Abstract
Background: Many of the foods abundant in the traditional Mediterranean diet, such as vegetables and fish, have been associated with slower cognitive decline.
Objective: We investigated whether adherence to a Mediterranean dietary pattern or to the Healthy Eating Index–2005 (HEI-2005) is associated with cognitive change in older adults.
Design: This article is based on analyses of data from an ongoing longitudinal study in adults aged ≥65 y known as the Chicago Health and Aging Project (CHAP). CHAP participants (2280 blacks and 1510 whites) with ≥2 cognitive assessments were evaluated for adherence to 1) the Mediterranean dietary pattern (MedDiet; maximum score: 55) and 2) the HEI-2005 (maximum score: 100). For both scoring systems, higher scores connote greater adherence. Cognitive function was assessed at 3-y intervals on the basis of a composite measure of global cognition. Linear mixed models were used to examine the association of dietary scores to change in cognitive function. Mean follow-up time was 7.6 y.
Results: Mean (±SD) scores for participants were 28.2 ± 0.1 for the MedDiet and 61.2 ± 9.6 for the HEI-2005. White participants had higher energy-adjusted MedDiet scores but lower HEI-2005 scores than did black participants. Higher MedDiet scores were associated with slower rates of cognitive decline (β = +0.0014 per 1-point increase, SEE = 0.0004, P = 0.0004) after adjustment for age, sex, race, education, participation in cognitive activities, and energy. No such associations were observed for HEI-2005 scores.
Conclusion: The Mediterranean dietary pattern as captured by the MedDiet scoring system may reduce the rate of cognitive decline with older age.
INTRODUCTION
The traditional Mediterranean diet pattern is characterized by an abundance of plant foods and wine, usually with meals. The health benefits of this pattern are many and include reduced risk of ischemic heart disease (1–3), cancers (4–7), all-cause mortality (8–10), and, more recently, of Alzheimer disease and cognitive decline (11–14).
Willett et al (15) were one of the first groups to promote this pattern as a model for healthy eating, but there have been many interpretations of the Mediterranean dietary pattern. Trichopoulou et al (9) developed a scale for adherence to this pattern in the Greek population, with a later revision to include fish. The scale varied from 0 to 9 in which a score of 1 was assigned to each of 9 components if the sex-specific median intake for the population was exceeded. Other Mediterranean diet indexes (Alternate Mediterranean Diet, MeDi, and other scores) used median scores in the study population to establish component cutoffs, even though the study populations were not Mediterranean (5, 11–14, 16, 17). In this study of the Chicago Health and Aging Project (CHAP), we score individual diets not in terms of the median intakes of the CHAP population but rather in relation to intakes of the Greek population by using the MedDiet scoring system developed by Panagiotakos et al (18).
We also describe the CHAP population sample in terms of another diet quality index, the Healthy Eating Index–2005 (HEI-2005). This index is based on the recommendations from the 2005 Dietary Guidelines for Americans (19). There are certain components common to both indexes (both emphasize whole grains or nonrefined grains, along with fruit and vegetables), but there are also differences. In the HEI-2005, there are no separate components for fish, poultry, alcohol (at least in terms of its beneficial constituents), or legumes, nuts, or beans. In the MedDiet scoring system, there are no components for dark-green and orange vegetables, sodium, saturated fat, or calories from alcohol, sugar, and fat. To our knowledge, there are no reports in which the HEI-2005 has been studied with respect to cognitive changes in a biracial community population of older adults.
SUBJECTS AND METHODS
Study population
Study subjects were participants in CHAP, an ongoing cohort study of older residents residing on the south side of Chicago. Beginning in 1993, a total of 6158 participated in 90-min in-home interviews that included 4 cognitive tests (79% participation overall). Follow-up interviews including cognitive assessments were conducted in 3-y cycles for all participants. Of the original baseline cohort, 951 died before follow-up, 255 had invalid dietary data, and 1201 with only 1 cognitive assessment. This left 3790 CHAP participants for analysis of cognitive change (648 participants had 5 cognitive assessments, 1167 had 4, 886 had 3, and 1089 had 2). This analytic sample reflects a mean of 7.6 y of follow-up. The Institutional Review Board of Rush University Medical Center approved the study, and all participants were given informed consent.
Dietary assessment
We used a modified version of the Harvard food-frequency questionnaire (FFQ) to measure usual intake of 139 food items and vitamin and mineral supplements over the past year. FFQs were distributed to study participants for completion and returned by mail, but approximately one-third requested interview administration. The validity and reproducibility of the questionnaire were assessed previously (20, 21).
The MedDiet score (and the MedDiet wine score) was adapted from that described by Panagiotakos et al (18) and is based on reported intakes of alcohol (wine only for the MedDiet wine score) and 10 food groups. Food items on the CHAP FFQ were assigned to food groups as follows: nonrefined cereals (3 items); potatoes (3 items); fruit (11 items); vegetables (16 items); legumes, nuts, and beans (7 items); fish (3 items); olive oil (2 items); red meat and meat products (11 items); poultry (4 items); and full-fat cheese and other dairy (6 items). Consumption of items characteristic of the Mediterranean pattern (eg, fruit, vegetables, legumes, olive oil, fish, potatoes, and nonrefined cereals) was assigned a score of 5 for at least daily consumption. There were 3 items that constitute nonrefined cereals or grains on the CHAP FFQ; these were as follows: hot breakfast cereal (eg, oatmeal); dark breads; and other grains, such as kasha, couscous, bulgar. Scores less than 5 were assigned to fewer servings proportionately. If these items were reported as being rarely consumed, then a score of 0 was assigned (see Table 1). For example, for the nonrefined cereals and breads score, a score of 5 would be assigned to participants who reported >32 servings/wk or >4.5 servings/d (the median reported for the Greek population). In contrast, for foods not consistent with the Mediterranean pattern (eg, red meat and meat products), the opposite scores were assigned (eg, a score of 0 for participants consuming these items >10 times/wk to a score of 5 for consumption of less than or equal to once per week). Alcohol intake was defined in 2 ways, yielding 2 overall scores: the MedDiet score and MedDiet wine score. These total scores differ only with respect to the alcohol component, which for the MedDiet score accounts for all forms of alcohol (for which the CHAP questionnaire had 3 items—beer, wine or wine coolers, and liquor (eg, vodka or rum) (18). The MedDiet wine score includes only wine intake (one item on the CHAP FFQ). We chose to create this additional score to emphasize the traditional alcoholic beverage of the Mediterranean region (9, 15). For each alcoholic beverage item, the number of servings was converted to milliliters of alcohol, in which a drink (5 oz of wine, 12 oz of beer, or 1.5 oz of hard liquor or a shot of 80-proof liquor) is equivalent to 17.7 mL of alcohol (22). A score of 0 for the alcohol or wine component was assigned for intakes >700 mL/d or none at all; intakes of <300 mL were assigned a 5, intakes of 300 mL were assigned a 4, and so forth. Once summed across all 11 components, the maximum number of points for the MedDiet score and the MedDiet wine score is 55. Thus, higher scores connote greater adherence to a Mediterranean diet.
TABLE 1.
MedDiet score component servings and corresponding component scores for 3790 Chicago Health and Aging Project (CHAP) participants at baseline1
| No. servings/wk for maximum score of 5 | No. of servings/wk reported by participants | Energy-adjusted scores |
|||||
| Components | All (n = 3790) | Whites (n = 1510) | Blacks (n = 2280) | Women (n = 2339) | Men (n = 1451) | ||
| Nonrefined cereals and breads2 | >32 | 6.4 (6.2, 6.6)3 | 1.5 (1.4, 1.5) | 1.3 (1.2, 1.3) | 1.6 (1.5, 1.6) | 1.4 (1.4, 1.5) | 1.4 (1.4, 1.5) |
| Potatoes2 | >18 | 2.3 (2.2, 2.3) | 1.1 (1.1, 1.1) | 1.2 (1.2, 1.2) | 1.0 (1.0, 1.0) | 1.1 (1.1, 1.1) | 1.1 (1.1, 1.1) |
| Fruit4 | >22 | 15.1 (14.8, 15.4) | 3.3 (3.3, 3.4) | 3.4 (3.3, 3.4) | 3.3 (3.2, 3.3) | 3.4 (3.3, 3.4) | 3.2 (3.1, 3.2) |
| Vegetables24 | >33 | 12.4 (12.2, 12.7) | 2.4 (2.3, 2.4) | 2.5 (2.4, 2.5) | 2.3 (2.2, 2.3) | 2.5 (2.4, 2.5) | 2.2 (2.2, 2.3) |
| Legumes, nuts, beans24 | >6 | 2.9 (2.8, 3.0) | 2.7 (2.6, 2.7) | 2.6 (2.5, 2.6) | 2.7 (2.7, 2.8) | 2.6 (2.6, 2.7) | 2.7 (2.7, 2.8) |
| Fish5 | >6 | 1.5 (1.5, 1.6) | 1.8 (1.8, 1.9) | 1.5 (1.5, 1.6) | 1.9 (1.9, 2.0) | 1.7 (1.7, 1.8) | 1.7 (1.6, 1.7) |
| Olive oil24 | ≥7 | 0.6 (0.6, 0.7) | 0.7 (0.6, 0.7) | 1.0 (0.9, 1.1) | 0.4 (0.4, 0.5) | 0.7 (0.7, 0.8) | 0.7 (0.6, 0.7) |
| Red meats4 | ≤1 | 3.6 (3.5, 3.7) | 3.3 (3.3, 3.4) | 3.4 (3.3, 3.5) | 3.3 (3.2, 3.3) | 3.5 (3.4, 3.5) | 3.2 (3.1, 3.2) |
| Poultry24 | ≤3 | 2.2 (2.2, 2.3) | 4.7 (4.6, 4.7) | 4.8 (4.7, 4.8) | 4.6 (4.6, 4.6) | 4.6 (4.6, 4.7) | 4.7 (4.7, 4.8) |
| Full-fat dairy | ≤10 | 1.8 (1.6, 1.9) | 5.0 (4.9, 5.0) | 5.0 (5.0, 5.0) | 5.0 (4.9, 5.0) | 5.0 (4.8, 5.0) | 4.9 (4.9, 5.0) |
| Wine only (mL)3 | <3006 | 9.1 (7.9, 10.3) | 1.0 (0.9, 1.1) | 1.7 (1.6, 1.8) | 0.5 (0.5, 0.6) | 1.0 (0.9, 1.0) | 1.0 (0.9, 1.2) |
| Alcohol (mL)24 | <3006 | 40.6 (37.4, 43.8) | 1.8 (1.8, 1.9) | 2.6 (2.5, 2.7) | 1.4 (1.3, 1.4) | 1.5 (1.4, 1.6) | 2.4 (2.2, 2.5) |
| MedDiet2 | NA | NA | 28.2 (28.1,28.4) | 29.2 (29.0, 29.4) | 27.6 (27.4, 27.7) | 28.1 (27.9, 28.3) | 28.4 (28.2, 28.6) |
| MedDiet wine24 | NA | NA | 27.4 (27.2, 27.5) | 28.3 (28.1, 28.6) | 26.8 (26.6, 26.9) | 27.5 (27.4, 27.7) | 27.1 (26.9, 27.4) |
Scores were calculated as described in reference 18. All components have a maximum score of 5. MedDiet wine score is a modification made by the present researchers. NA, not applicable.
Significant differences were observed on the basis of ANOVA between whites and blacks (P values from <0.01 to <0.0001).
Mean; 95% CI in parentheses (all such values).
Significant differences were observed on the basis of ANOVA between women and men (P values from <0.05 to <0.0001).
A significant interaction was observed for fish scores for sex and race (P = 0.045): white women (mean: 1.5; 95% CI: 1.5, 1.6), white men (mean: 1.6; 95% CI: 1.5, 1.7), black women (mean: 1.9; 95% CI: 1.9, 2.0), black men (mean: 1.9; 95% CI: 1.8, 1.9).
Alcohol and wine are not monotonic functions, and a score of 0 was assigned to those participants who reported no alcohol intake and to those who consumed >700 mL/wk.
The HEI-2005 is a 12-component measure of dietary quality developed by an interagency expert panel to reflect diet-related recommendations of the 2005 Dietary Guidelines (23). Coding of mixed food items (eg, pizza, baked lasagna) on the FFQ was performed as described by Guenther et al (23). Relative proportions of mixed dishes were based on values for dietary equivalents from MyPyramid Equivalents Database, 2.0 for USDA Survey Foods, 2003–2004 (24). Unlike the MedDiet scores, intakes of foods and nutrients are initially scored on a density basis—that is, as a ratio to energy intake. Adequacy of components (eg, food groups, oils) was based on the 1200- to 2400-kcal patterns (Table 2). For example, a score of 10 for sodium is assigned to intakes ≤0.7 g sodium/1000 kcal, whereas a score of 0 is assigned for intakes ≥2 g/1000 kcal. High scores for the latter 3 components (sodium, saturated fats, and calories from solid fats, alcoholic beverages, and added sugars) reflect lower intakes.
TABLE 2.
Healthy Eating Index–2005 (HEI-2005) and component scores for 3790 Chicago Health and Aging Project (CHAP) participants in the analytic sample
| Component scores (maximum points1) | No of servings: maximum score | All (n = 3790) | Whites (n = 1510) | Blacks (n = 2280) | Women (n = 2339) | Men (n = 1451) |
| Total fruit (5)23 | ≥0.8 cup equiv/1000 kcal | 4.4 (4.3, 4.4)4 | 4.5 (4.4, 4.5) | 4.3 (4.2, 4.3) | 4.4 (4.4, 4.5) | 4.2 (4.1, 4.3) |
| Whole fruit (5)23 | ≥0.4 cup equiv/1000 kcal | 4.3 (4.3, 4.3) | 4.4 (4.4, 4.5) | 4.2 (4.1, 4.2) | 4.4 (4.3, 4.4) | 4.2 (4.1, 4.2) |
| Total vegetables (5)5 | ≥1.1 cup equiv/1000 kcal | 3.6 (3.5, 3.6) | 3.8 (3.8, 3.9) | 3.4 (3.3, 3.4) | 3.7 (3.6, 3.7) | 3.4 (3.3, 3.5) |
| Dark-green and orange vegetables and legumes (5)23 | ≥0.4 cup equiv/1000 kcal | 2.3 (2.2, 2.3) | 2.1 (2.0, 2.1) | 2.4 (2.4, 2.5) | 2.4 (2.3, 2.5) | 2.1 (2.0, 2.2) |
| Total grains (5)2 | ≥3 oz equiv/1000 kcal | 4.8 (4.8, 4.9) | 4.9 (4.9, 4.9) | 4.8 (4.8, 4.8) | 4.8 (4.8, 4.8) | 4.8 (4.8, 4.9) |
| Whole grains (5)23 | ≥1.5 oz equiv/1000 kcal | 2.2 (2.2, 2.3) | 2.0 (1.9, 2.1) | 2.4 (2.3, 2.4) | 2.3 (2.2, 2.3) | 2.2 (2.1, 2.2) |
| Milk and milk products (10)23 | ≥1.3 cup equiv/1000 kcal | 5.0 (4.9, 5.1) | 5.9 (5.8, 6.1) | 4.3 (4.2, 4.4) | 5.1 (5.0, 5.2) | 4.8 (4.6, 4.9) |
| Meat and beans (10)23 | ≥2.5 oz equiv/1000 kcal | 8.8 (8.7, 8.8) | 8.5 (8.4, 8.6) | 9.0 (8.9, 9.1) | 8.6 (8.6, 8.7) | 9.0 (8.9, 9.1) |
| Oils (10)3 | ≥12 g/1000 kcal | 8.2 (8.1, 8.3) | 8.2 (8.1, 8.4) | 8.2 (8.1, 8.3) | 8.3 (8.2, 8.4) | 8.0 (7.9, 8.1) |
| Saturated fat (10)6 | ≤7% total kcal | 7.2 (7.1, 7.3) | 6.8 (6.6, 6.9) | 7.5 (7.4, 7.6) | 7.3 (7.1, 7.4) | 7.1 (6.9, 7.2) |
| Sodium (10)2 | ≤0.7 g/1000 kcal | 6.0 (6.0, 6.1) | 5.8 (5.7, 5.9) | 6.2 (6.1, 6.2) | 6.0 (5.9, 6.1) | 6.1 (6.0, 6.2) |
| Calories from solid fats, alcoholic beverages, and added sugars (20)23 | ≤20% total kcal | 4.5 (4.3, 4.7) | 2.5 (2.3, 2.8) | 5.8 (5.5, 6.1) | 4.4 (4.1, 4.6) | 4.7 (4.3, 5.0) |
| Total HEI-2005 (100)23 | — | 61.2 (60.9, 61.5) | 59.5 (59.0, 59.9) | 62.3 (61.9, 62.7) | 61.6 (61.2, 62.0) | 60.5 (60.0, 61.0) |
Maximum score achievable for that component with the HEI-2005 scoring system. Other than the last component, the other components of the HEI-2005 are weighted equally—each receiving a maximum of 10 points—with a few exceptions. Fruit, vegetables, and grains each have 2 components (total and a subgroup) with 5 points each; thus, these 3 food groups are effectively allotted 10 points each. There is a score of zero for no intake, and the scores increase as intakes increase up to the density standard. Scores for amounts between zero and the standard are prorated linearly; that is, the reported amount per 1000 calories is divided by the standard and multiplied by the total possible number of points (5 or 10). equiv, equivalents.
Significant differences were observed on the basis of ANOVA between whites and blacks (P values from <0.01 to <0.001).
Significant differences were observed on the basis of ANOVA between women and men (P values from <0.05 to <0.0001).
Mean score; 95% CI in parentheses for all 3790 CHAP participants on the basis of the completed food-frequency questionnaire (all such values).
Significant interaction for total vegetables for sex and race (P = 0.022): white women (mean: 3.9; 95% CI: 3.8, 4.0), white men (mean: 3.7; 95% CI: 3.6, 3.8), black women (mean: 3.5; 95% CI: 3.5, 3.6), black men (mean: 3.2; 95% CI: 3.1, 3.3).
Significant interaction for saturated fat for sex and race (P = 0.016): white women (mean: 6.8; 95% CI: 6.6, 6.9), white men (mean: 6.8; 95% CI: 6.6, 7.0), black women (mean: 7.6; 95% CI: 7.5, 7.7), black men (mean: 7.3; 95% CI: 7.1, 7.4).
Cognitive function assessment
CHAP population interviews included administration of 4 cognitive tests during in-home interviews at each cycle. Tests included the East Boston tests of immediate and delayed recall (25), the Mini-Mental State Examination (MMSE) (26), and the Symbol Digit Modalities Test (27). Scores on each were expressed as z scores and averaged for a global measure of cognitive function that was approximately normally distributed and which reduced the floor and ceiling effects and other measurement errors of the individual tests. In a previous factor analysis, all 4 tests loaded on a single factor that accounted for 74% of the variance (28).
Other covariates
Information on nondietary variables was collected at the participants' baseline interview. Age was computed from self-reported birth date and date of baseline cognitive assessment. Education was computed from self-reported highest grade or years of education. Assessment of depressive symptoms was made on the basis of the 10-item version of the Center for Epidemiologic Studies–Depression (CES-D) scale and was derived from the original 30-item version; it has acceptable reliability and a similar factor structure compared with the original version (29). A composite score reflecting the mean frequency of participation in cognitive activities (eg, reading newspapers, books, playing games or crossword puzzles, going to religious services) was constructed as described previously (30). Questions on cigarette smoking allowed for the computation of an indicator variable (never, former, or current smoker). Hypertension was based on self-reported history or measured systolic blood pressure ≥160 mm Hg and diastolic blood pressure ≥95 mm Hg. Stroke history was determined by self-report.
Statistics
Statistical analyses were performed by using SAS version 9.1 (SAS Institute Inc, Cary, NC). We used mixed-effects models to estimate the effect of adherence to the Mediterranean diet (MedDiet score) or to the HEI-2005 on the rates of within-person change in cognitive scores over time. Adjusted models included terms for age (y), sex, race, education (y), participation in cognitive activities and total energy intake (kcal; for MedDiet and MedDiet wine scores), and the interaction between time and each variable. Additional models to estimate the influence of adherence to MedDiet wine score on rates of within-person change in cognitive score over time also included total alcohol intake. The interaction terms with time represent the effects of the variables on the rate of change in cognitive scores. β Coefficients for the diet scores represent the associations between the dietary indexes at baseline on cognitive scores or rates of change in cognitive scores over time (interaction terms with time and dietary scores). A positive β coefficient for interaction terms with time and diet score reflects a reduction in the rate of decline in cognitive scores. Effect modification was examined by modeling 2- and 3-factor interaction terms among the covariates, the dietary quality score, and time. MedDiet, MedDiet wine, and HEI-2005 scores were also modeled in tertiles using the lowest tertile as the referent category.
RESULTS
In the leftmost column in Table 1, we specify the number of servings per week necessary for a perfect score of 5 for each component or food group of the MedDiet score, and in the next column the mean number of servings per week reported by CHAP participants based on responses to the FFQ are shown. Unadjusted MedDiet scores ranged from 12 to a high of 45 (Table 3). The highest MedDiet scores (indicating good adherence) were observed for fruit, red meats, poultry, full-fat dairy (the latter 3 components were reverse scored). Average total alcohol intake was 2.3 drinks/wk, and average wine intake was 0.5 drinks/wk. The mean (±SD) MedDiet score was 28.2 ± 0.1; for the MedDiet wine score the average was 27.4 ± 0.1.
TABLE 3.
Comparison of the demographic characteristics of the baseline Chicago Health and Aging Project (CHAP) analytic sample1
| Analytic sample for MedDiet scores |
||||
| Characteristic | All(n = 3790) | Lowest tertile: 12–25 (n = 1163) | Middle tertile: 26–29 (n = 1251) | Highest tertile: 30–45 (n = 1376) |
| Age (y) | 75.4 ± 6.22 | 75.9 ± 6.5 | 75.5 ± 6.3 | 74.9 ± 5.8 |
| Sex (% female) | 61.7 | 63.5 | 63.4 | 58.8 |
| Race (% black) | 60.2 | 69.4 | 63.3 | 49.3 |
| Smoking (% current) | 14.5 | 16.8 | 14.9 | 12.2 |
| Education (y) | 12.2 ± 3.7 | 11.3 ± 3.5 | 11.9 ± 3.6 | 13.2 ± 3.6 |
| BMI (kg/m2) | 27.1 ± 5.5 | 27.4 ± 5.9 | 27.3 ± 5.6 | 26.7 ± 5.1 |
| Stroke (%) | 7.3 | 8.2 | 8.0 | 5.9 |
| Hypertension (%) | 53.1 | 54.3 | 56.0 | 49.5 |
| Global cognitive score | 0.18 ± 0.73 | 0.04 ± 0.75 | 0.13 ± 0.74 | 0.36 ± 0.65 |
| Mini-Mental State Examination | 26.5 ± 4.1 | 25.9 ± 4.3 | 26.2 ± 4.3 | 27.3 ± 3.3 |
| CES-D (10-item) | 1.5 ± 1.9 | 1.7 ± 2.0 | 1.6 ± 2.0 | 1.2 ± 1.7 |
| Multivitamin use (%) | 34.0 | 27.4 | 32.0 | 41.5 |
The baseline sample consists of participants with valid food-frequency questionnaires and with ≥2 cognitive tests available (such tests conducted every 3 y). CES-D, Center for Epidemiologic Studies–Depression scale. Significant differences were observed across tertiles (P values from <0.05 to <0.0001 on the basis of ANOVA and chi-square tests).
Mean ± SD (all such values).
White participants had higher energy-adjusted MedDiet scores (29.2 ± 0.1) than did black participants (27.6 ± 0.1; Table 1). Black participants consumed more servings of fish and nonrefined cereals than did white participants but fewer servings of potatoes, vegetables, olive oil, poultry, wine, and alcohol. Score differences by race were small except for alcohol (or wine), reflecting the very low consumption by black CHAP participants. Although there were no differences in overall MedDiet scores between men and women, women consumed more fruit, vegetables, and olive oil but less red meat and alcohol than did men (Table 1). Although not shown, compared with nonsmokers, smokers had lower MedDiet and MedDiet wine scores.
The maximum attainable HEI-2005 score for each component is provided in parentheses in the leftmost column of Table 2, followed by the number of servings needed to receive the maximum score in the next column. Many CHAP participants were not adherent to the recommended number of servings of dark-green and orange vegetables (and legumes), whole grains, or milk and milk products. The poorest score (mean of 4.5 out of a possible 20) was seen for calories from solid fats, alcoholic beverages, and added sugars in whites (mean: 2.5) as compared with that in blacks (mean: 5.8; P < 0.0001). Blacks had higher HEI-2005 scores than did whites (62.3 ± 10.0 compared with 59.5 ± 8.8, P < 0.0001). Whites also had significantly poorer scores for dark-green and orange vegetables (and legumes), whole grains, and saturated fat components than did blacks. Total HEI-2005 scores were also higher for nonsmokers than for smokers (61.9 ± 9.7 compared with 58.2 ± 9.4) and for women compared with men (61.6 ± 9.6 compared with 60.5 ± 9.7).
The average age of CHAP adults was 75.4 ± 6.2 y, and 60.2% of the sample was black (Table 3). Mean cognitive score at baseline for the cohort of 3790 persons was 0.18 ± 0.73. In crude analyses, adherence to the Mediterranean dietary pattern was associated with being white, not currently smoking, higher educational level, lower body mass index, and greater multivitamin use. Individuals with higher MedDiet scores had lower prevalence of stroke, hypertension, and depression. Moreover, global cognitive scores were higher among those with higher MedDiet scores.
The overall annual rate of change in global cognitive score was a decline of 0.06 units per year (β = −0.06). In mixed models adjusted for age, sex, race, participation in cognitive activities, education, and total energy intake, both MedDiet scores and MedDiet wine scores were associated with higher baseline global cognitive scores (Table 4). Importantly, higher MedDiet scores and MedDiet wine scores were associated with reduced declines in cognitive function (β = 0.0014, SEE = 0.0004, P = 0.0004, and β=0.0014, SEE = 0.0004, P = 0.0009, respectively). Thus, if we were comparing 2 persons with MedDiet scores or MedDiet wine scores that were 10 points apart, the person with the higher scores would appear to perform as if she or he were 3 y younger cognitively. HEI-2005 scores were not associated with baseline cognitive score or rate of cognitive decline.
TABLE 4.
Estimated effects (β coefficients) of MedDiet scores, MedDiet wine scores, and Healthy Eating Index–2005 (HEI-2005) scores on global cognitive scores at baseline (cross-sectional) and on rates of change in global cognitive scores among 3790 Chicago Health and Aging Project (CHAP) participants followed for an average of 7.6 y1
| Cross-sectional model2 |
Rate of change model3 |
|||
| Score indicators | β (SEE) | P value | β (SEE) | P value |
| MedDiet score | 0.0070 (0.0022) | 0.0013 | 0.0014 (0.0004) | 0.0004 |
| MedDiet wine score | 0.0050 (0.0022) | 0.0231 | 0.0014 (0.0004) | 0.0009 |
| HEI-2005 score | −0.0011 (0.001) | 0.236 | 0.0002 (0.0002) | 0.214 |
Values are presented as β or regression coefficients (SEE) and the corresponding P value.
Values reflect adjustment for age, sex, race, education, participation in cognitive activities, and total energy intake in mixed linear models.
Scores were entered into the mixed models with adjustment for age, sex, race, education, participation in cognitive activities, total energy intake, and the interaction between time and each dietary quality score.
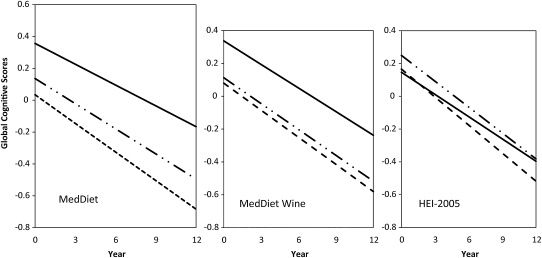
As shown in Figure 1, when we examined rate differences in cognitive decline as a function of these scores when expressed in tertiles, the upper tertile of MedDiet scores (scores of 30–45) was significantly associated with slower rates of change (β = 0.0171, SEE = 0.0046, P = 0.0002). Similarly, the highest tertile of MedDiet wine scores was also associated with slower rates of cognitive change (β = 0.0106, SEE = 0.0046, P = 0.021). When we modeled HEI-2005 scores into tertiles (Figure 1), there was no significant association observed with rates in cognitive change (β = 0.0056, SEE = 0.0043, P = 0.194 for the third tertile).
FIGURE 1.
Rates of changes in global cognitive scores over time as a function of MedDiet score tertiles (left), MedDiet wine score tertiles (middle), and Healthy Eating Index–2005 (HEI-2005) score tertiles (right). The first tertile is represented by dashed lines, the second by solid plus dashed lines, and the third by solid lines. Rates of change among Chicago Health and Aging Project (CHAP) participants were significantly associated with those whose MedDiet scores were in the highest or third tertile (solid lines; β = −0.0171, SEE = 0.0046, P = 0.0002) but not with those whose scores were in the second tertile (β = −0.0079, SEE = 0.0045, P = 0.075). Similarly, those whose MedDiet wine scores were in the third tertile experienced significantly slower rates of cognitive decline (β = −0.0106, SEE = 0.0046, P = 0.021) but not those whose scores were in the second tertile (β = −0.0063, SEE = 0.0045, P = 0.156). There were no significant associations in rates of change in cognitive scores between CHAP persons categorized by HEI-2005 tertiles (for the third tertile: β = −0.0171, SEE =0.0046, P = 0.0002; for the second tertile: β = −0.0021, SEE = 0.0042, P = 0.621).
When we repeated the analyses after exclusion of participants whose baseline cognitive scores were in the bottom 10% of the distribution, the multiple-adjusted estimates for MedDiet score (β = 0.0015, SEE = 0.0004, P = 0.0004) and for MedDiet wine score (β = 0.0013, SEE = 0.0004, P = 0.0022) changed little. Results were not affected when persons with heart disease or stroke were excluded (n = 139); the MedDiet and MedDiet wine scores continued to be significant predictors of cognitive change (β = 0.0015, SEE = 0.0004, P = 0.0003, and β = 0.0014, SEE = 0.0004, P = 0.0009, respectively). We observed no evidence for effect modification in the rate differences by age, sex, and race in separate multiple-adjusted models for any of the dietary adherence indexes.
We also examined whether adherence to such a dietary pattern was stable over time by computing the difference in MedDiet scores based on FFQs completed at baseline and at the next subsequent interview cycle. The mean difference in scores was close to 0 and not significant (−0.08, P = 0.32). For HEI-2005 scores between 2 consecutive cycles, the result was similar (mean difference of −0.30, P = 0.10).
DISCUSSION
In this biracial Midwest population of older adults, a dietary index based on the traditional Mediterranean diet was associated with slower rates of cognitive decline. By contrast, the HEI-2005 dietary quality index was not associated with cognitive change. To our knowledge, the HEI-2005 has not been examined in relation to cognitive change in older persons. The finding of association with the Mediterranean diet is consistent with earlier reports from a triethnic northern Manhattan cohort (11, 13, 14) in which higher Mediterranean type scores were associated with reduced risk of incident mild cognitive impairment and incident Alzheimer disease. In the Three City Study, higher Mediterranean-type diet scores were associated with higher MMSE scores at the end of 5 y, but changes in the other neurologic tests or in a global cognitive measure bore no significant associations with Mediterranean type scores (12). Comparisons of the Manhattan, Chicago, and French studies are difficult because in both the Manhattan and French studies median intakes of the study populations as opposed to intakes in Greece were used to assess adherence to the Mediterranean diet. In both the Manhattan and French reports, a score—the ratio of monounsaturated fats to saturated fat intakes—was used in lieu of the component score for olive oil. Benchmarking scores to population medians in France may approximate the traditional Mediterranean diet, but this approach is less likely to capture the Mediterranean pattern very effectively for the North Manhattan cohort, a diverse study population composed of Caribbean Hispanic, black, and white New Yorkers.
The findings reported here are consistent with some of our earlier findings—in particular, for individual foods such as vegetables and fish (31, 32). However, the computation of a summary score may not simply reflect associations of change in cognitive performance with individual components in an additive manner. An appealing feature of the selected dietary pattern approach (in contrast to single-nutrient methods) is that the lay public can more readily identify with target foods and beverages (rather than nutrients) to consume. Moreover, the Mediterranean pattern as reflected by the MedDiet and MedDiet wine scores incorporates a component not examined previously by our group—alcohol or wine consumption—in relation to cognitive decline. The putative role of moderate alcohol and, in particular, wine consumption for neuroprotection has strong epidemiologic support. On the basis of evidence from at least 3 European studies (nested case-control and longitudinal cohorts) wine intake has been related to incident dementia (33–35). However, not all studies have found that type of alcohol was predictive of this neuroprotection (36, 37). There is also strong biochemical evidence for the benefits of wine on neuropathology, which is largely attributed to reservatrol. The latter has been shown to reduce amyloid β (Aβ) production, promote proteosome clearance of Aβ (38), produce nonamyloidogenic Aβ cleavage products that induce genes critical for neuroprotection (39, 40), and activate the mammalian homolog of an NAD+ dependent deacetylase, sirtuin I (41).
In addition to the benefits of moderate wine or alcohol consumption, there is a biological basis for the protective role of the overall Mediterranean dietary pattern on rates of cognitive change over time. A myriad of studies—clinical trials and cohort—point to the value of such a dietary pattern in reducing markers of oxidative stress and in altering expression of anti- and proinflammatory markers thought to play a role in the pathogenesis of vascular diseases as well as Alzheimer disease (16, 42–44). Similar to previous reports, associations of MedDiet scores with cognitive change were only slightly attenuated when persons with stroke or heart disease were excluded (12–14). Cerebrovascular disease may often coexist in persons diagnosed with Alzhemier disease (45). It is possible that such a dietary pattern, particularly if maintained for years, may prevent or mitigate cerebrovascular pathology, which in turn alters clinical expression and detection of cognitive changes. Alternatively, this dietary pattern may influence β-amyloid or tau metabolism.
The present findings lend support to the premise that adherence to a Mediterranean diet as defined by MedDiet scores may afford some protection against cognitive decline in older black and white adults. Adherence to the HEI-2005 pattern scores was not related to cognitive change in CHAP participants. One possible explanation for the null finding for the HEI-2005 scores is that the HEI was designed for use with data obtained by the 24-h dietary recall method, which is a more detailed assessment of dietary intake than the FFQ method. However, the HEI-2005 scores of CHAP adults are similar to those reported for older adults in Continuing Survey of Food Intakes of Individuals 1994–96 (mean: 65.4) and in the National Health and Nutrition Examination Survey (NHANES) 2001–2002 (mean: 67.6) (46). Another more plausible explanation for why MedDiet scores but not those of the HEI-2005 were associated with slower cognitive decline is the emphasis on different dietary components. For example, the total MedDiet score attributes relatively fewer points for the red meat component and for the full-fat dairy component than does the HEI-2005 for the meats and beans component and for the milk and milk products components. The MedDiet attributes the highest points for moderate alcohol consumption and fewer points for no or greater consumption, whereas the HEI-2005 assigns more points for a lower proportion of calories from 3 sources—alcohol, solid fats, and added sugars. That component also receives a greater weight (20 out of 100 points) than do other components. In addition, the HEI-2005 includes components that have no reported association with cognition (eg, sodium, total grains). Another limitation with the sodium component of the HEI-2005 is the difficulty in capturing accurate estimates of sodium intake with a summary tool such as an FFQ. Thus, the emphasis of the HEI-2005 is not as relevant as the MedDiet to brain health.
Strengths of the present study include the prospective design, a biracial community sample, use of a well-validated dietary questionnaire, and use of multiple tests to measure cognitive function. Moreover, the present analyses included adjustment for or exclusion of many confounders. Because of the observational study design, we must caution against a causal interpretation of findings.
Acknowledgments
We gratefully acknowledge the study coordinators' efforts and their staff. We thank all of the CHAP participants for their willingness to participate in the study and to complete the food-frequency questionnaires, cognitive testing, and other surveys or tests.
The authors' responsibilities were as follows—CCT: design of the proposed study, initial coding of questionnaires to calculate scores, and manuscript preparation; MJK and HL: statistical analysis and programming; and MCM, DAE, and RSW: critical revisions of the manuscript. All authors contributed to the review of the manuscript. None of the authors had a conflict of interest to disclose.
REFERENCES
- 1.de Lorgeril M, Salen P. The Mediterranean-style diet for the prevention of cardiovascular diseases. Public Health Nutr 2006;9:118–23 [DOI] [PubMed] [Google Scholar]
- 2.Barzi F, Woodward M, Marfisi RM, et al. Mediterranean diet and all-causes mortality after myocardial infarction: results from the GISSI-Prevenzione trial. Eur J Clin Nutr 2003;57:604–11 [DOI] [PubMed] [Google Scholar]
- 3.Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009;119:1093–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosetti C, Gallus S, Trichopoulou A, et al. Influence of the Mediterranean diet on the risk of cancers of the upper aerodigestive tract. Cancer Epidemiol Biomarkers Prev 2003;12:1091–4 [PubMed] [Google Scholar]
- 5.Reedy J, Mitrou PN, Krebs-Smith SM, et al. Index-based dietary patterns and risk of colorectal cancer: the NIH-AARP Diet and Health Study. Am J Epidemiol 2008;168:38–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Lorgeril M, Salen P. Modified Cretan Mediterranean diet in the prevention of coronary heart disease and cancer: an update. World Rev Nutr Diet 2007;97:1–32 [DOI] [PubMed] [Google Scholar]
- 7.Dixon LB, Subar AF, Peters U, et al. Adherence to the USDA Food Guide, DASH eating plan, and Mediterranean dietary pattern reduces the risk of colorectal adenoma. J Nutr 2007;137:2443–50 [DOI] [PubMed] [Google Scholar]
- 8.Knoops KT, de Groot LC, Kromhout D, et al. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: the HALE project. JAMA 2004;292:1433–9 [DOI] [PubMed] [Google Scholar]
- 9.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med 2003;348:2599–608 [DOI] [PubMed] [Google Scholar]
- 10.Mitrou PN, Kipnis V, Thiébaut ACM, et al. Mediterranean dietary pattern and prediction of all-cause mortality in a US population: results from the NIH-AARP diet and health study. Arch Intern Med 2007;167:2461–8 [DOI] [PubMed] [Google Scholar]
- 11.Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer's disease. Ann Neurol 2006;59:912–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feart C, Samieri C, Rondeau V, et al. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA 2009;302:638–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Arch Neurol 2009;66:216–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer disease. JAMA 2009;302:627–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willett WC, Sacks F, Trichopoulou A, et al. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr 1995;61:1402S–6S [DOI] [PubMed] [Google Scholar]
- 16.Fung TT, McCullough ML, Newby PK, et al. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr 2005;82:163–73 [DOI] [PubMed] [Google Scholar]
- 17.Martinez-Gonzalez MA, Fernandez-Jarne E, Serrano-Martinez M, Marti A, Martinez JA, Martin-Moreno JM. Mediterranean diet and reduction in the risk of a first acute myocardial infarction: an operational healthy dietary score. Eur J Nutr 2002;41:153–60 [DOI] [PubMed] [Google Scholar]
- 18.Panagiotakos DB, Pitsavos C, Arvaniti F, Stefanadis C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev Med 2007;44:335–40 [DOI] [PubMed] [Google Scholar]
- 19.US Department of Health and Human Services, US Department of Agriculture Dietary guidelines for Americans. Washington, DC: US Department of Health and Human Services, US Department of Agriculture, 2005 [Google Scholar]
- 20.Morris MC, Tangney CC, Bienias JL, Evans DA, Wilson RS. Validity and reproducibility of a food frequency questionnaire by cognition in an older biracial sample. Am J Epidemiol 2003;158:1213–7 [DOI] [PubMed] [Google Scholar]
- 21.Tangney CC, Bienias JL, Evans DA, Morris MC. Reasonable estimates of serum vitamin E, vitamin C, and beta-cryptoxanthin are obtained with a food frequency questionnaire in older black and white adults. J Nutr 2004;134:927–34 [DOI] [PubMed] [Google Scholar]
- 22.Kloner RA, Rezkalla SH. To drink or not to drink? That is the question. Circulation 2007;116:1306–17 [DOI] [PubMed] [Google Scholar]
- 23.Guenther PM, Reedy J, Krebs-Smith SM. Development of the Healthy Eating Index-2005. J Am Diet Assoc 2008;108:1896–901 [DOI] [PubMed] [Google Scholar]
- 24.Bowman SA, Friday JE, Moshfegh A. My Pyramid Equivalents Database, 2.0 for USDA Survey Foods, 2003-2004. Food Surveys Research Group [serial online] September 2008. Available from: http://www.ars.usda.gov/ba/bhnr/fsrgn (cited 19 May 2009)
- 25.Scherr PA, Albert MS, Funkenstein HH, et al. Correlates of cognitive function in an elderly community population. Am J Epidemiol 1988;128:1084–101 [DOI] [PubMed] [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98 [DOI] [PubMed] [Google Scholar]
- 27.Smith A. Symbol Digit Modalities Test manual–revised. Los Angeles, CA: Western Psychological, 1984 [Google Scholar]
- 28.Wilson RS, Beckett LA, Bennett DA, Albert MS, Evans DA. Change in cognitive function in older persons from a community population: relation to age and Alzheimer disease. Arch Neurol 1999;56:1274–9 [DOI] [PubMed] [Google Scholar]
- 29.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health 1993;5:179–93 [DOI] [PubMed] [Google Scholar]
- 30.Wilson RS, Bennett DA, Bienias JL, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology 2002;59:1910–4 [DOI] [PubMed] [Google Scholar]
- 31.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology 2006;67:1370–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Fish consumption and cognitive decline with age in a large community study. Arch Neurol 2005;62:1849–53 [DOI] [PubMed] [Google Scholar]
- 33.Truelsen T, Thudium D. Grǿnbæk M. Amount and type of alcohol and the risk of dementia: the Copenhagen City Heart Study. Neurology 2002;59:1313–9 [DOI] [PubMed] [Google Scholar]
- 34.Mehlig K, Skoog I, Guo X, et al. Alcoholic beverages and incidence of dementia: 34-year follow-up of the prospective population study of women in Goteborg. Am J Epidemiol 2008;167:684–91 [DOI] [PubMed] [Google Scholar]
- 35.Orgogozo JM, Dartigues JF, Lafont S, et al. Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev Neurol (Paris) 1997;153:185–92 [PubMed] [Google Scholar]
- 36.Ruitenberg A, vanSwieten JC, Witteman JC, et al. Alcohol consumption and risk of dementia: the Rotterdam Study. Lancet 2002;359:281–6 [DOI] [PubMed] [Google Scholar]
- 37.Mukamal KJ, Kuller LH, Fitzpatrick AL, Longstreth WT, Mittleman MA, Siscovick DS. Prospective study of alcohol consumption and risk of dementia in older adults. JAMA 2003;289:1405–13 [DOI] [PubMed] [Google Scholar]
- 38.De Vrij FM, Fischer DF, van Leeuwen FW, Hol EM. Protein quality control in Alzheimer's disease by the ubiquitin proteasome system. Prog Neurobiol 2004;74:249–70 [DOI] [PubMed] [Google Scholar]
- 39.Karuppagounder SS, Pinto JT, Xu H, Chen HL, Beal MF, Gibson GE. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer's disease. Neurochem Int 2009;54:111–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vingtdeux V, Giliberto L, Zhao H, et al. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J Biol Chem 2010;285:9100–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science 2004;305:1010–3 [DOI] [PubMed] [Google Scholar]
- 42.Fito M, Guxens M, Corella D, et al. Effect of a traditional Mediterranean diet on lipoprotein oxidation: a randomized controlled trial. Arch Intern Med 2007;167:1195–203 [DOI] [PubMed] [Google Scholar]
- 43.Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA 2004;292:1440–6 [DOI] [PubMed] [Google Scholar]
- 44.Serrano-Martinez M, Palacios M, Martinez-Losa E, et al. A Mediterranean dietary style influences TNF-alpha and VCAM-1 coronary blood levels in unstable angina patients. Eur J Nutr 2005;44:348–54 [DOI] [PubMed] [Google Scholar]
- 45.Schneider JA, Boyle PA, Arvanitakis Z, Bienias JL, Bennett DA. Subcortical infarcts, Alzheimer's disease pathology, and memory function in older persons. Ann Neurol 2007;62:59–66 [DOI] [PubMed] [Google Scholar]
- 46.Juan W, Guenther PM, Kott PS. Diet quality of older Americans in 1994–96 and 2001–02 as measured by the Healthy Eating Index-2005. US Department of Agriculture Center for Nutrition Policy and Promotion Nutrition Insight 2008;41. Available from: http//:www.cnpp.usda.gov/Publications/NutritionInsights/Insight41.pdf (cited 5 May 2009) [Google Scholar]