Abstract
Disruption of circulating γδ T-cell populations is an early and common outcome of HIV infection. T-cell receptor (TCR)-γ2δ2 cells (expressing the Vγ2 and Vδ2 chains of the γδ TCR) are depleted, even though they are minimally susceptible to direct HIV infection, and exemplify indirect cell depletion mechanisms that are important in the progression to AIDS. Among individuals with common or normally progressing HIV disease, the loss of TCR-γ2δ2 cells has a broad impact on viral immunity, control of opportunistic pathogens and resistance to malignant disease. Advanced HIV disease can result in complete loss of TCR-γ2δ2 cells that are not recovered even during antiretroviral therapy with complete virus suppression. However, normal levels of TCR-γ2δ2 were observed among natural virus suppressors (low or undetectable virus without antiretroviral therapy) irrespective of their MHC haplotype, consistent with their disease-free status. The pattern of loss and recovery of TCR-γ2δ2 cells revealed their unique features and functional capacities, and encourage the development of immune-based therapies to activate and expand this T-cell subset. New research has identified drugs that might reconstitute the TCR-γ2δ2 population, recover their functional contributions, and improve control of HIV replication and disease. Here, we review research on HIV and TCR-γδ T cells to highlight the consequences of depleting this subset and the unique features of TCR-γδ biology that argue in favor of clinical strategies to reconstitute this T-cell subset in individuals with HIV/AIDS.
Keywords: γδ T cell, AIDS, bisphosphonates, cancer, HIV, IL-2, immunotherapy, phosphoantigen
T-cell receptors & cell types
Mammalian genomes encode two alternate sets of T-cell receptor (TCR) genes. Helper (CD4+) and cytotoxic (CD8+) T cells express a heterodimeric TCR composed of one α and one β chain. TCR-αβ cell clones recognize peptide epitopes associated with MHC class I or II molecules on cell surfaces. MHC recognition and specific patterns of transcription factor expression dictate whether individual clones develop into CD8+ or one of several CD4+ subsets [1].
The requirement for MHC in antigen presentation restricts TCR-αβ cells’ recognition to epitopic peptides that include anchor residues appropriate for binding to individual MHCs. This complex system contains 105–106 individual TCR-αβ cell clones in most adults that anticipate the vast array of environmental antigens [2–4]. Most of these clones may never be used, and the majority of TCR-αβ cells circulate as naive, antigen-inexperienced cells surviving via cytokine-dependent homeostatic proliferation. Cells responding to persistent viral agents are maintained as antigen-experienced memory that declines slowly with age. For example, T cells specific for cytomegalovirus become increasingly oligoclonal with age [5,6], although some clones survive at least for decades. The exquisite mechanisms for cell homeostasis and memory generation have been reviewed elsewhere [7].
Besides the familiar TCR-αβ found on MHC-restricted CD4+ and CD8+ T cells, another TCR exists that bypasses MHC interactions and shows less rigid compartmentalization into these lineages. Prior to the early steps of thymic education and TCR-αβ selection, primitive thymocytes may rearrange an alternate TCR chain designated Vδ, and divert to a pathway that results in expression of TCR-γδ, which is a heterodimer of γ and δ chains.
The human TCR-γδ chains are encoded by genomic loci, with only three Vδ chains and seven Vγ chains available for generating mature TCR that recognize a unique profile of non-peptidic antigens and do not require classical MHC class I or II presentation. These three features; fewer V genes, lack of MHC restriction and recognition of nonpeptidic antigens, are key to understanding the unique properties of TCR-γδ cells.
Individual Vδ+ subsets are enriched within distinct anatomic compartments. Vδ1+ cells are prominent in the intraepithelial layer of mucosal surfaces, where they respond to stress antigens on epithelial cells [8] and produce IL-10 but little or no IL-2, IL-4 or IFN-γ [9]. Vδ1 cells also appear in blood, but the intraepithelial and blood populations seem to mix little or not at all [10,11]. Cells expressing the Vδ2 chain are also found in the mucosa but seem to inhabit only the lamina propria layer. The Vδ2+ population is found mostly in blood and secondary lymphoid tissues; blood and lamina propria layer subsets interchange and have the same subset distribution [12,13]. Overall, TCR-γδ cells comprise 5–10% of circulating lymphocytes in healthy adults. Among most healthy adults, the ratio of Vδ2:Vδ1 cells in blood is approximately 3–10; mechanisms controlling this ratio are unknown. The Vδ2 cells were significantly more abundant in Caucasian compared with African–American healthy controls [14] and the Vδ2:Vδ1 ratios were inverted in healthy adults from Ghana [15], suggesting that environmental or genetic factors control the balance of subsets. Compensatory changes for Vδ2 and Vδ1 populations in blood suggest a homeostatic balancing mechanism that controls the overall blood count, similar to what was seen in mice [16].
Sensing of cell stress is an important role for TCR-γδ cells and provides a general context for understanding their properties (reviewed in [16,17]). Stress may result from cellular damage, neoplastic transformation or infection. In stressed cells, there is marked upregulation of MHC-associated molecules, including murine T10 or T22 and human nonpolymorphic CD1c [18,19]. Human Vγ2Vδ2 T cells are potently stimulated by isoprenoid pathway intermediates from bacteria [20,21]. Viral antigens, including herpesvirus glycoproteins [22,23] or unknown molecules induced by Listeria infection [24], may also signal stress and the capacity for TCR-γδ cells to discriminate transformed from normal cells.
Stress sensing may also involve MHC-like molecules including MICA or MICB [25] and the ULBP ligands [23] that bind to natural killer (NK) receptor molecules on TCR-γδ cells. Through combinations of ligands for TCR-γδ and NK-like receptors, γδ T cells recognize stressed cells and generate potent responses that include proinflammatory cytokine secretion and cytotoxicity.
Impacts of infectious diseases on TCR-γδ cells
HIV infection alters blood levels for both the Vδ1 and the Vδ2 subsets. The Vδ1 cells in blood express one of five Vγ chains (Vγ1.2, 1.3, 1.4, 1.5 or 1.8) [26]. Few antigen-specific responses have been reported for human Vδ1 cells, and there are few associations between specific Vγ chain use and function [27]. More recently, Vδ1 cell responses to Candida albicans, including production of IL-17 [28], and human herpes-virus-8 [29] have been reported. Additionally, a recent study in SIV-infected macaques argued that intestinal microbial translocation in pathogenically infected animals released stimulatory molecules into blood that triggered Vδ1 cell proliferation [30].
Activated Vδ1 cells can be cytotoxic effectors but have a narrow range of cell targets including T-cell leukemias [31,32]. Polyclonally activated Vδ1 cells from HIV-infected donors were also cytotoxic for normal CD4+ T cells [33]. The Vδ1 subset is often expanded in HIV-infected individuals [34,35], which inverts the Vδ2:Vδ1 cell ratio, possibly due to microbial products that accumulate in blood when mucosal boundaries fail and allow microbial translocation. If these same cells are cytotoxic for uninfected CD4 T cells, Vδ1 cell activation may promote CD4 cell depletion and HIV disease progression. Thus, Candida albicans, prominent pathogens in HIV-infected patients, have the potential to stimulate Vδ1 cells and promote disease progression. It may be important to prevent or reverse the accumulation of cytotoxic Vδ1 cells when considering potential therapeutic targets involving TCR-γδ T cells.
As noted earlier, Vδ2:Vδ1 cell ratios are inverted in patients with progressing HIV disease. We and others also showed that functional responses of Vδ2 cells were lost in HIV disease [36,37] and bulk depletion of this subset also contributed to inverting the ratio. Functional responses to phosphoantigens, a property of healthy Vδ2 cells, were lost even while the overall CD4+ T-cell count remained roughly in the normal range [37]. With detailed flow cytometry for Vδ2+ cells and molecular analyses of the TCR repertoire [38], we were able to show specific depletion of Vδ2+ cells in HIV disease. Loss of these Vδ2 cells was a significant factor in the inverted Vδ2:Vδ1 ratio [38,39]. Vδ2 cell depletion was observed consistently in patients with HIV disease, except for a group of natural virus suppressors (elite controllers) that are discussed later. A unique aspect of HIV disease is that cell depletion impacts one specific subfamily of Vδ2 cells expressing the Vγ2Vδ2 TCR (also known as Vγ9Vδ2). Among unrelated HIV-infected individuals, loss of Vγ2Vδ2 T cells is ubiquitous and the extent of depletion was related to disease progression in a population (southern Chinese former plasma donors) all infected with the same strain of HIV at roughly the same time [40].
We have tried to understand why TCR-γδ cell depletion is narrowly focused on one specific subfamily of cells expressing the Vγ2Vδ2 TCR. The stimulating antigens for Vγ2Vδ2 T cells are low-molecular-weight pyrophosphate intermediates of isoprenoid biosynthesis (generally termed phosphoantigens) [41]. Phosphoantigen-responsive cells preferentially express the Vγ2Vδ2 TCR and the γ chain uses only the Jγ1.2 joining segment (also known as JP) [42]. Since phosphoantigens are ubiquitous, coming from mammalian, bacterial or protozoan metabolism, the Vγ2-Jγ1.2 Vδ2+ subset is stimulated chronically throughout life and expands to dominate blood TCR-γδ cells in healthy adults [43]. Selection and expansion of cells expressing the Vγ2-Jγ1.2 chain rearrangement introduces a bias into the population that can be observed by spectratyping, a method that measures length diversity in a population of Vγ2 chain mRNA. The mRNA coding region length is impacted by size differences among individual J segments, deletions occurring during V-J rearrangement and the extent of nontemplated nucleotides added during recombination. For Vγ2 chains, the naive repertoire is best exemplified by cord blood where the unselected chains have lengths that center around 984 nucleotides (coding region length) and include all possible J segments (Figure 1). In healthy adults, the repertoire shifts because of strong selection for the Vγ2-Jγ1.2 rearrangement; the peak is now found between 990 and 996 nucleotides, and more than 75% of Vγ2 chains in this size range contain the Jγ1.2 segment [42]. With the onset of HIV infection, we observe specific loss of Vγ2-Jγ1.2 chains (which are most responsive to phosphoantigen stimulation) and a consequent shift of the spectratype back toward the naive or cord blood profile [42,44]. In adults, loss of Vγ2 chain lengths in the region of 990–996 nucleotides in length is diagnostic for HIV disease, and we used this metric to characterize infection, disease progression and outcomes of therapy.
Figure 1.
The distribution of Vγ2 chain lengths in many naive (cord blood), healthy or HIV-positive individuals shows the significant impact of infection on the T-cell receptor repertoire, including the preferential loss of antigen-responsive chains with lengths between 990 and 996 nucleotides.
Chronic exposure to microbial or host-derived phosphoantigens selects for Vγ2Vδ2 T cells and creates a highly biased repertoire of antigen-experienced cells. The circulating population contains few naive cells (CD27+/CD45RA+) [45], being mostly T central memory (CD27+/CD45RA-), and is functionally redundant. Redundancy means that a large collection of independent T-cell clones, each with the Vγ2-Jγ1.2 rearrangement but having distinct γ-chain CDR3 region sequences, all respond to phosphoantigens.
Phosphoantigen stimulation is required to maintain a bias toward Vγ2-Jγ1.2 chains and the predominance of the memory phenotype. It has been suggested that chronically stimulated cells are depleted during HIV infection because of virally induced expression of FasL or TNF-related apoptosis-inducing ligand and an exaggerated susceptibility of TCR-γδ cells to these killing mechanisms [46,47]. However, the mechanism for Vδ2 cell depletion in HIV disease remains enigmatic. Few of these cells express CD4 and they are poorly susceptible to direct HIV infection. The loss of Vδ2 cells is considered an indirect consequence of HIV disease, but one so efficient that Vγ2-Jγ1.2+ cells may no longer be found in perhipheral blood mononuclear cells from individuals with chronic, progressing HIV disease. Once the Vγ2Vδ2 subset is lost completely, these cells return slowly or not at all during antiretroviral therapy [39,48].
The loss, and in worst cases seeming extinction, of Vγ2Vδ2 T cells has several impacts on HIV disease. First, Vγ2Vδ2 cells normally produce copious amounts of proinflammatory cytokines IFN-γ and TNF-α that are important for promoting effective type 1 immunity against HIV. Second, these cells produce β-chemokines (e.g., macrophage inflammatory protein-1α [MIP1-α], macrophage inflammatory protein-1β [MIP1-β] and RANTES) that block virus attachment to the CCR5 co-receptor [49]. TCR-γ2δ2 cells also express CCR5 and might be altered by targeted drugs such as the CCR5 antagonist Maraviroc. Third, Vγ2Vδ2 T cells are potent effectors in antibody-directed cell cytotoxicity [50,51], a mechanism that is important for HIV inhibition by the fraction of circulating, virus-specific antibodies that fail to neutralize infection but still bind the envelope glycoprotein. Fourth, Vγ2Vδ2 cells may be directly cytotoxic for HIV-infected CD4 T cells [52]. Fifth, Vγ2Vδ2 T cells express the costimulatory ligand CD137L (4-1BBL), which engages the CD137 receptor, activates NK cells and increases their cytotoxicity [53]. Importantly, CD137L is also needed for CD4+ T-cell activation; T cells costimulated through CD137 have increased resistance to inhibition by regulatory T cells [54]. Thus, CD137L+ Vγ2Vδ2 T cells may be important costimulators for CD4 T-cell function. Last, Vγ2Vδ2 T cells have multiple effects on dendritic cell (DC) function. There are reciprocal interactions between TCR-γδ cells and DCs using both cytokine and contact-dependent mechanisms that increase expression levels of DC costimulatory molecules, promote TCR-γδ cell proliferation and increase the production of proinflammatory cytokines IFN-γ and TNF-α [55,56]. Through their capacity to activate NK cell cytotoxicity, TCR-γδ cells may play a crucial role in the mechanism for DC editing. The editing process removes immature, immunosuppressive DCs, which are MHC class I-negative and susceptible to activated NK cells [57], leaving lymph nodes with enriched populations of mature DCs that are potent for antigen presentation and T-cell activation. Considering the impact of Vγ2Vδ2 cells on these, and possibly other important mechanisms for viral immunity, it is clear that HIV-mediated depletion of Vγ2Vδ2 T cells is part of the mechanism for HIV evasion of host defenses and establishment of chronic, persistent infection with progressing disease.
One goal for immunotherapy targeted at TCR-γδ T cells is to prevent or reverse damage to the Vγ2Vδ2 subset and regain antiviral functions of this cell population. To establish the rationale for developing new immunotherapies targeted at TCR-γδ cells, we review the clinical research on TCR-γδ T cells.
Clinical aspects of TCR-γδ cells & HIV disease
T-cell receptor-γδ cells have been reported to play a role in resistance to viral [58], bacterial [59] and protozoan diseases, and some of these have been reviewed [60]. We also know that human Vδ2 are expanded to very high levels during convalescence from holoendemic malaria [61] or Francisella tularemia infection [62], showing that they respond directly to pathogens and disease. The original observations in patients suggested that HIV disease caused anergy in the Vγ2Vδ2 population, which explained the loss of functional responses to phosphoantigen. However, we subsequently showed that functional responses were lost when the Vγ2-Jγ1.2 subset was depleted, which explained why treatment did not lead to rapid recovery of the Vδ2 subset. These studies were performed with clinical specimens from HIV-infected patients who had received at most one antiretroviral drug with incomplete virus suppression during the early 1990s. The patients frequently had low CD4 cell counts and progressing disease with evidence of ongoing virus replication and infection [38]. Cross-sectional studies in these progressing patients showed severe depletion of Vγ2Vδ2 T cells [39]. Subsequent longitudinal studies examined patients who were among the first to switch from no or single drug therapy to combination antiretroviral therapy. Again, these individuals generally had low CD4 T-cell counts; Vγ2Vδ2 T cells were extremely low and did not recover during the 2.5-year interval of combination therapy [48]. Once Vγ2Vδ2 T cells were depleted severely, recovery of this population occurred slowly or not at all. These results were discouraging because most HIV patients in the early studies had too few Vγ2Vδ2 T cells to justify targeted immunotherapy. A later cross-sectional study that included patients treated sooner after infection and with better antiretroviral drugs gave an indication that Vγ2Vδ2 T cells could be recovered when virus was suppressed for a sufficiently long time [39]. The Vγ2Vδ2 cell levels and functional responses approached 50% of control levels after an average of 8.7 years of therapy and were higher in patients with greater than 350 CD4 T cells/mm3 [45].
Recent clinical studies provided additional support for a protective role of Vδ2 cells in HIV disease. We developed a cohort of HIV-infected patients with consistently low or undetectable vRNA levels without antiretroviral therapy and without disease progression (natural viral suppressors [NVS]) [63]. Similar patients have been called elite controllers, elite suppressors and HIV controllers at other institutions [64]. This subset of patients appears exceptional among long-term nonprogressors, because they have unique host immune and/or genetic factors that combine to suppress and control HIV. When Vδ2 cells from NVS donors were compared with age-, gender- and race-matched controls, the levels were similar in both groups, indicating that NVS patients are the only group of HIV-infected individuals with normal levels of Vδ2 cells [65]. However, a closer look at the repertoire of Vδ2 cells showed that HIV infection originally damaged the Vδ2 cell population but uniquely among NVS, the Vδ2 cells recovered to normal levels once viral replication was controlled [65]. NVS patients had normal levels of Vδ2 cells but the population was less complex (in terms of TCR repertoire) because there was an initial round of cell depletion that was similar in people with common HIV disease (requiring therapy) and the NVS group.
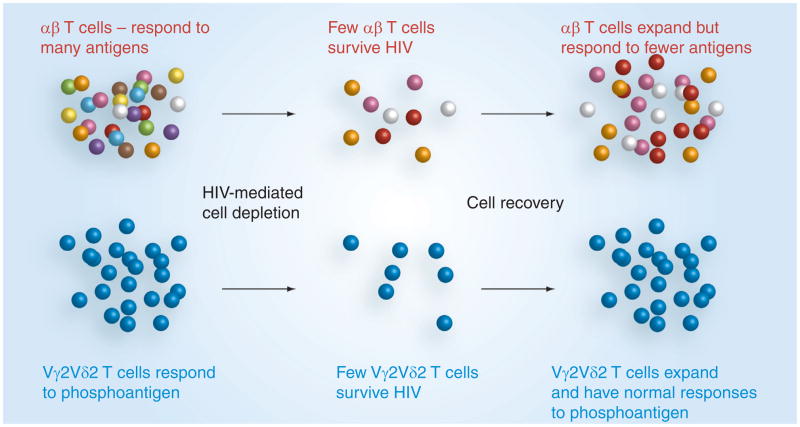
This pattern was familiar from early studies of CD4 T-cell populations in HIV disease. Antiretroviral therapy promotes an increase in CD4 cell count for most patients by expanding cell clones that remain after the initial onslaught of HIV [66]. Even though CD4 cell levels increase during therapy, some antigen responses are not recovered because those particular clones were already extinguished. The comparison between Vδ2 and CD4 T cells also highlights a key difference in the capacity of these two cell populations. Among the Vγ2Vδ2 cells, many distinct clones respond to the same phosphoantigen. CD4 T-cell populations are more complex, with fewer clones reacting to individual peptide epitopes [42]. There is a functional redundancy among Vδ2 cells such that a damaged population that expands to normal cell levels can approximate the function of a healthy, undamaged population (Figure 2). This represents a fundamental difference between TCR-αβ and TCR-γδ T cells. The TCR-αβ populations are highly complex with little redundancy and once depleted are difficult to recover. TCR-γδ cells, especially the Vγ2Vδ2 subset, are highly redundant and withstand HIV-mediated depletion while maintaining the capacity to expand and resume normal function once virus replication is contained. It is conceivable that treatments aiming to stimulate Vγ2Vδ2 T cells can be potent in HIV-infected individuals, as long as phosphoantigen-reactive cells have not been extinguished. Considering the trends to early initiation of antiretroviral therapy, an increasing proportion of patients should retain the response to phosphoantigen and the potential to benefit from targeted immunotherapy.
Figure 2. HIV depletes αβ T-cell clones and, despite increasing cell numbers during treatment, these clones and their functional responses are not recovered.
The redundancy of Vγ2Vδ2 T cells absorbs the initial impacts of HIV. When cell members are recovered during treatment, normal function also returns. Clonal depletion occurs in both types of T cell, but the impact on function is less for the Vγ2Vδ2 subset.
Immunotherapy strategies targeting Vγ2Vδ2 T cells
An important goal for research on NVS or elite controllers is to identify mechanisms of immunity unique to this group. Several genes, including alleles of MHC class I [67], have been associated with NVS status but novel therapeutic targets have not yet emerged from this research. Our finding that Vγ2Vδ2 cells are reconstituted to normal levels in the NVS group implies that targeted immunotherapy may be a strategy for recreating the immunity status of NVS and controlling HIV disease. One approach is to stimulate the Vγ2Vδ2 subset in HIV-infected individuals so that partial or complete reconstitution of preinfection cell levels will be attained, and then observe the impact on vRNA, CD4 counts or other markers of HIV disease progression.
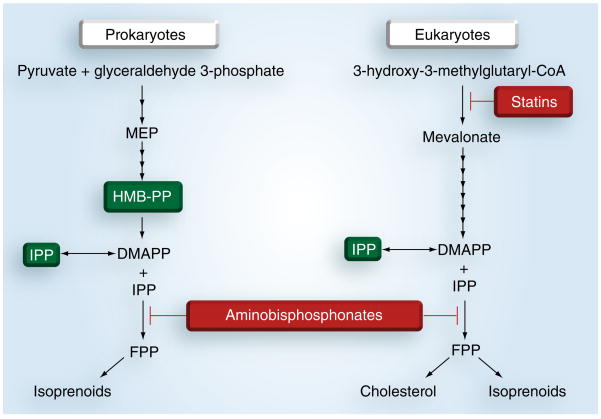
This takes advantage of the unique antigens for Vγ2Vδ2 T cells. Since they do not require MHC class I or II presentation, γδ T cells recognize a broad range of molecules, including nonpeptidic compounds. The Vγ2Vδ2 subset recognizes phosphoantigens, which are intermediates in microbial or host isoprenoid biosynthesis. This same pathway (leading to cholesterol biosynthesis in mammals) has been the target of multiple therapeutic drugs, including the class of bisphosphonates used to treat osteoporosis and cancer. Bisphosphonates are incorporated by myeloid lineage cells, including osteoclasts and DCs [68,69]. They block metabolic conversion of isopentenyl pyrophosphate (Figure 3), allowing this phosphoantigen to accumulate until stimulatory levels are reached [70]. In vitro, bisphosphonates added to peripheral blood mononuclear cells from healthy donors are potent stimulators of Vδ2 cell proliferation and effector function [71].
Figure 3. Vγ2Vδ2 T cells respond to intermediates (green) of bacterial or mammalian isoprenoid biosynthesis.
Pathway inhibitors (red) can block the production of stimulatory intermediates (statins) or cause them to accumulate (bisphosphonates).
Bisphosphonates are advancing as experimental treatments for human cancers with the specific objective of activating Vδ2 T cells, increasing tumor surveillance and promoting cytotoxic killing of malignant cells. The roles for Vδ2 T cells as anticancer agents have been reviewed elsewhere [72–75]. Normally, γδ T cells are active in tumor surveillance [76]; stimulation and expansion of these potent cells may contribute to improved outcomes in patients with malignancies. The earliest studies used pamidronate to treat myeloma [77,78]. Administration of pamidronate plus IL-2, which is needed to support cell division, increased circulating Vδ2 cells and produced objective clinical responses in multiple myeloma. Similar results were obtained with zoledronate (trade name Zometa®) plus IL-2 treatment for hormone-resistant prostate cancer [79]. In each case, the Vδ2 cell activation and expansion were correlated directly with objective clinical responses to cancer. Whether we might substitute other cytokines (e.g., IL-7 or IL-15) for IL-2 has not been explored in patients. In our hands, IL-15 can replace IL-2 for most proliferation or cytokine studies, but may have different effects on cytotoxicity [Unpublished Data].
Bisphosphonates have been used safely in HIV-infected patients for the treatment of HIV-related osteoporosis, and they are generally considered safe and effective first-line therapies for HIV-related bone disorders [80]. Until recently, studies have not looked at the in vivo effects of bisphosphonates on TCR-γδ cells in HIV-infected patients. Zoledronate, a newer bisphosphonate, plus IL-2 were given together to patients with early HIV infection [81], with a resultant marked expansion of the Vδ2+ subset and a shift of cells into the effector memory (CD27−/CD45RA−) subset. The treatment protocol was completed without significant adverse events. However, in that study there were no attempts to evaluate treatment impacts on vRNA or CD4 levels. By defining appropriate clinical groups for Vγ2Vδ2 T-cell-targeted therapy, early-stage safety studies may be completed to support more extensive trials on the utility of Vδ2 cell-targeted therapy. The guiding concept is that bisphosphonate/IL-2 treatment will elevate Vδ2 cells, thereby replicating the circumstance in our NVS group and leading to improved ‘natural’ control over HIV disease. Other potential benefits, including improved tumor immunity and better control of opportunistic pathogens, are desirable consequences of this approach.
Obstacles & opportunities for immunotherapy targeting Vγ2Vδ2 T cells
As described earlier, our strategy proposes bisphosphonate/IL-2 therapy as an adjunct HIV treatment with the goal of reconstituting Vδ2 cell functions and increasing host control over HIV disease. This direction presents an enormous opportunity for advancing the agenda of novel approaches to long-term HIV treatment and control. Successful outcomes might include slower disease progression, reduced comorbidities (especially cancer), and lowered or modified requirements for antiretroviral therapy. However, we recognize two specific obstacles to progress that may require additional investigation.
The possibility always exists that new treatments will elicit significant adverse events (SAEs). Bisphosphonates alone are generally safe, with a high therapeutic index for their licensed indications in bone resorption and malignant disease. The significant complications of esophageal erosion and osteonecrosis of the jaw have declined with newer drugs and delivery routes, but are not yet eliminated. There are clear health benefits for bisphosphonate use in HIV-infected patients, especially improved bone mineral density [4,80]. Even among HIV-negative individuals, zoledronate was associated with lower death rates for elderly hip fracture patients with improved recovery from pneumonia and lowered arthrosclerosis [82].
Published studies on bisphosphonate/IL-2 therapy for cancer reported relatively minor adverse effects including fever, injection site soreness, nausea and diarrhea [79]; no SAEs were reported. Especially for prostate cancer, there was a direct correlation between Vδ2 cell responses to zoledronate/IL-2 and objective clinical responses. Thus, positive therapeutic impacts of bisphosphonate/IL-2 treatment were observed in patients with advanced cancer without SAEs. In HIV disease, we must be aware of the potential for immune reconstruction syndrome [83]. Activated TCRγδ cells will produce proinflammatory cytokines and caution must be taken to minimize or manage the potential consequences of immune reconstitution.
A more substantial obstacle may be the capacity to achieve and sustain durable responses to bisphosphonate/IL-2 therapy. Repeated drug treatments in cancer patients showed a pattern of declining responses during extended dosing schedules [79]. The declining responses were attributed to the development of anergy, although formal tests for anergy have not yet been reported. In the context of HIV infection, the potential to develop anergy means we are confronted with a potential short-term therapy window for a long-term, chronic disease. Our laboratories are defining protocols for Vδ2 cell activation, including adding immunomodulators such as rapamycin that increase the yield of cells and potentially modulate the onset of anergy [84]. Both of these potential obstacles, the risk for SAEs and anergy, are being addressed but will not be resolved without definitive human clinical trials. Perhaps the more critical questions focus on the rationale and clinical objectives for human trials.
To provide context to the work on Vδ2 cell responses, consider the impact of antiretroviral therapy on CD4 T cells. The initial effect of HIV infection is a progressive reduction in CD4 cell counts. When a threshold value around 200 CD4 T cells/mm3 is reached, there is a sharp increase in opportunistic infections and cancer. With the onset of potent, combination antiretroviral therapy that achieves substantial and durable virus suppression, most patients begin to recover CD4 cell counts. In our studies, Vδ2 levels were not well-associated with CD4 count among HIV-infected individuals with long-term treatment, although Vδ2 levels were generally higher for HIV patients with greater than 350 CD4 T cells/mm3 [45]. Thus, we cannot expect conventional antiretroviral therapy to repair or reconstitute the Vδ2 cell population and additional strategies, including bisphosphonates plus IL-2 treatment, may be required. Clearly, it is important to pursue human clinical trials to determine the responsiveness of Vδ2 cells in HIV-infected patients, address safety concerns and improve the rationale for interventional studies designed to assess the impact of targeted immunotherapy on HIV disease.
Conclusion
γδ T cells are dramatically impacted by HIV infection, including expansion of the Vδ1 subset and depletion of the Vδ2 subset. Substantial functional deficits also result, especially in the Vδ2 cell population. As reviewed here, new research into this cell population has provided insights into how HIV effects on γδ cells are correlated with disease progression. New findings in this field have identified novel approaches for potential treatment targets that could help to correct these deficits and achieve stable, long-term virus control. By focusing on improving the host’s response to chronic HIV infection, new pathways for achieving control of HIV infection may be realized.
Future perspective
The challenges for HIV therapy are to adopt sustainable antiretroviral regimens that are suitable for long-term use and have acceptable toxicity profiles, or to focus on modifying host responses to improve natural control over virus replication and disease. New antiretroviral therapies continue to emerge in a remarkable joining of science and industry that has developed safe, effective and low-toxicity drugs with the capacity for potent virus suppression by multiple mechanisms [85]. We envision similar opportunities for virus and disease control through manipulation of host immunity.
Several approaches are possible for HIV immunotherapy. One might imagine therapeutic vaccination, seeking to elicit specific immune responses to viral components with the goal of reconstituting a capacity for immune suppression of virus. Therapeutic vaccination might be augmented by high-efficiency delivery methods, novel adjuvants, costimulatory molecules and informed selection of virus targets. Delivery methods may include recombinant viruses, bacteria, nanoparticles or other novel formulations. When combined with appropriate adjuvants and costimulatory molecules, these components of a vaccine dose are intended to manipulate innate immunity in order to improve the acquired immune response against specific viral antigens. Thus, the field of HIV immunotherapy is approaching the problem of modifying host immunity through manipulation of basic immunoregulatory circuits, but progress is slowed by the vast array of potential choices of vaccine components and the nature of HIV-mediated destruction of immune cells, particularly the critical CD4+ T-cell subsets.
Targeted activation of γδ T cells is an alternative to traditional immunotherapy for HIV. This strategy seeks to increase the impact of γδ T-cell viral immunity and to ‘tune’ the immune response by promoting DC maturation and polarization to type 1 responses. The ability to administer low-toxicity drugs, including bisphosphonates plus moderate doses of IL-2, allows us to favor beneficial antiviral immunity and overcome the immune deviation that has been noted in HIV disease [86]. This seemingly subtle approach to treating HIV disease may be of critical importance for improving virus control. The ultimate objective would be to reconstitute a favorable environment of potent antiviral immunity and reduce the need for conventional antiretroviral therapy.
The proposal to modulate γδ T-cell activity as an approach to HIV treatment does not stand in isolation. Our concepts and plans for clinical studies are facilitated by the evolving standard of care that affords HIV-infected patients access to potent antiretroviral therapy at an earlier stage in disease. By treating earlier, patients retain greater proportions of their immune capacity, especially in the γδ T-cell population. The redundant nature of this T-cell subset provides normal functionality even after an HIV-mediated depletion and subsequent immune reconstitution, which is not uniformly true for CD4+ T-cell responses. The γδ T-cell response to phosphoantigen is ubiquitous and one of few approaches for activating a specific T-cell subset in every person, with the capacity to inhibit HIV disease and the valuable additional benefit of stimulating a potent arm in the tumor surveillance mechanism. This critical tool may become an important adjunct therapy in HIV disease, especially as we face the problems of chronic antiretroviral therapy and increasing risk for cancer.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
The authors have recieved NIH funding for this work and PHS grants CA113261, CA142458 and AI077394 (C David Pauza). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Viret C, Janeway CA., Jr MHC and T cell development. Rev Immunogenet. 1999;1(1):91–104. [PubMed] [Google Scholar]
- 2.Casrouge A, Beaudoing E, Dalle S, Pannetier C, Kanellopoulos J, Kourilsky P. Size estimate of the αβ TCR repertoire of naive mouse splenocytes. J Immunol. 2000;164(11):5782–5787. doi: 10.4049/jimmunol.164.11.5782. [DOI] [PubMed] [Google Scholar]
- 3.Arstila TP, Casrouge A, Baron V, Even J, Kanellopoulos J, Kourilsky P. A direct estimate of the human ab T cell receptor diversity. Science. 1999;286(5441):958–961. doi: 10.1126/science.286.5441.958. [DOI] [PubMed] [Google Scholar]
- 4.Bolland MJ, Grey AB, Horne AM, et al. Annual zoledronate increases bone density in highly active antiretroviral therapy-treated human immunodeficiency virus-infected men: a randomized controlled trial. J Clin Endocrinol Metab. 2007;92(4):1283–1288. doi: 10.1210/jc.2006-2216. [DOI] [PubMed] [Google Scholar]
- 5.Wedderburn LR, Patel A, Varsani H, Woo P. The developing human immune system: T-cell receptor repertoire of children and young adults shows a wide discrepancy in the frequency of persistent oligoclonal T-cell expansions. Immunology. 2001;102(3):301–309. doi: 10.1046/j.1365-2567.2001.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Day EK, Carmichael AJ, ten Berge IJ, Waller EC, Sissons JG, Wills MR. Rapid CD8+ T cell repertoire focusing and selection of high-affinity clones into memory following primary infection with a persistent human virus: human cytomegalovirus. J Immunol. 2007;179(5):3203–3213. doi: 10.4049/jimmunol.179.5.3203. [DOI] [PubMed] [Google Scholar]
- 7.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 8.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial γδ T cells. Science. 1998;279(5357):1737–1740. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 9.Kapp JA, Kapp LM, McKenna KC, Lake JP. γδ T-cell clones from intestinal intraepithelial lymphocytes inhibit development of CTL responses ex vivo. Immunology. 2004;111(2):155–164. doi: 10.1111/j.0019-2805.2003.01793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gould DS, Ploegh HL, Schust DJ. Murine female reproductive tract intraepithelial lymphocytes display selection characteristics distinct from both peripheral and other mucosal T cells. J Reprod Immunol. 2001;52(1–2):85–99. doi: 10.1016/s0165-0378(01)00110-3. [DOI] [PubMed] [Google Scholar]
- 11.Poles MA, Barsoum S, Yu W, et al. Human immunodeficiency virus type 1 induces persistent changes in mucosal and blood γδ T cells despite suppressive therapy. J Virol. 2003;77(19):10456–10467. doi: 10.1128/JVI.77.19.10456-10467.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacDougall A, Enders P, Hatfield G, Pauza D, Rakasz E. Vγ2 TCR repertoire overlap in different anatomical compartments of healthy, unrelated rhesus macaques. J Immunol. 2001;166(4):2296–2302. doi: 10.4049/jimmunol.166.4.2296. [DOI] [PubMed] [Google Scholar]
- 13.Rakasz E, MacDougall AV, Zayas MT, et al. γδ T cell receptor repertoire in blood and colonic mucosa of rhesus macaques. J Med Primatol. 2000;29(6):387–396. doi: 10.1111/j.1600-0684.2000.290602.x. [DOI] [PubMed] [Google Scholar]
- 14▪.Cairo C, Armstrong CL, Cummings JS, et al. Impact of age, gender, and race on circulating γδ T cells. Hum Immunol. 2010;71(10):968–975. doi: 10.1016/j.humimm.2010.06.014. First report of racial differences that impact baseline levels of Vγ2Vδ2 T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15▪.Hviid L, Akanmori BD, Loizon S, et al. High frequency of circulating γδ T cells with dominance of the v(δ)1 subset in a healthy population. Int Immunol. 2000;12(6):797–805. doi: 10.1093/intimm/12.6.797. Population differences affect the Vγ2:Vδ1 cell ratios even in the absence of overt infectious disease. [DOI] [PubMed] [Google Scholar]
- 16.Hayday AC. γδ T cells and the lymphoid stress-surveillance response. Immunity. 2009;31(2):184–196. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Bonneville M, O’Brien RL, Born WK. γδ T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010;10(7):467–478. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- 18.Adams EJ, Strop P, Shin S, Chien Y-H, Garcia KC. An autonomous CDR3δ is sufficient for recognition of the nonclassical MHC class I molecules T10 and T22 by γδ T cells. Nat Immunol. 2008;9(7):777–784. doi: 10.1038/ni.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chien YH, Konigshofer Y. Antigen recognition by γδ T cells. Immunol Rev. 2007;215:46–58. doi: 10.1111/j.1600-065X.2006.00470.x. [DOI] [PubMed] [Google Scholar]
- 20.Constant P, Davodeau F, Peyrat MA, et al. Stimulation of human γδ T cells by nonpeptidic mycobacterial ligands. Science. 1994;264(5156):267–270. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka Y, Morita CT, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature. 1995;375(6527):155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 22.Scotet E, David-Ameline J, Peyrat MA, et al. T cell response to Epstein–Barr virus transactivators in chronic rheumatoid arthritis. J Exp Med. 1996;184(5):1791–1800. doi: 10.1084/jem.184.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinle A, Li P, Morris DL, et al. Interactions of human NKG2D with its ligands MICA, MICB, and homologs of the mouse RAE-1 protein family. Immunogenetics. 2001;53(4):279–287. doi: 10.1007/s002510100325. [DOI] [PubMed] [Google Scholar]
- 24.Aydintug MK, Roark CL, Chain JL, Born WK, O’Brien RL. Macrophages express multiple ligands for γδ TCRs. Mol Immunol. 2008;45(11):3253–3263. doi: 10.1016/j.molimm.2008.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bauer S, Groh V, Wu J, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285(5428):727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 26.Strauss WM, Quertermous T, Seidman JG. Measuring the human T cell receptor γ-chain locus. Science. 1987;237(4819):1217–1219. doi: 10.1126/science.3498213. [DOI] [PubMed] [Google Scholar]
- 27.Leslie DS, Vincent MS, Spada FM, et al. CD1-mediated γ/δ T cell maturation of dendritic cells. J Exp Med. 2002;196(12):1575–1584. doi: 10.1084/jem.20021515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fenoglio D, Poggi A, Catellani S, et al. Vδ1 T lymphocytes producing IFN-γ and IL-17 are expanded in HIV-1-infected patients and respond to Candida albicans. Blood. 2009;113(26):6611–6618. doi: 10.1182/blood-2009-01-198028. [DOI] [PubMed] [Google Scholar]
- 29.Barcy S, De Rosa SC, Vieira J, et al. γδ+ T cells involvement in viral immune control of chronic human herpesvirus 8 infection. J Immunol. 2008;180(5):3417–3425. doi: 10.4049/jimmunol.180.5.3417. [DOI] [PubMed] [Google Scholar]
- 30.Harris LD, Klatt NR, Vinton C, et al. Mechanisms underlying γδ T cell subset perturbations in SIV-infected Asian rhesus macaques. Blood. 2010 doi: 10.1182/blood-2010-05-283549. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamb LS, Jr, Musk P, Ye Z, et al. Human γδ(+) T lymphocytes have in vitro graft vs leukemia activity in the absence of an allogeneic response. Bone Marrow Transplant. 2001;27(6):601–606. doi: 10.1038/sj.bmt.1702830. [DOI] [PubMed] [Google Scholar]
- 32.Meeh PF, King M, O’Brien RL, et al. Characterization of the γδ T cell response to acute leukemia. Cancer Immunol Immunother. 2006;55(9):1072–1080. doi: 10.1007/s00262-005-0094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sindhu ST, Ahmad R, Morisset R, Ahmad A, Menezes J. Peripheral blood cytotoxic γδ T lymphocytes from patients with human immunodeficiency virus type 1 infection and AIDS lyse uninfected CD4+ T cells, and their cytocidal potential correlates with viral load. J Virol. 2003;77(3):1848–1855. doi: 10.1128/JVI.77.3.1848-1855.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rossol R, Dobmeyer JM, Dobmeyer TS, et al. Increase in Vδ1+ γδ T cells in the peripheral blood and bone marrow as a selective feature of HIV-1 but not other virus infections. Br J Haematol. 1998;100(4):728–734. doi: 10.1046/j.1365-2141.1998.00630.x. [DOI] [PubMed] [Google Scholar]
- 35.Wesch D, Hinz T, Kabelitz D. Analysis of the TCR Vγ repertoire in healthy donors and HIV-1-infected individuals. Int Immunol. 1998;10(8):1067–1075. doi: 10.1093/intimm/10.8.1067. [DOI] [PubMed] [Google Scholar]
- 36▪.Poccia F, Boullier S, Lecoeur H, et al. Peripheral Vγ9/Vδ2 T cell deletion and anergy to nonpeptidic mycobacterial antigens in asymptomatic HIV-1-infected persons. J Immunol. 1996;157(1):449–461. Confirmatory report about HIV impacts on γδ T cells. [PubMed] [Google Scholar]
- 37▪.Wallace M, Scharko AM, Pauza CD, et al. Functional γδ T-lymphocyte defect associated with human immunodeficiency virus infections. Mol Med. 1997;3(1):60–71. Discusses in situ impacts on Vγ2Vδ2 functional responses to phosphoantigen. [PMC free article] [PubMed] [Google Scholar]
- 38▪▪.Enders PJ, Yin C, Martini F, et al. HIV-mediated γδ T cell depletion is specific for Vγ2+ cells expressing the Jγ1.2 segment. AIDS Res Hum Retroviruses. 2003;19(1):21–29. doi: 10.1089/08892220360473934. First demonstration that depletion (not anergy) of cells expressing Vγ2-Jδ1.2 chains was responsible for the loss of phosphoantigen responses in HIV disease. [DOI] [PubMed] [Google Scholar]
- 39▪▪.Bordon J, Evans PS, Propp N, Davis CE, Jr, Redfield RR, Pauza CD. Association between longer duration of HIV-suppressive therapy and partial recovery of the Vγ2 T cell receptor repertoire. J Infect Dis. 2004;189(8):1482–1486. doi: 10.1086/382961. Effective antiretroviral therapy initiated prior to the extensive loss of Vγ2δ2 T cells, slowed cell depletion and allowed for partial reconstitution. [DOI] [PubMed] [Google Scholar]
- 40▪.Li H, Peng H, Ma P, et al. Association between Vγ2Vδ2 T cells and disease progression after infection with closely related strains of HIV in China. Clin Infect Dis. 2008;46(9):1466–1472. doi: 10.1086/587107. Clinical studies in a group infected with the same HIV strains at roughly the same time revealed strong correlations between Vγ2Vδ2 cell levels and disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sireci G, Champagne E, Fournie JJ, Dieli F, Salerno A. Patterns of phosphoantigen stimulation of human Vγ9/Vδ2 T cell clones include Th0 cytokines. Hum Immunol. 1997;58(2):70–82. doi: 10.1016/s0198-8859(97)00211-5. [DOI] [PubMed] [Google Scholar]
- 42.Evans PS, Enders PJ, Yin C, Ruckwardt TJ, Malkovsky M, Pauza CD. In vitro stimulation with a non-peptidic alkylphosphate expands cells expressing Vγ2-Jγ1.2/Vδ2 T-cell receptors. Immunology. 2001;104(1):19–27. doi: 10.1046/j.0019-2805.2001.01282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parker CM, Groh V, Band H, et al. Evidence for extrathymic changes in the T cell receptor γ/δ repertoire. J Exp Med. 1990;171(5):1597–1612. doi: 10.1084/jem.171.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hebbeler AM, Cairo C, Cummings JS, Pauza CD. Individual Vγ2-Jγ1.2+ T cells respond to both isopentenyl pyrophosphate and Daudi cell stimulation: generating tumor effectors with low molecular weight phosphoantigens. Cancer Immunol Immunother. 2007;56(6):819–829. doi: 10.1007/s00262-006-0235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45▪▪.Cummings JS, Cairo C, Armstrong C, Davis CE, Pauza CD. Impacts of HIV infection on Vγ2Vδ2 T cell phenotype and function: a mechanism for reduced tumor immunity in AIDS. J Leukoc Biol. 2008;84(2):371–379. doi: 10.1189/jlb.1207847. HIV infection removes the memory subset of Vγ2Vδ2 cells and reduces the capacity for tumor cell cytotoxicity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martini F, Urso R, Gioia C, et al. γδ T-cell anergy in human immunodeficiency virus-infected persons with opportunistic infections and recovery after highly active antiretroviral therapy. Immunology. 2000;100(4):481–486. doi: 10.1046/j.1365-2567.2000.00068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poccia F, Gougeon ML, Agrati C, et al. Innate T-cell immunity in HIV infection: the role of Vγ9Vδ2 T lymphocytes. Curr Mol Med. 2002;2(8):769–781. doi: 10.2174/1566524023361880. [DOI] [PubMed] [Google Scholar]
- 48▪.Hebbeler AM, Propp N, Cairo C, et al. Failure to restore the Vγ2-Jγ1.2 repertoire in HIV-infected men receiving highly active antiretroviral therapy (HAART) Clin Immunol. 2008;128(3):349–357. doi: 10.1016/j.clim.2008.04.008. Advanced HIV disease extinguished the Vγ2-Jδ1.2+ subset and they were not recovered during a subsequent 2.5 years of highly active antiretroviral therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tikhonov I, Deetz CO, Paca R, et al. Human Vγ2Vδ2 T cells contain cytoplasmic RANTES. Int Immunol. 2006;18(8):1243–1251. doi: 10.1093/intimm/dxl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Z, Freedman MS. CD16+ γδ T cells mediate antibody dependent cellular cytotoxicity: potential mechanism in the pathogenesis of multiple sclerosis. Clin Immunol. 2008;128(2):219–227. doi: 10.1016/j.clim.2008.03.513. [DOI] [PubMed] [Google Scholar]
- 51.Gertner-Dardenne J, Bonnafous C, Bezombes C, et al. Bromohydrin pyrophosphate enhances antibody-dependent cell-mediated cytotoxicity induced by therapeutic antibodies. Blood. 2009;113(20):4875–4884. doi: 10.1182/blood-2008-08-172296. [DOI] [PubMed] [Google Scholar]
- 52.Wallace M, Malkovsky M, Carding SR. γ/δ T lymphocytes in viral infections. J Leukoc Biol. 1995;58(3):277–283. doi: 10.1002/jlb.58.3.277. [DOI] [PubMed] [Google Scholar]
- 53.Maniar A, Zhang X, Lin W, et al. Human γδ T lymphocytes induce robust NK cell mediated antitumor cytotoxicity through CD137 engagement. Blood. 2010;116(10):1726–1733. doi: 10.1182/blood-2009-07-234211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robertson SJ, Messer RJ, Carmody AB, Mittler RS, Burlak C, Hasenkrug KJ. CD137 costimulation of CD8+ T cells confers resistance to suppression by virus-induced regulatory T cells. J Immunol. 2008;180(8):5267–5274. doi: 10.4049/jimmunol.180.8.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Conti L, Casetti R, Cardone M, et al. Reciprocal activating interaction between dendritic cells and pamidronate-stimulated γδ T cells: role of CD86 and inflammatory cytokines. J Immunol. 2005;174(1):252–260. doi: 10.4049/jimmunol.174.1.252. [DOI] [PubMed] [Google Scholar]
- 56.Martino A, Casetti R, D’Alessandri A, Sacchi A, Poccia F. Complementary function of γδ T-lymphocytes and dendritic cells in the response to isopentenyl-pyrophosphate and lipopolysaccharide antigens. J Clin Immunol. 2005;25(3):230–237. doi: 10.1007/s10875-005-4080-8. [DOI] [PubMed] [Google Scholar]
- 57.Della Chiesa M, Romagnani C, Thiel A, Moretta L, Moretta A. Multidirectional interactions are bridging human NK cells with plasmacytoid and monocyte-derived dendritic cells during innate immune responses. Blood. 2006;108(12):3851–3858. doi: 10.1182/blood-2006-02-004028. [DOI] [PubMed] [Google Scholar]
- 58.Sciammas R, Bluestone JA. TCRγδ cells and viruses. Microbes Infect. 1999;1(3):203–212. doi: 10.1016/s1286-4579(99)80035-5. [DOI] [PubMed] [Google Scholar]
- 59.Kaufmann SH, Schaible UE. Antigen presentation and recognition in bacterial infections. Curr Opin Immunol. 2005;17(1):79–87. doi: 10.1016/j.coi.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 60.Hansen DS, D’Ombrain MC, Schofield L. The role of leukocytes bearing natural killer complex receptors and killer immunoglobulin-like receptors in the immunology of malaria. Curr Opin Immunol. 2007;19(4):416–423. doi: 10.1016/j.coi.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 61.Dieli F, Troye-Blomberg M, Farouk SE, Sireci G, Salerno A. Biology of γδ T cells in tuberculosis and malaria. Curr Mol Med. 2001;1(4):437–446. doi: 10.2174/1566524013363627. [DOI] [PubMed] [Google Scholar]
- 62.Poquet Y, Kroca M, Halary F, et al. Expansion of Vγ9 Vδ2 T cells is triggered by Francisella tularensis-derived phosphoantigens in tularemia but not after tularemia vaccination. Infect Immun. 1998;66(5):2107–2114. doi: 10.1128/iai.66.5.2107-2114.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sajadi MM, Constantine NT, Mann DL, et al. Epidemiologic characteristics and natural history of HIV-1 natural viral suppressors. J Acquir Immune Defic Syndr. 2009;50(4):403–408. doi: 10.1097/QAI.0b013e3181945f1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Migueles SA, Connors M. Long-term nonprogressive disease among untreated HIV-infected individuals: clinical implications of understanding immune control of HIV. JAMA. 2010;304(2):194–201. doi: 10.1001/jama.2010.925. [DOI] [PubMed] [Google Scholar]
- 65▪▪.Riedel DJ, Sajadi MM, Armstrong CL, et al. Natural viral suppressors of HIV-1 have a unique capacity to maintain γδ T cells. AIDS. 2009;23(15):1955–1964. doi: 10.1097/QAD.0b013e32832ff1ff. First study of Vγ2Vδ2 T cells in natural viral suppressors or elite controllers, showing they are unique in the ability to reconstitute this subset to normal levels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lane HC. The generation of CD4 T lymphocytes in patients with HIV infection. J Biol Regul Homeost Agents. 1995;9(3):107–109. [PubMed] [Google Scholar]
- 67.Pereyra F, Addo MM, Kaufmann DE, et al. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis. 2008;197(4):563–571. doi: 10.1086/526786. [DOI] [PubMed] [Google Scholar]
- 68.Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998;13(4):581–589. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- 69.Rogers MJ, Watts DJ, Russell RG. Overview of bisphosphonates. Cancer. 1997;80(8 Suppl):1652–1660. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1652::aid-cncr15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 70.Roelofs AJ, Jauhiainen M, Monkkonen H, Rogers MJ, Monkkonen J, Thompson K. Peripheral blood monocytes are responsible for γδ T cell activation induced by zoledronic acid through accumulation of IPP/DMAPP. Br J Haematol. 2009;144(2):245–250. doi: 10.1111/j.1365-2141.2008.07435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Das H, Wang L, Kamath A, Bukowski JF. Vγ2Vδ2 T-cell receptor-mediated recognition of aminobisphosphonates. Blood. 2001;98(5):1616–1618. doi: 10.1182/blood.v98.5.1616. [DOI] [PubMed] [Google Scholar]
- 72.Caccamo N, Dieli F, Meraviglia S, Guggino G, Salerno A. γδ T cell modulation in anticancer treatment. Curr Cancer Drug Targets. 2010;10(1):27–36. doi: 10.2174/156800910790980188. [DOI] [PubMed] [Google Scholar]
- 73.Caccamo N, Meraviglia S, Cicero G, et al. Aminobisphosphonates as new weapons for γδ T cell-based immunotherapy of cancer. Curr Med Chem. 2008;15(12):1147–1153. doi: 10.2174/092986708784310468. [DOI] [PubMed] [Google Scholar]
- 74.Dieli F, Caccamo N, Meraviglia S. Advances in immunotherapy of castration-resistant prostate cancer: bisphosphonates, phosphoantigens and more. Curr Opin Investig Drugs. 2008;9(10):1089–1094. [PubMed] [Google Scholar]
- 75.Rey J, Veuillen C, Vey N, Bouabdallah R, Olive D. Natural killer and γδ T cells in haematological malignancies: enhancing the immune effectors. Trends Mol Med. 2009;15(6):275–284. doi: 10.1016/j.molmed.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 76.Ferrarini M, Ferrero E, Dagna L, Poggi A, Zocchi MR. Human γδ T cells: a nonredundant system in the immune-surveillance against cancer. Trends Immunol. 2002;23(1):14–18. doi: 10.1016/s1471-4906(01)02110-x. [DOI] [PubMed] [Google Scholar]
- 77▪.Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of γδ T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96(2):384–392. Initial report on bisphosphonate (pamidronate)/IL-2 therapy for prostate cancer with positive outcomes. [PubMed] [Google Scholar]
- 78.Wilhelm M, Kunzmann V, Eckstein S, et al. γδ T cells for immune therapy of patients with lymphoid malignancies. Blood. 2003;102(1):200–206. doi: 10.1182/blood-2002-12-3665. [DOI] [PubMed] [Google Scholar]
- 79▪.Dieli F, Vermijlen D, Fulfaro F, et al. Targeting human γδ T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007;67(15):7450–7457. doi: 10.1158/0008-5472.CAN-07-0199. Initial report on bisphosphonate (pamidronate)/IL-2 therapy for myeloma with positive outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang J, Meixner L, Fernandez S, McCutchan JA. A double-blinded, randomized controlled trial of zoledronate therapy for HIV-associated osteopenia and osteoporosis. AIDS. 2009;23(1):51–57. doi: 10.1097/QAD.0b013e32831c8adc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81▪▪.Poccia F, Gioia C, Martini F, et al. Zoledronic acid and interleukin-2 treatment improves immunocompetence in HIV-infected persons by activating Vγ9Vδ2 T cells. AIDS. 2009;23(5):555–565. doi: 10.1097/QAD.0b013e3283244619. First use of zoledronate/IL-2 treatment in HIV-infected individuals, showing increased Vγ2Vδ2 cell levels without significant adverse events. [DOI] [PubMed] [Google Scholar]
- 82.Colon-Emeric CS, Mesenbrink P, Lyles KW, et al. Potential mediators of the mortality reduction with zoledronic acid after hip fracture. J Bone Miner Res. 2010;25(1):91–97. doi: 10.1359/jbmr.090704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Muller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(4):251–261. doi: 10.1016/S1473-3099(10)70026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li H, Pauza CD. Rapamycin increases the yield and effector function of human γδ T cells stimulated in vitro. Cancer Immunol Immunother. 2010 doi: 10.1007/s00262-010-0945-7. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Broder S. Twenty-five years of translational medicine in antiretroviral therapy: promises to keep. Sci Transl Med. 2010;2(39):39ps33. doi: 10.1126/scitranslmed.3000749. [DOI] [PubMed] [Google Scholar]
- 86.Maggi E, Mazzetti M, Ravina A, et al. Ability of HIV to promote a TH1 to TH0 shift and to replicate preferentially in TH2 and TH0 cells. Science. 1994;265(5169):244–248. doi: 10.1126/science.8023142. [DOI] [PubMed] [Google Scholar]