Abstract
Leukocytes, containing myeloperoxidase (MPO), produce the reactive chlorinating species, HOCl, and they have important roles in the pathophysiology of cardiovascular disease. Leukocyte-derived HOCl can target primary amines, alkenes and vinyl ethers of lipids, resulting in chlorinated products. Plasmalogens are vinyl ether-containing phospholipids that are abundant in tissues of the cardiovascular system. The HOCl oxidation products derived from plasmalogens are α-chlorofatty aldehyde and unsaturated molecular species of lysophosphatidylcholine. α-chlorofatty aldehyde is the precursor of both α-chlorofatty alcohol and α-chlorofatty acid. Both α-chlorofatty aldehyde and α-chlorofatty acid accumulate in activated neutrophils and have disparate chemotactic properties. In addition, α-chlorofatty aldehyde increases in activated monocytes, human atherosclerotic lesions and rat infarcted myocardium. This article addresses the pathways for the synthesis of these lipids and their biological targets.
Keywords: atherosclerosis, fatty acid, fatty aldehyde, lipid, monocyte, myeloperoxidase, myocardial ischemia, neutrophil, plasmalogen
This article focuses on the oxidation of plasmalogen phospholipids by hypochlorous acid (HOCl) produced by the activity of myeloperoxidase (MPO). The immediate oxidation product of plasmalogens targeted by HOCl is α-chlorofatty aldehyde, which gives rise to a family of chlorinated lipids. The relevance of plasmalogens in the cardiovascular system, as well as the rationale for considering plasmalogens as targets of MPO-derived HOCl produced from leukocytes, are discussed below.
Plasmalogens are a predominant phospholipid present in the plasma membrane of tissues in the cardiovascular system
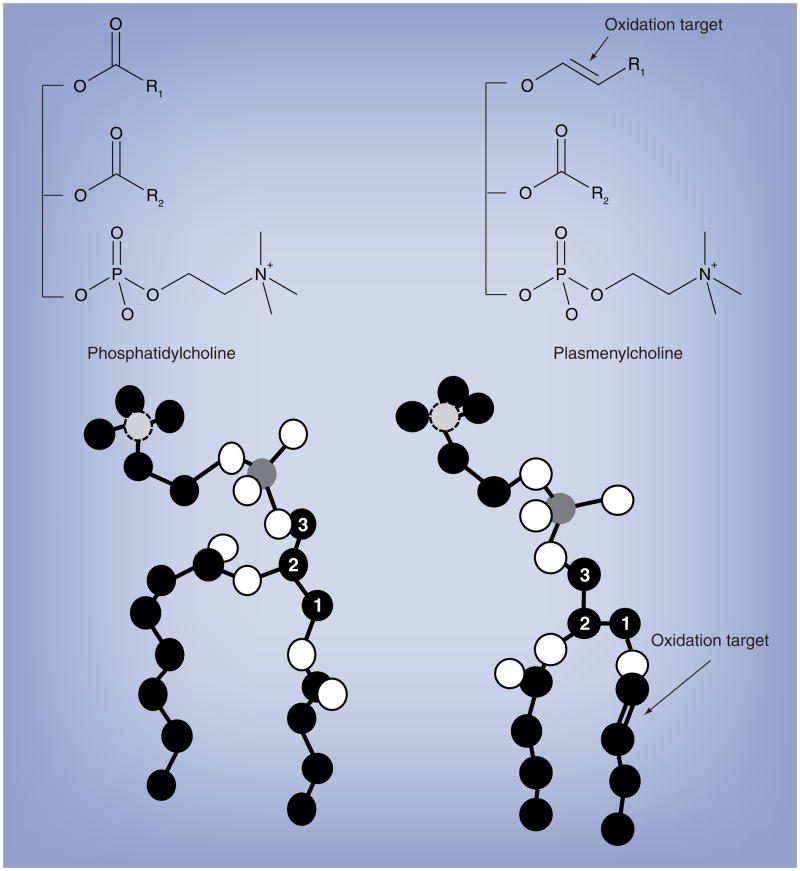
Plasmalogens were originally described as the aldehyde-like lipid of the plasma, which contain a vinyl ether-linked aliphatic chain attached to the sn-1 position of the glycerol backbone. Chemically, this vinyl ether-linkage is a masked aldehyde that is acid labile (pH ≤ 2). Figure 1 shows the molecular structure of a plasmalogen that was determined by NMR studies; the arrow indicates the plasmalogen vinyl ether bond [1]. From a biophysical perspective, plasmalogens pack tighter in membranes and the orientation of the polar head group of the plasmalogen is more perpendicular with the plane of the bilayer compared with diacyl molecular species [1]. This orientation may provide accessibility for phospholipase A2, which could target plasmalogens that contain arachidonic acid residues esterified at the sn-2 carbon of its glycerol backbone for hydrolysis [1].
Figure 1. Chemical and molecular structures of phosphatidylcholine and plasmenylcholine.
The diacyl choline glycerophospholipid (phosphatidylcholine, left structure) and plasmalogen choline glycerophospholipid (plasmenylcholine, right structure) are shown, which are components of the bilayer of biological membranes. Carbon, oxygen, phosphorous and nitrogen are shown as black filled circle, white filled circle, dark gray filled circle and light gray filled circle with dashed line, respectively. The numbered carbon atoms represent the sn carbons of the glycerol backbone. The vinyl ether bond of plasmalogens, which is targeted by oxidants is indicated by the arrow. R1 and R2 indicate aliphatic groups.
Plasmalogens are the predominant molecular subclass of the ethanolamine and choline glycerophospholipids in human myocardium [2]. At a subcellular level, plasmalogens have been shown to be the predominant phospholipid in the sarcolemma of canine cardiac myocytes [3]. In addition to their enrichment in plasma membranes, plasmalogens are highly abundant in lipid rafts [3–5]. Tissues associated with the cardiovascular system and various cell types, including endothelial cells and smooth muscle cells, have highly abundant pools of plasmalogens [2,3,6–9]. Circulating neutrophils, monocytes, as well as lipoproteins, are enriched with plasmalogens [10–13].
Considerable interest has been directed toward the role of plasmalogens in the cardiovascular system, which stem from:
The enrichment of arachidonic acid residues that are esterified to the sn-2 position of the glycerol backbone;
Their potential to generate other cardioactive lipids, such as platelet-activating factor, diglycerides and lysophospholipids;
The suggestion that they are antioxidants.
In the vascular wall, arachidonic acid mobilization is regulated by phospholipases which regulate arachidonic acid for eicosanoid production. Plasmalogens containing arachidonic acid are an important substrate for the phospholipases that are activated in Ang II-stimulated aortic smooth muscle [9]. Lysophospholipids are particularly important in both ischemic myocardium due to their arrhythmogenic properties [14,15] and atherosclerosis due to their role as proatherogenic molecules [16–18]. Furthermore, lysoplasmenylethanolamine is an acyl acceptor for transacylation reactions with 1-O-alkyl-2-acyl-sn-glycero-3-phosphocholine, which is a key mechanism for the production of platelet-activating factor [19–21]. Plasmalogens also serve as substrates for phospholipase C, which catalyzes the production of plasmalogenic diglycerides [22–24]. Plasmalogenic diglycerides (e.g., 1-O-alk-1′-enyl-2-acyl-sn-glycerol) have been suggested to play key roles in the regulation of several protein kinase C isozymes [25,26].
The masked aldehyde is considered to be relatively enzymatically inert. However, it is accessible in the hydrophilic plane of membrane bilayers and can be targeted by oxidants (Figure 1). Zoeller and coworkers have shown that plasmalogens protect cells from free radical damage through putative anti-oxidant properties [8,27,28]. Plasmalogens have been proposed to serve as a trap for reactive oxygen species. The intermediates of reactive oxygen species targeting the plasmalogen vinyl ether include a dioxetane and ene intermediates, which degrade to generate fatty aldehydes, 1-formyl- and 1-lysophospholipids [28–31]. Vance proposed that plasmalogens, which are synthesized in the liver and secreted as complexed with lipoproteins, serve as endogenous plasma antioxidants [13]. The products from the unmasking of plasmalogens by free radical reactive oxygen species (e.g., lysophospholipids, fatty aldehydes and α-hydroxyfatty aldehydes) do not likely accumulate to sufficient levels to exert cytotoxic effects, and thus this antioxidant role of plasmalogens is protective to the cell. The targeting of plasmalogens by oxidants led to the hypothesis that plasmalogens may be important targets for the two-electron oxidant HOCl that is produced by proinflammatory leukocytes. The targeting of plasmalogens by HOCl, and the generation of a family of chlorinated lipids, as well as the role of these lipids in cardiovascular disease, will be the focus of this article.
Role of MPO-containing leukocytes in innate immunity
Myeloperoxidase plays a central role in the generation of leukocyte-derived oxidants that combat invading pathogens [32,33]. Recently, the biological roles of the oxidation reactions mediated by MPO have been reviewed [34,35]. MPO amplifies the potential of the oxidants released by phagocytes by converting hydrogen peroxide to the potent oxidant, HOCl [36]. HOCl and its conjugate base (OCl−), as well as chlorine gas that is in equilibrium with HOCl, are collectively the major reactive chlorinating species (RCS) produced by MPO [36,37]. HOCl is in equilibrium with secondary chlorinating intermediates, such as N-monochloramines [38]. HOCl has potent microbicidal and cytotoxic properties, due to its chemical reactivity, which, in respect to lipids, predominantly includes chlorination of amines, alkenes and vinyl ethers [39–43]. There are many targets for HOCl-mediated oxidation, and the targets that are oxidized will depend on their abundance in the microenvironment in which HOCl is present, as well as their relative chemical reactivity with HOCl. Thiols (e.g., glutathione) and thioethers (e.g., methionine) are the most chemically reactive groups that are oxidized by HOCl [44]. The role of MPO-derived oxidants in microbial killing has been reviewed recently [34]. Although decreased microbial killing in the presence of peroxidase inhibitors suggests a role for MPO-derived oxidants in killing, it should be appreciated that the heme poisons employed as peroxidase inhibitors are not specific for MPO [45]. Other support for the role of MPO-derived oxidants as microbicidal agents includes the observation that MPO−/− mice have decreased survival rates when challenged with microbial burdens [46]. On the other hand, MPO-deficient humans do not have a significantly increased risk of prolonged microbial infection [47]. HOCl may not be the primary MPO-derived oxidant that targets microbes. It has been predicted that the majority of phagosomal HOCl reacts with neutrophil granule targets to yield chloramines, which may be the primary RCS-targeting microorganisms [48,49]. It should also be appreciated that MPO can use other anions to produce additional hypohalous acids that may compete with chlorine under some conditions. For example, thiocyanate and chloride are competing substrates used by MPO, and thus, in smokers, it is feasible that hypothiocyanous acid is produced at significant levels by neutrophils in the lung [50]. In addition to RCS and hypothiocyanous acid, other oxidants produced by MPO include, but are not limited to, reactive nitrating species and reactive brominating species [34,35].
Cells of the innate immune system that contain MPO are involved in wound repair mechanisms, including lesions present in atherosclerosis, as well as in ischemia-reperfusion and infarcted myocardium. The targeting of biomolecules by RCS, while potent in killing many invading organisms and having a role in wound repair, also results in the production of chlorinated biomolecules that remain in the host until metabolically cleared.
MPO-containing monocytes & macrophages are important cellular mediators of human atherosclerosis
Atherosclerosis is a chronic inflammatory disease of the vascular wall. Early in the atherosclerotic process, proinflammatory monocytes are recruited to the subintimal layer of the vascular wall, differentiate into macrophages and propagate a local inflammatory process in the vascular wall. The uptake of modified LDL by macrophages leading to foam cell formation is an important mechanism during the early stages of atherosclerosis [51–53]. Considerable attention has been given to delineating the oxidants and oxidized products that mediate atherosclerotic lesion formation and propagation. Multiple independent studies have supported a role for MPO in human atherosclerosis [54–57]. MPO likely has a role in the early development of atheromas as well as in the mature plaque [58–61]. Libby and coworkers demonstrated that MPO accumulates in the shoulder regions of mature human atherosclerotic plaques [58]. MPO may play a role in plaque rupture and acute coronary syndromes in the mature plaque since HOCl produced by MPO activates the metalloproteinase, matrilysin, in vitro [62]. MPO colocalizes with this matrix metalloproteinase in tissue sections from vulnerable plaques, suggesting a role for MPO in plaque rupture and acute coronary syndromes [58,62]. Multiple MPO-derived oxidation products have been found in human atherosclerotic lesions, including 3-chlorotyrosine and 3-nitrotyrosine [55,63,64]. 3-nitrotyrosine in atherosclerotic lesions can be produced from MPO oxidants, but is also largely attributed to peroxynitrite-mediated oxidation [64]. Hazen and coworkers demonstrated that reactive nitrogen species produced by MPO oxidize LDL, leading to the modification of polyunsaturated aliphatic residues associated with choline glycerophospholipids present in LDL [59–61]. This modified LDL can contribute to foam cell formation owing to its unregulated uptake compared with unmodified LDL by macrophages. In addition, Heinecke and others have provided multiple levels of evidence showing an important HDL modification by MPO-generated oxidants resulting in HDL dysfunction in reverse cholesterol transport. Both 3-chlorotyrosine and 3-nitrotyrosine are found in HDL present in both human and murine atherosclerotic lesions [63–65]. Levels of 3-chlorotyrosine and 3-nitrotyrosine in HDL are elevated in humans with coronary artery disease [63–65]. Proteomic studies have revealed tyrosine and methionine residues that are chlorinated and oxidized in the major apoprotein of HDL, apoA-1 [63,65–67]. Recent studies demonstrate that chlorination, and not nitration of apoA-1 by HOCl, impairs a key mechanism for cholesterol efflux (i.e., apoA-1 interaction with ATP-binding cassette transporter) [68]. Thus, MPO may have a critical role in both LDL modification leading to foam cell development, and HDL chlorination leading to reduced removal of cholesterol through reverse cholesterol transport. With respect to the central theme of this review, it should be noted that the MPO-derived oxidation product of plasmalogen, α-chlorofatty aldehyde, accumulates 1400-fold in human atherosclerotic lesions compared with normal vascular tissue [69].
While strong evidence supports the role of MPO in human atherosclerosis, the involvement of MPO in mouse models of atherosclerosis is poorly understood since murine macrophages lack MPO [46]. Furthermore, murine monocyte MPO levels are a tenth of that in humans [46], and plasmalogen oxidation products of MPO-derived HOCl are not detectable in phorbol ester-stimulated mouse monocytes in comparison with that in human monocytes [70]. Studies using MPO−/− mice crossed with apoE−/− mice failed to show a reduction in mouse atherosclerosis in the absence of MPO [46]. However, careful examination of wild-type mouse atherosclerotic lesions revealed that macrophages do not possess MPO [46]. Using the LDL receptor (LRLR)−/− mouse subjected to a western-type (proatherogenic) diet, LeBoeuf and coworkers demonstrated that in a mouse atherosclerosis model, transgenic mice expressing human MPO in their macrophages have a twofold increase in atherosclerotic lesion area compared with mice that do not express human MPO in their macrophages [71]. Furthermore, Reynolds et al. crossed mice overexpressing both the -463G and -463A alleles of human MPO with LDLR−/− mice, which resulted in increased aortic atherosclerosis compared with control LDLR−/− mice [72]. Taken together, MPO likely has a role in human atherosclerosis, and enhancing the MPO content in the monocytes and macrophages of mice with human MPO results in the appearance of MPO-containing cells in atherosclerotic lesions that accelerate the pathophysiological sequelae of murine atherosclerosis.
Plasma MPO levels have been shown to correlate with the risk of coronary artery disease. MPO deficiency in humans is associated with increased susceptibility to fungal and yeast infections, but markedly reduced incidence of cardiovascular disease [73]. Functional polymorphisms in the promoter region of MPO that result in decreased MPO expression have also been associated with decreased risk for cardiovascular disease in several independent studies [73–75]. Plasma levels of 3-chlorotyrosine and 3-nitrotyrosine are elevated in the plasma and serum proteins of patients with established cardiovascular disease [63–65]. However, unlike plasma levels of MPO, plasma levels of 3-chlorotyrosine and 3-nitrotyrosine do not predict mortality in patients following myocardial infarction and they have not been shown to be a prognostic indicator of future adverse coronary events [76].
MPO-containing neutrophils are important cellular mediators of the pathophysiological sequelae of myocardial ischemia
Neutrophils may impact postischemic contractile function both through their localized degranulation at the endothelial surface and through their infiltration of postischemic myocardium as part of the wound repair process [77–80]. Neutrophil MPO release is increased in subjects with acute coronary syndromes, and serum MPO levels provide a prognostic indication of acute coronary syndromes, as well as adverse coronary events [81–83]. Furthermore, circulating MPO levels from patients with ST-elevation myocardial infarction are elevated to a greater extent in the coronary circulation than in the systemic circulation [84]. Interestingly, another recent study demonstrated that atrial and plasma MPO levels are elevated in patients with atrial fibrillation, and further demonstrated that MPO−/− mice are protected from electrically stimulated atrial fibrillation [85]. Activated neutrophils are known to mediate endothelial dysfunction via secretion of proteolytic enzymes, such as elastase, and oxygen radicals, which can have short-term and long-term effects on endothelial function [77]. Activation of NADPH oxidase during the respiratory burst in neutrophils results in the production of superoxide anions that, through the concerted action of superoxide dismutase, dismutate to produce hydrogen peroxide. Similar to monocytes and macrophages, MPO activity in neutrophils amplifies the oxidizing potential of hydrogen peroxide by using it as a substrate to produce HOCl [36], which may have an important role in the pathophysiology of both myocardial ischemia and atrial fibrillation.
Neutrophil infiltration into previously ischemic zones is considered to be an important mechanism that, in part, mediates myocardial reperfusion injury [86–89]. Myocardial damage in response to ischemia-reperfusion is reduced in the hearts of animals that are either rendered neutropenic, pretreated with antibodies that block neutrophil–endothelial cell interactions, or genetically modified to reduce neutrophil infiltration [88,90–92]. It is likely that some degree of myocardial damage during reperfusion following ischemia is mediated by neutrophil-derived free radicals [93,94]. For example, in isolated perfused hearts, the addition of neutrophils during reperfusion following ischemia leads to the production of oxygen free radicals and attenuated recovery [95,96].
The role of MPO-derived products in ischemic-reperfusion myocardium is partially understood. For example, MPO−/− mice have an increased risk of ventricular rupture following left anterior descending coronary artery (LAD) ligation compared with wild-type mice [97]. The increased propensity of ventricular rupture in the MPO−/− mouse subjected to LAD ligation may involve decreased inhibition of plasminogen activator inhibitor 1. Other studies have suggested that MPO-derived products that have been measured to date (e.g., glycine and threonine oxidation products) do not impact postischemic function, but have a role in ventricular remodeling [98]. On the other hand, other MPO-oxidation products, including the chlorinated lipid, 2-chlorohexadecanal [99], have been shown to directly impact ventricular function. Studies have also demonstrated that HOCl added to isolated heart perfusates, elicits contractile dysfunction and arrhythmias, possibly by targeting adrenergic receptors and sodium–potassium ATPase [100–102].
Identification of chlorinated lipids derived from phospholipids
Phospholipids, fatty acids, sterols and sphingolipids have several targets that are susceptible to HOCl oxidation. The alkenes in aliphatic residues of esterified and nonesterified fatty acids, as well as the double bond in cholesterol are oxidized to chlorohydrins [37,39,103–108]. Another lipidic target of HOCl is the primary amine groups present in ethanolamine and serine glycerophospholipids. These primary amine-containing phospholipids are oxidized to chloramines, dichlorinated amines, chorimines and nitriles [109,110]. Several structures have recently been proposed that include chlorohydrins of the alkene of the sphingosine backbone in reactions of sphingomyelin with HOCl [111]. In addition, the amine of sphingosine is susceptible to HOCl oxidation, resulting in the production of a nitrile and 2-hexadecenal [112]. Although alkenes and primary amines are susceptible to HOCl oxidation, only the chlorohydrin of lysophosphatidylcholine has been identified in vivo in human atherosclerotic lesions [108]. Historically, attention has been directed to chlorohydrins and chloramine products produced from HOCl oxidation of lipids; however, this article focuses on the HOCl oxidation of the vinyl ether of plasmalogens leading to the production of a family of chlorinated lipids that have been identified in vivo.
α-chlorofatty aldehyde is an oxidation product of plasmalogen reactions with HOCl
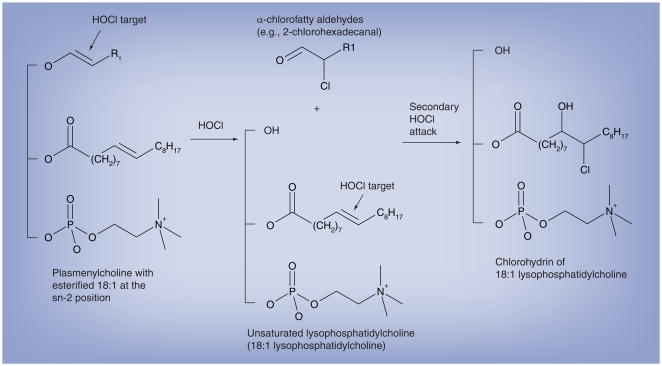
Based on the vulnerable nature of the vinyl ether bond of plasmalogens to oxidation, we tested the possibility that this bond is a target for HOCl. Initial experiments demonstrated that the relatively water-soluble plasmalogen, lysoplasmenylcholine, was indeed targeted by either HOCl or chlorine gas [42]. The product of this reaction was the α-chlorofatty aldehyde, 2-chlorohexadecanal, which was confirmed by mass spectrometry and NMR. In additon, the production of this chlorinated aldehyde was dependent on targeting the masked aldehyde, vinyl ether bond of plasmalogens, since HOCl did not react with hexadecanal to yield 2-chlorohexadecanal. Subsequent analyses were performed on the HOCl oxidation products of plasmenylcholine containing a mono-unsaturated fatty acid at the sn-2 position (e.g., 1-O-hexadec-1′-enyl-2-octadec-9′-enoyl-sn-glycero-3-phosphocholine) [42]. These analyses demonstrated that the two predominant oxidation products are α-chlorofatty aldehyde and the lysophosphatidylcholine, 2-octadec-9′-enoyl-sn-glycero-3-phosphocholine. Further examination has shown that unsaturated lysophosphatidylcholines can also be targeted by secondary HOCl attack, leading to the production of chlorohydrins of unsaturated lysophosphatidylcholines [107]. Figure 2 summarizes reactions that occur between plasmalogens and HOCl, leading to the production of α-chlorofatty aldehyde. The plasmalogen vinyl ether bond is a preferred target for HOCl oxidation compared with alkenes, but excess HOCl compared with plasmalogen vinyl ether bond targets leads to the complete degradation of vinyl ether containing plasmalogens and subsequent targeting of sn-2 aliphatic alkenes [107]. It should be noted that Leßig and coworkers demonstrated that excess supraphysiological levels of HOCl (5 mM) leads to complete degradation of plasmalogens with glycerophosphorylcholine product [113].
Figure 2. Chlorinated lipid products derived from HOCl targeting plasmenylcholine.
The vinyl ether bond of plasmalogens is targeted by HOCl resulting in the release of the masked aldehyde as an α-chlorofatty aldehyde. The other product of this reaction is an unsaturated molecular species of lysophosphatidylcholine. The alkene bond in the unsaturated molecular species of lysophosphatidylcholine is targeted by HOCl to yield a chlorohydrin molecular species. The vinyl ether bond is a preferred target for HOCl compared with the alkene, but the alkene is targeted following the removal of vinyl ether residues from the initial attack.
HOCl: Hypochlorous acid.
The characterization of the product of HOCl targeting of plasmalogens led to several methods that were developed to both purify and quantify 2-chlorohexadecanal. For example, thin-layer chromatography with silica gel G as a solid phase and petroleum ether/ethyl ether/acetic acid (90/10/1) separates α-chlorofatty aldehydes (retention factor [Rf] = 0.46) from nonhalogenated fatty aldehydes and fatty acids (Rf = 0.57 and 0.19, respectively) [42]. The confirmation of the structure of 2-chlorohexadecanal using gas chromatography–mass spectrometry following derivatization to its pentafluorobenzyl oxime also proved to be an extremely sensitive analytical tool to detect α-chlorofatty aldehydes [42,69,70,99,114]. Utilizing a deuterated internal standard analog (e.g., 2-chloro-[7,7,8,8-d4]-hexadecanal), these molecules could be readily quantified in biological samples employing selected ion monitoring and negative ion-chemical ionization mass spectrometry (e.g., [69,114]).
Studies by other laboratory groups confirmed the production of chlorinated fatty aldehydes from plasmalogens. Malle and coworkers demonstrated that lipoprotein-associated plasmalogens are targeted by HOCl, resulting in 2-chlorohexadecanal production [115]. Davies and coworkers [116] demonstrated that the rate constant for HOCl targeting the vinyl ether bond of plasmalogens is two orders of magnitude faster than the targeting of alkenes. These studies strongly suggest that plasmalogens are preferred targets of leukocyte-derived HOCl and supports the notion that these molecules are produced in biological samples.
Do plasmalogen-derived HOCl oxidation products accumulate in biological samples?
Human neutrophils and monocytes are enriched with MPO that converts hydrogen peroxide to HOCl during activation [38,117]. In addition, neutrophils contain robust plasmalogen pools that potentially could be oxidized by MPO-derived HOCl. Thus, it was not surprising that phorbol ester- and fMLP-activated human neutrophils accumulate the α-chlorofatty aldehyde, 2-chloro-hexadecanal [114]. Following activation, neutrophil 2-chlorohexadecanal levels increase over the first 30 min and then decrease, which may reflect either metabolism or Schiff base adduct formation of the aldehyde with primary amines. These studies also suggested that neutrophil-derived HOCl target endothelial cell plasmalogens, and thus neutrophil-derived HOCl has the potential to impact neighboring cells and in the case of the vascular wall they may impact endothelial function. Neutrophil 2-chlorohexadecanal production is blocked by heme enzyme inhibitors including azide and 3-aminotriazole, which inhibit MPO activity [118,119]. The dependence of 2-chlorohexadecanal production on MPO activity has also been supported by the attenuation of 2-chlorohexadecanal accumulation in peritoneal lavage from MPO-knockout mice compared with wild-type mice subjected to peritonitis protocols (e.g., thioglycolate recruitment for 1 day followed by zymosan activation) [69]. It should be appreciated that although mouse neutrophil MPO content (per neutrophil) is five- to ten-fold less than that in human [120,121], the neutrophil-enriched peritoneal lavage under these conditions contains 2-chlorohexadecanal [69]. Similarly, phorbol ester stimulated human monocytes produce 2-chloro-hexadecanal [70]. These studies also demonstrated that monocyte-derived HOCl could target lipoprotein-associated plasmalogens in addition to monocyte-associated plasmalogens, resulting in increased production of 2-chlorooctadecanal in comparison with 2-chlorohexadecanal [70]. This later finding was of particular importance since both 16 and 18 carbon plasmalogens are found in human LDL. Unsaturated lysophosphatidylcholine species were also increased in LDL exposed to activated monocytes. Further comparisons were made between human monocytes and mouse monocytes, which confirmed that α-chlorofatty aldehydes were produced in the human but not in the mouse monocyte, which is devoid of MPO activity [70].
α-chlorofatty aldehyde is a precursor of other chlorinated lipids including α-chlorofatty acid
With the discovery that the masked aldehyde of plasmalogens is targeted by HOCl, yielding a halogenated aldehyde, it became important to determine whether this chlorinated aldehyde was of biological relevance, or whether it is metabolized to functionally relevant molecules. One potential mechanism is that 2-chlorohexadecanal could elicit functional changes in targeted cells through Schiff base adduct formation with primary amines of proteins and lipids. Schiff base adduct formation could potentially alter membrane dynamics and protein function. 2-chlorohexadecanal Schiff base adducts have been identified with ethanolamine glycerophospholipids and lysine [122]. Schiff base stabilization with cyanoborohydride resulted in an adduct containing an unsaturated carbon–carbon bond from the elimination of HCl [122]. Overall, these findings suggest that Schiff base adduct formation with 2-chlorohexadecanal may be an important mechanism by which 2-chlorohexadecanal either elicits cell injury or mediates signaling pathways.
Other studies have explored the metabolism of 2-chlorohexadecanal. Wildsmith et al. used radiolabeled, as well as stable isotope-labeled, 2-chlorohexadecanal to demonstrate that neutrophils and endothelial cells can metabolize 2-chlorohexadecanal to 2-chlorohexadecanol (an α-chlorofatty alcohol) and 2-chloro-hexadecanoic acid (an α-chlorofatty acid) [123]. Although 2-chlorohexadecanal could be metabolized within these cells, it appears that they actively secrete the metabolites of 2-chloro-hexadecanal rather than accumulate them within the cell. Some radiolabeled 2-chloro-hexadecanal is also incorporated into complex lipids such as triglycerides and phospholipids, and it is likely that this represents the esterification of the oxidation product, 2-chloro-hexadecanoic acid, into these pools. It should be appreciated that fatty aldehyde is an intermediate of the fatty acid–fatty alcohol cycle, which regulates cellular levels of fatty alcohol and fatty acid that can be used for lipid biosynthesis (particularly ether-linked lipid biosynthesis that is dependent of fatty alcohol as a precursor) [124]. Studies using a cell culture model of fatty aldehyde dehydrogenase (FALDH; one of the enzymes of the fatty acid–fatty alcohol cycle) deficiency, FAA.K1A, demonstrate that 2-chlorohexadecanal oxidation to 2-chlorohexadecanoic acid was dependent on FALDH activity [125]. In addition, neutrophils were shown to oxidize and reduce endogenously produced 2-chlorohexadecanal, leading to the release of 2-chlorohexadecanoic acid and 2-chlorohexadecanol from activated neutrophils [125]. Taken together, these cell-based experiments indicate that plasmalogen targeting by HOCl leads not only to the production of 2-chlorohexadecanal, but a family of chlorinated lipids produced from the unmasking of plasmalogen vinyl ether aliphatic group.
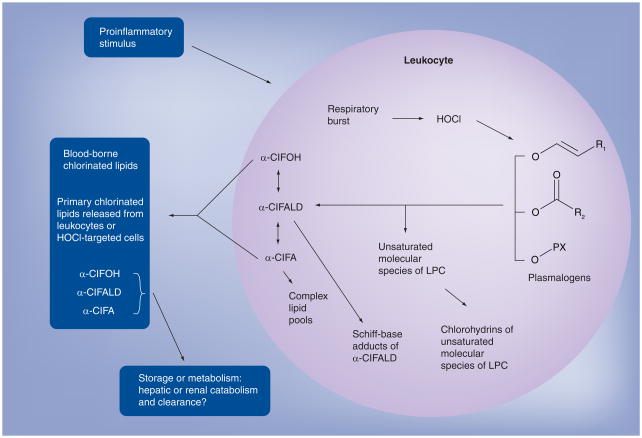
The family of chlorinated lipids derived from HOCl targeting the plasmalogen vinyl ether bond is shown in Figure 3. The two immediate products of HOCl-targeting plasmalogens, α-chlorofatty aldehyde and lysophosphatidylcholine, are the precursors for this family, which include chlorohydrins of unsaturated molecular species of lysophosphatidylcholine, α-chlorofatty alcohol, α-chlorofatty acid and complex lipids (e.g., triglycerides and phosphatidylcholines containing esterified residues of α-chlorofatty acid). Other nonchlorinated products include the Schiff base adducts of α-chlorofatty aldehyde. Also as shown in Figure 3, chlorinated lipids may be released from sites of inflammation and enter the circulatory system. Identifying the mechanisms responsible for the clearance of these chlorinated lipids will be important to determine in future studies since metabolic clearance may be critical in reversing biological effects of these lipids.
Figure 3. Family of chlorinated lipids derived from HOCl targeting plasmenylcholine.
Chlorinated lipids are produced from HOCl targeting of plasmalogens. The overall metabolic fate of these chlorinated lipids remains to be resolved, but it is known that they are readily released from cells, and plasma levels of α-chlorofatty acid are detected in rodent models of inflammation.
α-ClFA: α-chlorofatty acid; α-ClFALD: α-chlorofatty aldehyde; α-ClFOH: α-chlorofatty alcohol; LPC: Lysophosphatidylcholine.
HOCl: Hypochlorous acid.
Although it is not the focus of this review, it should be noted that many of the chlorinated oxidation products shown in Figure 3, are also present as brominated oxidation products derived from oxidation of the vinyl ether bond of plasmalogens by hypobromous acid. MPO can use physiological levels of bromide to produce hypobromous acid, and eosinophil peroxidase selectively uses bromide to produce hypobromous acid [36,126,127]. Both phorbol ester-activated neutrophils and eosinophils in the presence of physiological levels of bromide produce 2-bromohexadecanal [128,129]. Thus, it is seems likely that brominated lipid products may be produced by analogous pathways as those shown in Figure 3, and these lipid species potentially may have roles in inflammation.
Lipoprotein plasmalogen targeting by HOCl & chlorinated lipid accumulation during atherosclerosis
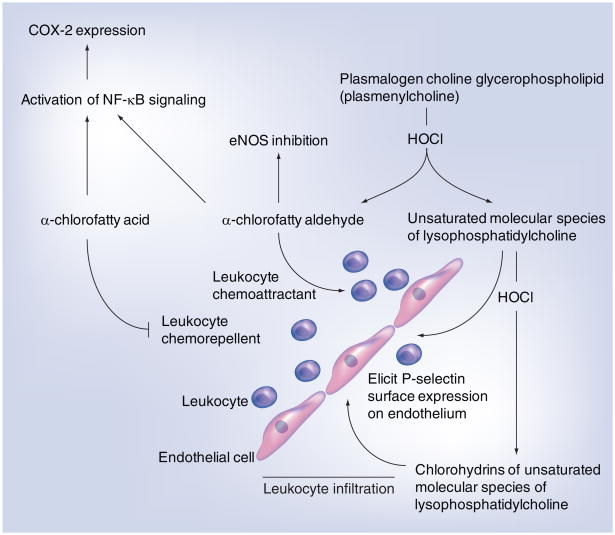
As previously mentioned, monocyte-derived HOCl can target LDL-associated plasmalogen, leading to the production of 2-chlorohexadecanal and unsaturated molecular species of lysophosphatidylcholine [70]. The implication of this finding is that HOCl produced by MPO present in developing atherosclerotic lesions could lead to the accumulation of 2-chlorohexadecanal and unsaturated molecular species in the vascular wall. Human atherosclerotic tissue displays a 1400- and 34-fold increase in 2-chlorohexadecanal and unsaturated molecular species of lysophosphatidylcholine, respectively, in comparison with normal human aorta [69]. In addition, recent studies demonstrated that brain plasmalogens are targeted by HOCl in mice treated with lipopolysaccharide, leading to the transient accumulation of α-chlorofatty aldehydes in the brain [130]. These HOCl-derived plasmalogen oxidation products may augment inflammatory mechanisms by the concerted chemoattraction of phagocytes by 2-chlorohexadecanal [114] and the stimulation of membrane surface expression of phagocyte-tethering proteins (e.g., P-selectin) on endothelial cells by unsaturated molecular species of lysophosphatidylcholine [69]. Thus, in vitro experiments have suggested that through the attack of the vinyl ether bond of plasmenylcholine, two products are made that concertedly attract phagocytes (2-chlorohexadecanal) and facilitate the tethering of phagocytes to endothelium (unsaturated molecular species of lysophosphatidylcholine) (Figure 4). With the discovery that 2-chlorohexadecanal can be metabolized to 2-chlorohexadecanoic acid and 2-chlorohexadecanol, it remains to be determined if these metabolites also increase in atherosclerotic lesions and whether they have proatherosclerotic properties.
Figure 4. Known targets of chlorinated lipids derived from HOCl targeting plasmenylcholine.
HOCl and eNOS are reactive chlorinating species and endothelial nitric oxide synthase, respectively. Flattened and rounded cells represent endothelial cells and leukocytes, respectively, as indicated.
eNOS: Endothelial nitric oxide synthase; HOCl: Hypochlorous acid.
Myocardial plasmalogen targeting by HOCl during ischemia: functional ramifications of myocardial chlorinated lipid accumulation
Neutrophil infiltration into myocardial infarct zones not only contributes to the removal of cellular debris from necrosed tissue, but possibly also leads to additional damage to viable tissue. Since activated neutrophils produce HOCl and accumulate 2-chlorohexadecanal, it seems likely that neutrophil infiltration that occurs in myocardial infarcts could lead to the accumulation of chlorinated lipids in infarct zones. Studies from our laboratory demonstrated that 2-chlorohexadecanal accumulates in rat hearts subjected to LAD occlusion [99]. Heart tissue from rats subjected to surgical infarction had elevated levels of 2-chlorohexadecanal and neutrophil infiltration levels compared with heart tissue from rats subjected to sham surgery. To further support the role of neutrophils in 2-chlorohexadecanal accumulation in infarcted hearts, it was shown that infarcted heart tissue from rats rendered neutropenic had reduced levels of 2-chlorohexadecanal and reduced neutrophil infiltration compared with infarcted heart tissue from rats that had normal levels of circulating neutrophils [99]. In addition, perfusing hearts with concentrations of 2-chlorohexadecanal found in infarcted myocardium elicited cardiac injury and resulted in antichronotropic and anti-ionotropic effects on the isolated perfused rat heart [99]. These studies were performed prior to the discovery of the metabolites of 2-chlorohexadecanal, and thus, future experiments should shed light on the role of 2-chlorohexadecanal metabolites in postischemic contractile dysfunction.
Other related oxidation products of plasmalogen targeting by HOCl include unsaturated molecular species of lysophosphatidylcholine & chlorohydrins of lysophosphatidylcholine
Chemical reactions of HOCl with plasmenylcholine lead to unsaturated lysophosphatidylcholine molecular species to be produced, which are susceptible to secondary HOCl targeting, thus leading toward the production of chlorohydrins of unsaturated lysophosphatidylcholines [107]. Others have attributed chemical treatment of tissues with HOCl and subsequent lipid chlorohydrin product to several biological consequences, including red blood cell and endothelial cell lysis, as well as decreased contractile tension in isolated cardiac papillary muscle preparations [131,132]. Despite the properties that have been attributed to lipid chlorohydrins, the demonstration of their presence in vivo is limited. Using tandem mass spectrometry techniques, we demonstrated that chlorohydrins of unsaturated lysophosphatidylcholines accumulate in human atherosclerotic tissue [108]. Furthermore, as shown with other lipid chlorohydrins, the chlorohydrins of unsaturated lysophosphatidylcholine likely enhance the tethering of proinflammatory leukocytes to regions of inflammation since they cause P-selectin surface expression on endothelial cells [108]. In summary, considerable interest has been directed toward the oxidation of the alkene bond yielding chlorohydrins and the potential that they have as biological mediators, but these putative mediators need to be thoroughly characterized and quantified in vivo.
Summary of conditions in which chlorinated lipids are produced
Table 1 summarizes the levels of chlorinated lipids that have been determined in activated cells as well as under proinflammatory conditions in vivo. Estimates of the concentration of chlorinated lipids have been calculated under some conditions. For example, the 2-chlorohexadecanal levels reach concentrations as high as 90 μM in phorbol ester-stimulated human neutrophils, while the 2-chlorohexadecanal concentration in infarct rat myocardium is estimated to be approximately 1 μM [99,114]. In human atherosclerotic lesions, 2-chlorohexadecanal levels of approximately 10 μM have been calculated [69]. The metabolites of α-chlorofatty aldehyde have only recently been examined under conditions of leukocyte activation. Plasma and phorbol ester-stimulated neutrophil levels of 2-chlorohexadecanoic acid are approximately 1 nM and approximately 10 μM, respectively [125].
Table 1.
Summary of chlorinated lipids found in leukocytes and in vivo.
| Chlorinated lipid | Tissue/cell | Chlorinated lipid level-condition | Ref. |
|---|---|---|---|
| α-chlorofatty aldehyde | Human neutrophils | Not detected in unstimulated cells 35 pmol/106 PMA-stimulated cells |
[114] |
| Human monocytes | 0.4 pmol/106 cells (unstimulated) 6.9 pmol/106 PMA-stimulated cells |
[70] | |
| Human aorta | 0.0015 pmol/nmol inorganic phosphate in normal tissue 2.08 pmol/nmol inorganic phosphate in atherosclerotic tissue |
[69] | |
| Rat heart | 0.3 pmol/μmol inorganic phosphate: no surgical intervention 4.5 pmol/μmol inorganic phosphate: 24 h LAD occlusion 1.3 pmol/μmol inorganic phosphate: 24 h sham surgery (pericarditis) |
[99] | |
| α-chlorofatty acid | Human neutrophils | 1.2 pmol/106 cells (unstimulated) 13 pmol/106 PMA-stimulated cells |
[125] |
| Mouse BALF | 11.3 fmol/ml: control 45.3 fmol/ml: Sendai virus-treated mice |
[125] | |
| Mouse plasma | 0.8 pmol/ml: control 1.4 pmol/ml: sendai virus-treated mice |
[125] | |
| α-chlorofatty alcohol | Human neutrophils | 0.3 pmol/106 cells (unstimulated) 2.3 pmol/106 PMA: stimulated cells |
[125] |
| LPC chlorohydrins | Human aorta | 0.09 pmol/mg wet weight: normal 7.28 pmol/mg wet weight: atherosclerotic |
[108] |
LAD: Left anterior descending coronary artery; PMA: Phorbol myristate acetate.
Biological targets of chlorinated lipids
Of the chlorinated lipids, 2-chlorohexadecanal is best understood in respect to biological properties. Functionally, 2-chlorohexadecanal causes myocardial injury and reduces ventricular performance [99]. Several studies demonstrated biochemical mechanisms that may have an important role in the functional consequences of increased levels of 2-chlorohexadecanal. Marsche and coworkers demonstrated that HDL-associated 2-chlorohexadecanal alters nitric oxide production in endothelial cells through a reduction in endothelial nitric oxide synthase (eNOS) activity [115] (Figure 4). These investigators have suggested that 2-chlorohexadecanal reduced eNOS levels in endothelial cells through a mechanism involving mRNA instability. This action of 2-chlorohexadecanal on endothelial cell nitric oxide production has been proposed to be important in the proinflammatory mechanisms occurring in ischemia-reperfusion injury, glomerulosclerosis and atherosclerosis [115]. Our laboratory’s studies with human coronary artery endothelial cells revealed that both 2-chlorohexadecanal and 2-chlorohexadecanoic acid increase COX-2 expression and prostacyclin production through a mechanism that is likely mediated by an increase in NFκB signaling [133]. It is interesting to consider that 2-chlorohexadecanal decreases the production of the vasodilator, nitric oxide, while increasing the production of another vasodilator, prostacyclin. However, since 2-chlorohexadecanal increases NFκB signaling [133], it is possible that other proinflammatory pathways may be activated that have not yet been revealed.
2-chlorohexadecanal is a chemoattractant for neutrophils [114] and, interestingly, its metabolite, 2-chlorohexadecanoic acid, has chemorepellant properties [125]. Thus, it can be envisaged that 2-chlorohexadecanal may be produced as a result of infiltration and activation of neutrophils, and participate in the recruitment of additional neutrophils. However, the accumulation of the 2-chlorohexadecanal metabolite, 2-chlorohexadecanoic acid, may have an important role in shutting down further neutrophil recruitment. It should also be re-emphasized that in concert with 2-chlorohexadecanal production, unsaturated molecular species of lysophosphatidylcholine (and perhaps their chlorohydrins) are produced, which further enhance neutrophil infiltration by stimulating the surface expression of P-selectin (Figure 4) [69,133].
The biological roles of the other chlorinated lipids remain to be revealed. As the actions of these compounds are revealed, the relationship of each compound to their precursors and metabolites will need to be addressed. It is possible that specific chlorinated metabolites are the active components of the family of chlorinated lipids that are produced as a result of HOCl-targeting plasmalogens. For example, both 2-chlorohexadecanal and 2-chlorohexadecanoic acid increase endothelial COX-2 levels and it is quite possible that 2-chloro-hexadecanal is not the active compound that elicits COX-2 expression, but rather, its fatty acid metabolite could stimulate the pathway [125,133]. Furthermore, the physiological consequences of 2-chlorohexadecanal Schiff base adducts need to be determined. It is possible that Schiff base adduct formation represents a nonspecific mechanism whereby 2-chlorohexadecanal could elicit injury and 2-chlorohexadecanal oxidation to 2-chloro-hexadecanoic acid may be important in reducing injury mediated by the Schiff base adducts.
Conclusion
While the production of RCS by leukocytes is an important mechanism in innate immunity to fend off invading micro-organisms, the collateral damage of these RCS attacking host tissues and biomolecules may be important in the early stages and subsequent sequelae of cardiovascular disease. There are multiple targets that HOCl may attack in host tissues. While some of these targets are more chemically reactive for HOCl oxidation (e.g., glutathione and methionine), the plasmalogen vinyl ether bond is one of the more reactive lipidic targets and its oxidation by HOCl leads to the production of chlorinated lipids that have been identified in vivo. Over the past decade, considerable advances have been made showing that this family of chlorinated lipids are produced as a result of the activation of leukocytes containing MPO. The initial products of plasmalogen oxidation by HOCl, α-chlorofatty aldehyde and unsaturated molecular species of lysophosphatidylcholine (LPC), accumulate in response to leukocyte activation. Collectively, they have biological properties that enhance the recruitment and infiltration of leukocytes to sites of inflammation. This may represent one of the more important roles of plasmalogen targeting by HOCl. Recent studies demonstrated that α-chlorofatty aldehyde is readily oxidized to α-chlorofatty acid, which can serve as a chemorepellent. It is possible that, as α-chloro-fatty acid levels exceed α-chlorofatty aldehyde levels, this metabolic pathway may contribute to resolution of the neutrophil infiltration by limiting further recruitment of neutrophils.
Our understanding of the role of chlorinated lipids in physiological and pathophysiological conditions is at the early stages, and these molecules may prove to be extremely important in the pathophysiological sequelae of diseases, including atherosclerosis and ischemic heart disease. Recent studies demonstrated that these chlorinated lipids accumulate in these diseases, yet the family of chlorinated lipid metabolites needs to be elucidated and the role of these metabolites as mediators of disease remains to be understood.
Future perspective
The discovery that chlorinated lipids are produced in vivo is a rather recent finding. Metabolites of α-chlorofatty aldehyde have been partially revealed, but delineating the specific metabolic pathways associated with the metabolites will provide important information as to whether metabolites are the active compounds that elicit cell responses (e.g., cell injury and cardiac contractile dysfunction). Pathways associated with the elimination of toxic chlorinated lipids from the body also remain to be elucidated. Some of the potential metabolic pathways may include dechlorination of either α-chlorofatty aldehyde, α-chlorofatty acid or α-chlorofatty alcohol. It seems likely that α-chlorofatty acid may be the key metabolite that may be further metabolized. Chlorine at the α-position is expected to prevent mitochondrial β-oxidation, but it is possible that these compounds may undergo ω-oxidation, which might then be cleared from the body or have important biological properties. Alternatively, α-chlorofatty acid may be stored in complex lipid pools as an esterified fatty acid. If α-chlorofatty acid has important biological roles, then this storage may provide an important means of turning off functional pathways modulated by α-chlorofatty acid. In addition, the release of α-chlorofatty acid from esterified pools could potentially be regulated by specific lipases (e.g., triglyceride lipases or phospholipases) that could be tightly regulated.
A critical area that needs to be addressed in the future is determining the targets of the different chlorinated lipids that are produced in vivo. Defining the targets will go hand-in-hand with delineating the critical metabolites of chlorinated lipids. While some signaling pathways have been identified, it is important to determine if there are specific receptors for these lipids and what role they might have in physiological responses. Insights into the receptors, targets and signaling mechanisms impacted by chlorinated lipids could lead to the development of new therapeutic targets for diseases such as atherosclerosis and postischemic contractile dysfunction.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
This research was supported by NIH grants HL074214, HL088073, HL098907 and RR019232, as well as Grant-in-Aid 0650044Z from the American Heart Association. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Han XL, Gross RW. Plasmenylcholine and phosphatidylcholine membrane bilayers possess distinct conformational motifs. Biochemistry. 1990;29(20):4992–4996. doi: 10.1021/bi00472a032. [DOI] [PubMed] [Google Scholar]
- 2.Hazen SL, Hall CR, Ford DA, Gross RW. Isolation of a human myocardial cytosolic phospholipase A2 isoform. Fast atom bombardment mass spectroscopic and reverse-phase high pressure liquid chromatography identification of choline and ethanolamine glycerophospholipid substrates. J Clin Invest. 1993;91(6):2513–2522. doi: 10.1172/JCI116487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gross RW. High plasmalogen and arachidonic acid content of canine myocardial sarcolemma: a fast atom bombardment mass spectroscopic and gas chromatography-mass spectroscopic characterization. Biochemistry. 1984;23(1):158–165. doi: 10.1021/bi00296a026. [DOI] [PubMed] [Google Scholar]
- 4.Pike LJ, Han X, Chung KN, Gross RW. Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: a quantitative electrospray ionization/mass spectrometric analysis. Biochemistry. 2002;41:2075–2088. doi: 10.1021/bi0156557. [DOI] [PubMed] [Google Scholar]
- 5.Han X, Gross RW. Electrospray ionization mass spectroscopic analysis of human erythrocyte plasma membrane phospholipids. Proc Natl Acad Sci USA. 1994;91(22):10635–10639. doi: 10.1073/pnas.91.22.10635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Post JA, Verkleij AJ, Roelofsen B, Op de Kamp JA. Plasmalogen content and distribution in the sarcolemma of cultured neonatal rat myocytes. FEBS Lett. 1988;240(1–2):78–82. doi: 10.1016/0014-5793(88)80343-0. [DOI] [PubMed] [Google Scholar]
- 7.Gross RW. Identification of plasmalogen as the major phospholipid constituent of cardiac sarcoplasmic reticulum. Biochemistry. 1985;24(7):1662–1668. doi: 10.1021/bi00328a014. [DOI] [PubMed] [Google Scholar]
- 8.Zoeller RA, Grazia TJ, LaCamera P, Park J, Gaposchkin DP, Farber HW. Increasing plasmalogen levels protects human endothelial cells during hypoxia. Am J Physiol Heart Circ Physiol. 2002;283(2):H671–H679. doi: 10.1152/ajpheart.00524.2001. [DOI] [PubMed] [Google Scholar]
- 9.Ford DA, Gross RW. Plasmenylethanolamine is the major storage depot for arachidonic acid in rabbit vascular smooth muscle and is rapidly hydrolyzed after angiotensin II stimulation. Proc Natl Acad Sci USA. 1989;86(10):3479–3483. doi: 10.1073/pnas.86.10.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malavolta M, Bocci F, Boselli E, Frega NG. Normal phase liquid chromatography-electrospray ionization tandem mass spectrometry analysis of phospholipid molecular species in blood mononuclear cells: Application to cystic fibrosis. J Chromatogr B. 2004;810(2):173–186. doi: 10.1016/j.jchromb.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Chilton FH, Connell TR. 1-ether-linked phosphoglycerides. Major endogenous sources of arachidonate in the human neutrophil. J Biol Chem. 1988;263(11):5260–5265. [PubMed] [Google Scholar]
- 12.Chilton FH, Murphy RC. Remodeling of arachidonate-containing phosphoglycerides within the human neutrophil. J Biol Chem. 1986;261(17):7771–7777. [PubMed] [Google Scholar]
- 13.Vance JE. Lipoproteins secreted by cultured rat hepatocytes contain the antioxidant 1-alk-1-enyl-2-acyl-glycerophosphoethanolamine. Biochim Biophys Acta. 1990;1045(2):128–134. doi: 10.1016/0005-2760(90)90141-j. [DOI] [PubMed] [Google Scholar]
- 14.Corr PB, Saffitz JE, Sobel BE. Lysophospholipids, long chain acylcarnitines and membrane dysfunction in the ischaemic heart. Basic Res Cardiol. 1987;82(Suppl 1):199–208. doi: 10.1007/978-3-662-08390-1_24. [DOI] [PubMed] [Google Scholar]
- 15.Corr PB, Gross RW, Sobel BE. Amphipathic metabolites and membrane dysfunction in ischemic myocardium. Circ Res. 1984;55(2):135–154. doi: 10.1161/01.res.55.2.135. [DOI] [PubMed] [Google Scholar]
- 16.Kohno M, Yokokawa K, Yasunari K, et al. Induction by lysophosphatidylcholine, a major phospholipid component of atherogenic lipoproteins, of human coronary artery smooth muscle cell migration. Circulation. 1998;98(4):353–359. doi: 10.1161/01.cir.98.4.353. [DOI] [PubMed] [Google Scholar]
- 17.Quinn MT, Parthasarathy S, Steinberg D. Lysophosphatidylcholine: a chemotactic factor for human monocytes and its potential role in atherogenesis. Proc Natl Acad Sci USA. 1988;85(8):2805–2809. doi: 10.1073/pnas.85.8.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sartipy P, Hurt-Camejo E. Modification of plasma lipoproteins by group IIa phospholipase A(2): possible implications for atherogenesis. Trends Cardiovasc Med. 1999;9(8):232–238. doi: 10.1016/s1050-1738(00)00030-x. [DOI] [PubMed] [Google Scholar]
- 19.McHowat J, Kell PJ, O’Neill HB, Creer MH. Endothelial cell paf synthesis following thrombin stimulation utilizes Ca(2+)-independent phospholipase A(2) Biochemistry. 2001;40(49):14921–14931. doi: 10.1021/bi0156153. [DOI] [PubMed] [Google Scholar]
- 20.Uemura Y, Lee TC, Snyder F. A coenzyme A-independent transacylase is linked to the formation of platelet-activating factor (PAF) by generating the lyso-PAF intermediate in the remodeling pathway. J Biol Chem. 1991;266(13):8268–8272. [PubMed] [Google Scholar]
- 21.Venable ME, Nieto ML, Schmitt JD, Wykle RL. Conversion of 1-O-[3H] alkyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine to lyso platelet-activating factor by CoA-independent transacylase in membrane fractions of human neutrophils. J Biol Chem. 1991;266(28):18691–18698. [PubMed] [Google Scholar]
- 22.Ford DA, Gross RW. Identification of endogenous 1-O-alk-1′-enyl-2-acyl-sn-glycerol in myocardium and its effective utilization by choline phosphotransferase. J Biol Chem. 1988;263(6):2644–2650. [PubMed] [Google Scholar]
- 23.Ford DA, Gross RW. Differential accumulation of diacyl and plasmalogenic diglycerides during myocardial ischemia. Circ Res. 1989;64(1):173–177. doi: 10.1161/01.res.64.1.173. [DOI] [PubMed] [Google Scholar]
- 24.Wolf RA, Gross RW. Identification of neutral active phospholipase C which hydrolyzes choline glycerophospholipids and plasmalogen selective phospholipase A2in canine myocardium. J Biol Chem. 1985;260(12):7295–7303. [PubMed] [Google Scholar]
- 25.Ford DA, Gross RW. Activation of myocardial protein kinase C by plasmalogenic diglycerides. Am J Physiol. 1990;258(1 Pt 1):C30–C36. doi: 10.1152/ajpcell.1990.258.1.C30. [DOI] [PubMed] [Google Scholar]
- 26.Ford DA, Miyake R, Glaser PE, Gross RW. Activation of protein kinase C by naturally occurring ether-linked diglycerides. J Biol Chem. 1989;264(23):13818–13824. [PubMed] [Google Scholar]
- 27.Morand OH, Zoeller RA, Raetz CR. Disappearance of plasmalogens from membranes of animal cells subjected to photosensitized oxidation. J Biol Chem. 1988;263(23):11597–11606. [PubMed] [Google Scholar]
- 28.Zoeller RA, Morand OH, Raetz CR. A possible role for plasmalogens in protecting animal cells against photosensitized killing. J Biol Chem. 1988;263(23):11590–11596. [PubMed] [Google Scholar]
- 29.Zoeller RA, Lake AC, Nagan N, Gaposchkin DP, Legner MA, Lieberthal W. Plasmalogens as endogenous antioxidants: somatic cell mutants reveal the importance of the vinyl ether. Biochem J. 1999;338 (Pt 3):769–776. [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson DH, Inerowicz HD, Grove J, Sarna T. Structural characterization of plasmenylcholine photooxidation products. Photochem Photobiol. 2003;78(4):323–330. doi: 10.1562/0031-8655(2003)078<0323:scoppp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 31.Stadelmann-Ingrand S, Favreliere S, Fauconneau B, Mauco G, Tallineau C. Plasmalogen degradation by oxidative stress: production and disappearance of specific fatty aldehydes and fatty α-hydroxyaldehydes. Free Radic Biol Med. 2001;31(10):1263–1271. doi: 10.1016/s0891-5849(01)00720-1. [DOI] [PubMed] [Google Scholar]
- 32.Klebanoff SJ, Waltersdorph AM, Rosen H. Antimicrobial activity of myeloperoxidase. Methods Enzymol. 1984;105:399–403. doi: 10.1016/s0076-6879(84)05055-2. [DOI] [PubMed] [Google Scholar]
- 33.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6(3):173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 34.Davies MJ, Hawkins CL, Pattison DI, Rees MD. Mammalian heme peroxidases: From molecular mechanisms to health implications. Antioxid Redox Signal. 2008;10(7):1199–1234. doi: 10.1089/ars.2007.1927. [DOI] [PubMed] [Google Scholar]
- 35.van der Veen BS, de Winther MP, Heeringa P. Myeloperoxidase: molecular mechanisms of action and their relevance to human health and disease. Antioxid Redox Signal. 2009;11(11):2899–2937. doi: 10.1089/ars.2009.2538. [DOI] [PubMed] [Google Scholar]
- 36.Harrison JE, Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976;251(5):1371–1374. [PubMed] [Google Scholar]
- 37.Hazen SL, Hsu FF, Duffin K, Heinecke JW. Molecular chlorine generated by the myeloperoxidase-hydrogen peroxide-chloride system of phagocytes converts low density lipoprotein cholesterol into a family of chlorinated sterols. J Biol Chem. 1996;271(38):23080–23088. doi: 10.1074/jbc.271.38.23080. [DOI] [PubMed] [Google Scholar]
- 38▪.Lampert MB, Weiss SJ. The chlorinating potential of the human monocyte. Blood. 1983;62(3):645–651. Presents an important characterization of the role of myeloperoxidase (MPO) in monocytes. [PubMed] [Google Scholar]
- 39▪.Heinecke JW, Li W, Mueller DM, Bohrer A, Turk J. Cholesterol chlorohydrin synthesis by the myeloperoxidase-hydrogen peroxide-chloride system: potential markers for lipoproteins oxidatively damaged by phagocytes. Biochemistry. 1994;33(33):10127–10136. doi: 10.1021/bi00199a041. One of the first reports suggesting that chlorinated lipids could be produced by targeting by MPO-derived reactive chlorinating species (RCS) [DOI] [PubMed] [Google Scholar]
- 40.Thomas EL, Jefferson MM, Grisham MB. Myeloperoxidase-catalyzed incorporation of amines into proteins: role of hypochlorous acid and dichloramines. Biochemistry. 1982;21(24):6299–6308. doi: 10.1021/bi00267a040. [DOI] [PubMed] [Google Scholar]
- 41▪.Winterbourn CC, van den Berg JJ, Roitman E, Kuypers FA. Chlorohydrin formation from unsaturated fatty acids reacted with hypochlorous acid. Arch Biochem Biophys. 1992;296(2):547–555. doi: 10.1016/0003-9861(92)90609-z. One of the first reports suggesting that chlorinated lipids could be produced by targeting by MPO-derived RCS. [DOI] [PubMed] [Google Scholar]
- 42▪▪.Albert CJ, Crowley JR, Hsu FF, Thukkani AK, Ford DA. Reactive chlorinating species produced by myeloperoxidase target the vinyl ether bond of plasmalogens: Identification of 2-chlorohexadecanal. J Biol Chem. 2001;276(26):23733–23741. doi: 10.1074/jbc.M101447200. First demonstration that plasmalogens are targeted by MPO-derived RCS, and the characterization of the oxidation products. In addition, this study develops analytical tools to quantify chlorinated lipids. [DOI] [PubMed] [Google Scholar]
- 43.Pattison DI, Hawkins CL, Davies MJ. Hypochlorous acid-mediated oxidation of lipid components and antioxidants present in low-density lipoproteins: absolute rate constants, product analysis, and computational modeling. Chem Res Toxicol. 2003;16(4):439–449. doi: 10.1021/tx025670s. [DOI] [PubMed] [Google Scholar]
- 44.Pattison DI, Davies MJ. Reactions of myeloperoxidase-derived oxidants with biological substrates: gaining chemical insight into human inflammatory diseases. Curr Med Chem. 2006;13(27):3271–3290. doi: 10.2174/092986706778773095. [DOI] [PubMed] [Google Scholar]
- 45▪.Klebanoff SJ. Myeloperoxidase: contribution to the microbicidal activity of intact leukocytes. Science. 1970;169(950):1095–1097. doi: 10.1126/science.169.3950.1095. First demonstration of the critical role of MPO in microbe killing. [DOI] [PubMed] [Google Scholar]
- 46.Brennan ML, Anderson MM, Shih DM, et al. Increased atherosclerosis in myeloperoxidase-deficient mice. J Clin Invest. 2001;107(4):419–430. doi: 10.1172/JCI8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thong YH. How important is the myeloperoxidase microbicidal system of phagocytic cells? Med Hypotheses. 1982;8(3):249–254. doi: 10.1016/0306-9877(82)90120-7. [DOI] [PubMed] [Google Scholar]
- 48.Chapman AL, Hampton MB, Senthilmohan R, Winterbourn CC, Kettle AJ. Chlorination of bacterial and neutrophil proteins during phagocytosis and killing of staphylococcus aureus. J Biol Chem. 2002;277(12):9757–9762. doi: 10.1074/jbc.M106134200. [DOI] [PubMed] [Google Scholar]
- 49.Winterbourn CC, Hampton MB, Livesey JH, Kettle AJ. Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome: implications for microbial killing. J Biol Chem. 2006;281(52):39860–39869. doi: 10.1074/jbc.M605898200. [DOI] [PubMed] [Google Scholar]
- 50.van Dalen CJ, Whitehouse MW, Winterbourn CC, Kettle AJ. Thiocyanate and chloride as competing substrates for myeloperoxidase. Biochem J. 1997;327(Pt 2):487–492. doi: 10.1042/bj3270487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berliner JA, Heinecke JW. The role of oxidized lipoproteins in atherogenesis. Free Radic Biol Med. 1996;20(5):707–727. doi: 10.1016/0891-5849(95)02173-6. [DOI] [PubMed] [Google Scholar]
- 52.Marathe GK, Prescott SM, Zimmerman GA, McIntyre TM. Oxidized LDL contains inflammatory PAF-like phospholipids. Trends Cardiovasc Med. 2001;11(3–4):139–142. doi: 10.1016/s1050-1738(01)00100-1. [DOI] [PubMed] [Google Scholar]
- 53.Witztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991;88(6):1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54▪▪.Daugherty A, Dunn JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest. 1994;94(1):437–444. doi: 10.1172/JCI117342. First demonstration that MPO is involved in human atherosclerosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hazen SL, Heinecke JW. 3-chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J Clin Invest. 1997;99(9):2075–2081. doi: 10.1172/JCI119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hazell LJ, Arnold L, Flowers D, Waeg G, Malle E, Stocker R. Presence of hypochlorite-modified proteins in human atherosclerotic lesions. J Clin Invest. 1996;97(6):1535–1544. doi: 10.1172/JCI118576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malle E, Waeg G, Schreiber R, Grone EF, Sattler W, Grone HJ. Immunohistochemical evidence for the myeloperoxidase/H2O2/halide system in human atherosclerotic lesions: colocalization of myeloperoxidase and hypochlorite-modified proteins. Eur J Biochem. 2000;267(14):4495–4503. doi: 10.1046/j.1432-1327.2000.01498.x. [DOI] [PubMed] [Google Scholar]
- 58.Sugiyama S, Okada Y, Sukhova GK, Virmani R, Heinecke JW, Libby P. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol. 2001;158(3):879–891. doi: 10.1016/S0002-9440(10)64036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Podrez EA, Schmitt D, Hoff HF, Hazen SL. Myeloperoxidase-generated reactive nitrogen species convert LDL into an atherogenic form in vitro. J Clin Invest. 1999;103(11):1547–1560. doi: 10.1172/JCI5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Podrez EA, Poliakov E, Shen Z, et al. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. J Biol Chem. 2002;277(41):38503–38516. doi: 10.1074/jbc.M203318200. [DOI] [PubMed] [Google Scholar]
- 61.Podrez EA, Poliakov E, Shen Z, et al. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J Biol Chem. 2002;277(41):38517–38523. doi: 10.1074/jbc.M205924200. [DOI] [PubMed] [Google Scholar]
- 62.Fu X, Kassim SY, Parks WC, Heinecke JW. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7). A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J Biol Chem. 2001;276(44):41279–41287. doi: 10.1074/jbc.M106958200. [DOI] [PubMed] [Google Scholar]
- 63.Zheng L, Nukuna B, Brennan ML, et al. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004;114(4):529–541. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pennathur S, Bergt C, Shao B, et al. Human atherosclerotic intima and blood of patients with established coronary artery disease contain high density lipoprotein damaged by reactive nitrogen species. J Biol Chem. 2004;279(41):42977–42983. doi: 10.1074/jbc.M406762200. [DOI] [PubMed] [Google Scholar]
- 65.Bergt C, Pennathur S, Fu X, et al. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc Natl Acad Sci USA. 2004;101(35):13032–13037. doi: 10.1073/pnas.0405292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shao B, Belaaouaj A, Verlinde CL, Fu X, Heinecke JW. Methionine sulfoxide and proteolytic cleavage contribute to the inactivation of cathepsin G by hypochlorous acid: an oxidative mechanism for regulation of serine proteinases by myeloperoxidase. J Biol Chem. 2005;280(32):29311–29321. doi: 10.1074/jbc.M504040200. [DOI] [PubMed] [Google Scholar]
- 67.Shao B, Bergt C, Fu X, et al. Tyrosine 192 in apolipoprotein A-I is the major site of nitration and chlorination by myeloperoxidase, but only chlorination markedly impairs ABCA1-dependent cholesterol transport. J Biol Chem. 2005;280(7):5983–5993. doi: 10.1074/jbc.M411484200. [DOI] [PubMed] [Google Scholar]
- 68.Shao B, Tang C, Heinecke JW, Oram JF. Oxidation of apolipoprotein A-I by myeloperoxidase impairs the initial interactions with ABCA1 required for signaling and cholesterol export. J Lipid Res. 2010;51(7):1849–1858. doi: 10.1194/jlr.M004085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69▪▪.Thukkani AK, McHowat J, Hsu FF, Brennan ML, Hazen SL, Ford DA. Identification of α-chloro fatty aldehydes and unsaturated lysophosphatidylcholine molecular species in human atherosclerotic lesions. Circulation. 2003;108(25):3128–3133. doi: 10.1161/01.CIR.0000104564.01539.6A. First demonstration that chlorinated lipids are present in vivo during human disease. Both α-chlorofatty aldehyde and unsaturated molecular species of lysophophatidylcholine are elevated in human atherosclerotic tissue. [DOI] [PubMed] [Google Scholar]
- 70.Thukkani AK, Albert CJ, Wildsmith KR, et al. Myeloperoxidase-derived reactive chlorinating species from human monocytes target plasmalogens in low density lipoprotein. J Biol Chem. 2003;278(38):36365–36372. doi: 10.1074/jbc.M305449200. [DOI] [PubMed] [Google Scholar]
- 71.McMillen TS, Heinecke JW, LeBoeuf RC. Expression of human myeloperoxidase by macrophages promotes atherosclerosis in mice. Circulation. 2005;111(21):2798–2804. doi: 10.1161/CIRCULATIONAHA.104.516278. [DOI] [PubMed] [Google Scholar]
- 72.Castellani LW, Chang JJ, Wang X, Lusis AJ, Reynolds WF. Transgenic mice express human mpo -463G/A alleles at atherosclerotic lesions, developing hyperlipidemia and obesity in -463G males. J Lipid Res. 2006;47(7):1366–1377. doi: 10.1194/jlr.M600005-JLR200. [DOI] [PubMed] [Google Scholar]
- 73.Kutter D, Devaquet P, Vanderstocken G, Paulus JM, Marchal V, Gothot A. Consequences of total and subtotal myeloperoxidase deficiency: risk or benefit? Acta Haematologica. 2000;104(1):10–15. doi: 10.1159/000041062. [DOI] [PubMed] [Google Scholar]
- 74.Nikpoor B, Turecki G, Fournier C, Theroux P, Rouleau GA. A functional myeloperoxidase polymorphic variant is associated with coronary artery disease in French–Canadians. Am Heart J. 2001;142(2):336–339. doi: 10.1067/mhj.2001.116769. [DOI] [PubMed] [Google Scholar]
- 75.Pecoits-Filho R, Stenvinkel P, Marchlewska A, et al. A functional variant of the myeloperoxidase gene is associated with cardiovascular disease in end-stage renal disease patients. Kidney Int Suppl. 2003;84:S172–S176. doi: 10.1046/j.1523-1755.63.s84.32.x. [DOI] [PubMed] [Google Scholar]
- 76.Mocatta TJ, Pilbrow AP, Cameron VA, et al. Plasma concentrations of myeloperoxidase predict mortality after myocardial infarction. J Am Coll Cardiol. 2007;49:1993–2000. doi: 10.1016/j.jacc.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 77.Jordan JE, Zhao Z-Q, Vinten-Johansen J. The role of neutrophils in myocardial ischemia-reperfusion injury. Cardiovasc Res. 1999;43(4):860–878. doi: 10.1016/s0008-6363(99)00187-x. [DOI] [PubMed] [Google Scholar]
- 78.Sheridan F, Dauber I, McMurtry I, Lesnefsky E, Horwitz L. Role of leukocytes in coronary vascular endothelial injury due to ischemia and reperfusion. Circ Res. 1991;69(6):1566–1574. doi: 10.1161/01.res.69.6.1566. [DOI] [PubMed] [Google Scholar]
- 79.Pabla R, Buda AJ, Flynn DM, et al. Nitric oxide attenuates neutrophil-mediated myocardial contractile dysfunction after ischemia and reperfusion. Circ Res. 1996;78(1):65–72. doi: 10.1161/01.res.78.1.65. [DOI] [PubMed] [Google Scholar]
- 80.Engler R, Covell J. Granulocytes cause reperfusion ventricular dysfunction after 15-minute ischemia in the dog. Circ Res. 1987;61(1):20–28. doi: 10.1161/01.res.61.1.20. [DOI] [PubMed] [Google Scholar]
- 81.Baldus S, Heeschen C, Meinertz T, et al. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003;108(12):1440–1445. doi: 10.1161/01.CIR.0000090690.67322.51. [DOI] [PubMed] [Google Scholar]
- 82.Brennan ML, Penn MS, Van Lente F, et al. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2003;349(17):1595–1604. doi: 10.1056/NEJMoa035003. [DOI] [PubMed] [Google Scholar]
- 83.Zhang R, Brennan ML, Fu X, et al. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA. 2001;286(17):2136–2142. doi: 10.1001/jama.286.17.2136. [DOI] [PubMed] [Google Scholar]
- 84.Marshall CJ, Nallaratnam M, Mocatta T, et al. Factors influencing local and systemic levels of plasma myeloperoxidase in ST-segment elevation acute myocardial infarction. Am J Cardiol. 2010;106(3):316–322. doi: 10.1016/j.amjcard.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 85.Rudolph V, Andrié RP, Rudolph TK, et al. Myeloperoxidase acts as a profibrotic mediator of atrial fibrillation. Nature Med. 2010;16(4):470–474. doi: 10.1038/nm.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lucchesi BR. Modulation of leukocyte-mediated myocardial reperfusion injury. Ann Rev Physiol. 1990;52:561–576. doi: 10.1146/annurev.ph.52.030190.003021. [DOI] [PubMed] [Google Scholar]
- 87.Mehta J, Dinerman J, Mehta P, et al. Neutrophil function in ischemic heart disease. Circulation. 1989;79(3):549–556. doi: 10.1161/01.cir.79.3.549. [DOI] [PubMed] [Google Scholar]
- 88.Hayasaki T, Kaikita K, Okuma T, et al. CC chemokine receptor-2 deficiency attenuates oxidative stress and infarct size caused by myocardial ischemia-reperfusion in mice. Circulation J. 2006;70(3):342–351. doi: 10.1253/circj.70.342. [DOI] [PubMed] [Google Scholar]
- 89.Ockaili R, Natarajan R, Salloum F, et al. HIF-1 activation attenuates postischemic myocardial injury: role for heme oxygenase-1 in modulating microvascular chemokine generation. Am J Physiol Heart Circ Physiol. 2005;289(2):H542–H548. doi: 10.1152/ajpheart.00089.2005. [DOI] [PubMed] [Google Scholar]
- 90.Litt MR, Jeremy RW, Weisman HF, Winkelstein JA, Becker LC. Neutrophil depletion limited to reperfusion reduces myocardial infarct size after 90 minutes of ischemia. Evidence for neutrophil-mediated reperfusion injury. Circulation. 1989;80(6):1816–1827. doi: 10.1161/01.cir.80.6.1816. [DOI] [PubMed] [Google Scholar]
- 91.Palazzo AJ, Jones SP, Anderson DC, Granger DN, Lefer DJ. Coronary endothelial P-selectin in pathogenesis of myocardial ischemia-reperfusion injury. Am J Physiol. 1998;275(5 Pt 2):H1865–H1872. doi: 10.1152/ajpheart.1998.275.5.H1865. [DOI] [PubMed] [Google Scholar]
- 92.Palazzo AJ, Jones SP, Girod WG, Anderson DC, Granger DN, Lefer DJ. Myocardial ischemia-reperfusion injury in CD18- and ICAM-1-deficient mice. Am J Physiol. 1998;275(6 Pt 2):H2300–H2307. doi: 10.1152/ajpheart.1998.275.6.H2300. [DOI] [PubMed] [Google Scholar]
- 93.Hansen PR. Role of neutrophils in myocardial ischemia and reperfusion. Circulation. 1995;91(6):1872–1885. doi: 10.1161/01.cir.91.6.1872. [DOI] [PubMed] [Google Scholar]
- 94.Chen SY, Hsiao G, Hwang HR, Cheng PY, Lee YM. Tetramethylpyrazine induces heme oxygenase-1 expression and attenuates myocardial ischemia/reperfusion injury in rats. J Biomed Sci. 2006;13(5):731–740. doi: 10.1007/s11373-006-9098-2. [DOI] [PubMed] [Google Scholar]
- 95.Duilio C, Ambrosio G, Kuppusamy P, DiPaula A, Becker LC, Zweier JL. Neutrophils are primary source of O2 radicals during reperfusion after prolonged myocardial ischemia. Am J Physiol Heart Circ Physiol. 2001;280(6):H2649–H2657. doi: 10.1152/ajpheart.2001.280.6.H2649. [DOI] [PubMed] [Google Scholar]
- 96.Shandelya SM, Kuppusamy P, Weisfeldt ML, Zweier JL. Evaluation of the role of polymorphonuclear leukocytes on contractile function in myocardial reperfusion injury. Evidence for plasma-mediated leukocyte activation. Circulation. 1993;87(2):536–546. doi: 10.1161/01.cir.87.2.536. [DOI] [PubMed] [Google Scholar]
- 97.Askari AT, Brennan ML, Zhou X, et al. Myeloperoxidase and plasminogen activator inhibitor-1 play a central role in ventricular modeling after myocardial infarction. J Exp Med. 2003;197:615–624. doi: 10.1084/jem.20021426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vasilyev N, Williams T, Brennan M-L, et al. Myeloperoxidase-generated oxidants modulate left ventricular remodeling but not infarct size after myocardial infarction. Circulation. 2005;112(18):2812–2820. doi: 10.1161/CIRCULATIONAHA.105.542340. [DOI] [PubMed] [Google Scholar]
- 99▪▪.Thukkani AK, Martinson BD, Albert CJ, Vogler GA, Ford DA. Neutrophil-mediated accumulation of 2-ClHDA during myocardial infarction: 2-ClHDA-mediated myocardial injury. Am J Physiol Heart Circ Physiol. 2005;288:H2955–H2964. doi: 10.1152/ajpheart.00834.2004. Demonstration that α-chlorofatty aldehydes accumulate in infarcted myocardium and elicit myocardial contractile dysfunction. [DOI] [PubMed] [Google Scholar]
- 100.Kato K, Shao Q, Elimban V, Lukas A, Dhalla NS. Mechanism of depression in cardiac sarcolemmal Na+-K+-ATPase by hypochlorous acid. Am J Physiol. 1998;275(3 Pt 1):C826–C831. doi: 10.1152/ajpcell.1998.275.3.C826. [DOI] [PubMed] [Google Scholar]
- 101.Okabe E, Takahashi S, Norisue M, et al. The effect of hypochlorous acid and hydrogen peroxide on coronary flow and arrhythmogenesis in myocardial ischemia and reperfusion. Eur J Pharmacol. 1993;248(1):33–39. doi: 10.1016/0926-6917(93)90022-i. [DOI] [PubMed] [Google Scholar]
- 102.Persad S, Elimban V, Siddiqui F, Dhalla NS. Alterations in cardiac membrane β-adrenoceptors and adenylyl cyclase due to hypochlorous acid. J Molec Cell Cardiol. 1999;31(1):101–111. doi: 10.1006/jmcc.1998.0847. [DOI] [PubMed] [Google Scholar]
- 103.Arnhold J, Osipov AN, Spalteholz H, Panasenko OM, Schiller J. Effects of hypochlorous acid on unsaturated phosphatidylcholines. Free Radic Biol Med. 2001;31(9):1111–1119. doi: 10.1016/s0891-5849(01)00695-5. [DOI] [PubMed] [Google Scholar]
- 104.Robaszkiewicz A, Greig FH, Pitt AR, Spickett CM, Bartosz G, Soszynski M. Effect of phosphatidylcholine chlorohydrins on human erythrocytes. Chem Phys Lipids. 2010;163(7):639–647. doi: 10.1016/j.chemphyslip.2010.05.201. [DOI] [PubMed] [Google Scholar]
- 105.Spickett CM. Chlorinated lipids and fatty acids: an emerging role in pathology. Pharmacol Ther. 2007;115(3):400–409. doi: 10.1016/j.pharmthera.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 106.Carr AC, van den Berg JJ, Winterbourn CC. Chlorination of cholesterol in cell membranes by hypochlorous acid. Arch Biochem Biophys. 1996;332(1):63–69. doi: 10.1006/abbi.1996.0317. [DOI] [PubMed] [Google Scholar]
- 107.Messner MC, Albert CJ, Hsu FF, Ford DA. Selective plasmenylcholine oxidation by hypochlorous acid: formation of lysophosphatidylcholine chlorohydrins. Chem Phys Lipids. 2006;144(1):34–44. doi: 10.1016/j.chemphyslip.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 108▪▪.Messner MC, Albert CJ, McHowat J, Ford DA. Identification of lysophosphatidylcholine–chlorohydrin in human atherosclerotic lesions. Lipids. 2008;43:243–249. doi: 10.1007/s11745-008-3151-z. First demonstration that chlorohydrins are present in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Flemmig J, Spalteholz H, Schubert K, Meier S, Arnhold J. Modification of phosphatidylserine by hypochlorous acid. Chem Phys Lipids. 2009;161(1):44–50. doi: 10.1016/j.chemphyslip.2009.06.144. [DOI] [PubMed] [Google Scholar]
- 110.Kawai Y, Kiyokawa H, Kimura Y, Kato Y, Tsuchiya K, Terao J. Hypochlorous acid-derived modification of phospholipids: characterization of aminophospholipids as regulatory molecules for lipid peroxidation. Biochemistry. 2006;45(47):14201–14211. doi: 10.1021/bi0610909. [DOI] [PubMed] [Google Scholar]
- 111.Nusshold C, Kollroser M, Kofeler H, et al. Hypochlorite modification of sphingomyelin generates chlorinated lipid species that induce apoptosis and proteome alterations in dopaminergic PC12 neurons in vitro. Free Radic Biol Med. 2010;48(12):1588–1600. doi: 10.1016/j.freeradbiomed.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brahmbhatt VV, Hsu FF, Kao JL, Frank EC, Ford DA. Novel carbonyl and nitrile products from reactive chlorinating species attack of lysosphingolipid. Chem Phys Lipids. 2007;145(2):72–84. doi: 10.1016/j.chemphyslip.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 113.Leßig J, Schiller J, Arnhold J, Fuchs B. Hypochlorous acid-mediated generation of glycerophosphocholine from unsaturated plasmalogen glycerophosphocholine lipids. J Lipid Res. 2007;48:1316–1324. doi: 10.1194/jlr.M600478-JLR200. [DOI] [PubMed] [Google Scholar]
- 114.Thukkani AK, Hsu FF, Crowley JR, Wysolmerski RB, Albert CJ, Ford DA. Reactive chlorinating species produced during neutrophil activation target tissue plasmalogens: production of the chemoattractant, 2-chlorohexadecanal. J Biol Chem. 2002;277(6):3842–3849. doi: 10.1074/jbc.M109489200. [DOI] [PubMed] [Google Scholar]
- 115.Marsche G, Heller R, Fauler G, et al. 2-chlorohexadecanal derived from hypochlorite-modified high-density lipoprotein-associated plasmalogen is a natural inhibitor of endothelial nitric oxide biosynthesis. Arterioscler Thromb Vasc Biol. 2004;24(12):2302–2306. doi: 10.1161/01.ATV.0000148703.43429.25. [DOI] [PubMed] [Google Scholar]
- 116.Skaff O, Pattison DI, Davies MJ. The vinyl ether linkages of plasmalogens are favored targets for myeloperoxidase-derived oxidants: a kinetic study. Biochemistry. 2008;47:8237–8245. doi: 10.1021/bi800786q. [DOI] [PubMed] [Google Scholar]
- 117.Albrich JM, McCarthy CA, Hurst JK. Biological reactivity of hypochlorous acid: implications for microbicidal mechanisms of leukocyte myeloperoxidase. Proc Natl Acad Sci USA. 1981;78(1):210–214. doi: 10.1073/pnas.78.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Heim WG, Appleman D, Pyfrom HT. Effects of 3-amino-1,2,4-triazole (AT) on catalase and other compounds. Am J Physiol. 1956;186(1):19–23. doi: 10.1152/ajplegacy.1956.186.1.19. [DOI] [PubMed] [Google Scholar]
- 119.Kukreja RC, Weaver AB, Hess ML. Stimulated human neutrophils damage cardiac sarcoplasmic reticulum function by generation of oxidants. Biochim Biophys Acta. 1989;990(2):198–205. doi: 10.1016/s0304-4165(89)80034-0. [DOI] [PubMed] [Google Scholar]
- 120.Podrez EA, Abu-Soud HM, Hazen SL. Myeloperoxidase-generated oxidants and atherosclerosis. Free Radic Biol Med. 2000;28(12):1717–1725. doi: 10.1016/s0891-5849(00)00229-x. [DOI] [PubMed] [Google Scholar]
- 121.Rausch PG, Moore TG. Granule enzymes of polymorphonuclear neutrophils: a phylogenetic comparison. Blood. 1975;46(6):913–919. [PubMed] [Google Scholar]
- 122.Wildsmith KR, Albert CJ, Hsu FF, Kao JL-F, Ford DA. Myeloperoxidase-derived 2-chlorohexadecanal forms Schiff bases with primary amines of ethanolamine glycerophospholipids and lysine. Chem Phys Lipids. 2006;139:157–170. doi: 10.1016/j.chemphyslip.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 123▪▪.Wildsmith KR, Albert CJ, Anbukumar DS, Ford DA. Metabolism of myeloperoxidase-derived 2-chlorohexadecanal. J Biol Chem. 2006;281:16849–16860. doi: 10.1074/jbc.M602505200. Presents the discovery that α-chlorofatty aldehyde is metabolized to α-chlorofatty acid and α-chlorofatty alcohol. Expansion of the chlorinated lipids to the level of describing it as a chlorinated lipidome. [DOI] [PubMed] [Google Scholar]
- 124.Rizzo WB, Craft DA, Dammann AL, Phillips MW. Fatty alcohol metabolism in cultured human fibroblasts. Evidence for a fatty alcohol cycle. J Biol Chem. 1987;262(36):17412–17419. [PubMed] [Google Scholar]
- 125.Anbukumar DS, Shornick LP, Albert CJ, et al. Chlorinated lipid species in activated human neutrophils: lipid metabolites of 2-chlorohexadecanal. J Lipid Res. 2010;51:1085–1092. doi: 10.1194/jlr.M003673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Thomas EL, Bozeman PM, Jefferson MM, King CC. Oxidation of bromide by the human leukocyte enzymes myeloperoxidase and eosinophil peroxidase. Formation of bromamines. J Biol Chem. 1995;270(7):2906–2913. doi: 10.1074/jbc.270.7.2906. [DOI] [PubMed] [Google Scholar]
- 127.Weiss SJ, Test ST, Eckmann CM, Roos D, Regiani S. Brominating oxidants generated by human eosinophils. Science. 1986;234(4773):200–203. doi: 10.1126/science.3018933. [DOI] [PubMed] [Google Scholar]
- 128.Albert CJ, Crowley JR, Hsu FF, Thukkani AK, Ford DA. Reactive brominating species produced by myeloperoxidase target the vinyl ether bond of plasmalogens: disparate utilization of sodium halides in the production of α-halo fatty aldehydes. J Biol Chem. 2002;277(7):4694–4703. doi: 10.1074/jbc.M110875200. [DOI] [PubMed] [Google Scholar]
- 129.Albert CJ, Thukkani AK, Heuertz RM, Slungaard A, Hazen SL, Ford DA. Eosinophil peroxidase-derived reactive brominating species target the vinyl ether bond of plasmalogens generating a novel chemoattractant, α-bromo fatty aldehyde. J Biol Chem. 2003;278(11):8942–8950. doi: 10.1074/jbc.m211634200. [DOI] [PubMed] [Google Scholar]
- 130.Ullen A, Fauler G, Kofeler H, et al. Mouse brain plasmalogens are targets for hypochlorous acid-mediated modification in vitro and in vivo. Free Radic Biol Med. 2010 doi: 10.1016/j.freeradbiomed.2010.08.025. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Carr AC, Vissers MC, Domigan NM, Winterbourn CC. Modification of red cell membrane lipids by hypochlorous acid and haemolysis by preformed lipid chlorohydrins. Redox Report. 1997;3(5–6):263–271. doi: 10.1080/13510002.1997.11747122. [DOI] [PubMed] [Google Scholar]
- 132.Iwase H, Yamada Y, Uemura H, et al. Effect of monochlorohydrins of linoleic acid on guinea-pig cardiac papillary muscles. Biochem Biophys Res Comm. 1997;231(2):295–298. doi: 10.1006/bbrc.1997.6090. [DOI] [PubMed] [Google Scholar]
- 133.Messner MC, Albert CJ, Ford DA. 2-chlorohexadecanal and 2-chlorohexadecanoic acid induce COX-2 expression in human coronary artery endothelial cells. Lipids. 2008;43(7):581–588. doi: 10.1007/s11745-008-3189-y. [DOI] [PMC free article] [PubMed] [Google Scholar]