Abstract
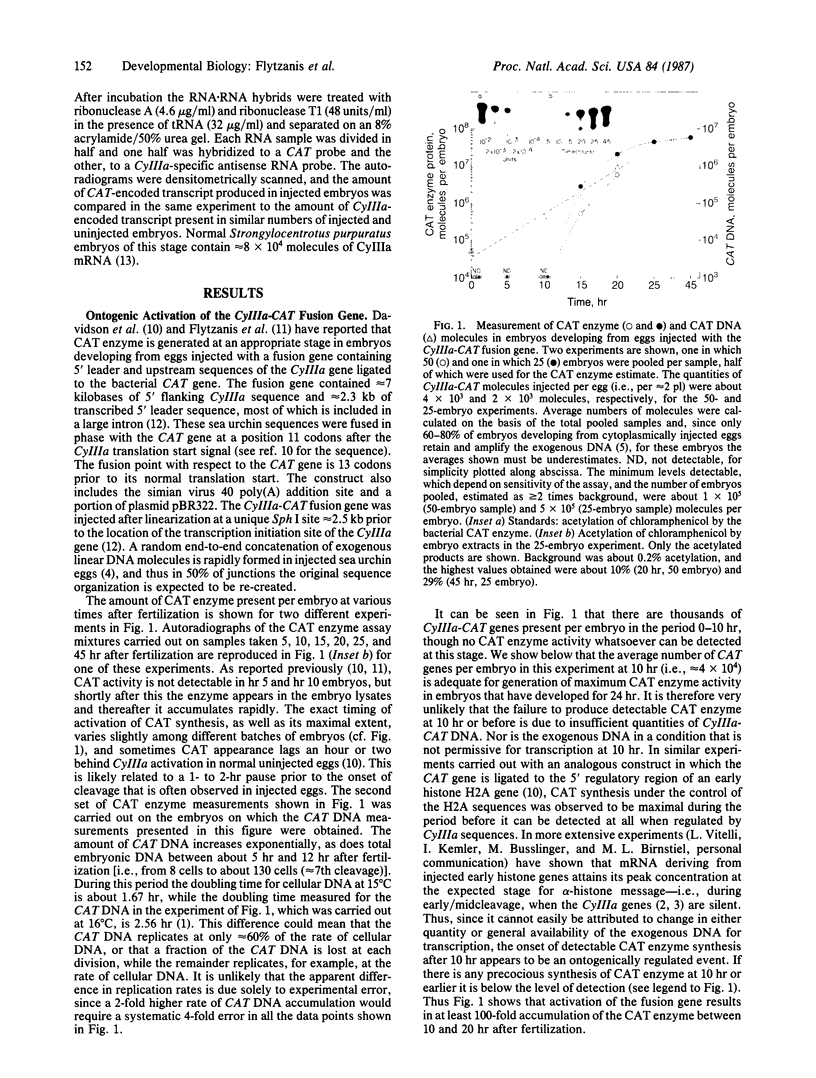
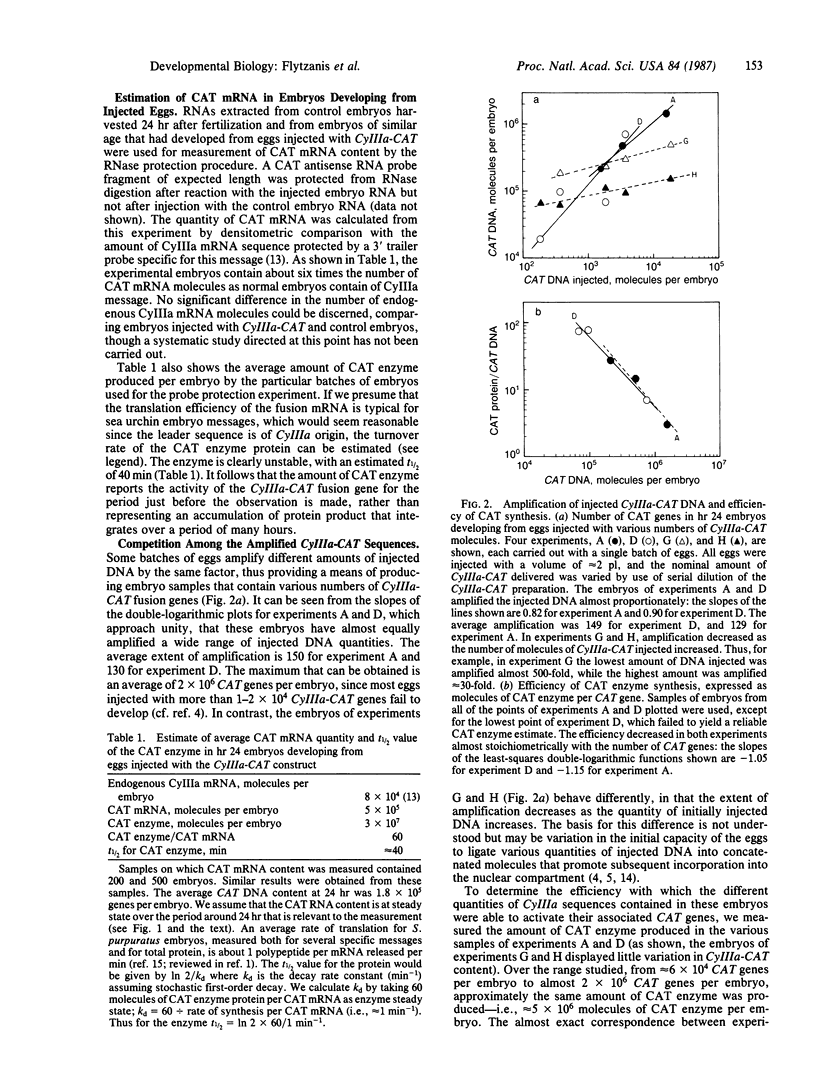
Regulatory sequences of a sea urchin cytoskeletal actin gene (CyIIIa) were ligated to the bacterial gene coding for chloramphenicol acetyltransferase (CAT; acetyl-CoA:chloramphenicol O3-acetyltransferase, EC 2.3.1.28) and the construct was injected into unfertilized sea urchin eggs. CAT activity is detected at the early blastula stage, when transcripts of the endogenous CyIIIa gene normally appear. Our measurements show that during activation the amount of CAT enzyme increases at least 100-fold; that there are present in late blastula stage embryos about 5 X 10(5) molecules of CAT mRNA (i.e., approximately 6 times the number of endogenous CyIIIa mRNAs); and that within the range studied the amount of CAT enzyme produced is independent of the number of CyIIIa-CAT genes incorporated per embryo, probably because the genes are present in excess of factors required for their activation. Activation of the CyIIIa-CAT construct is seriously inhibited, or abolished, by successive deletions of upstream CyIIIa sequences.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunk C. F., Jones K. C., James T. W. Assay for nanogram quantities of DNA in cellular homogenates. Anal Biochem. 1979 Jan 15;92(2):497–500. doi: 10.1016/0003-2697(79)90690-0. [DOI] [PubMed] [Google Scholar]
- Cox K. H., Angerer L. M., Lee J. J., Davidson E. H., Angerer R. C. Cell lineage-specific programs of expression of multiple actin genes during sea urchin embryogenesis. J Mol Biol. 1986 Mar 20;188(2):159–172. doi: 10.1016/0022-2836(86)90301-3. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Flytzanis C. N., Lee J. J., Robinson J. J., Rose S. J., 3rd, Sucov H. M. Lineage-specific gene expression in the sea urchin embryo. Cold Spring Harb Symp Quant Biol. 1985;50:321–328. doi: 10.1101/sqb.1985.050.01.041. [DOI] [PubMed] [Google Scholar]
- Flytzanis C. N., McMahon A. P., Hough-Evans B. R., Katula K. S., Britten R. J., Davidson E. H. Persistence and integration of cloned DNA in postembryonic sea urchins. Dev Biol. 1985 Apr;108(2):431–442. doi: 10.1016/0012-1606(85)90046-6. [DOI] [PubMed] [Google Scholar]
- Forbes D. J., Kirschner M. W., Newport J. W. Spontaneous formation of nucleus-like structures around bacteriophage DNA microinjected into Xenopus eggs. Cell. 1983 Aug;34(1):13–23. doi: 10.1016/0092-8674(83)90132-0. [DOI] [PubMed] [Google Scholar]
- Goustin A. S. Two temporal phases for the control of histone gene activity in cleaving sea urchin embryos (S. purpuratus). Dev Biol. 1981 Oct 15;87(1):163–175. doi: 10.1016/0012-1606(81)90069-5. [DOI] [PubMed] [Google Scholar]
- Hough-Evans B. R., Wold B. J., Ernst S. G., Britten R. J., Davidson E. H. Appearance and persistence of maternal RNA sequences in sea urchin development. Dev Biol. 1977 Oct 1;60(1):258–277. doi: 10.1016/0012-1606(77)90123-3. [DOI] [PubMed] [Google Scholar]
- Lee J. J., Calzone F. J., Britten R. J., Angerer R. C., Davidson E. H. Activation of sea urchin actin genes during embryogenesis. Measurement of transcript accumulation from five different genes in Strongylocentrotus purpuratus. J Mol Biol. 1986 Mar 20;188(2):173–183. doi: 10.1016/0022-2836(86)90302-5. [DOI] [PubMed] [Google Scholar]
- Lee J. J., Shott R. J., Rose S. J., 3rd, Thomas T. L., Britten R. J., Davidson E. H. Sea urchin actin gene subtypes. Gene number, linkage and evolution. J Mol Biol. 1984 Jan 15;172(2):149–176. doi: 10.1016/s0022-2836(84)80035-2. [DOI] [PubMed] [Google Scholar]
- McMahon A. P., Flytzanis C. N., Hough-Evans B. R., Katula K. S., Britten R. J., Davidson E. H. Introduction of cloned DNA into sea urchin egg cytoplasm: replication and persistence during embryogenesis. Dev Biol. 1985 Apr;108(2):420–430. doi: 10.1016/0012-1606(85)90045-4. [DOI] [PubMed] [Google Scholar]
- McMahon A. P., Novak T. J., Britten R. J., Davidson E. H. Inducible expression of a cloned heat shock fusion gene in sea urchin embryos. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7490–7494. doi: 10.1073/pnas.81.23.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shott R. J., Lee J. J., Britten R. J., Davidson E. H. Differential expression of the actin gene family of Strongylocentrotus purpuratus. Dev Biol. 1984 Feb;101(2):295–306. doi: 10.1016/0012-1606(84)90143-x. [DOI] [PubMed] [Google Scholar]




