the margins were either positive or of unknown status. At mastectomy, 47% of the patients had lymph node involvement ranging from no nodes to three, whereas 53% had more advanced disease with involvement of four or more nodes. The median number of recovered axillary lymph nodes was 13. A total of 77 patients (40%) received a taxane as a component of their chemotherapy.
With respect to the course of postmastectomy radiation therapy, 80% of patients were treated with an accelerated hyperfractionated schedule that involved twice-daily 1.5-Gy fractions. The 20% of patients who were treated with once-daily 2-Gy fractions either were treated during the early years of this series or were unable to receive twice-daily treatments, mainly for logistical reasons that made it difficult to come to the hospital twice a day. The standard total dose before 1986 was 60 Gy, whereas the standard dose from 1986 to the end of the study period was 66 Gy. We compared the locoregional treatment outcomes according to total dose (≤60 Gy vs. >60 Gy). Of the patients, 79 (41%) received ≤60 Gy, and their median total dose was 60 Gy (range, 43.5–60 Gy). The 113 (59%) patients in the higher-dose group received a median dose of 66 Gy (range, 61–72 Gy). In the 60-Gy treatment group, 58% were treated with twice-daily treatment and 42% with once-daily treatment. In the 66-Gy group, 96% were treated with twice-daily treatment.
Locoregional control for patients who completed the planned treatment
In an effort to determine variables that were associated with locoregional recurrence after postmastectomy radiation, we evaluated the relationship between patient, tumor, and treatment-related factors and LRC in the 192 patients who completed the planned course of treatment. Data from this analysis are shown in Table 2. The variable with the strongest association with LRC was clinical response to neoadjuvant chemotherapy. The 5-year LRC rate was 95% for the 42 patients with a clinical CR, 86% for the 111 patients experiencing a PR, and 51% in the 30 patients with less than a PR (p <0.0001). Clinical response to neoadjuvant chemotherapy was also the strongest predictor of both OS and DMFS. Of patients who had a clinical CR, 70% were free of metastatic disease and 73% were alive at 5 years, whereas of those who had a PR, 42% were metastasis free and 51% were alive at 5 years. Patients with less than a PR had a particularly poor prognosis; all 30 of these patients experienced distant recurrence by 5 years, and only 12% survived 5 years (p < 0.0001 for all comparisons).
Table 2.
Univariate analysis of variables associated with locoregional control for patients who were able to complete the planned course of treatment
| Variable | No. of patients | 5-year LRC | p Value |
|---|---|---|---|
| Response to chemotherapy*,† | |||
| CR | 42 | 95% | |
| PR | 111 | 86% | <0.0001 |
| NR | 30 | 51% | |
| Surgical margin status† | |||
| Negative | 125 | 91% | 0.0005 |
| Positive/unknown | 60 | 68% | |
| No. of nodes pathologically positive | |||
| 0–3 | 90 | 94% | 0.006 |
| ≥4 | 102 | 74% | |
| Taxane used | |||
| Yes | 77 | 92% | 0.04 |
| No | 115 | 79% | |
| Age (y) | |||
| ≤45 | 70 | 79% | 0.10 |
| >45 | 122 | 86% | |
| XRT dose | |||
| Median 60 Gy | 79 | 78% | 0.17 |
| Median 66 Gy | 113 | 88% | |
| XRT fractionation | |||
| Once daily | 38 | 81% | 0.98 |
| Twice daily | 154 | 84% | |
Abbreviations: CR = complete response; NR = less than a partial response; PR = partial response; XTR = radiotherapy.
Excludes 9 patients who did not receive neoadjuvant chemotherapy.
Factors that remained significant on multivariate analysis.
Other factors significantly associated with LRC included surgical margin status, number of pathologically positive lymph nodes, and whether a patient received taxane chemotherapy. Patients with negative margins had an LRC rate of 91% at 5 years, whereas those with positive or unknown margin status had an LRC rate of 68% (p = 0.0005). Patients with lymph node involvement of no to three nodes had a better LRC rate than patients with four or more involved lymph nodes (94% vs. 74%, respectively; p = 0.006). Patients who received a taxane in addition to doxorubicin-based chemotherapy had a better 5-year LRC rate than patients who did not receive a taxane (92% vs. 79%, respectively; p = 0.04).
Of the 192 patients, 184 were able to complete the planned course of treatment received neoadjuvant chemotherapy, mastectomy, and postmastectomy radiation. Among these 184 patients, 29 had no residual invasive disease within the breast or lymph nodes (pathological complete response [pCR]); 64 had residual disease in the breast but no positive lymph nodes (n = 13) or one to three positive lymph nodes after chemotherapy (n = 51); and 91 had four or more positive lymph nodes after chemotherapy. The respective 5-year rates of LRC were 96% in the pCR group vs. 83% in the non-pCR group with positive lymph nodes ranging from none to three and 76% in the group with four or more positive lymph nodes (p = 0.019).
Adjuvant chemotherapy was used in 152 of 184 patients treated with neoadjuvant chemotherapy and appeared to have no impact on LRC (85% yes vs. 84% no, p = 0.975).
Multivariate analysis, which incorporated all variables associated with LRC, found two factors that remained independently significant: namely, clinical response to chemotherapy and surgical margin status.
Effect of radiation fractionation schedule and total dose
In the group of 192 patients who completed the course of planned treatment, the actuarial 5-year LRC rates for patients treated with once-daily or twice-daily fractionation did not differ significantly (81% vs. 84%, respectively; p = 0.98); but the small number of patients treated with once-daily fractionation limits the significance of this comparison. Similarly there was no statistical difference in LRC according the mean total radiation dose. At 5 years, the LRC rate of the patients who received a mean total dose of 60 Gy was 78%, whereas that for the patients that received a mean total dose of 66 Gy was 88% (p = 0.17).
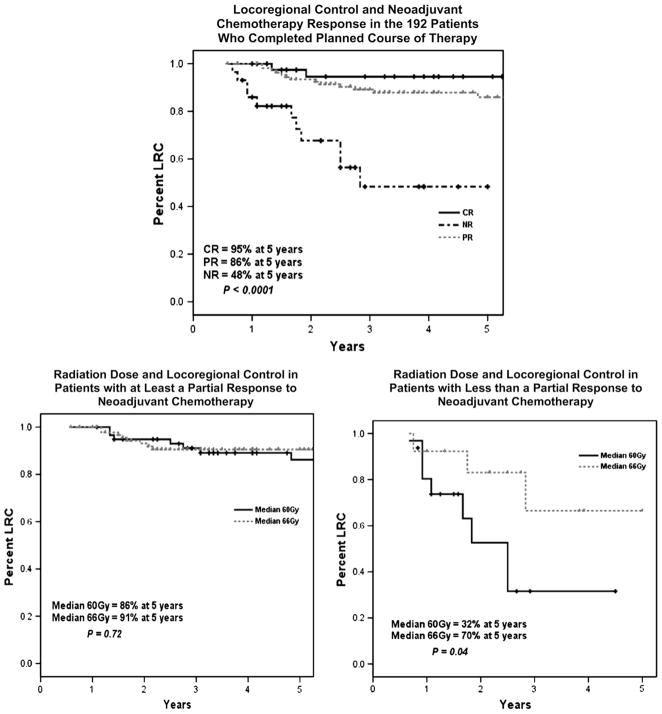
Although dose did not appear to have an independent significant effect on LRC rates, further analyses were performed to determine if higher dosages may benefit particular subsets. Figure 2 shows the effect of radiation dose according to response to neoadjuvant chemotherapy. For patients who experienced a clinical CR or PR, the dose of postmastectomy radiation did not have a statistically significant impact on LRC; 86% of patients treated to 60 Gy maintained LRC at 5 years, whereas 91% of patients treated to 66 Gy maintained LRC over the same interval (p = 0.72). For patients whose disease did not have a PR to chemotherapy, a higher dose was associated with a significant benefit in LRC. Of these patients, 70% who received 66 Gy had LRC at 5 years, whereas 32% of those who received only 60 Gy had LRC at 5 years (p = 0.04).
Fig. 2.
(Top) Locoregional control (LRC) as a function of response to neoadjuvant chemotherapy. (Bottom left) LRC in patients who experienced at least a PR to chemotherapy as a function of radiation dose (n = 94 for dose 66 Gy, n = 59 for 60 Gy). (Bottom right) LRC in patients who experienced less than a PR to chemotherapy as a function of radiation dose (n = 14 for dose 66 Gy, n = 16 for 60 Gy).
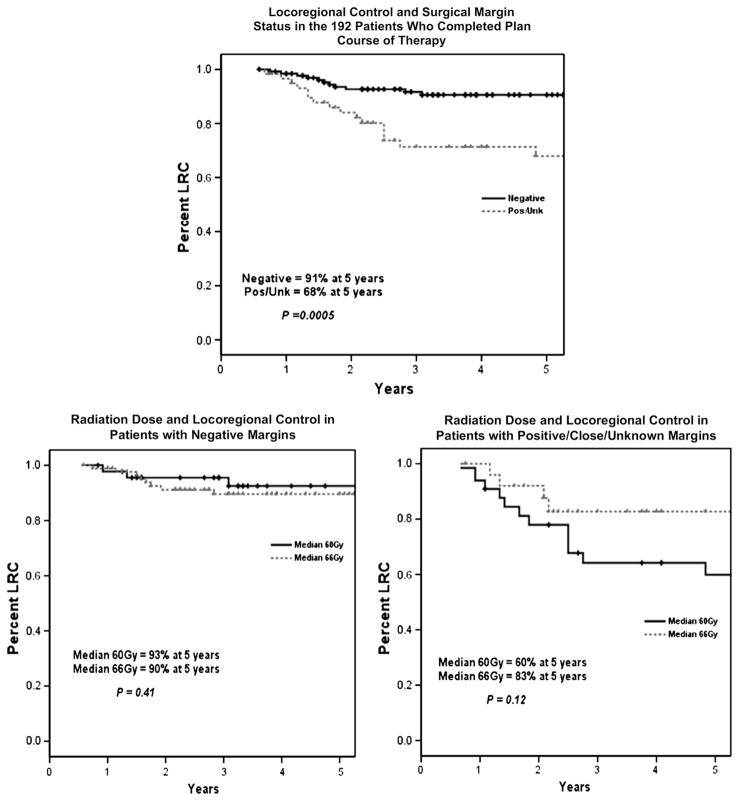
Figure 3 shows the effect of margin status on LRC and stratifies the patients by this parameter to assess the impact of radiation dose in these subsets. Patients with negative surgical margins did not appear to benefit from higher radiation doses; the 5-year LRC rate was 93% in patients treated to 60 Gy and 90% in patients treated to 66 Gy (p = 0.41). For patients whose surgical margins were close, positive, or unknown, however, the 5-year LRC rates were 60% in patients treated to 60 Gy and 83% in patients treated to 66 Gy (p = 0.12).
Fig. 3.
(Top) Locoregional control (LRC) as a function of surgical margin status. (Bottom left) LRC in patients with negative surgical margins as a function of radiation dose (n = 87 for dose 66 Gy, n = 46 for 60 Gy). (Bottom right) LRC in patients with positive or unknown surgical margins as a function of radiation dose (n = 26 for dose 66 Gy, n = 33 for 60 Gy).
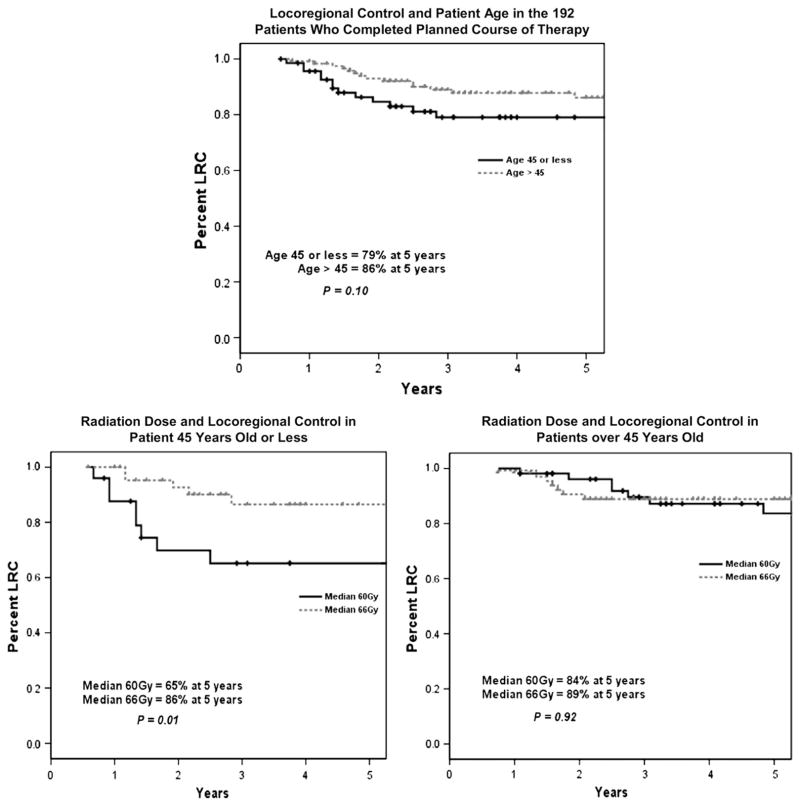
Figure 4 shows that LRC was marginally better in the group of women >45 years of age (86% 5-year LRC rate) than in those ≤45 years (79%), although this difference was not statistically significant. Nonetheless, in assessing the effect of dose in these two groups, the patients ≤45 years of age did appear to have a significant improvement in 5-year LRC with a higher dose of postmastectomy radiation (86% LRC in the 66-Gy group vs. 65% LRC in the 60-Gy group; p = 0.01).
Fig. 4.
(Top) Locoregional control (LRC) as a function of age (≤45 years vs. >45 years). (Bottom left) LRC in patients aged ≤45 years as a function of radiation dose (n = 45 for dose 66 Gy, n = 25 for 60 Gy). (Bottom right) LRC in patients aged >45 years as a function of radiation dose (n = 68 for dose 66 Gy, n = 54 for 60 Gy).
For each curve shown in Figs. 3 to 5, we also analyzed the effect of once-daily vs. twice-daily fractionation for the 60-Gy dose level and found no clinically apparent or statistical difference between these two fractional schemes. There were too few patients treated once daily to 66 Gy to allow any such comparison within the 66-Gy dose level. We also analyzed the effect of dose on patients with four or more positive lymph nodes after chemotherapy and did not find a statistically significant difference in outcome in LRC in this subgroup.
Fig. 5.
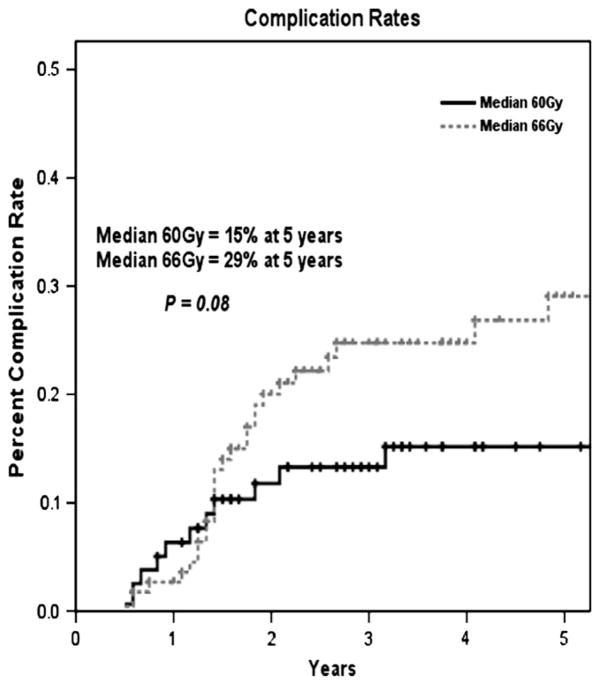
Actuarial Grade 3 to 4 complication rates in patients treated to a lower radiation dose of 60 Gy (n = 79) vs. a higher dose of 66 Gy (n = 113).
Complications
Table 3 details the types and incidences of late Grade 3 to 4 complications in patients treated to 60 or 66 Gy. Of the 79 patients treated to a median dose of 60 Gy, 12 developed at least one Grade 3 to 4 late complication, whereas of the 113 patients treated to a median dose of 66 Gy, 28 had at least one such complication. The most common Grade 3 to 4 late side effects were severe chest wall fibrosis (n = 10), symptomatic lymphedema (n = 12), and severe telangiectasia (n = 9). The major difference between the two dose groups was in the development of symptomatic lymphedema (n = 2 in the 60-Gy group, n = 10 in the 66-Gy group). Moreover, 2 patients who received the higher dose developed brachial plexopathy. Both of these patients had supraclavicular lymphadenopathy at diagnosis. Brachial plexopathy was not observed in the lower-dose group. Figure 5 shows the actuarial risk of developing a Grade 3 to 4 late complication as a function of radiation dose (29% in the 66-Gy group vs. 15% in the 60-Gy group, p = 0.08). The effect of fractionation schedule (once-daily vs. twice-daily treatment) within the 66-Gy dose arm could not be evaluated because 96% of the patients treated to 66 Gy received twice-daily fractionation.
Table 3.
Grade 3 to 4 late complications observed for all patients completing combined-modality therapy as planned*
| Complication | Median 60 Gy (n = 79) |
Median 66 Gy (n = 113) |
||
|---|---|---|---|---|
| Gr 3 | Gr 4 | Gr 3 | Gr 4 | |
| Rib fracture | 0 | 1 | 0 | 2 |
| Lymphedema | 2 | 0 | 10 | 0 |
| Fibrosis | 3 | 0 | 7 | 0 |
| Telangiectasia | 4 | 0 | 5 | 0 |
| Lung | 2 | 0 | 0 | 0 |
| Necrosis/ulceration | 0 | 1 | 0 | 3 |
| Brachial plexopathy | 0 | 0 | 0 | 2 |
| Infection/pain | 0 | 0 | 2 | 0 |
| Total | 11 | 2 | 24 | 7 |
Abbreviation: Gr = Grade.
Data are numbers of patients with complication (by grade).
DISCUSSION
This study updates nearly 30 years of our institution’s experience of locoregional management of nonmetastatic IBC and defines factors associated with outcome. With a total of 256 patients included in the analysis, this study is one of the largest reported series of locoregional treatment outcomes for patients with this disease. The intended goal of treatment for all patients in this series was to deliver combined-modality therapy consisting of doxorubicin-based chemotherapy, mastectomy, and postmastectomy radiation. Of the patients, 75% were able to complete this treatment as intended. The high rate of distant metastatic disease and the poor OS rate among patients who were unable to receive the intended treatment highlights the need for new systemic treatment strategies for this cohort.
Patients who were able to complete the entire course of planned treatment that included neoadjuvant chemotherapy, mastectomy and postmastectomy radiation had a 5-year OS rate of 51%—an encouraging result given that IBC was once considered a uniformly fatal disease (1). For such patients, the achievement of LRC becomes a matter of greater importance than in those with chemo-refractory disease. Indeed, several previous studies have confirmed that, in patients who respond to modern chemotherapy, there is an association between achievement of durable LRC and survival (8–10). Locoregional recurrence in patients with IBC is invariably associated with distant dissemination and death from disease (11–13).
Over the last three decades we have refined the radiation therapy of IBC at our institution in an attempt to improve LRC rate. Early in that period, we moved from a once-daily fractionation schema to an accelerated hyperfractionated approach (15, 19); later we escalated the total radiation dose from 60 Gy to 66 Gy (8). In our previous report updating our experience with IBC, Liao et al. (8) reviewed the results of 115 patients with IBC and concluded that escalating the dose from 60 Gy to 66 Gy delivered by twice-daily fractionation significantly improved the 5-year LRC rate (58% vs. 84%, respectively; p = 0.04).
With a greater number of patients and more mature follow-up, we now confirm that treatment to a chest-wall cumulative dose of 66 Gy is of clinical value for patients with a poor response to chemotherapy, patients with close or positive surgical margins, and possibly patients <45 years of age. Importantly, we also found that an excellent LRC rate was achieved with lower morbidity with a dose of 60 Gy for patients without any of these features. Stratification of patients according to these features may be warranted in that there was a trend for increased significant late toxicity associated with dose escalation to 66 Gy. From these data, we conclude that dose escalation should be reserved for patients with the high-risk features just described (patients with a poor response to chemotherapy; patients with close or postive surgical margins; and patients <45 years of age).
More than 80% of the patients in this series were treated with an accelerated hyperfractionated (twice-daily) schedule. Only those patients treated in the early years of this series or those who were highly selected received once-daily treatment. Because of these confounding biases, we could not draw meaningful conclusions about the superiority of once-daily vs. twice-daily fractionation strategies. In addition, the bolus schedule used to enhance the skin dose could not be quantitatively evaluated. Although all patients had bolus used for a component of treatment, the scheduling for the later half of the treatment course was customized to ensure a brisk skin reaction without requiring a treatment break.
This study was limited by a relatively small sample size, particularly within the subset analyses. This provided low statistical power to detect differences in outcomes. In addition, this was a retrospective analysis, and treatment decisions concerning radiation dose levels were not determined by randomization. The outcomes that we report according to radiation dose levels may therefore have been affected by confounding biases. Accordingly, these data should be considered as hypothesis generating and would best be con-firmed by a prospective controlled trial.
CONCLUSION
In conclusion, based on the study findings, combined-modality therapy using doxorubicin-based chemotherapy, mastectomy, and postmastectomy radiation provides reasonable LRC and 5-year survival rates for patients with IBC who successfully complete the planned treatment course. The LRC and survival outcomes remain poor for those who do not have a sufficient response to neoadjuvant chemotherapy to allow completion of this planned sequence of treatment. An aggressive locoregional treatment strategy, including radiation therapy to the chest wall and draining lymphatics to 51 Gy followed by a chest wall boost dose to 66 Gy, may be justified for patients with a poor response to neoadjuvant chemotherapy, those with positive or unknown surgical margin status, and those <45 years of age. Patients ≥45 years of age who have a good response to neoadjuvant chemotherapy, receive a taxane along with their doxorubicin-based chemotherapy, have negative surgical margins, and have fewer than four involved lymph nodes can be treated to a lower total dose of 60 Gy. It is our hope that, as new targeted agents such as trastuzumab and lapatanib (20) are incorporated into the treatment strategy for this disease, further improvements in response rates, LRC, and, ultimately, survival will be achieved.
Acknowledgments
Supported in part by the Nellie B. Connally Breast Cancer Research Fund, the Arlette and William Coleman Foundation, and National Cancer Institute grants CA16672 and T32CA77050.
Footnotes
Presented in part at the 48th Annual Meeting of the American Society of Therapeutic Radiology and Oncology (ASTRO), Philadelphia, PA, November 5–9, 2006.
Conflict of interest: none.
References
- 1.Bozzetti F, Saccozzi R, De Lena M, et al. Inflammatory cancer of the breast: Analysis of 114 cases. J Surg Oncol. 1981;18:355–361. doi: 10.1002/jso.2930180405. [DOI] [PubMed] [Google Scholar]
- 2.Haagenson CD. Diseases of the breast. Philadelphia: WB Saunders; 1971. [Google Scholar]
- 3.Wingo PA, Jamison PM, Young JL, et al. Population-based statistics for women diagnosed with inflammatory breast cancer (United States) Cancer Causes Control. 2004;15:321–328. doi: 10.1023/B:CACO.0000024222.61114.18. [DOI] [PubMed] [Google Scholar]
- 4.Cristofanilli M, Valero V, Buzdar AU, et al. Inflammatory breast cancer (IBC) and patterns of recurrence: Understanding the biology of a unique disease. Cancer. 2007;110:1436–1444. doi: 10.1002/cncr.22927. [DOI] [PubMed] [Google Scholar]
- 5.Fields JN, Perez CA, Kuske RR, et al. Inflammatory carcinoma of the breast: Treatment results on 107 patients. Int J Radiat Oncol Biol Phys. 1989;17:249–255. doi: 10.1016/0360-3016(89)90436-7. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Angulo AM, Hennessy BT, Broglio K, et al. Trends for inflammatory breast cancer: Is survival improving? Oncologist. 2007;12:904–912. doi: 10.1634/theoncologist.12-8-904. [DOI] [PubMed] [Google Scholar]
- 7.Krutchik AN, Buzdar AU, Blumenschein GR, et al. Combined chemoimmunotherapy and radiation therapy of inflammatory breast carcinoma. J Surg Oncol. 1979;11:325–332. doi: 10.1002/jso.2930110407. [DOI] [PubMed] [Google Scholar]
- 8.Liao Z, Strom EA, Buzdar AU, et al. Locoregional irradiation for inflammatory breast cancer: Effectiveness of dose escalation in decreasing recurrence. Int J Radiat Oncol Biol Phys. 2000;47:1191–1200. doi: 10.1016/s0360-3016(00)00561-7. [DOI] [PubMed] [Google Scholar]
- 9.Liauw SL, Benda RK, Morris CG, et al. Inflammatory breast carcinoma: Outcomes with trimodality therapy for nonmetastatic disease. Cancer. 2004;100:920–928. doi: 10.1002/cncr.20083. [DOI] [PubMed] [Google Scholar]
- 10.Panades M, Olivotto IA, Speers CH, et al. Evolving treatment strategies for inflammatory breast cancer: A population-based survival analysis. J Clin Oncol. 2005;23:1941–1950. doi: 10.1200/JCO.2005.06.233. [DOI] [PubMed] [Google Scholar]
- 11.Fleming RY, Asmar L, Buzdar AU, et al. Effectiveness of mastectomy by response to induction chemotherapy for control in inflammatory breast carcinoma. Ann Surg Oncol. 1997;4:452–461. doi: 10.1007/BF02303668. [DOI] [PubMed] [Google Scholar]
- 12.Hasbini A, Le Pechoux C, Roche B, et al. Alternating chemotherapy and hyperfractionated accelerated radiotherapy in non-metastatic inflammatory breast cancer [Article in French] Cancer Radiother. 2000;4:265–273. doi: 10.1016/s1278-3218(00)80004-9. [DOI] [PubMed] [Google Scholar]
- 13.Thomas F, Arriagada R, Spielmann M, et al. Pattern of failure in patients with inflammatory breast cancer treated by alternating radiotherapy and chemotherapy. Cancer. 1995;76:2286–2290. doi: 10.1002/1097-0142(19951201)76:11<2286::aid-cncr2820761116>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 14.Barker JL, Nelson AJ, Montague ED. Inflammatory carcinoma of the breast. Radiology. 1976;121:173–176. doi: 10.1148/121.1.173. [DOI] [PubMed] [Google Scholar]
- 15.Thoms WW, Jr, McNeese MD, Fletcher GH, et al. Multimodal treatment for inflammatory breast cancer. Int J Radiat Oncol Biol Phys. 1989;17:739–745. doi: 10.1016/0360-3016(89)90060-6. [DOI] [PubMed] [Google Scholar]
- 16.Thames HD, Jr, Peters LJ, Withers HR, et al. Accelerated fractionation vs. hyperfractionation: Rationales for several treatments per day. Int J Radiat Oncol Biol Phys. 1983;9:127–138. doi: 10.1016/0360-3016(83)90089-5. [DOI] [PubMed] [Google Scholar]
- 17.Bristol IJ, Buchholz TA. Inflammatory breast cancer: Current concepts in local management. Breast Dis. 2005;22:75–83. doi: 10.3233/bd-2006-22109. [DOI] [PubMed] [Google Scholar]
- 18.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 19.Barker JL, Montague ED, Peters LJ. Clinical experience with irradiation of inflammatory carcinoma of the breast with and without elective chemotherapy. Cancer. 1980;45:625–629. doi: 10.1002/1097-0142(19800215)45:4<625::aid-cncr2820450402>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 20.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]