Abstract
Background: Zinc is involved in many essential cellular functions, including DNA repair and immune system maintenance. Although experimental evidence supports a role for zinc in prostate carcinogenesis, epidemiologic data are inconsistent; no data on cancer-specific survival have been reported.
Objective: Our objective was to determine whether dietary zinc assessed near the time of prostate cancer diagnosis is associated with improved disease-specific survival.
Design: This population-based cohort consists of 525 men aged <80 y from Örebro County, Sweden, with a diagnosis of prostate cancer made between 1989 and 1994. Study participants completed self-administered food-frequency questionnaires, and zinc intake was derived from nutrient databases. Cox proportional hazards regression was used to estimate multivariate hazard ratios (HRs) and 95% CIs for time to death from prostate cancer as well as death from all causes through February 2009 by quartile (Q) of dietary zinc intake. Models were also stratified by disease stage at diagnosis (localized or advanced).
Results: With a median follow-up of 6.4 y, 218 (42%) men died of prostate cancer and 257 (49%) died of other causes. High dietary zinc intake was associated with a reduced risk of prostate cancer–specific mortality (HRQ4 vs Q1: 0.64; 95% CI: 0.44, 0.94; P for trend = 0.05) in the study population. The association was stronger in men with localized tumors (HR: 0.24; 95% CI: 0.09, 0.66; P for trend = 0.005). Zinc intake was not associated with mortality from other causes.
Conclusion: These results suggest that high dietary intake of zinc is associated with lower prostate cancer–specific mortality after diagnosis, particularly in men with localized disease.
INTRODUCTION
Prostate cancer is the most commonly diagnosed cancer among men in many industrialized nations and is a leading cause of cancer death. Because the incidence of prostate cancer is greatly influenced by the diagnostic intensity in the population, comparison of trends in prostate cancer mortality may provide greater clues about the etiology of clinically relevant disease. The ≈12-fold difference in international prostate cancer mortality rates between low-risk countries in Asia and high-risk countries such as Sweden (1) suggests that environmental components, such as diet, may account for some of the observed variation (2, 3). The identification of chemopreventive factors for prostate cancer death could make marked improvements in the health of men. However, current evidence regarding the association between specific dietary factors and prostate cancer–specific survival is lacking (4, 5).
Zinc is an essential element with antioxidant properties that is involved in a range of cellular functions, including DNA repair and apoptosis. Zinc helps in the maintenance of the immune system (6), and at moderate concentrations may reduce inflammation and oxidative stress (7–12). The concentration of zinc in prostate tissue is higher than that in any other tissue in the body; however, the influence of dietary zinc intake on these concentrations is unknown (13, 14). Zinc concentrations in prostate tumors appear to be lower than those in adjacent normal tissue, because malignant cells lose the ability to accumulate zinc (14, 15). Greater local zinc depletion in prostate tumors has been associated with a higher Gleason score (16). An inverse association between zinc intake and prostate cancer has long been suspected, and physiologic and experimental evidence supports the hypothesis (17), although the results of epidemiologic studies have been mixed (13, 18–20).
For men, the US and Nordic Nutrition recommendations are 11 and 9 mg/d, respectively (21, 22). Some zinc supplements have amounts that are magnitudes higher than those that can be achieved through diet alone. There may be a limit to the benefits of zinc, because daily long-term intakes >150 mg have been associated with adverse health effects, including reduced immune function (23).
We examined the association between zinc and all-cause and cancer-specific mortality in a study of Swedish men with prostate cancer followed for an average of 7.6 y. The original case-control study from which our study population is drawn found no association between dietary zinc and incidence of total or advanced prostate cancer (24). Sweden has one of the highest prostate cancer mortality rates in the world (19.3/100,000 in 2007), accounting for 22% of cancer deaths in men (25). To our knowledge, our study is the first to examine the dietary intake of zinc in relation to prostate cancer–specific mortality.
SUBJECTS AND METHODS
Study design
The study population consists of prostate cancer patients recruited as part of a population-based case-control study in Örebro County, Sweden (24, 26, 27). Eligible cases were men with newly diagnosed prostate cancer who were born in Sweden, living in Örebro, and aged <80 y during 2 recruitment periods: January 1989 to September 1991 and May 1992 to July 1994. The final enrollment of 525 cases resulted in an 80.6% case participation rate. Twelve percent of eligible cases refused participation, whereas 7% were either too ill to participate or died before the interview (26). All cases were confirmed by a study pathologist. Prostate cancer screening did not occur in this population at the time of the study; as a result, most cases were diagnosed due to prostate-related symptoms.
Tumors were graded by using the TNM classification system and were defined as localized if confined to the prostate (T1-T2/M0) and as advanced (stage T3-T4/M0 or T1-T4/M1) if tumors progressed through the capsule or metastasized. Information on primary prostate cancer treatment was obtained through a review of medical records. Skeletal scintigraphy and radiography were used to assess the presence of skeletal metastases (26). The study was approved by the ethical review board of Uppsala University, Sweden (28).
Exposure assessment
Dietary information was collected through self-administered food-frequency questionnaires distributed to participants before they received a final diagnosis of prostate cancer. Most men completed the questionnaires just before or within 3 mo of diagnosis. In-person interviews were conducted in subjects recruited between January 1989 and September 1991 to assess nondietary factors such as family history of cancer and smoking and to make anthropomorphic measurements. Questionnaire data for cases diagnosed during the second study period (May 1992 to July 1994) were obtained through self-administered questionnaires received by mail and completed by phone if needed. Body mass index (BMI) was calculated by using clinical measurements.
The food-frequency questionnaire included 68 food items common to the Swedish diet. Frequencies of consumption were multiplied by standard portion sizes based on the 1988 Swedish National Food Administration handbook and on the nutrient composition of foods specific to Sweden to determine daily energy and nutrient intakes (29). Through this method, the nutrient content of individual food sources was also calculated, including zinc and iron. In the original case-control study, the questionnaire was validated in 87 control subjects who completed four 1-wk dietary records 3–4 mo apart. Pearson's correlation coefficients between energy-adjusted nutrients assessed from the questionnaires and dietary records ranged from 0.2 to 0.6, including 0.5 for energy intake; correlations for zinc and iron were not reported (26).
Outcome ascertainment
All Swedish residents are assigned a national registration number—a unique identifier that permits linkage across nationwide health registries. Deaths were identified through linkage to the Swedish Cause of Death Registry—a nationwide register with >99% coverage and a reported high reliability for prostate cancer death (28, 30). A committee of study urologists (OA, S-OA, and J-EJ) confirmed the cause of death through a review of medical records.
Statistical analysis
Cases were followed from diagnosis until the date of death from prostate cancer or censored at the date of death from other causes or the end of follow-up (1 February 2009). Dietary intakes of log10-transformed zinc were adjusted for nonalcohol energy intake by using the residual method, regressing total caloric intake against zinc intake (31). Energy-adjusted zinc values were then categorized into quartiles based on the distribution in the study population.
Survival analyses were conducted by using Cox proportional hazards regression to estimate multivariate hazard ratios (HRs) and 95% CIs on associations of time to death from prostate cancer associated with dietary zinc intake, with a comparison of each of the top 3 quartiles with the lowest quartile. To assess potential competing risks from other causes of death, secondary analyses estimated HRs for time to death from other causes as well as total mortality associated with zinc intake. Multivariate models were adjusted for total energy intake, age at diagnosis (41–64, 65–69, 70–74, or 75–79 y), family history of prostate cancer (yes or no), primary treatment (hormones, prostatectomy, other treatment, or watchful waiting), smoking status (never, former, or current), calendar year of diagnosis (1989–1991 or 1992–1994), tumor differentiation (well, moderately, or poorly differentiated), and World Health Organization categories of BMI (in kg/m2) (32), with underweight and normal-weight participants combined in the reference category. Four participants missing information on height and BMI were assigned the median values. Tests for linear trend across categories were conducted by modeling the median value of each nutrient quartile as a continuous variable in a multivariate model. A P value <0.05 from the Wald test indicated statistical significance. In addition to a main-effects analysis, the models were stratified by localized or advanced stage at diagnosis to examine whether associations between zinc and prostate cancer mortality differed according to clinical stage. We also conducted a sensitivity analysis to reassess the associations between zinc and prostate cancer survival, excluding deaths that occurred during the first 2 y of follow-up.
The proportional hazards assumption was tested by creating an interaction term between zinc intake (median of each quartile) and follow-up time (continuous) and adding it to the multivariable model of the main effect of zinc. The interaction term was not statistically significant; thus, we concluded that the proportional hazards assumption was met.
We examined a potential nonlinear relation between zinc and prostate cancer survival by fitting a restricted cubic spline with 3 knots to a Cox proportional hazards regression model using a continuous variable for energy-adjusted zinc and adjusting for all other covariates. The model was analyzed by using the macro designed by Govindarajulu et al (33), reporting the P value from the likelihood ratio test for nonlinearity. All analyses were conducted by using SAS version 9.1 (SAS Institute, Cary, NC).
We explored the contribution of specific zinc sources in our study population by calculating the zinc content of food groups: grains, meat (beef and pork, excluding poultry), fish, and dairy products. Those food groups contributing ≥20% of total zinc intake were added to a multivariate Cox model including zinc to determine whether the effects of zinc on prostate cancer survival were independent of specific food sources. Because the study population consumes large amounts of meat, an important contributor of dietary zinc, we explored potential main effects of meat intake (beef and pork, excluding poultry) separately in multivariate Cox models adjusted for other covariates, excluding zinc from the models. Furthermore, because interactions between iron and zinc have been observed in humans (34), and many dietary sources of zinc also contain iron, we investigated the main effect of dietary iron intake on prostate cancer–specific mortality and modeled the association of dietary zinc and prostate cancer death with additional adjustment for iron and meat intake.
RESULTS
During up to 20 y of follow-up (median: 6.4 y; range: 0.1–20 y), 218 (42%) men had died of prostate cancer and 257 (49%) from other causes. In this cohort, 295 men (56%) were diagnosed at an advanced stage. Selected characteristics of the study population are shown in Table 1. Men who eventually died of their disease more often received their diagnosis at a later clinical stage and had more poorly differentiated tumors. Men in all 3 outcome groups had similar mean dietary zinc intakes, just >14 mg/d.
TABLE 1.
Selected characteristics of the Örebro prostate cancer study population by outcome1
| All cases2 (n = 525) | Prostate cancer death (n = 218) | Other death (n = 257) | |
| Age at diagnosis (y) | 70.7 ± 5.93 | 69.6 ± 6.3 | 72.5 ± 4.8 |
| Follow-up time (y) | 7.6 ± 5.5 | 5.0 ± 3.7 | 7.8 ± 4.9 |
| Year of diagnosis [n (%)] | |||
| 1989–1991 | 256 (48.8) | 106 (48.6) | 120 (46.7) |
| 1992–1994 | 269 (51.2) | 112 (51.4) | 137 (53.3) |
| BMI at diagnosis [n (%)] | |||
| ≤24.9 kg/m2 | 218 (41.6) | 92 (42.2) | 107 (41.7) |
| 25–29.9 kg/m2 | 250 (47.6) | 102 (46.8) | 122 (47.5) |
| ≥30 kg/m2 | 53 (10.1) | 22 (10.1) | 26 (10.1) |
| Missing | 4 (0.80) | 2 (0.90) | 2 (0.80) |
| Smoking status [n (%)] | |||
| Never smoker | 154 (2.3) | 70 (32.1) | 66 (25.7) |
| Former smoker | 205 (39.1) | 80 (36.7) | 105 (40.9) |
| Current smoker | 137 (26.1) | 55 (25.2) | 72 (28.0) |
| Missing | 29 (5.5) | 13 (6.0) | 14 (5.5) |
| Family history, father or brother [n (%)] | |||
| Yes | 61 (11.6) | 22 (10.1) | 31 (12.1) |
| No | 464 (88.4) | 196 (89.9) | 226 (87.9) |
| Tumor differentiation, WHO classification [n (%)] | |||
| Good | 273 (52.0) | 67 (30.7) | 166 (64.6) |
| Moderate | 192 (36.6) | 104 (47.7) | 80 (31.1) |
| Poor | 60 (11.4) | 47 (21.6) | 11 (4.3) |
| Tumor stage [n (%)] | |||
| T0/T1 | 134 (25.5) | 16 (7.3) | 94 (36.6) |
| T2 | 96 (18.3) | 28 (12.8) | 54 (21.0) |
| T3 | 172 (32.8) | 71 (32.6) | 90 (35.0) |
| T4/M1 | 123 (23.4) | 103 (47.3) | 19 (7.4) |
| Treatment [n (%)] | |||
| Watchful waiting | 350 (66.7) | 120 (55.0) | 205 (79.8) |
| Hormone therapy | 128 (24.4) | 86 (39.4) | 36 (14.0) |
| Prostatectomy | 22 (4.2) | 4 (1.8) | 8 (3.1) |
| Other treatment | 25 (4.8) | 8 (3.7) | 8 (3.1) |
| Dietary intake | |||
| Nonalcohol energy (kcal/d) | 2101 ± 567 | 2094 ± 588 | 2118 ± 552 |
| Zinc (mg/d) | 14.2 ± 4.1 | 14.2 ± 4.2 | 14.3 ± 4.1 |
| Red meat (g/d) | 139 ± 79 | 133 ± 69 | 144 ± 87 |
WHO, World Health Organization.
Fifty men remained alive at the end of follow-up, 1 February 2009.
Mean ± SD (all such values).
Results of the survival analysis are shown in Table 2. Dietary zinc intake was associated with lower prostate cancer mortality, with a comparison of men in the highest quartile (>15.6 mg/d) with those in the lowest quartile of zinc intake (HR: 0.64; 95% CI: 0.44, 0.94; P for trend = 0.05). Zinc intake did not appear to significantly reduce the risk of death from other causes (HR: 0.92; 95% CI: 0.64, 1.33; P for trend = 0.66), although there was a 22% nonsignificant reduction in risk of all-cause mortality. On stratification by stage at diagnosis (Table 3), the protective association with high zinc intake appeared restricted to men in whom the diagnosis of prostate cancer was made at an early stage. In men with localized tumors, those in the highest quartile of zinc intake were 76% less likely to die of their disease (95% CI: 0.09, 0.66; P for trend = 0.005) than were men in the lowest quartile. In comparison, the HR was attenuated for those in whom the diagnosis of prostate cancer was made at an advanced stage (HR: 0.71; 95% CI: 0.46, 1.11; P for trend = 0.25). No association was made between dietary zinc and mortality from other causes after stratification by stage. No significant associations were observed between zinc intake and prostate cancer–specific mortality when results were stratified by level of differentiation (good compared with moderate/poor) at diagnosis (data not shown).
TABLE 2.
Results of survival analysis for the association between quartile (Q) of zinc intake and prostate cancer–specific, other-cause, and all-cause mortality in a prospective cohort of 525 prostate cancer cases
| Hazard ratio (95% CI) |
||||
| Mortality and quartile of intake | Zinc intake1 | No. of events | Model 12 | Model 23 |
| mg/d | ||||
| Prostate cancer mortality (n = 218) | ||||
| Q1 | 9.0–12.8 | 60 | 1.00 (referent) | 1.00 (referent) |
| Q2 | 12.8–14.1 | 46 | 0.68 (0.46, 1.00) | 0.67 (0.45, 1.01) |
| Q3 | 14.1–15.6 | 56 | 0.84 (0.58, 1.22) | 0.83 (0.57, 1.23) |
| Q4 | 15.6–20.1 | 56 | 0.64 (0.44, 0.94) | 0.64 (0.42, 0.98) |
| P for trend | 0.05 | 0.08 | ||
| Other-cause mortality (n = 257) | ||||
| Q1 | 9.0–12.8 | 61 | 1.00 (referent) | 1.00 (referent) |
| Q2 | 12.8–14.1 | 73 | 0.98 (0.69, 1.38) | 1.00 (0.70, 1.42) |
| Q3 | 14.1–15.6 | 63 | 0.97 (0.67, 1.40) | 1.01 (0.69, 1.48) |
| Q4 | 15.6–20.1 | 60 | 0.92 (0.64, 1.33) | 0.99 (0.66, 1.48) |
| P for trend | 0.66 | 0.98 | ||
| All-cause mortality (n = 475) | ||||
| Q1 | 9.0–12.8 | 121 | 1.00 (referent) | 1.00 (referent) |
| Q2 | 12.8–14.1 | 119 | 0.82 (0.63, 1.06) | 0.83 (0.64, 1.08) |
| Q3 | 14.1–15.6 | 119 | 0.92 (0.71, 1.19) | 0.94 (0.72, 1.23) |
| Q4 | 15.6–20.1 | 116 | 0.78 (0.60, 1.02) | 0.82 (0.61, 1.10) |
| P for trend | 0.13 | 0.30 | ||
Energy-adjusted values.
Cox proportional hazards model adjusted for age at diagnosis, total energy, family history of prostate cancer, primary treatment, differentiation, smoking status, calendar year of diagnosis, and BMI.
Model additionally adjusted for dietary iron.
TABLE 3.
Results of survival analysis by clinical stage subgroups for the association between quartile (Q) of zinc intake and prostate cancer-specific, other-cause, and all-cause mortality in a prospective cohort of 525 prostate cancer cases
| Localized stage (n = 230)1 |
Advanced stage (n = 295)2 |
||||||
| Hazard ratio (95% CI) |
Hazard ratio (95% CI) |
||||||
| Mortality and quartile of intake | Zinc intake3 | No. of events | Model 14 | Model 25 | No. of events | Model 14 | Model 25 |
| mg/d | |||||||
| Prostate cancer mortality (n = 218) | |||||||
| Q1 | 9.0–12.8 | 18 | 1.00 (referent) | 1.00 (referent) | 42 | 1.00 (referent) | 1.00 (referent) |
| Q2 | 12.8–14.1 | 10 | 0.38 (0.16, 0.89) | 0.40 (0.17, 0.94) | 36 | 0.73 (0.46, 1.15) | 0.70 (0.43, 1.13) |
| Q3 | 14.1–15.6 | 10 | 0.52 (0.23, 1.21) | 0.58 (0.24, 1.39) | 46 | 0.94 (0.61, 1.45) | 0.90 (0.57, 1.43) |
| Q4 | 15.6–20.1 | 6 | 0.24 (0.09, 0.66) | 0.30 (0.10, 0.92) | 50 | 0.71 (0.46, 1.11) | 0.67 (0.41, 1.10) |
| P for trend | 0.005 | 0.03 | 0.25 | 0.22 | |||
| Other-cause mortality (n = 257) | |||||||
| Q1 | 9.0–12.8 | 38 | 1.00 (referent) | 1.00 (referent) | 23 | 1.00 (referent) | 1.00 (referent) |
| Q2 | 12.8–14.1 | 38 | 1.01 (0.63, 1.34) | 1.02 (0.63, 1.65) | 35 | 0.90 (0.51, 1.58) | 0.94 (0.53, 1.67) |
| Q3 | 14.1–15.6 | 37 | 0.83 (0.51, 1.34) | 0.85 (0.52, 1.41) | 26 | 1.33 (0.74, 2.40) | 1.41 (0.76, 2.64) |
| Q4 | 15.6–20.1 | 35 | 0.88 (0.54, 1.44) | 0.93 (0.54, 1.63) | 25 | 0.94 (0.51, 1.75) | 1.01 (0.52, 1.97) |
| P for trend | 0.46 | 0.65 | 0.81 | 0.66 | |||
| All-cause mortality (n = 475) | |||||||
| Q1 | 9.0–12.8 | 56 | 1.00 (referent) | 1.00 (referent) | 65 | 1.00 (referent) | 1.00 (referent) |
| Q2 | 12.8–14.1 | 48 | 0.78 (0.52, 1.17) | 0.80 (0.53, 1.20) | 71 | 0.83 (0.58, 1.17) | 0.83 (0.58, 1.19) |
| Q3 | 14.1–15.6 | 47 | 0.73 (0.48, 1.10) | 0.77 (0.50, 1.18) | 72 | 1.07 (0.76, 1.52) | 1.08 (0.75, 1.56) |
| Q4 | 15.6–20.1 | 41 | 0.65 (0.42, 1.01) | 0.74 (0.45, 1.20) | 75 | 0.78 (0.55, 1.12) | 0.79 (0.53, 1.16) |
| P for trend | 0.04 | 0.18 | 0.39 | 0.47 | |||
Defined as stage T0–T2/M0.
Defined as stage T3–T4/M0 or T0–T4/M1.
Energy-adjusted values.
Cox proportional hazards model adjusted for age at diagnosis, total energy, family history of prostate cancer, primary treatment, differentiation, smoking status, calendar year of diagnosis, and BMI.
Model additionally adjusted for dietary iron.
A sensitivity analysis among 440 men (169 cancer deaths) was conducted, excluding deaths that occurred during the first 2 y of follow-up, because those individuals likely suffered from the most severe disease and thus may not be representative of all cases. The association between zinc intake and disease-specific mortality was of a similar magnitude, yet slightly more significant in this analysis (HR: 0.59; 95% CI: 0.39, 0.90; P for trend = 0.02). Results stratified by stage (44 localized and 125 advanced) were similar to those of the full analysis.
Among individual foods, grains contributed 36% of total dietary zinc in the study population, with crisp and whole-meal breads alone contributing >20%. Meat (beef and pork, excluding poultry) contributed 32% of total dietary zinc. Dairy products contributed 22% of dietary zinc, with the remainder coming from fish, fruit, vegetables, and other sources. Poultry accounted for <1% of total dietary zinc, because >25% of the study population reported no poultry intake.
When the models were adjusted for total grains, meat (beef and pork), and dairy products, no appreciable change in the association between the highest quartile of zinc intake and total prostate cancer–specific mortality was observed after adjustment for any of the 3 food groups. A slightly stronger association between zinc and survival was seen among men in whom the diagnosis was made at an early stage after adjustment for meat (HR: 0.22; 95% CI: 0.07, 0.66) and grains (HR: 0.19; 95% CI: 0.07, 0.53), but not after adjustment for dairy products. All associations between zinc and survival remained nonsignificant in men in whom the diagnosis was made at an advanced stage. Meat intake alone (beef and pork), without adjustment for zinc, was not associated with disease-specific mortality overall (HR: 0.94; 95% CI: 0.61, 1.44) or with mortality in men in whom the diagnosis was made at an early or advanced stage. However, the highest quartile of meat intake was associated with an increased risk of death from other causes in men in whom the diagnosis of prostate cancer was made at an advanced stage (HR: 2.29; 95% CI: 1.20, 4.35). The association between meat and other-cause mortality appeared stronger after adjustment for zinc and iron (HR: 2.86; 95% CI: 1.36, 6.03).
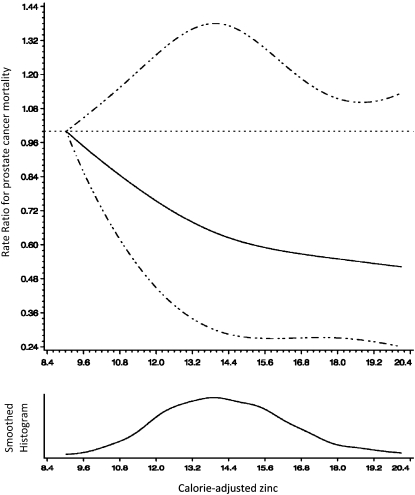
Because meat (beef and pork) is also a major source of iron, and high iron concentrations can impede zinc absorption (34), we further adjusted the zinc models for dietary iron (energy-adjusted Spearman r = 0.53). The study population reported high iron intakes (mean: 13 mg/d), which were greater than the US recommended intake of 8 mg/d and the Nordic recommended intake of 9 mg/d for men. The association between zinc and overall prostate cancer–specific mortality remained unchanged after adjustment for dietary iron (Table 2), whereas the estimate was slightly attenuated in men with localized disease (Table 3; HR: 0.30; 95% CI: 0.10, 0.92). Effect estimates remained nonsignificant in men in whom the diagnosis was made at an advanced stage. On its own, dietary iron was not significantly associated with overall prostate cancer–specific mortality in all patients (HRQ4 vs Q1: 0.85; 95% CI: 0.58, 1.26) or in men with advanced disease. There was a suggested inverse association in men with localized disease (HRQ4 vs Q1: 0.29; 95% CI: 0.10, 0.84); however, the estimate failed to maintain statistical significance after adjustment for zinc (HR: 0.47; 95% CI: 0.14, 1.58). The cubic spline regression (Figure 1) supports a linear association between zinc intake and prostate cancer–specific mortality (P for nonlinearity = 0.69).
FIGURE 1.
Representation of a cubic spline regression model for energy-adjusted zinc (continuous) and prostate cancer–specific mortality (33). Adjusted for age, BMI, smoking status, family history of prostate cancer, treatment, and year of diagnosis. The dotted lines represent CIs.
DISCUSSION
The results of this study in Swedish men suggest that high zinc intakes, within the range of intakes obtained through dietary sources, may be associated with lower disease-specific mortality, specifically in men with localized tumors. This finding is noteworthy in that the study population had negligible supplement use and infrequent prostate cancer screening at the time of diagnosis.
Eleven epidemiologic studies to date have looked at the relation between zinc and prostate cancer, assessing both dietary and supplemental intakes of zinc (13, 18–20, 26, 35–40). Most studies of zinc have focused on cancer incidence, and the results have been largely inconsistent, with little support for the protective association suggested by experimental evidence. Of the studies that assessed dietary zinc, an Italian hospital-based case-control study observed a significant risk of total and advanced prostate cancer in the highest quintile of intake (>15.65 mg/d) compared with the lowest quintile (18). Other studies found no association (26, 36); however, high dietary zinc intakes modestly reduced the risk of high-grade disease in the Prostate Cancer Prevention Trial (40). The original Swedish case-control study from which our study population is drawn found no association between energy-adjusted dietary zinc and the incidence of total or advanced prostate cancer (24).
Our study did not assess zinc intake at the higher intakes attained through supplementation. Of the studies that assessed supplemental zinc intake, the Health Professionals Follow-Up Study (HPFS) observed a significant association with incidence of advanced prostate cancer only in men consuming >100 mg/d (relative risk: 2.29; 95% CI: 1.06, 4.95) or with ≥10 y of supplement use (relative risk: 2.37; 95% CI: 1.42, 3.95) (13). In contrast, a recent analysis of the Vitamins and Lifestyle (VITAL) cohort found a nonsignificant decreased risk of advanced cancer in men taking >15 mg Zn/d as supplements for 10 y (HR: 0.34; 95% CI: 0.13, 1.09) (20). Both studies failed to find an association with dietary zinc. A case-control study from Washington state also found a suggested protective association among daily zinc supplement users (odds ratio: 0.55; 95% CI: 0.30, 1.00) (35). Although the epidemiologic evidence points to a potential adverse association for advanced prostate cancer with high supplemental intakes of zinc, the effect of dietary zinc intake remains unclear.
There are possible explanations for the differences between our study and previous epidemiologic analyses of zinc and prostate cancer. Zinc may play a role in determining outcome after a diagnosis of prostate cancer, but not in the development of disease. Because our study was the first to look at survival, these findings require confirmation in other populations. Second, many studies assess total zinc intake as a combination of both diet and supplements. Individuals who take zinc supplements, under most circumstances, will have much higher zinc intakes than those with exclusive dietary sources. Third, the men in our study population had a wide range of intakes, including higher intakes, with 80% reporting intakes above the US recommended intake of 11 mg/d (41). It is also possible that the sources of zinc in our study population, primarily grains and red meat, are different from the sources of zinc in other populations. Specific food sources of zinc may be important, because zinc from meat is more readily bioavailable than is zinc from vegetarian sources (6, 42). Common vegetarian sources of zinc, including legumes and whole grains, also contain phytic acid, which inhibits zinc bioavailability (42).
Our study suggests that zinc contributes to improved survival only in men with early-stage cancers, which is consistent with earlier reports from this study population that intake of vitamin B-6 was more strongly linked with prostate cancer survival among localized cancers (28). These findings suggest that the outcome of early-stage prostate cancer may still be amenable to modification by nutritional factors, whereas advanced-stage disease may have progressed beyond the point of nutritional influence.
Our study was the first to take dietary iron into account when evaluating the association between zinc and prostate cancer. We noticed a slight weakening of the associations between zinc and disease-specific survival after adjustment for iron intake. However, we were unable to separate the effects of more easily absorbed heme iron from nonheme iron. Heme iron catalyzes oxidative reactions that can lead to cellular and tissue damage and may contribute to a variety of diseases, including prostate cancer (43). A recent analysis in the National Institutes of Health–AARP cohort found a positive association between heme iron, but not total dietary iron, and total and advanced prostate cancer (44). Future studies should investigate the possible interactions of zinc and iron concerning prostate cancer survival and attempt to separate the effects of heme iron from those of total dietary iron.
Our study had several advantages. We had complete follow-up in our study population and were able to assess vital status for each participant with up to 20 y of follow-up. Because our study was conducted before the onset of prostate cancer screening, we had an adequate number of advanced stage tumors, which allowed us sufficient power to stratify our analysis by stage at diagnosis. Furthermore, high average zinc intakes in this population allowed us to explore a range of dietary intakes that has been difficult to attain in other study populations.
We cannot rule out the possibility of inaccurate recall of dietary patterns because of the close proximity in time between completion of the questionnaires and diagnosis of prostate cancer. However, because we only included prostate cancer cases in this analysis, recall bias was not an issue; rather, if there was inaccurate recall of dietary intake, we could expect a similar level of error among all participants. From the results of the sensitivity analysis, it appears unlikely that men with more severe disease recalled diet differently than did men with less severe disease, because the association with zinc remained largely unchanged after the exclusion of men who died within the first 2 y of follow-up. Other limitations of the study included a single assessment of dietary intake, which may not be representative of long-term dietary habits. However, the men were asked to recall their regular diet of the previous year in an attempt to exclude recent effects of illness. Our study also assessed disease characteristics only at diagnosis; thus, we are unable to assess changes to these factors over time. In addition, the relatively few prostate cancer deaths among localized cases resulted in wide CIs for stratified analyses.
The study population was recruited in Sweden in the late 1980s to early 1990s in a clinical environment that is quite different from current US clinical practice. Curative treatments such as radical prostatectomy and radiation therapy were not widely used in Sweden or other Nordic countries until the 1990s (45, 46). Reflecting this trend, a high proportion of men of all cancer stages in our study population (66.7%) chose or were recommended for watchful waiting after a prostate cancer diagnosis.
Dietary zinc does not appear to be related to causes of death other than prostate cancer in this population. Because we did not have specific causes of death for those men who did not die of prostate cancer, the analysis of other-cause mortality served to assess the influence of competing risks in the survival analysis. Because no clear associations were observed between zinc and other-cause mortality, we do not believe that competing risks and informative censoring were major concerns in our analysis.
A protective role of zinc against prostate cancer has long been suspected because of support from biological and experimental evidence; however, such a role has not been clearly confirmed in epidemiologic studies. Most of these studies have examined zinc in relation to the risk of developing prostate cancer rather than in relation to endpoints of disease progression or survival. Our findings of an inverse association between dietary zinc and prostate cancer–specific mortality suggest that zinc may play an important role in prostate cancer outcomes, particularly in men with localized disease. Because of the current lack of corroborative evidence, our study results are not sufficient to recommend zinc supplements to men after a prostate cancer diagnosis. However, these findings should encourage future studies of zinc and prostate cancer to include survival endpoints in an attempt to confirm our conclusions.
Acknowledgments
The authors' responsibilities were as follows—MME: analyzed the data, wrote the paper, and had primary responsibility for the final content; J-EJ, S-OA, and OA: designed the research, collected the data, and provided significant consultation; NH and AW: collected the data; and KF, JLK, ELG, and LAM: revised the manuscript and provided significant advice and consultation. All authors read and approved the final manuscript. The authors reported no conflicts of interest.
REFERENCES
- 1.Ferlay J, Shin H, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008, cancer incidence and mortality worldwide: IARC CancerBase no. 10. Lyon, France: International Agency for Research on Cancer, 2010. Available from: http://globocan.iarc.fr (cited 15 September 2010) [Google Scholar]
- 2.Hsing AW, Tsao L, Devesa SS. International trends and patterns of prostate cancer incidence and mortality. Int J Cancer 2000;85:60–7 [DOI] [PubMed] [Google Scholar]
- 3.Shimizu H, Ross RK, Bernstein L, Yatani R, Henderson BE, Mack TM. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br J Cancer 1991;63:963–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan JM, Gann PH, Giovannucci EL. Role of diet in prostate cancer development and progression. J Clin Oncol 2005;23:8152–60 [DOI] [PubMed] [Google Scholar]
- 5.Chan JM, Holick CN, Leitzmann MF, et al. Diet after diagnosis and the risk of prostate cancer progression, recurrence, and death (United States). Cancer Causes Control 2006;17:199–208 [DOI] [PubMed] [Google Scholar]
- 6.Rink L, Gabriel P. Zinc and the immune system. Proc Nutr Soc 2000;59:541–52 [DOI] [PubMed] [Google Scholar]
- 7.Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr 1998;68:447S–63S [DOI] [PubMed] [Google Scholar]
- 8.Feng P, Li T, Guan Z, Franklin RB, Costello LC. The involvement of Bax in zinc-induced mitochondrial apoptogenesis in malignant prostate cells. Mol Cancer 2008;7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prasad AS. Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Exp Gerontol 2008;43:370–7 [DOI] [PubMed] [Google Scholar]
- 10.Prasad AS, Beck FW, Bao B, et al. Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. Am J Clin Nutr 2007;85:837–44 [DOI] [PubMed] [Google Scholar]
- 11.De Marzo AM, Platz EA, Sutcliffe S, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer 2007;7:256–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palapattu GS, Sutcliffe S, Bastian PJ, et al. Prostate carcinogenesis and inflammation: emerging insights. Carcinogenesis 2005;26:1170–81 [DOI] [PubMed] [Google Scholar]
- 13.Leitzmann MF, Stampfer MJ, Wu K, Colditz GA, Willett WC, Giovannucci EL. Zinc supplement use and risk of prostate cancer. J Natl Cancer Inst 2003;95:1004–7 [DOI] [PubMed] [Google Scholar]
- 14.Zaichick VYe, Sviridova TV, Zaichick SV. Zinc in the human prostate gland: normal, hyperplastic and cancerous. Int Urol Nephrol 1997;29:565–74 [DOI] [PubMed] [Google Scholar]
- 15.Costello LC, Feng P, Milon B, Tan M, Franklin RB. Role of zinc in the pathogenesis and treatment of prostate cancer: critical issues to resolve. Prostate Cancer Prostatic Dis 2004;7:111–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cortesi M, Fridman E, Volkov A, et al. Clinical assessment of the cancer diagnostic value of prostatic zinc: a comprehensive needle-biopsy study. Prostate 2008;68:994–1006 [DOI] [PubMed] [Google Scholar]
- 17.Costello LC, Franklin RB, Feng P, Tan M, Bagasra O. Zinc and prostate cancer: a critical scientific, medical, and public interest issue (United States). Cancer Causes Control 2005;16:901–15 [DOI] [PubMed] [Google Scholar]
- 18.Gallus S, Foschi R, Negri E, et al. Dietary zinc and prostate cancer risk: a case-control study from Italy. Eur Urol 2007;52:1052–6 [DOI] [PubMed] [Google Scholar]
- 19.Key TJ, Silcocks PB, Davey GK, Appleby PN, Bishop DT. A case-control study of diet and prostate cancer. Br J Cancer 1997;76:678–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez A, Peters U, Lampe JW, White E. Zinc intake from supplements and diet and prostate cancer. Nutr Cancer 2009;61:206–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nordic Council of Ministers Nordic nutrition recommendations 2004: integrating nutrition and physical activity. 4th ed Copenhagen, Denmark: Nordic Council of Ministers, 2004 [Google Scholar]
- 22.Institute of Medicine, Food and Nutrition Board Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, molybdenum, nickel, silicon, vanadium and zinc. Washington, DC: National Academy Press, 2001 [PubMed] [Google Scholar]
- 23.Santillo VM, Lowe FC. Role of vitamins, minerals and supplements in the prevention and management of prostate cancer. Int Braz J Urol 2006;32:3–14 [DOI] [PubMed] [Google Scholar]
- 24.Andersson SO, Baron J, Bergstrom R, Lindgren C, Wolk A, Adami HO. Lifestyle factors and prostate cancer risk: a case-control study in Sweden. Cancer Epidemiol Biomarkers Prev 1996;5:509–13 [PubMed] [Google Scholar]
- 25.Engholm G, Ferlay J, Christensen N, et al. NORDCAN: Cancer Incidence, Mortality, Prevalence and Prediction in the Nordic Countries, version 3.5. Association of the Nordic Cancer Registries. Copenhagen, Denmark: Danish Cancer Society, 2009 [Google Scholar]
- 26.Andersson SO, Wolk A, Bergstrom R, et al. Energy, nutrient intake and prostate cancer risk: a population-based case-control study in Sweden. Int J Cancer 1996;68:716–22 [DOI] [PubMed] [Google Scholar]
- 27.Chan JM, Giovannucci E, Andersson SO, Yuen J, Adami HO, Wolk A. Dairy products, calcium, phosphorous, vitamin D, and risk of prostate cancer (Sweden). Cancer Causes Control 1998;9:559–66 [DOI] [PubMed] [Google Scholar]
- 28.Kasperzyk JL, Fall K, Mucci LA, et al. One-carbon metabolism-related nutrients and prostate cancer survival. Am J Clin Nutr 2009;90:561–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bergstroumlm L. Food database PC version 1992. Uppsala, Sweden: Swedish Food Administration, 1992. (Report no. 14) [Google Scholar]
- 30.Fall K, Stromberg F, Rosell J, Andren O, Varenhorst E. Reliability of death certificates in prostate cancer patients. Scand J Urol Nephrol 2008;42:352–7 [DOI] [PubMed] [Google Scholar]
- 31.Willett W. Nutritional Epidemiology, 2nd ed. New York: Oxford University Press, 1998 [Google Scholar]
- 32.World Health Organization Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. WHO Technical Report Series 854. Geneva, Switzerland: World Health Organization, 1995 [PubMed] [Google Scholar]
- 33.Govindarajulu US, Spiegelman D, Thurston SW, Ganguli B, Eisen EA. Comparing smoothing techniques in Cox models for exposure-response relationships. Stat Med 2007;26:3735–52 [DOI] [PubMed] [Google Scholar]
- 34.Whittaker P. Iron and zinc interactions in humans. Am J Clin Nutr 1998;68(suppl):442S–6S [DOI] [PubMed] [Google Scholar]
- 35.Kristal AR, Stanford JL, Cohen JH, Wicklund K, Patterson RE. Vitamin and mineral supplement use is associated with reduced risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 1999;8:887–92 [PubMed] [Google Scholar]
- 36.Lee MM, Wang RT, Hsing AW, Gu FL, Wang T, Spitz M. Case-control study of diet and prostate cancer in China. Cancer Causes Control 1998;9:545–52 [DOI] [PubMed] [Google Scholar]
- 37.Vlajinac HD, Marinkovic JM, Ilic MD, Kocev NI. Diet and prostate cancer: a case-control study. Eur J Cancer 1997;33:101–7 [DOI] [PubMed] [Google Scholar]
- 38.West DW, Slattery ML, Robison LM, French TK, Mahoney AW. Adult dietary intake and prostate cancer risk in Utah: a case-control study with special emphasis on aggressive tumors. Cancer Causes Control 1991;2:85–94 [DOI] [PubMed] [Google Scholar]
- 39.Kolonel LN, Yoshizawa CN, Hankin JH. Diet and prostatic cancer: a case-control study in Hawaii. Am J Epidemiol 1988;127:999–1012 [DOI] [PubMed] [Google Scholar]
- 40.Kristal AR, Arnold KB, Neuhouser ML, et al. Diet, supplement use, and prostate cancer risk: results from the Prostate Cancer Prevention Trial. Am J Epidemiol 2010;172:566–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.US Department of Agriculture, Agricultural Research Service USDA National Nutrient Database for Standard Reference, release 21. Nutrient Data Laboratory Home Page, 2008. Available from: http://www.ars.usda.gov/ba/bhnrc/ndl (cited August 2009)
- 42.Hunt JR. Bioavailability of iron, zinc, and other trace minerals from vegetarian diets. Am J Clin Nutr 2003;78(suppl):633S–9S [DOI] [PubMed] [Google Scholar]
- 43.Tappel A. Heme of consumed red meat can act as a catalyst of oxidative damage and could initiate colon, breast and prostate cancers, heart disease and other diseases. Med Hypotheses 2007;68:562–4 [DOI] [PubMed] [Google Scholar]
- 44.Sinha R, Park Y, Graubard BI, et al. Meat and meat-related compounds and risk of prostate cancer in a large prospective cohort study in the United States. Am J Epidemiol 2009;170:1165–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adolfsson J, Garmo H, Varenhorst E, et al. Clinical characteristics and primary treatment of prostate cancer in Sweden between 1996 and 2005. Scand J Urol Nephrol 2007;41:456–77 [DOI] [PubMed] [Google Scholar]
- 46.Kvale R, Auvinen A, Adami HO, et al. Interpreting trends in prostate cancer incidence and mortality in the five Nordic countries. J Natl Cancer Inst 2007;99:1881–7 [DOI] [PubMed] [Google Scholar]