Abstract
Background: The Women's Health Initiative Dietary Modification (DM) Trial was a randomized controlled trial that compared the effects of a low-fat (≤20% of total energy) or a usual diet in relation to chronic disease risk in postmenopausal women.
Objective: We characterized long-term body-composition changes associated with the DM trial and potential modifiers of these associations.
Design: In the DM trial, 48,835 women aged 50–79 y were randomly assigned to intervention (40%) or comparison (60%) groups. We studied a subset with whole-body dual-energy X-ray absorptiometry scans at baseline and during follow-up. Changes in fat mass (FM), lean mass (LM), and percentage body fat between the intervention (n = 1580) and comparison (n = 2731) groups at years 1, 3, and 6 were compared. By using generalized estimating equations, we calculated overall differences between groups and tested for interactions with age, diabetes, race-ethnicity (white, black, and Hispanic), body mass index (BMI), and hormone therapy (HT).
Results: The intervention women experienced significantly greater reductions in percentage body fat, FM, and LM at years 1 and 3 than did women in the comparison group (all P < 0.05). At year 6, only the FM change was significantly different between groups. Overall, the intervention was associated with reductions in percentage body fat (−0.8%; 95% CI: −1.0%, −0.6%), FM (−1.1 kg; 95% CI: −1.3, −0.8 kg), and LM (−0.17 kg; 95% CI: −0.28, −0.06 kg) during follow-up (all P < 0.003). Intervention associations varied by race-ethnicity, BMI, diabetes, and HT and remained significant after adjustment for physical activity.
Conclusion: This intervention was associated with modest long-term body-composition changes; the findings were more robust in years 1 and 3. This trial was registered at clinicaltrials.gov as NCT00000611.
See corresponding editorial on page 481
INTRODUCTION
The Dietary Modification (DM) Trial of the Women's Health Initiative (WHI) study was a randomized controlled trial of postmenopausal women that compared a low-fat dietary intervention with a usual diet in relation to breast and colon cancer and coronary heart disease (CHD) (1). Although this dietary modification intervention was hypothesized to reduce the risk of breast and colorectal cancer and CHD, after ≈8 y of follow-up the results from the intent-to-treat analyses suggested that the reduction in fat and concomitant increases in fruit, vegetable, and grain intakes did not significantly alter the risk of incident benign proliferative breast disease (2), invasive breast cancer (3), invasive colorectal cancer (4), treated diabetes (5), or CHD or stroke (6), although a long-term reduced risk of ovarian cancer (7) was found. Also, significant interaction between the intervention and the percentage of energy from fat at baseline was reported; women with a high percentage of energy from fat at baseline who greatly reduced their fat intake during the intervention had a reduced risk of invasive breast cancer (4).
The intervention was associated with significant changes in body weight (8) and endogenous estrogen concentrations (3). Compared with women not assigned to the intervention, women in the intervention group lost 2.2 kg more in the first year and maintained lower weight during an average of 7.5 y of follow-up (8). Similarly, women in the intervention group were less likely to experience increases in waist circumference during follow-up, although mean changes in waist-to-hip ratios were not significantly different between the intervention and comparison groups. Notably, the effects of the intervention on changes in other body-composition traits, such as whole-body lean and fat mass, have not been assessed previously in this cohort, but may help to inform the chronic disease findings and be of interest because weight and body mass index (BMI) may be imprecise estimates of body fat content, particularly across ethnic groups (9–12) and in postmenopausal women (13). Furthermore, data describing body composition changes with weight loss are limited in older adults (14).
Dual-energy X-ray absorptiometry (DXA), which directly assesses bone mineral content and the soft tissue surrounding the bone by measuring the amounts of fat and lean tissue, can be used to characterize body composition and provide precise estimation of fat, bone, and bone-free lean mass (15–17). By using longitudinal DXA measurements collected from a subset of women enrolled in the WHI DM trial, we investigated the relation between the low-fat dietary intervention and short- and long-term changes in whole-body percentage body fat and fat and lean mass measurements. We also assessed the effect modification of this relation by other baseline factors, including age, self-reported race-ethnicity, BMI, hormone therapy (HT), and treated type 2 diabetes status.
SUBJECTS AND METHODS
Subjects
The design and rationale of the WHI DM clinical trial were described previously (1, 3, 18, 19). A total of 48,835 postmenopausal women aged 50–79 y at enrollment in 1993–1998, were randomly assigned to a low-fat dietary intervention group (40%) or usual diet comparison group (60%) at 40 sites around the United States (20). Randomization was performed by using a permuted block algorithm and was stratified by clinical center and age group (3). Exclusion criteria for the DM trial included a history of breast, colorectal, and other cancers except for nonmelanoma skin cancer in the previous 10 y, medical conditions predictive of a survival time of <3 y, type I diabetes mellitus, or a high risk of lack of retention or intervention nonadherence (18). Women were also excluded if they 1) reported consumption of <600 kcal/d or >5000 kcal/d; 2) consumed a diet with <32% of total energy from fat, as estimated by food-frequency questionnaire before randomization; or 3) reported consuming ≥10 main meals/wk prepared outside of the home (18).
Demographic and personal characteristics, medication use, physical measurements (height, weight, and waist and hip circumferences), and self-reported medical history were collected at baseline (18). Body mass index (BMI) was calculated as measured mass (in kg)/measured height squared (in m). We defined a history of treated type 2 diabetes (T2D) as the self-report of the use of antidiabetic pills at any time or the use of injectable insulin for T2D at baseline. A proportion of women in the DM trial were also randomly assigned to a WHI Hormone Therapy (HT) trial at baseline; details about eligibility and treatments were published previously (21). Briefly, women underwent a 3-mo HT washout period and were randomly assigned to either estrogen alone (women with prior hysterectomy), estrogen plus progestin (women with intact uterus), or placebo. In the current analyses, a total of 476 women (15.6%) also participated in the HT trial. However, women not enrolled in the HT trial may have been using HT at baseline. Thus, we used 2 variables to describe hormone use: 1) among women enrolled in the HT trial, we included women randomly assigned to the HT arms only; and 2) all HT use including use as a part of the HT trial or baseline use in women not participating in the HT trial. The physical activity level, described as weekly energy expenditure, was calculated by using a standardized classification system (22) based on self-reported physical activity data collected from personal habit questionnaires at baseline and years 1, 3, and 6. All participants provided written informed consent. WHI protocol and consent forms were reviewed and approved by the Institutional Review Boards at each of the participating institutions.
Low-fat dietary intervention and usual diet comparison
The dietary intervention was designed to promote dietary change with the goals of reducing total fat intake to 20% of total energy, increasing vegetable and fruit intakes to ≥5 servings/d and increasing grain intake to ≥6 servings/d (1). Women in the intervention group received individual fat gram goals and participated in an intensive behavioral modification program (18) consisting of 18 group sessions in the first year and quarterly maintenance sessions until the trial ended in 2005. The intervention did not include reduced energy intake or weight-loss goals. Women randomly assigned to the comparison group were asked to maintain their usual diet by not changing their current normal eating patterns and were given a copy of Nutrition and Your Health: Dietary Guidelines for Americans (23). Neither group was asked to make changes in exercise or other health-related behaviors, although both groups received general health-related materials including exercise tips. Dietary intake for all DM participants was monitored by using the WHI food-frequency questionnaire, which was administered to all participants at baseline and year 1 and thereafter to a rotating sample of one-third of participants every 3 y.
DXA scans
The present study consists of a subset of women enrolled in the DM trial who received whole-body DXA scans at the 3 clinical centers where DXA was performed: Birmingham, AL; Tucson/Phoenix, AZ; and Pittsburgh, PA (n = 1580 in the intervention group and 2371 in the comparison group). With use of the same standard protocol at all sites, whole-body scans (including the head) using fan-beam mode were obtained from Hologic QDR scanners (QDR 2000, 2000+, or 4500W; Hologic, Waltham, MA) at randomization and during the follow-up visits at years 1, 3, 6, and 9 (24). Scanner performance was monitored longitudinally by using spine and whole-body phantom scans. Quality-control procedures implemented at the University of California, San Francisco, DXA Coordinating Center included investigation of unacceptable scans, outliers, and periodic review of random scans. In vivo cross-calibration was performed at 2 sites to convert QDR4500 to QDR2000-equivalent values when 2 QDR 2000 scanners were retired. These correction factors and adjustments for longitudinal changes in scanner performance were applied to participant scan results.
Self-identified white, black, and Hispanic women with ≥2 whole-body scan measurements (one at baseline randomization and one or more at any of the year 1, 3, or 6 follow-up visits) were included in these analyses (overall n = 1217 in the intervention group and n = 1836 in the comparison group). Women of other race-ethnicities were excluded because of very small numbers. The body-composition traits evaluated included percentage body fat, whole-body fat mass (in g), and whole-body lean mass (in g). One woman in the comparison group who had a very low percentage body fat (11.4%) but a high BMI (36.4) at baseline and was found to have more concordant BMI and body fat measures during follow-up was excluded from analyses.
Statistical analysis
To evaluate the association between the DM intervention and changes in percentage body fat, total fat, and lean body mass at years 1, 3, and 6, we used 2 sample t tests to compare both mean measurements at each of the visits and mean changes from baseline at each of the visits in the intervention and comparison groups. In sensitivity analyses, we compared measurements between groups using the Wilcoxon's rank-sum (Mann-Whitney) test, which does not require normality assumptions, and repeated analyses using loge-transformed measurements; the results were similar and are not shown. We also assessed the overall change in body composition traits (ie, the population-averaged effect of the intervention on change in body-composition traits) using a generalized estimating equation (GEE) model with an unstructured correlation matrix and semirobust SEs to account for the correlation between the repeated change measurements (25). We report β coefficients and 95% CIs. Models were adjusted for visit year and scanner ID; a total of 7 scanners were used at the 4 study sites. Although women were randomly assigned at each clinical site, additional adjustment for baseline characteristics was explored. To assess whether the association between the dietary intervention and change in DXA measurements varied by visit year or by baseline characteristics—including age, race-ethnicity, BMI, T2D status, and postmenopausal HT—GEE models were extended to include (multiplicative scale) interaction terms, and formal tests of interactions were conducted. All analyses were performed by using Stata version 10.1 statistical software (2009; Stata Corporation, College Station, TX).
RESULTS
Baseline demographic, medical and lifestyle characteristics were similar between the intervention and comparison groups with available DXA measurements at each of the time points (data not shown) and between all women contributing to any of the analyses (Table 1). The one exception was that women in the intervention group were slightly more likely to report at baseline a history of diabetes requiring prescription medication than were women in the comparison group (chi-square test, P = 0.03). Women in the intervention and comparison groups had mean ages of 62 and 62 y and mean BMIs of 29.1 and 29.3, respectively, and most (≥78%) described their race-ethnicity as white. The prevalence of current cigarette smoking was relatively low in the intervention and comparison groups: 8% and 6%, respectively. For the numbers of women contributing to the analyses at each year, see Supplemental Table 1 under “Supplemental data” in the online issue.
TABLE 1.
Study population baseline characteristics1
| Baseline characteristic | Intervention (n = 1217) | Comparison (n = 1836) |
| Age (y) | 62.2 ± 7.12 | 62.3 ± 7.2 |
| 50–54 y [n (%)] | 203 (17) | 303 (17) |
| 55–59 y [n (%)] | 268 (22) | 406 (22) |
| 60–64 y [n (%)] | 280 (23) | 418 (23) |
| 65–69 y [n (%)] | 251 (21) | 373 (20) |
| 70–79 y [n (%)] | 215 (18) | 336 (18) |
| Race-ethnicity [n (%)] | ||
| Black or African American | 215 (18) | 309 (17) |
| Hispanic/Latina | 47 (4) | 89 (5) |
| White, not of Hispanic origin | 955 (79) | 1438 (78) |
| Highest level of education [n (%)] | ||
| <High school diploma | 78 (7) | 132 (7) |
| High school diploma or GED | 283 (23) | 403 (22) |
| >High school | 492 (41) | 740 (40) |
| College graduate or higher | 356 (30) | 552 (30) |
| BMI category [n (%)] | ||
| Underweight, 17.8–18.4 kg/m2 | 3 (<1) | 1 (<1) |
| Normal, 18.5–24.9 kg/m2 | 301 (25) | 469 (26) |
| Overweight, 25.0–29.9 kg/m2 | 449 (37) | 654 (36) |
| Obesity class I, 30.0–34.9 kg/m2 | 273 (23) | 445 (24) |
| Obesity class II, 35.0–39.9 kg/m2 | 136 (11) | 193 (11) |
| Obesity class III, ≥40 kg/m2 | 52 (4) | 72 (4) |
| Height (cm) | 161.9 ± 6.1 | 161.8 ± 6.1 |
| Weight (kg) | 76.2 ± 15.4 | 76.7 ± 15.5 |
| BMI (kg/m2) | 29.1 ± 5.6 | 29.3 ± 5.6 |
| Waist-to-hip ratio | 0.81 ± 0.07 | 0.81 ± 0.07 |
| Weekly energy expenditure (METs)3 | 5.0 (1.0–12.8) | 5.8 (0.8–13.5) |
| Current smoker [n (%)] | 91 (8) | 115 (6) |
| HT arms of HT trial [n (%)]4 | 155 (52) | 92 (51) |
| HT use [n (%)] | 595 (49) | 929 (51) |
| Treated for type 2 diabetes [n (%)] | 94 (8) | 105 (6)5 |
Data for women contributing to any of the analyses are shown; includes women with baseline data who also had data at years 1, 3, or 6. GED, General Equivalency Diploma; METs, metabolic equivalents; HT, hormone therapy.
Mean ± SD (all such values).
Values are medians; interquartile ranges in parentheses. Baseline physical activity data were available only for a subset of participants: n = 867 (intervention group) and 1265 (comparison group).
Percentage of the total number of women included in these analyses who were also enrolled in the HT trial (n = 476).
Significantly different from the intervention group, P = 0.03 (chi-square test).
DXA measurements at baseline
At baseline, mean (±SD) percentage body fat measurements did not differ significantly (P = 0.96) between the intervention (45.2 ± 6.6%) and comparison (45.2 ± 6.8%) groups (see Supplemental Table 2 under “Supplemental data” in the online issue). Mean fat and lean masses (see Supplemental Table 2 under “Supplemental data” in the online issue) and BMI (not shown) also were not significantly different between the groups at baseline (all P > 0.8).
TABLE 2.
Overall changes in dual-energy X-ray measurements during follow-up in women in the intervention group compared with the comparison group
| Crude model1 |
Model 22 |
Model 33 |
Model 44 |
|||||
| Measure | β (95% CI) | P value | β (95% CI) | P value | β (95% CI) | P value | β (95% CI) | P value |
| Body fat (%) | −0.8 (−1.0, −0.6) | <0.001 | −0.8 (−1.0,-0.6) | <0.001 | −0.4 (−0.5, −0.2) | <0.001 | −0.5 (−0.7, −0.2) | <0.001 |
| Fat mass (g) | −1070.5 (−1309.3, −831.7) | <0.001 | −1066.5 (−1303.4, −829.5) | <0.001 | −357.9 (−531.6, −184.2) | <0.001 | −682.3 (−1014.5, −350.1) | <0.001 |
| Lean mass (g) | −169.2 (−277.0, −61.3) | 0.002 | −169.1 (−276.2, −61.9) | 0.002 | −25.5 (−129.1, 78.1) | 0.63 | −189.2 (−335.8, −42.7) | 0.011 |
Generalized estimating equation (GEE) model adjusted for scanner ID and visit year.
GEE model adjusted for scanner ID, visit year, baseline age (continuous), and study site.
GEE model adjusted for scanner ID, visit year, baseline age (continuous), study site, and change in BMI.
GEE model adjusted for scanner ID, visit year, baseline age (continuous), study site, and change in physical activity. A smaller subset had longitudinal physical activity data: n = 812 (intervention group) and 1220 (comparison group).
Change in DXA measurements over time
Change in percentage body fat was positively correlated with change in fat mass (ρ = 0.89, P < 0.001). Much weaker, although significant, correlations were found for lean and fat mass change (ρ = 0.14) and for lean mass and percentage body fat change (ρ = −0.23), which was inversely correlated. Change in BMI was strongly correlated with change in fat mass (ρ = 0.74); weaker correlations were found for changes in percentage body fat (ρ = 0.57) and lean mass (ρ = 0.34).
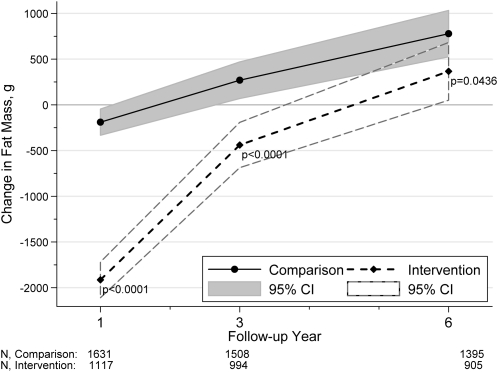
Changes in DXA measurements in the intervention and comparison groups are shown in the figures. Percentage body fat decreased in both groups from baseline to year 1; women in the intervention group lost significantly more percentage body fat (P < 0.001; Figure 1). Between baseline and year 3, women in the intervention group lost percentage body fat, whereas women in the comparison group gained percentage body fat; the difference in change between the groups was modest (<1%) but statistically significant (P < 0.0001). By year 6, mean percentage body fat had increased from baseline in both groups; although the women in the intervention group gained slightly less, their change from baseline was no longer significantly different from the change observed in women in the comparison group (P = 0.057). Fat mass changes from baseline followed patterns similar to those for percentage body fat; the largest differences were observed during the first year of follow-up, with women in the intervention group losing 1.72 ± 0.12 kg (mean ± SE) more than women in the comparison group (Figure 2). Lean mass decreased in both groups during follow-up (Figure 3), with women in the intervention group losing significantly more in years 1 (P = 0.004) and 3 (P = 0.038), but not in year 6 (P = 0.076).
FIGURE 1.
Mean changes in percentage body fat from baseline to years 1, 3, and 6. P values (2-sided t test) indicate the difference between the intervention and comparison groups at years 1, 3, and 6. With the use of generalized estimating equation models, interaction between the intervention and visit year was tested. The interaction term was significant, P < 0.001 (Wald test), suggesting that the association between the intervention and change in percentage body fat varied over time.
FIGURE 2.
Mean changes in fat mass from baseline to years 1, 3, and 6. P values (2-sided t test) indicate the difference between the intervention and comparison groups at years 1, 3, and 6. With the use of generalized estimating equation models, interaction between the intervention and visit year was tested. The interaction term was significant, P < 0.001 (Wald test), suggesting that the association between the intervention and change in fat mass varied over time.
FIGURE 3.
Mean changes in lean mass from baseline to years 1, 3, and 6. P values (2-sided t test) indicate the difference between the intervention and comparison groups at years 1, 3, and 6. With the use of generalized estimating equation models, interaction between the intervention and visit year was tested. We found that the association between the intervention and change in lean mass did not significantly vary over time, P = 0.92 (Wald test).
By using GEE, we compared overall mean changes in percentage body fat, total fat mass, and total lean mass during follow-up between the intervention and comparison groups (Table 2). Although a group-by-time interaction is evident in Figures 1 and 2, the GEE models in Table 2 show composite results over 6 y of follow-up; we chose not to stratify results by visit year because the overall change was of interest. Women in the intervention group lost significantly more percentage body fat, fat mass, and lean mass than did women in the comparison group (all P < 0.003). Adjustment for visit year, or the randomization variables study site and age category, had little effect on the estimates. A finer adjustment for age, with the use of a continuous variable instead of a categorical variable, slightly attenuated the estimates (except for percentage body fat), but the P values did not change. Because proper randomization typically ensures equal distribution of characteristics that may confound observed associations, additional adjustments for other baseline characteristics had little, if any, effect on the estimates and were not included in the final models (data not shown). To determine whether the intervention was associated with DXA measurements above and beyond weight changes captured in BMI, we also adjusted GEE models for changes in BMI during the same time periods (Table 2). As expected, adjustment for BMI greatly attenuates the association; however, the intervention was still associated with small decreases in percentage body fat and total fat mass, perhaps not captured in the cruder BMI measurement. Similarly, we tested for associations between the intervention and DXA measurements above and beyond self-reported changes in physical activity (Table 2) for the same time period. Intervention associations with percentage body fat and fat mass were attenuated by adjustment for changes in physical activity, whereas lean mass associations became slightly stronger; all remained statistically significant.
Effect modification of the dietary intervention
Changes in total percentage body fat and fat mass associated with the intervention significantly varied by self-reported race-ethnicity (P < 0.01 for both) and treated diabetes status (P < 0.01 and P = 0.04, respectively) (Tables 3 and 4). Significant decreases in percentage body fat and fat mass were observed in 1) white women, but not in black or Hispanic women, and 2) women without treated diabetes, but not in women with treated diabetes. Intervention-related changes in percentage body fat significantly varied by baseline BMI category (P = 0.0004). The largest losses in percentage body fat associated with the intervention were found in normal-weight women. Fat mass estimates followed similar trends (Table 4), but the interaction was not significant (P = 0.24). Investigation of baseline percentage body fat or the fat mass interaction instead of BMI category yielded results consistent with the BMI interaction findings (data not shown).
TABLE 3.
Overall changes in percentage body fat in women in the intervention group compared with the comparison group, stratified by baseline characteristics1
| Baseline characteristic | Difference in percentage body fat (95% CI)2 | P value | P for interaction |
| % | |||
| Age3 | 0.18 | ||
| 50–54 y | −0.32 (−0.76, 0.12) | 0.15 | |
| 55–59 y | −0.71 (−1.09, −0.34) | <0.001 | |
| 60–64 y | −0.98 (−1.35, −0.61) | <0.001 | |
| 65–69 y | −0.83 (−1.23, −0.44) | <0.001 | |
| 70–79 y | −0.98 (−1.42, −0.55) | <0.001 | |
| Race-ethnicity | 0.0015 | ||
| Blacks | −0.19 (−0.59, 0.21) | 0.36 | |
| Hispanics | −0.12 (−0.96, 0.72) | 0.79 | |
| Whites | −0.95 (−1.16, −0.74) | <0.001 | |
| Treated diabetes | 0.007 | ||
| No | −0.85 (−1.04, −0.66) | <0.001 | |
| Yes | 0.18 (−0.54, 0.91) | 0.62 | |
| BMI4 | 0.0004 | ||
| Normal | −1.38 (−1.80, −0.97) | <0.001 | |
| Overweight | −0.87 (−1.17, −0.56) | <0.001 | |
| Obesity class I | −0.41 (−0.76, −0.06) | 0.021 | |
| Obesity class II | −0.03 (−0.55, 0.49) | 0.91 | |
| Obesity class III | −0.56 (−1.24, 0.12) | 0.11 | |
| HT use | 0.37 | ||
| No | −0.86 (−1.12, −0.60) | <0.001 | |
| Yes | −0.70 (−0.96, −0.43) | <0.001 | |
| HT use (HT trial only) | 0.40 | ||
| No | −0.38 (−1.04, 0.28) | 0.26 | |
| Yes | −0.78 (−1.47, −0.09) | 0.028 |
HT, hormone therapy.
β Values (and 95% CIs) from generalized estimating equation models reflect the mean change in percentage body fat from baseline in the intervention group compared with the comparison group during follow-up, stratified by the above baseline characteristics.
With age as a continuous variable, the interaction term was nearly significant (P = 0.041).
The small numbers of underweight women were combined with normal-weight women for these analyses.
TABLE 4.
Overall change in fat mass in women in the intervention group compared with the comparison group, stratified by baseline characteristics1
| Baseline characteristics | Difference in fat mass (95% CI)2 | P value | P for interaction |
| g | |||
| Age | 0.36 | ||
| 50–54 y | −544.03 (−1106.23, 18.17) | 0.058 | |
| 55–59 y | −1110.84 (−1645.56, −576.12) | <0.001 | |
| 60–64 y | −1138.46 (−1636.21, −640.71) | <0.001 | |
| 65–69 y | −1244.10 (−1756.64, −731.55) | <0.001 | |
| 70–79 y | −1257.75 (−1796.17, −719.34) | <0.001 | |
| Race-ethnicity | 0.0008 | ||
| Blacks | −268.8 (−852.3, 314.6) | 0.37 | |
| Hispanics | −53.1 (−1064.5, 958.3) | 0.92 | |
| Whites | −1317.3 (−1587.3, −1047.4) | <0.001 | |
| Treated diabetes | 0.04 | ||
| No | −1143.5 (−1390.7, −896.2) | <0.001 | |
| Yes | −91.70 (−1065.2, 881.82) | 0.85 | |
| BMI3 | 0.24 | ||
| Normal | −1254.89 (−1631.43, −878.34) | <0.001 | |
| Overweight | −1315.85 (−1683.23, −948.46) | <0.001 | |
| Obesity class I | −774.44 (−1294.08, −254.81) | 0.003 | |
| Obesity class II | −424.12 (−1401.44, 553.21) | 0.40 | |
| Obesity class III | −1568.095 (−3087.46, −48.73) | 0.04 | |
| HT use | 0.02 | ||
| No | −1349.50 (−1689.46, −1009.54) | <0.001 | |
| Yes | −796.17 (−1131.70, −460.64) | <0.001 | |
| HT use (HT trial only) | 0.26 | ||
| No | −1304.34 (−2167.98, −440.71) | 0.003 | |
| Yes | −568.29 (−1550.81, 414.23) | 0.26 |
HT, hormone therapy.
β Values (and 95% CIs) from generalized estimating equation models reflect the mean change in fat mass from baseline in the intervention group compared with the comparison group during follow-up, stratified by the above baseline characteristics.
The small numbers of underweight women were combined with normal-weight women for these analyses.
No significant interactions between the intervention and baseline characteristics were seen for changes in lean body mass, with the exception of HT use: P = 0.049 for all users and P < 0.001 for HT trial new users only (Table 5). In stratified analyses, HT use, whether broad or limited to HT trial new users, was not associated with changes in lean mass, whereas significant losses in lean mass were observed in non-HT users. Similar HT trends were observed for fat mass changes, but not for percentage body fat, in which no significant interaction was detected.
TABLE 5.
Overall changes in lean mass in women in the intervention group compared with the comparison group, stratified by baseline characteristics1
| Baseline characteristics | Difference in lean mass (95% CI)2 | P value | P for interaction |
| g | |||
| Age | 0.88 | ||
| 50–54 y | −118.79 (−397.65, 160.07) | 0.40 | |
| 55–59 y | −236.63 (−474.05, 0.78) | 0.05 | |
| 60–64 y | −28.64 (−259.25, 201.97) | 0.81 | |
| 65–69 y | −331.01 (−548.20, −113.82) | 0.003 | |
| 70–79 y | −112.65 (−348.58, 123.27) | 0.35 | |
| Race-ethnicity | 0.11 | ||
| Blacks | 94.44 (−211.24, 400.11) | 0.55 | |
| Hispanics | 8.88 (−491.13, 508.88) | 0.97 | |
| Whites | −230.11 (−346.32, −113.91) | <0.001 | |
| Treated diabetes | 0.62 | ||
| No | −149.02 (−257.31, −40.73) | 0.007 | |
| Yes | −289.22 (−829.77, 251.32) | 0.29 | |
| BMI3 | 0.089 | ||
| Normal | 27.45 (−130.22, 185.12) | 0.73 | |
| Overweight | −282.43 (−442.30, −122.57) | 0.001 | |
| Obesity class I | −165.09 (−413.29, 83.12) | 0.19 | |
| Obesity class II | −305.86 (−752.33, 140.61) | 0.18 | |
| Obesity class III | 0.74 (−712.22, 713.70) | 0.998 | |
| HT use | 0.049 | ||
| No | −271.63 (−423.77, −119.49) | <0.001 | |
| Yes | −55.32 (−207.76, 97.13) | 0.48 | |
| HT use (HT trial only) | <0.001 | ||
| No | −1059.64 (−1465.72, −653.56) | <0.001 | |
| Yes | 365.78 (−48.63, 780.18) | 0.08 |
HT, hormone therapy.
β Values (and 95% CIs) from generalized estimating equation models reflect the mean change in lean mass from baseline in the intervention group compared with the comparison group during follow-up, stratified by the above baseline characteristics.
The small numbers of underweight women were combined with normal-weight women for these analyses.
DISCUSSION
To our knowledge, this study is one of the first to describe long-term body-composition changes accompanying minor weight loss in postmenopausal women. The DM intervention was associated with modest long-term body-composition changes, although intervention effects were more robust in years 1 and 3 after randomization. In absolute terms, women lost more fat than lean mass. Although women in the intervention group lost more fat mass at all time points than did women in the comparison group, they also lost more lean mass. Lean mass losses are generally not desirable because of their associations with loss of function and disability (26); however, lean mass changes in the intervention group on average were small. A more refined measure of fat-free mass change including skeletal muscle mass may be more clinically relevant (27), but was not available.
Few studies have characterized longer-term (>12 mo) body-composition changes associated with low-fat dietary interventions (28–30); however, in general, modest differences in long-term weight change (<3 kg) between intervention groups are reported (30). In a small study that assessed body-composition changes associated with a low-fat diet (15% of total energy from fat) in healthy women (mean age: 46 y), body fat decreased by 1.4% at year 1 (31). This reduction is comparable with our findings at 1 y, although the WHI fat recommendation was less stringent. The modest decreases in lean mass and increases in percentage body fat and fat mass that we observed in women in the comparison group are also consistent with reported body-composition changes with aging in the literature. Increasing age is associated with loss of lean mass (32) and specifically skeletal muscle mass, which comprises ≈50% of lean mass in healthy men and women (33, 34). Longitudinal studies have found that lean tissue mass losses in older adults are accompanied by fat mass gains in the absence of weight change (32, 34). A recent study contrasts cohort and age-related changes in body composition in adults aged 70–79 y (33). Although the age range is limited, younger cohorts had greater percentage body fat than older cohorts. During 5 y of follow-up, investigators found that percentage body fat initially increased with age (as a result of decreases in lean mass and increases in fat mass) and then leveled. An important consequence of body-composition changes with aging is that older adults will tend to have a higher percentage body fat than younger adults with the same BMI, which underlies the importance of body-composition assessment or consideration of the age-dependent context of BMI (35). Furthermore, the study highlights the advantage of considering changes in a longitudinal study, such as ours. Because individual trajectories over time are considered, analyses are not confounded by cohort effects that may be present in cross-sectional comparisons of groups differing by age.
Interestingly, we found no robust evidence of effect modification by age, although intervention associations with changes in percentage body fat and total fat mass were somewhat weaker in the youngest age group. In contrast, intervention associations differed significantly by treated diabetes status and race-ethnicity. White, but not black or Hispanic, women experienced significant losses in percentage body fat and fat mass. Similarly, women without treated diabetes experienced significant losses, whereas treated patients with diabetes did not. In part, differences in adherence between subgroups may have accounted for these findings (20); in sensitivity analyses, adherent black women had modest, but significant, reductions in percentage body fat and fat mass. Given the smaller numbers, we may have had a reduced power to detect associations in Hispanic women and women with diabetes; however, coefficients for these subgroups were closer to the null, which suggested little or no association.
Postmenopausal HT also appeared to modify intervention associations with fat and lean mass, with HT users experiencing smaller reductions than nonusers. These findings are not unexpected; long-term HT use has been associated with changes in muscle composition and increases in power (36), yet a recent WHI study found no evidence of HT treatment effects on change in performance-based measures of physical function (37). Results were generally similar for both definitions of HT use (trial use only compared with all users), although interactions influencing lean mass were more pronounced in the HT trial group. Differential HT interactions could have been due to cessation of therapy or reduced adherence in the nontrial users. However, confirmation of these exploratory results is needed.
Several limitations of our analysis deserve mention. The results may not be generalizable to populations differing by cohort age, health status, or other factors. Our analysis was secondary, because weight loss was not a primary trial outcome or even an intervention goal and was limited to women who had scans at baseline and at least once during a mean follow-up of 8.1 y. Although randomized, women were not blinded to the intervention. Women in the comparison group may have made adjustments to other lifestyle factors; these modifications may have biased our findings toward the null. Indeed, we did observe small decreases in percentage body fat and fat mass between baseline and year 1 in the comparison group. Conversely, nontargeted dietary or lifestyle changes resulting from the intervention could also have influence the findings. Changes in physical activity may potentially confound results because of their strong association with weight (38); adjustment for physical activity changes during follow-up did attenuate our findings, but they remained significant. In addition, women may not have been compliant. DM trial adherence was assessed previously by using different approaches (3, 20). A subset of women in the intervention group reported mean decreases in dietary fat as a percentage of total energy of 11%, 10%, and 8% in years 1, 3, and 6 of the trial, respectively (3). These data also suggest a small reduction in energy consumption in the intervention group (3), which is consistent with the modest weight loss in women in the intervention group during the trial (8). Furthermore, they suggest that, in practice, the intervention may have been modestly hypocaloric. Using a different measure of adherence based on regular participation in the DM group sessions and attendance at yearly exams (3), we conducted sensitivity analyses in adherent women and found slightly more robust percentage body fat and fat mass results. Several studies have validated DXA for the assessment of body-composition changes (39–41), and recent developments in scanners and software have resulted in improved precision and image resolution. However, DXA has some limitations in obese individuals, in whom it may be less precise (15), and in characterizations of lean tissue mass (42, 43). Thus, our BMI interaction findings should be interpreted cautiously given the small numbers in some categories and potential for increased measurement error. Lean tissue mass includes body water, so that changes in hydration may affect estimates (44). We expect this error to be nondifferential, but acknowledge that hydration differences resulting from nutritional changes may have biased the observed lean mass differences.
Despite these limitations, this longitudinal study, which was conducted in a large well-characterized cohort, has considerable strengths. Multiple DXA measurements collected using a standardized clinical protocol allowed us to investigate long-term body-composition changes. We have presented within-individual changes in body composition with aging (in the comparison group only) and changes associated with the intervention. The large sample size provided ample power to detect small differences and to investigate potential modifiers of the associations. We identified subgroups of women with varying responses; these subgroups may be targeted for additional research or modified interventions. Furthermore, these data contribute to future research on age-related changes in body composition and relations between health status and weight change in older adults. Indeed, our study is unique in having characterized long-term body-composition changes associated with a full-scale public health–oriented dietary intervention. Promotion of small changes in diet and exercise, decreases in body fat, or even prevention of weight gain have been discussed as important public health strategies for combating the obesity epidemic (45, 46). Our analysis is particularly informative on this standpoint; we describe an intervention that was significantly associated with these key outcomes (decreases in percentage body fat and fat mass) in an ethnically diverse population of US postmenopausal women.
Supplementary Material
Acknowledgments
We gratefully acknowledge the WHI participants and investigators and extend special thanks to Mary Pettinger and Ann Schwartz for advice on WHI data and DXA quality assurance, respectively.
The authors’ responsibilities were as follows—CLC: study design, statistical analysis, interpretation of data, and manuscript preparation; UP, MLN, LT, BH, and JW-W: study design, interpretation of data, and critical revision of the manuscript; CK and RP: study design, assistance with statistical analysis and interpretation of data; and MA, SAAB, LS, MV, and NB: interpretation of data and critical revision of the manuscript. BH serves as a consultant and lectures for Merck/Schering-Plough and receives research support in the form of donation of medications from Merck/Schering-Plough. None of the other authors reported a personal or financial conflict of interest.
REFERENCES
- 1.Ritenbaugh C, Patterson RE, Chlebowski RT, et al. The Women's Health Initiative Dietary Modification trial: overview and baseline characteristics of participants. Ann Epidemiol 2003;13(suppl):S87–97 [DOI] [PubMed] [Google Scholar]
- 2.Rohan TE, Negassa A, Caan B, et al. Low-fat dietary pattern and risk of benign proliferative breast disease: a randomized, controlled dietary modification trial. Cancer Prev Res (Phia) 2008;1:275–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prentice RL, Caan B, Chlebowski RT, et al. Low-fat dietary pattern and risk of invasive breast cancer: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006;295:629–42 [DOI] [PubMed] [Google Scholar]
- 4.Beresford SA, Johnson KC, Ritenbaugh C, et al. Low-fat dietary pattern and risk of colorectal cancer: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006;295:643–54 [DOI] [PubMed] [Google Scholar]
- 5.Tinker LF, Bonds DE, Margolis KL, et al. Low-fat dietary pattern and risk of treated diabetes mellitus in postmenopausal women: the Women's Health Initiative randomized controlled dietary modification trial. Arch Intern Med 2008;168:1500–11 [DOI] [PubMed] [Google Scholar]
- 6.Howard BV, Van Horn L, Hsia J, et al. Low-fat dietary pattern and risk of cardiovascular disease: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006;295:655–66 [DOI] [PubMed] [Google Scholar]
- 7.Prentice RL, Thomson CA, Caan B, et al. Low-fat dietary pattern and cancer incidence in the Women's Health Initiative Dietary Modification Randomized Controlled Trial. J Natl Cancer Inst 2007;99:1534–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howard BV, Manson JE, Stefanick ML, et al. Low-fat dietary pattern and weight change over 7 years: the Women's Health Initiative Dietary Modification Trial. JAMA 2006;295:39–49 [DOI] [PubMed] [Google Scholar]
- 9.Evans EM, Rowe DA, Racette SB, Ross KM, McAuley E. Is the current BMI obesity classification appropriate for black and white postmenopausal women? Int J Obes (Lond) 2006;30:837–43 [DOI] [PubMed] [Google Scholar]
- 10.Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat percent relationship. Obes Rev 2002;3:141–6 [DOI] [PubMed] [Google Scholar]
- 11.Rush EC, Goedecke JH, Jennings C, et al. BMI, fat and muscle differences in urban women of five ethnicities from two countries. Int J Obes (Lond) 2007;31:1232–9 [DOI] [PubMed] [Google Scholar]
- 12.Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord 1998;22:1164–71 [DOI] [PubMed] [Google Scholar]
- 13.Blew RM, Sardinha LB, Milliken LA, et al. Assessing the validity of body mass index standards in early postmenopausal women. Obes Res 2002;10:799–808 [DOI] [PubMed] [Google Scholar]
- 14.Bales CW, Buhr GT. Body mass trajectory, energy balance, and weight loss as determinants of health and mortality in older adults. Obes Facts 2009;2:171–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laskey MA. Dual-energy X-ray absorptiometry and body composition. Nutrition 1996;12:45–51 [DOI] [PubMed] [Google Scholar]
- 16.Jensen MD, Kanaley JA, Roust LR, et al. Assessment of body composition with use of dual-energy x-ray absorptiometry: evaluation and comparison with other methods. Mayo Clin Proc 1993;68:867–73 [DOI] [PubMed] [Google Scholar]
- 17.Haarbo J, Gotfredsen A, Hassager C, Christiansen C. Validation of body composition by dual energy X-ray absorptiometry (DEXA). Clin Physiol 1991;11:331–41 [DOI] [PubMed] [Google Scholar]
- 18.The Women's Health Initiative Study Group Design of the Women's Health Initiative clinical trial and observational study. Control Clin Trials 1998;19:61–109 [DOI] [PubMed] [Google Scholar]
- 19.Hays J, Hunt JR, Hubbell FA, et al. The Women's Health Initiative recruitment methods and results. Ann Epidemiol 2003;13(suppl):S18–77 [DOI] [PubMed] [Google Scholar]
- 20.Tinker LF, Rosal MC, Young AF, et al. Predictors of dietary change and maintenance in the Women's Health Initiative Dietary Modification Trial. J Am Diet Assoc 2007;107:1155–66 [DOI] [PubMed] [Google Scholar]
- 21.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA 2002;288:321–33 [DOI] [PubMed] [Google Scholar]
- 22.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000;32(suppl):S498–504 [DOI] [PubMed] [Google Scholar]
- 23.US Department of Agriculture Dietary guidelines for Americans. 4th ed Washington, DC: USDA, 1995. (Home and Garden Bulletin 232.) [Google Scholar]
- 24.Jackson RD, LaCroix AZ, Cauley JA, McGowan J. The Women's Health Initiative calcium-vitamin D trial: overview and baseline characteristics of participants. Ann Epidemiol 2003;13(suppl):S98–106 [DOI] [PubMed] [Google Scholar]
- 25.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986;42:121–30 [PubMed] [Google Scholar]
- 26.Miller SL, Wolfe RR. The danger of weight loss in the elderly. J Nutr Health Aging 2008;12:487–91 [DOI] [PubMed] [Google Scholar]
- 27.Visser M. Towards a definition of sarcopenia–results from epidemiologic studies. J Nutr Health Aging 2009;13:713–6 [DOI] [PubMed] [Google Scholar]
- 28.Astrup A, Toubro S, Raben A, Skov AR. The role of low-fat diets and fat substitutes in body weight management: what have we learned from clinical studies? J Am Diet Assoc 1997;97(Suppl):S82–7 [DOI] [PubMed] [Google Scholar]
- 29.Willett WC, Manson JE, Stampfer MJ, et al. Weight, weight change, and coronary heart disease in women. Risk within the 'normal’ weight range. JAMA 1995;273:461–5 [DOI] [PubMed] [Google Scholar]
- 30.Willett WC. Is dietary fat a major determinant of body fat? Am J Clin Nutr 1998;67(suppl):556S–62S [DOI] [PubMed] [Google Scholar]
- 31.Kasim SE, Martino S, Kim PN, et al. Dietary and anthropometric determinants of plasma lipoproteins during a long-term low-fat diet in healthy women. Am J Clin Nutr 1993;57:146–53 [DOI] [PubMed] [Google Scholar]
- 32.Gallagher D, Ruts E, Visser M, et al. Weight stability masks sarcopenia in elderly men and women. Am J Physiol Endocrinol Metab 2000;279:E366–75 [DOI] [PubMed] [Google Scholar]
- 33.Ding J, Kritchevsky SB, Newman AB, et al. Effects of birth cohort and age on body composition in a sample of community-based elderly. Am J Clin Nutr 2007;85:405–10 [DOI] [PubMed] [Google Scholar]
- 34.Raguso CA, Kyle U, Kossovsky MP, et al. A 3-year longitudinal study on body composition changes in the elderly: role of physical exercise. Clin Nutr 2006;25:573–80 [DOI] [PubMed] [Google Scholar]
- 35.Flegal KM, Shepherd JA, Looker AC, et al. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr 2009;89:500–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ronkainen PH, Kovanen V, Alen M, et al. Postmenopausal hormone replacement therapy modifies skeletal muscle composition and function: a study with monozygotic twin pairs. J Appl Physiol 2009;107:25–33 [DOI] [PubMed] [Google Scholar]
- 37.Michael YL, Gold R, Manson JE, et al. Hormone therapy and physical function change among older women in the Women's Health Initiative: a randomized controlled trial. Menopause 2010;17:295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DiPietro L. Physical activity, body weight, and adiposity: an epidemiologic perspective. Exerc Sport Sci Rev 1995;23:275–303 [PubMed] [Google Scholar]
- 39.Visser M, Pahor M, Tylavsky F, et al. One- and two-year change in body composition as measured by DXA in a population-based cohort of older men and women. J Appl Physiol 2003;94:2368–74 [DOI] [PubMed] [Google Scholar]
- 40.Houtkooper LB, Going SB, Sproul J, Blew RM, Lohman TG. Comparison of methods for assessing body-composition changes over 1 y in postmenopausal women. Am J Clin Nutr 2000;72:401–6 [DOI] [PubMed] [Google Scholar]
- 41.Visser M, Fuerst T, Lang T, Salamone L, Harris TB. Validity of fan-beam dual-energy X-ray absorptiometry for measuring fat-free mass and leg muscle mass. Health, Aging, and Body Composition Study–Dual-Energy X-ray Absorptiometry and Body Composition Working Group. J Appl Physiol 1999;87:1513–20 [DOI] [PubMed] [Google Scholar]
- 42.Clasey JL, Hartman ML, Kanaley J, et al. Body composition by DEXA in older adults: accuracy and influence of scan mode. Med Sci Sports Exerc 1997;29:560–7 [DOI] [PubMed] [Google Scholar]
- 43.Roubenoff R, Kehayias JJ, Dawson-Hughes B, Heymsfield SB. Use of dual-energy x-ray absorptiometry in body-composition studies: not yet a “gold standard”. Am J Clin Nutr 1993;58:589–91 [DOI] [PubMed] [Google Scholar]
- 44.Woodrow G. Body composition analysis techniques in the aged adult: indications and limitations. Curr Opin Clin Nutr Metab Care 2009;12:8–14 [DOI] [PubMed] [Google Scholar]
- 45.Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science 2003;299:853–5 [DOI] [PubMed] [Google Scholar]
- 46.Swinburn BA, Caterson I, Seidell JC, James WP. Diet, nutrition and the prevention of excess weight gain and obesity. Public Health Nutr 2004;7(1A):123–46 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.