Abstract
Study Objectives:
A significant portion of US military personnel are returning from deployment with trauma-related sleep disturbance, and disrupted sleep has been proposed as a mechanism for the development of medical conditions in those with posttraumatic stress disorder (PTSD). Although individuals with PTSD may realize improved sleep with either PTSD treatment or CBT for insomnia, many continue to experience residual sleep difficulties. Newly developed interventions designed to address nightmares are effective to this end, but often do not fully remove all aspects of PTSD-related sleep difficulties when used in isolation. A combined intervention involving both a nightmare-specific intervention and CBT for insomnia may lead to more marked reductions in PTSD-related sleep disturbances.
Methods:
Twenty-two veterans meeting criteria for PTSD were enrolled in the study. A combined intervention comprised of CBT for insomnia and imagery rehearsal therapy was evaluated against a usual care comparison group.
Results:
Intent-to-treat analyses revealed medium to large treatment effect sizes for all sleep diary outcomes, and very large treatment effects for insomnia severity, sleep quality, and PTSD symptoms.
Conclusions:
Findings demonstrate that an intervention targeting trauma-specific sleep disturbance produces large short-term effects, including substantial reductions in PTSD symptoms and insomnia severity. Future research should focus on the optimal approach to the treatment of comorbid PTSD and sleep disturbance in terms of sequencing, and should assure that sleep-focused interventions are available and acceptable to our younger veterans, who were more likely to drop out of treatment.
Citation:
Ulmer CS; Edinger JD; Calhoun PS. A multi-component cognitive-behavioral intervention for sleep disturbance in veterans with PTSD: a pilot study. J Clin Sleep Med 2011;7(1):57-68.
Keywords: PTSD, insomnia, nightmares, imagery rehearsal therapy, nightmare rescripting, cognitive-behavioral therapy for insomnia, exposure, sleep quality
More than 1.6 million US military personnel deployed to Afghanistan and Iraq from 2001 to 2008. Approximately 21% of the soldiers in the wars in Iraq (Operation Iraqi Freedom: OIF) and Afghanistan (Operation Enduring Freedom: OEF) will receive a diagnosis of PTSD following their service.1 Most of these veterans (70% to 91%) are likely to report difficulty initiating or maintaining sleep.2 Still others, who do not meet full diagnostic criteria for PTSD, will endorse sleep disturbance. All of these veterans will add to the existing population of veterans from earlier eras of military service, who continue to experience trauma-related sleep disturbance. Although findings have been mixed in terms of objective evidence of sleep disturbance in those with PTSD, recent studies have bolstered the evidence that persons with PTSD have objectively measured sleep complaints3,4 that warrant treatment attention. Whereas standard cognitive-behavioral PTSD treatment may facilitate improved sleep quality,5 evidence suggests that as many of 50% of patients who achieve PTSD remission following treatment continue to experience residual insomnia.6,7 Given the burden of PTSD on our veterans and the systems responsible for providing their health care, interventions addressing their sleep disturbances are vitally important for ameliorating current distress and preventing the consequent adverse health sequelae in the longer term.
BRIEF SUMMARY
Current Knowledge/Study Rationale: CBT for Insomnia results in improved sleep across a variety of populations. Imagery Rehearsal Therapy (IRT) has been shown effective in those with post-traumatic stress disorder (PTSD). Addressing the sleep complaints of Veterans with PTSD may be optimized by combining CBT for Insomnia with IRT for nightmares.
Study Impact: Pilot study findings suggest that this combined intervention is promising for improving sleep and reducing PTSD symptoms in Veterans with PTSD. Given the increasing number of military personnel returning from deployment with PTSD and trauma-related sleep disturbance, trauma-specific sleep interventions are imminently needed.
Insomnia and nightmares are the two primary complaints of veterans with PTSD. Cognitive-behavioral therapy (CBT) for insomnia enjoys substantial empirical support for treating primary and comorbid insomnia.8,9 In veterans, a secondary analysis of data acquired from a larger treatment study showed that the subset with PTSD displayed pre-to-post treatment reductions in sleep onset latency and wake after sleep onset, and increased sleep efficiency and sleep quality in response to CBT for insomnia.10 PTSD patients showed a better overall subjective and objective response to CBT than they did to sleep hygiene education. In spite of these positive outcomes, PTSD patients did not remit from insomnia following treatment in either treatment condition. The findings of these secondary analyses suggest that, although CBT for insomnia may be helpful for veterans with PTSD, interventions targeting the specific sleep concerns of this population are needed.
Although most individuals with PTSD are likely to experience insomnia, trauma-related sleep disturbance diverges from classic insomnia by virtue of several distinguishing factors. While insomniacs look favorably upon sleep, those with trauma-related sleep disturbance are likely to view sleep as a necessary evil. Most veterans with PTSD experience hypervigilance; the need to remain aware of their surroundings at all times. Sleeping is an inherently contradictory experience to vigilance. Thus, veterans with PTSD are often sleep-avoidant, and this avoidance is further reinforced by the aversiveness of the nightmares that accompany sleep. Finally, the sleep architecture of individuals with PTSD differs from both normal sleepers and insomniacs, particularly with respect to the duration and onset of REM sleep.3 Taken together, these observations suggest that trauma-related sleep disturbance is an inherently different and more complex phenomenon than insomnia that is unlikely to remit using existing insomnia treatment approaches.
Several studies have presented data on behavioral treatments for nightmares in PTSD populations. Imagery rehearsal therapy (IRT), first presented in the empirical literature by Kellner and colleagues,11,12 was designed to reduce nightmare frequency and severity using “rescripting” of nightmares. Krakow and colleagues presented a more intensive conceptualization of this protocol in which nightmares are described as learned behaviors that can be relearned through the use of enhanced imagery skills.13 Imagery rehearsal therapy has been empirically validated and has produced favorable findings in terms of nightmares and PTSD symptoms in female sexual assault victims14 and deployed US army soldiers.15 IRT was combined with CBT for insomnia in two studies; a group-based intervention for crime victims with PTSD,16 and a single session behavioral protocol for adult victims of violent crime.17 Both produced improvements in nightmares, sleep quality and PTSD symptoms. Even more recently, Nappi and colleagues published a study18 wherein IRT was employed as part of a VA clinical service. Treatment effect sizes (Cohen’s d) for nightmares, insomnia severity, and PTSD symptoms in the 35 veterans completing the course ranged from 0.16 (sleep quality) to 1.03 (PTSD symptoms).
Davis and colleagues developed a variation of IRT for nightmares that incorporates some standard treatment aspects of CBT-based PTSD treatment.19 Exposure, rescripting, and relaxation therapy (ERRT) was evaluated in a clinical trial of trauma-exposed adults (81.6% female, 67.3% meeting criteria for PTSD), and produced treatment effect sizes for nightmares, PTSD symptoms, self-reported sleep problems, and restfulness at one-week post-intervention ranged from 0.10 to 0.97 (Cohen’s d). Swanson and colleagues20 have since tested a combined version of ERRT and components of CBT for insomnia in 10 veterans with PTSD in a 10-session group therapy format. Treatment effect sizes for sleep and nightmares in this study ranged from 0.46 to 1.14 (Cohen’s d).
Given that CBT for insomnia and IRT have both been effective in reducing the sleep complaints of veterans with PTSD, our primary objective for the study was to assess the feasibility of a multi-component cognitive-behavioral sleep intervention for PTSD (SIP) using a randomized trial design. Our secondary purpose was to compare the intervention to usual care in terms of differential symptoms of sleep disturbance at post-intervention. We hypothesized that the combined effects of CBT for insomnia and IRT for nightmares would produce significantly greater improvements in sleep disturbance than usual care alone.
METHODS
Study Design
This study used a randomized parallel group experimental design. Participants were randomly assigned to either the intervention condition or a usual care condition, and were informed that the purpose of the study was to test the effectiveness of an intervention for improving sleep disturbance in veterans with PTSD. The study was approved by the institutional review board of our VA medical center, and all who enrolled provided written informed consent.
Participants
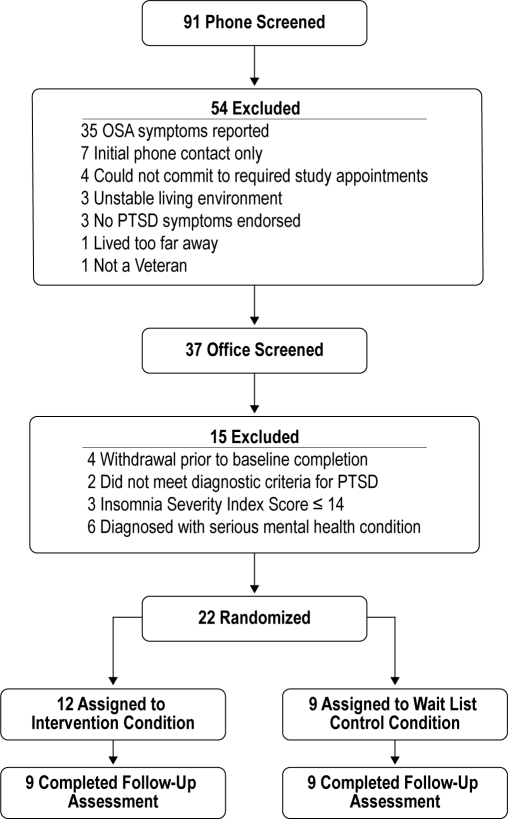
Study participants were recruited between 1/1/2008 and 12/31/2009 using flyers placed throughout the VA hospital and a community Veteran Center, and through letters sent to recently deployed veterans who had enrolled in a VA research registry and who had agreed to be recontacted for participation in VA research. To be considered for the study, veterans had to: (1) provide written informed consent; (2) meet DSM-IV-R criteria for a diagnosis of PTSD; (3) screen positive for an insomnia disorder on the Duke Structured Sleep Interview for Sleep Disorders; (4) score > 14 on the Insomnia Severity Index; and endorse nightmares on the PCL-M or the CAPs. Patients who screened positive on the DSISD for symptoms of sleep apnea, narcolepsy, restless legs syndrome, or circadian disorders, and those with active drug or alcohol abuse or dependence were excluded. All participants were required to have a PTSD diagnosis established using the Clinician-Administered PTSD Scale (CAPS)21 or the SCID. Of the 93 self-referred prospective study participants, 15 men and 7 women met study selection criteria, completed baseline procedures, and were subsequently randomized to treatment conditions (Figure 1). Of the 22 who were ultimately randomized to condition, PTSD diagnosis was established in 20 using the CAPs and in 2 using the SCID.
Figure 1.
Study participant flow chart
The average age of participants was 45.96 (SD 11.06). Most participants (73%) were either African American (N = 8) or Caucasian (N = 8).The demographic and mental health characteristics of participants are outlined in Table 1. Enrolled study participants were allowed to be engaged in mental health treatment during the study period. Table 2 outlines the specific type and frequency of mental health contacts received by each study participant during the study period.
Table 1.
Baseline demographic and treatment characteristics
| Difference Statistics |
||||
|---|---|---|---|---|
| SIP | Usual Care | F | p | |
| Age (Mean Years39) | 47.00 (9.47) | 50.22 (11.62) | 0.42 | 0.53 |
| Time since Trauma (Mean Years 39) | 12.51 (14.47) | 20.22 (16.95) | 1.00 | 0.33 |
| χ2 | p | |||
| Gender (% Male) | 66.7 | 55.6 | 0.23 | 0.63 |
| Race | 0.00 | 1.00 | ||
| % African American | 33.3 | 33.3 | ||
| % Caucasian | 33.3 | 33.3 | ||
| % Other | 33.3 | 33.3 | ||
| Working? (% Yes) | 33.3 | 67.7 | 2.00 | 0.16 |
| Era of Service | 3.72 | 0.29 | ||
| % Vietnam Era | 11.1 | 22.2 | ||
| % Post-Vietnam Era | 22.2 | 11.1 | ||
| % Persian Gulf War | 22.2 | 55.6 | ||
| % OEF/OIF | 44.4 | 11.1 | ||
| Antidepressant use (% at baseline) | 55.6 | 55.6 | 0.00 | 1.00 |
| Anxiolytic/hypnotic use (% at baseline) | 66.7 | 44.4 | 0.90 | 0.34 |
| Mental health treatment during study (%) | 33.3 | 55.6 | 0.90 | 0.34 |
| Medication management during study (%) | 77.8 | 44.4 | 2.1 | 0.15 |
Table 2.
Frequency and type of mental health treatment during the study period
| Subject # | Usual Care |
|---|---|
| 10 | 5 sessions of Cognitive Processing Therapy for PTSD |
| 1 30-minute session of medication management/supportive therapy | |
| 15 | 1 individual therapy session |
| 17 | None |
| 39 | 2 Women’s Trauma Group sessions |
| 1 individual therapy session | |
| 48 | None |
| 49 | 3 Women’s Trauma Group sessions |
| 3 30-minute medication management/supportive therapy | |
| 50 | 2 30-minute medication management/supportive therapy |
| 2 Mental Health Wellness Group sessions | |
| 2 Smoking Cessation Group sessions | |
| 83 | None |
| 87 | 2 individual therapy sessions |
| SIP | |
| 4 | None |
| 9 | 4 individual therapy sessions |
| 19 | 12 sessions of Anger Management Group Treatment |
| 1 30-minute medication management/supportive therapy | |
| 24 | 1 30-minute medication management/supportive therapy |
| 25 | 1 30-minute medication management/supportive therapy |
| 76 | 8 Individual Dialectical Behavior Therapy Group session |
| 6 Seeking Safety Treatment sessions | |
| 2 30-minute medication management/supportive therapy | |
| 82 | 2 30-minute medication management/supportive therapy |
| 84 | 2 30-minute medication management/supportive therapy |
| 88 | 1 30-minute medication management/supportive therapy |
SIP, Sleep Intervention for PTSD
Measures
Screening and Outcome Measures
Duke Structured Interview for Sleep Disorders (DSISD):
The DSISD is an instrument developed to assist in ascertaining DSM-IV and International Classification of Sleep Disorders sleep disorder diagnoses.22 The DSISD includes questions that incorporate criteria for ascertaining sleep disorders within both the DSM-IV and the recently updated ICSD sleep disorder nosologies. The instrument has acceptable reliability and discriminant validity, and is effective for discerning the types of sleep disorders that would disqualify participants for this study.
Folstein Mini-Mental Status Exam:
The Folstein Mini-Mental Status Exam (MMSE)23 was administered to all study candidates using standard administration/scoring procedures. The MMSE was used to identify and exclude individuals who have cognitive deficits that would preclude their ability to provide informed consent or to fully participate in an interactive treatment process. As consistent with clinical applications for dementia screening, those with a score < 24 were excluded from the study.
Insomnia Severity Index (ISI):
The ISI24 is a 7-item questionnaire that provides a global measure of perceived insomnia severity. Each item is rated on a 5-point Likert scale, and the total score ranges from 0-28. The following guidelines are recommended for interpreting the total score: 0-7 (no clinical insomnia), 8-14 (subthreshold insomnia), 15-21 (insomnia of moderate severity), and 22-28 (severe insomnia). The ISI has adequate psychometric properties, has been validated against diary and polysomnographic measures of sleep,25 and has been shown sensitive to therapeutic changes in several of our treatment studies of insomnia. The ISI was used to determine treatment eligibility, to assess treatment outcome, and to determine clinical significance of study findings.
Electronic Sleep Diary:
Subjective sleep estimates were obtained using a hand-held computer (PDA) containing an interactive program that automates the collection of subjective sleep data. The PDA device was programmed to elicit daily responses from participants and electronically record multiple days of subjective sleep information, in addition to the number and severity of nightmares for the previous night. Five variables of interest were calculated or obtained from electronic sleep diaries, as follows: total sleep time (TST); sleep onset latency (SOL); wake after sleep onset (WASO); sleep efficiency % (SE); and nightmare frequency (NM frequency).
The Pittsburgh Sleep Quality Index (PSQI):
The PSQI26 is a self-rating scale that yields a quantitative index of general sleep quality/disturbances. The PSQI is composed of 4 open-ended questions and 19 self-rated items (0-3 scale) assessing sleep quality and disturbances over a 1-month interval, and yields a global score of sleep quality, ranging from 0 to 21. A PSQI cut-off score of 5 is a sensitive and specific value for predicting both clinical and laboratory assessments of sleep. The PSQI is a widely used and valid instrument in clinical sleep research. The Pittsburgh Sleep Quality Index Addendum for PTSD (PSQI-A)27 was used to assess PTSD-related sleep quality, and has acceptable psychometric properties. A cut-off score of 4 was found to have excellent sensitivity and specificity for discriminating those without PTSD from those with PTSD.
PTSD Checklist-Military Version (PCL-M):
The PCL-M28 is a 17-item self-report measure reflecting the degree to which veterans were bothered by each of 17 DSM-IV symptoms of PTSD in the past month. It is rated on a 5-point scale ranging from 1 (“not at all”) to 5 (“extremely”). In Vietnam veterans, a PCL-M score ≥ 50 was found to be highly predictive of PTSD diagnosis using the SCID.29 The PCL-M was used to assess treatment outcome and as an indicator of clinical significance of study findings.
Patient Health Questionnaire (PHQ-2):
The PHQ-230 is a 2-item depression screening instrument that assesses frequency of depressed mood and anhedonia on a scale of 0 (not at all) to 3 (nearly every day). In a primary care population, the PHQ-2 has a sensitivity of 86% and specificity of 78% for detecting major depression, with a score ≥ 2 correctly categorizing the greatest number of those with major depression.
Procedure
Patients expressing interest in the study were first screened for study eligibility by phone, with the exception of a few veterans who presented to the office of the PI in response to flyers. A more rigorous study screening assessment was conducted in person. Those meeting study eligibility criteria were enrolled in the study, completed a baseline demographics questionnaire, and then conducted one week of sleep monitoring at home using the electronic sleep diary. Upon returning with study equipment, they completed a baseline packet of questionnaires assessing study outcome variables. They were then randomized to condition and to therapist. Those in the intervention condition were scheduled for their first session of SIP within 3 weeks and completed 6 bi-weekly intervention sessions over the following 12 weeks. Following their sixth session of SIP, they were again sent home with equipment to monitor sleep and then completed the post-intervention questionnaire packet upon their return. The usual care control condition procedures were identical, excepting that they did not receive the intervention. All usual care participants were offered the opportunity to receive SIP at the end of the study. All participants were paid $40 for each of the 2 assessment periods and $15 for study screening.
Therapists and Treatments
Treatment was conducted by the PI (CSU) and the co-investigator (JDE), who are both licensed clinical psychologists. Of the 13 randomized to the intervention condition, CSU treated 5 and JDE treated 8.
Usual Care/Usual Care Control:
Veterans seeking assistance for sleep disturbance and PTSD symptoms at the VA are often treated for their symptoms by their primary care provider (PCP). PCPs may provide hypnotics, antidepressants, anxiolytics, and mood stabilizing medications to address veteran symptoms, in addition to referring the veteran to the mental health clinic.
Sleep Intervention for PTSD (SIP):
Patients in the SIP condition were eligible to receive the same elements as the Usual Care patients. In addition, these patients received 6 bi-weekly 1-h individual sessions with the interventionist, including 3 sessions of cognitive-behavioral therapy (CBT) for insomnia and 3 sessions of imagery rehearsal therapy (IRT), in that order. CBT for insomnia consisted of a prescription for an individually tailored behavioral regimen based upon sleep restriction theory, stimulus control, standard sleep hygiene recommendations, and the identification and restructuring of dysfunctional beliefs and attitudes regarding sleep. The IRT component included education about the role of learning in nightmares, visual imagery skill building, and specific instructions on how to rescript nightmares. Participants were instructed to change the nightmare in any way that they liked and to practice this technique for ≥ 15 min/day, and to practice rescripting no more than 2 new dreams per week.
Analyses
Baseline group differences were assessed using standard χ2 procedures for categorical measures, and the F distribution for continuous variables.
Intent–to–Treat Outcome Analyses
For all intent-to-treat outcomes, linear mixed-effects models (PROC MIXED in SAS) were used to determine expected mean values at each time point and to test hypotheses of group differences. The model included time and the group-by-time interaction; an unstructured covariance matrix was used to account for the within-patient correlation over time. All available data, including data from participants who subsequently discontinued the study, were used for the longitudinal analyses. Mixed-effects models assume non-informative dropout, meaning that the probability of dropout may depend on covariates or a participants’ previous responses but not on current or future responses.31 A p value < 0.05 was considered statistically significant. Due to a PDA programming error, we lost a significant portion of the data for nightmare severity, so this variable was not included in analyses.
Completer Outcome Analyses
To analyze outcomes for completers only, a series of ANOVAs (analysis of variances) involving one between-subjects factor (SIP + Usual Care vs. Usual Care Only) and one within-subjects factor (time) were used to assess for group by time interactions. Since one study participant in the intervention group did not provide post-intervention sleep log data, these analyses were conducted on 9 usual care participants and 8 intervention participants. A p value < 0.05 was considered statistically significant.
Effect Size Calculations
Effect sizes were calculated for each outcome by subtracting post-intervention values for the usual care group from post-intervention values for the treatment group, and then dividing by the pooled baseline standard deviation.
Test of Clinical Significance
To assess the clinical significance of our findings, we employed the criteria used by Morin and colleagues,32 with remission from insomnia defined as a post-intervention ISI score ≤ 7 (no insomnia), and response defined as an 8-point drop in ISI score (a categorical change) from pre- to post-assessment. As discussed above, remission from PTSD was defined as a PCL-M score ≤ 49; sleep quality remission was defined as a post-intervention PSQI score ≤ 5; and remission from PTSD-specific disruptive nocturnal behaviors was defined as a PSQI-A post-intervention score < 4.There are no established criteria for sleep diary values reflecting “normal” sleep in those with PTSD. However, a criterion of < 31 min of WASO or SOL was found to discriminate those with insomnia from normal sleepers.22 Since individuals with PTSD endorse high levels of insomnia, we utilized this criterion as being reflective of normal WASO and SOL at post-intervention. Treatment response status was evaluated using χ2 analyses, wherein the frequency of responders and remitters, as defined above, were compared across conditions.
RESULTS
Treatment Completers versus Drop-Outs
Four of the 22 randomized study participants dropped out of the intervention group prior to completing the study. There were no dropouts from the usual care group. The 4 participants who did not complete the study were all male OEF/OIF veterans. They were also younger (F1,20 = 7.47, p = 0.01, Drop Out M = 34, SD = 2.94, Completer M = 48.61, SD = 10.42), had greater sleep onset latency (F1,20 = 5.76, p = 0.03, Drop Out M = 83.93, SD = 52.66, Completer M = 42.95, SD = 25.15), reported less restful sleep on diaries (F1,20 = 5.51, p = 0.03, Drop Out M = 2.5, SD = 0.37, Completer M = 4.08, SD = 1.31), and had poorer sleep quality (higher PSQI scores) (F1,19 = 4.69, p = 0.04, poorer sleep quality) than study completers at baseline. Two veterans dropped out due to work conflicts, one because of the distance from his residence to the VA, and one was lost to follow-up. Those not completing the study did not otherwise differ from completers on demographic characteristics or outcome measures.
Baseline Comparisons
Groups were compared on baseline demographic and outcome characteristics using univariate ANOVA analyses and χ2 analyses, as appropriate, for those completing the study. Groups did not differ on age, ethnicity, or gender distribution, time since trauma, or the number of Criterion A traumatic events (Table 1). In addition, participants did not differ across groups on any baseline outcome variables (Table 3). Three variables were considered as possible covariates: age, time since trauma, and baseline anxiolytic/hypnotic medication use. Since none of these variables correlated with baseline outcome measures, they were not included as covariates in analyses.
Table 3.
Baseline sleep characteristics
| Measure | SIP |
Usual Care |
Difference Statistic |
|||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | F | p | |
| TST | 315.45 | 70.13 | 311.99 | 70.71 | 0.01 | 0.91 |
| WASO | 85.07 | 62.11 | 103.83 | 28.31 | 0.71 | 0.41 |
| SOL | 50.30 | 39.50 | 50.54 | 27.02 | 0.00 | 0.99 |
| SE | 70.52 | 14.89 | 66.47 | 8.47 | 0.54 | 0.47 |
| NM Frequency | 0.72 | 0.43 | 0.59 | 0.68 | 0.32 | 0.56 |
| ISI | 22.77 | 4.57 | 22.00 | 3.97 | 0.17 | 0.69 |
| PCL-M | 63.08 | 12.47 | 63.44 | 11.66 | 0.01 | 0.95 |
| PHQ | 3.77 | 1.74 | 3.89 | 2.09 | 0.02 | 0.89 |
| PSQI | 14.17 | 3.27 | 14.33 | 3.35 | 0.01 | 0.91 |
| PSQI-A | 10.00 | 4.10 | 10.00 | 5.29 | 0.00 | 1.00 |
Therapist Effects
A series of repeated measures ANOVAs were conducted to assess for therapist effects for each outcome measure (Outcome Measure × Group × Therapist). No therapist effects or group by therapist effects were found for any outcome measures.
Mental Health Treatment Group Effects
Chi-square analyses were used to assess for group differences on treatment engagement during the study protocol. Groups did not differ on the percentage engaged in mental health treatment (χ2 = 0.90, p = 0.34) or medication management (χ2 = 2.10, p = 0.15) during the study period (See Table 2).
Sleep Diary Outcomes
Intent-to-treat statistical analyses showed a significant group by time interaction in favor of SIP over the usual care condition for all sleep diary outcomes (Table 4). SIP produced significantly greater baseline to post-intervention improvements in sleep diary measures of TST (F1,21 6.33, p = 0.02), WASO (F1,21 5.91, p = 0.02), SOL (F1,21 10.17, p = 0.004), SE (F1,21 14.61, p = 0.001), and nightmare frequency (F1,21 5.03, p = 5.03, p = 0.04) (Table 4). The effect sizes for all sleep diary outcomes fell in the “medium” to “large” range using Cohen’s original conceptualization of these terms.33
Table 4.
Comparison of predicted means, standard error (SE) values and treatment effect sizes for intent-to-treat groups on sleep diary outcomes
| Measure | Baseline | Post-Intervention Predicted |
Difference Statistic | Effect Size | ||
|---|---|---|---|---|---|---|
| SIP Group | TAU Group | F | Cohen’ s d | |||
| TST (Hours) | M | 5.23 | 6.47 | 5.26 | 6.33* | 1.06 |
| SE | 0.24 | 0.37 | 0.35 | |||
| WASO (Minutes) | M | 92.75 | 42.03 | 88.00 | 5.91* | -0.90 |
| SE | 10.87 | 14.47 | 13.73 | |||
| SOL (Minutes) | M | 50.40 | 22.39 | 45.03 | 10.17** | -0.66 |
| SE | 7.30 | 6.55 | 6.32 | |||
| SE (Percentage) | M | 68.86 | 86.89 | 70.91 | 14.61** | 1.27 |
| SE | 2.68 | 3.24 | 3.07 | |||
| NM Frequency (Mean per Night) | M | 0.67 | 0.51 | 0.83 | 5.03* | -0.60 |
| SE | 0.11 | 0.18 | 0.17 | |||
p < 0.05
p < 0.01
Completer analyses revealed a significant group by time interaction for nightmare frequency (F1,15 = 4.96, p = 0.04), with those in the intervention group reporting a significantly greater reduction in nightmares from baseline to post-intervention than the usual care group (Table 5). Trends toward significance were found for total sleep time (F1,15 = 3.54, p = 0.08) and sleep efficiency percentage (F1,15 = 3.70, p = 0.07), with the intervention group reporting more total sleep time and a higher sleep efficiency at post-intervention relative to usual care. Effect sizes for all completers-only sleep log outcomes fell in the “large” range.
Table 5.
Comparison of means, standard error (SE) values and treatment effect sizes for participants with complete baseline and post-intervention data on sleep log outcomes
| Measure | Baseline | Post-Intervention |
ANOVA Statistics |
|||||
|---|---|---|---|---|---|---|---|---|
| SIP | TAU | GROUP (A) | TIME (B) | A × B | A × B | |||
| Sleep Log Variable | N = 8 | N = 9 | F | F | F | Cohen's d | ||
| TST (Hours) | M | 5.27 | 6.56 | 5.24 | 2.11 | 4.08 | 3.54 | 1.06 |
| SE | 0.29 | 0.46 | 0.35 | |||||
| WASO (Minutes) | M | 88.93 | 30.95 | 92.58 | 8.54* | 4.31* | 1.14 | -1.37 |
| SE | 10.58 | 7.34 | 18.28 | |||||
| SOL (Minutes) | M | 42.95 | 12.45 | 45.11 | 6.52* | 8.17* | 2.68 | -1.30 |
| SE | 5.93 | 2.11 | 8.92 | |||||
| SE (Percentage) | M | 69.98 | 89.58 | 69.92 | 8.51* | 9.99** | 3.70 | 1.48 |
| SE | 3.12 | 2.06 | 4.24 | |||||
| NM Frequency (Mean per Night) | M | 0.63 | 0.27 | 0.73 | 0.95 | 0.15 | 4.96* | -0.89 |
| SE | 0.53 | 0.26 | 0.94 | |||||
p < 0.05
p < 0.01
Questionnaire Outcomes
Intent-to-treat statistical analyses showed a significant group by time interaction in favor of SIP over the usual care condition for most questionnaire outcomes. SIP produced significantly greater improvements in insomnia severity (ISI) (F1,21 11.80, p = 0.003), PTSD symptoms (PCL-M) (F1,21 22.72, p = 0.0001), and sleep quality (PSQI) (F1,21 17.31, p = 0.0005) (Table 6). Groups did not differ at post-intervention on depression (PHQ-2) or the PTSD-specific sleep quality measure (PSQI-A). The effect size for all significant questionnaire outcomes fell in the “large” range, and in the “small to moderate” range for nonsignificant questionnaire outcomes.
Table 6.
Comparison of predicted means, standard error (SE) values, and treatment effect sizes for intent-to-treat groups on questionnaire outcomes
| Measure | Baseline | Post-Intervention Predicted |
Difference Statistic | Effect Size | ||
|---|---|---|---|---|---|---|
| SIP Group | TAU Group | F | Cohen’s d | |||
| ISI | M | 22.46 | 12.45 | 21.58 | 11.80** | -2.15 |
| SE | 0.91 | 1.90 | 1.90 | |||
| PCL-M | M | 63.23 | 45.25 | 66.08 | 22.72** | -1.76 |
| SE | 2.53 | 3.51 | 3.51 | |||
| PHQ | M | 3.81 | 3.45 | 4.06 | 0.86 | -0.34 |
| SE | 0.39 | 0.54 | 0.54 | |||
| PSQI | M | 14.24 | 9.31 | 14.47 | 17.31** | -1.60 |
| SE | 0.70 | 1.07 | 1.07 | |||
| PSQI-A | M | 10.00 | 7.74 | 9.11 | 0.76 | -0.30 |
| SE | 0.96 | 1.36 | 1.36 | |||
p < 0.05
p < 0.01
ANOVAs were then conducted to assess for group differences for those with complete baseline and post-intervention data on the amount of change in their scores on self-report questionnaires (ISI, PCL-M, PHQ, PSQI, and PSQI-A) from baseline to post-intervention (Table 7). ANOVAs revealed a significant time by group interaction for the ISI (F3,14 = 9.88, p = 0.006), the PCL-M (F3,14 = 17.63, p = 0.001), and the PSQI (F3,14 = 15.91, p = 0.001), with those in the intervention condition faring better at post-intervention than the usual care condition. Groups did not differ significantly in depression (PHQ) or trauma-related sleep quality (PSQI-A) change from baseline to post-intervention. Effect sizes for all significant completers-only questionnaire outcomes fell in the “very large” (> 1.50) range, and in the “moderate” range for nonsignificant questionnaire outcomes.
Table 7.
Comparison of means, standard error (SE) values and treatment effect sizes for participants with complete baseline and post-intervention data on outcome questionnaires
| Measure | Baseline | Post-Intervention |
ANOVA Statistics |
|||||
|---|---|---|---|---|---|---|---|---|
| SIP | TAU | GROUP (A) | TIME (B) | A × B | A × B | |||
| Questionnaires | N = 9 | N = 9 | F | F | F | Cohen’s d | ||
| ISI | M | 22.22 | 12.44 | 21.44 | 5.23* | 12.34** | 9.88** | -2.17 |
| SE | 0.98 | 2.43 | 1.25 | |||||
| PCL-M | M | 62.39 | 44.00 | 66.22 | 5.29* | 9.24** | 17.63** | -1.85 |
| SE | 2.83 | 4.99 | 2.99 | |||||
| PHQ | M | 3.61 | 3.11 | 4.11 | 0.93 | 0.00 | 0.38 | −0.55 |
| SE | 0.43 | 0.72 | 0.54 | |||||
| PSQI | M | 13.67 | 8.22 | 14.56 | 6.47* | 13.21** | 15.91** | -2.05 |
| SE | 0.73 | 1.41 | 1.09 | |||||
| PSQI-A | M | 9.28 | 6.56 | 9.11 | 0.93 | 3.18 | 0.47 | -0.58 |
| SE | 1.04 | 1.54 | 1.76 | |||||
p < 0.05
p < 0.01
Clinical Significance Findings
Sleep Diary Outcomes
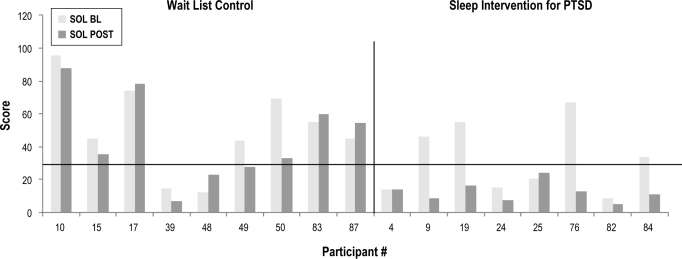
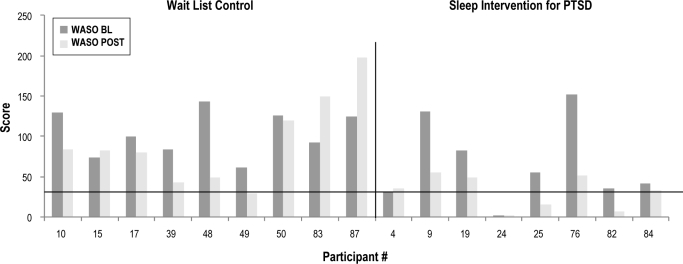
Post-assessment sleep diary data was not available for 1 participant in the intervention group. Figures 2 and 3 depict baseline to post-assessment change in SOL and WASO in those providing complete sleep diary data. As depicted, 4 subjects in the intervention group had baseline SOL values < 31 min, and one subject had a baseline WASO value < 31 min. In the wait-list group, 2 participants had SOL values < 31 min. Baseline SOL and WASO values did not differ between groups when considering only those participants with complete sleep diary data and baseline SOL and WASO values above the criterion level for “normal” sleep. At post-intervention, however, all of the intervention group participants (4/4 or 100%) in this subsample had achieved “normal” SOL as compared to only 14% of usual care participants (1/7) (χ2 = 7.5, p = 0.02). Groups did not differ at post-intervention on the percentage of WASO remitters (χ2 = 0.79, p = 0.55), with 1 of 9 remitting in the wait list group and 2 of 7 in the intervention group.
Figure 2.
Sleep onset latency
Figure 3.
Wake after sleep onset
Questionnaire Outcomes
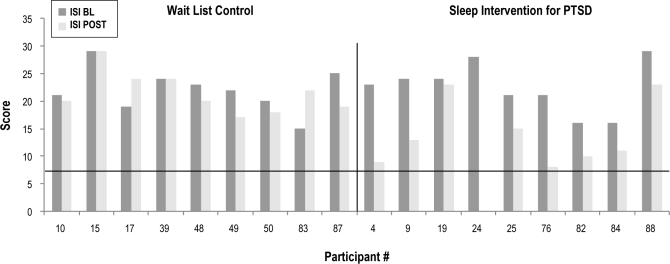
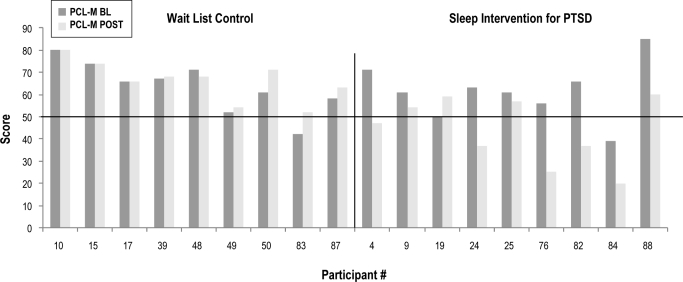
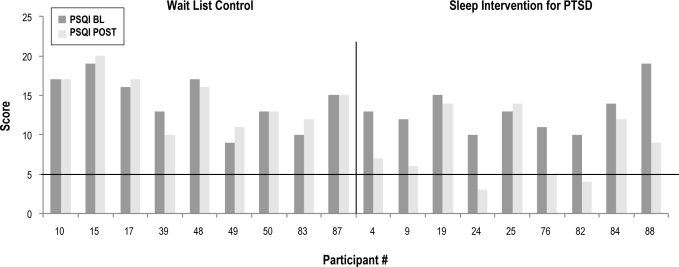
Figures 4, 5, and 6 depict baseline to post-intervention change in ISI, PCL-M, and PSQI scores, respectively. At post-intervention, one participant in the intervention group remitted from insomnia, (1/9 or 11%), and 4 were insomnia responders (4/9 or 44.4%). As depicted in Figure 5, 1 participant in each group had a baseline PCL-M score < 50. Baseline PCL-M values did not differ between groups when considering only those participants with complete sleep diary data and baseline PCL-M values above the PTSD screening cutoff of 50 (F = 0.17, p = 0.69). At post-intervention, however, half of the intervention group participants in this subsample (4/8 or 50%) had remitted from PTSD as compared to none of the usual care participants (0/8) (χ2 = 5.33, p = 0.04). In terms of sleep quality, groups did not differ at post-intervention on the percentage of sleep quality remitters, with 3 (3/9 or 33.3%) of those in the intervention group remitting, and none of those in the usual care group (0/9 or 0%). Finally, with regard to PTSD-specific disruptive nocturnal behaviors, groups did not differ at post-intervention with 3 (3/9 or 33.3%) of those in the intervention group remitting and one (1/9 or 11.1%) in the usual care group (χ2 = 1.29, p = 0.26).
Figure 4.
Insomnia severity
Figure 5.
PTSD symptoms
Figure 6.
Sleep quality
DISCUSSION
The primary purpose of our study was to assess the feasibility of SIP using a randomized trial design. Although the study revealed the need for slight procedural changes to be incorporated into a larger trial, the overall findings suggest that the intervention is feasible and generally acceptable to veterans with PTSD. Our secondary purpose was to compare the intervention to usual care in terms of differential symptoms of sleep disturbance at post-intervention. We hypothesized that this combined intervention, in addition to usual care, would produce better outcomes than usual care alone. Our hypothesis was supported in terms of both average improvement across individual measures and the clinical significance of our findings. Intent-to-treat analyses revealed medium to large effect sizes (group differences) for all sleep diary outcomes, and very large treatment effects (Cohen’s d > 1.50) for insomnia severity, sleep quality, and PTSD symptoms. In contrast, none of the participants in the usual care group responded or remitted from insomnia or PTSD, and did not improve from baseline on sleep quality. SIP did not produce a treatment effect for depression, and although SIP is not designed to treat depression, this finding was surprising in light of the treatment effect on correlated issues: insomnia and PTSD. We suspect that the failure to find a significant effect for depression may be related to the restricted total score range (0-6) for the depression measure employed in this study (PHQ-2).
In spite of large effect sizes, remission rates were lower than hoped for measures of both insomnia (11%) and sleep quality (33%). In fact, it is particularly surprising that remission rates for sleep targets were lower than for PTSD (50%). Comparison of PSQI and ISI items across groups does not reveal a consistent indicator of residual symptomatology that might explain the low remission rates for insomnia and sleep quality. However, of all sleep log variables assessed, the treatment effect sizes were lowest for nightmare frequency. Since there was no follow-up assessment included in the design of this pilot study, it remains unknown if the IRT skills acquired during the intervention might have resulted in better outcomes at a later follow-up assessment, or if decrements in clinical improvements would be revealed over time. However, it is plausible that residual nightmares in the treatment group might explain the low remission rates for insomnia and sleep quality. This explanation is also consistent with our finding of greater remission rates for SOL than WASO on sleep logs.
Randomized controlled trials of sleep-focused interventions in veterans with PTSD are largely absent from the empirical literature. However, our findings are generally consistent with the available studies addressing post-trauma sleep disturbance, excepting the larger effect size produced by SIP relative to other interventions. SIP produced significant treatment effects in the same domains as those found in the clinical outcomes of IRT for veterans described by Nappi and colleagues,18 including nightmares, insomnia severity, and PTSD symptoms, with the exception that we also found a significant treatment effect for sleep quality. Our findings are also generally consistent with those of Davis et al.19 ERRT resulted in a significant improvement in depression, however, whereas depression did not improve significantly with SIP, as discussed above. Insomnia was not assessed, and sleep diaries were not included in the study by Davis et al., so no comparisons can be made here.
SIP differs from previously assessed interventions in at least two areas. First, exposure is not an intended mechanism of change in SIP, in contrast with empirically based PTSD treatments and ERRT. In spite of differences in treatment protocols, however, SIP (50%) and ERRT (46%) produced similar rates of PTSD remission. Our finding of a large treatment effect for PTSD symptoms challenges the notion that exposure is a necessary component of PTSD and nightmare treatment, since SIP produced significant reductions in PTSD symptoms and nightmare frequency but involves very limited exposure to nightmare content.
Second, SIP incorporates an intervention that specifically targets insomnia and is heavily focused on regulation of erratic sleep schedules. Given the importance of homeostatic and circadian mechanisms on sleep and their role in sleep quality, it is our belief that behavioral sleep targets should be addressed first to provide the foundation for addressing other aspects of sleep disturbance (e.g., nightmares). Addressing nightmares without first targeting behaviors that serve to maintain insomnia is likely to impair the effectiveness of the nightmare intervention since sleep-disruptive behaviors may persist. However, in the absence of research showing a sequence effect, this assertion remains an empirical question.
As consistent with expectations for an intervention targeting sleep disturbance, SIP had the greatest impact on self-reported insomnia severity. SIP’s potent effect on PTSD symptoms, however, was a less expected and arguably, one of the more interesting findings of our study. The second largest treatment effect for SIP was found in the domain of PTSD symptoms (Cohen’s d = 1.76), and half of those in the intervention group scored in the subclinical PTSD screening range at post-intervention. This finding is consistent with the suggestion of some researchers, that sleep plays a significant role in the development and/or maintenance of PTSD.34–38
Krakow and colleagues have observed that many individuals with post-trauma symptoms, would prefer to address their insomnia first, then nightmares, and then PTSD, as applicable,39 and their observation is consistent with our clinical experience of PTSD patients seeking treatment for sleep disturbance. Acceptability of treatment is a highly relevant topic for a mental health condition for which avoidance is a primary cognitive feature. We did not assess the relative acceptability of sleep treatment versus PTSD treatment. However, if our clinical experience on this topic is based in fact, that those who are struggling with PTSD are more likely to seek and complete treatment for sleep disturbance, then it may be prudent to promote sleep disturbance interventions as a first-line PTSD treatment. Our evidence, along with the reports of other researchers, suggests that about half of participants could remit from PTSD following treatment for sleep disturbance.
As noted above, in spite of differences in approach, these interventions all facilitate a reduction in both nightmare frequency and PTSD symptoms. One explanation for this consistent finding across approaches is that ERRT, IRT, and SIP all promote an observer stance towards dream content. Several participants in our study reported a significant shift in nightmare frequency with the realization that they could alter the course of the dream while it was occurring. These participants described a shift from being part of the dream to being an observer of the dream. These reports are consistent with a shift towards metacognitive insight, as described by Teasdale,40 wherein “A distinction is made between metacognitive knowledge (knowing that thoughts are not necessarily always accurate) and metacognitive insight (experiencing thoughts as events in the field of awareness, rather than as direct readouts on reality)(pg. 146).” Recent findings of a study examining brain activity during self-related awareness tasks found that, by simply placing one’s attention on feelings and emotions one could modulate amygdala activation, thereby initiating the emotion regulation process.41 These authors propose that making oneself aware of emotions may provide a therapeutic distance that facilitates emotion regulation. Similarly, rather than promoting avoidance, IRT and similar approaches promote a stance of “observer” versus “experiencer” of affectively charged dream content.
Limitations
The findings of our study should be viewed in light of the limitations inherent to a pilot study. Namely, our sample size was very small, so we cannot be certain that the large effect sizes seen in this trial would be seen in a larger trial of SIP. Also, this study did not include an active control condition, and no follow-up data was collected. Thus, we cannot determine the effect of nonspecific factors, such as treatment expectancies and therapeutic alliance, and we cannot assure that our post-intervention outcomes translate to sustained beneficial effects. Davis et al.19 found that nightmare frequency improved from the post-intervention assessment to the follow-up assessments. Since we did not conduct a clinical assessment of PTSD using the CAPs at the 12-week assessment, we cannot assure that those screening negative for PTSD diagnosis following the intervention (PCL-M < 50) actually remitted from PTSD. Finally, we excluded veterans who screened positive for sleep apnea, since apnea produces its own sleep deprivation that would be unaddressed by our intervention. The inclusion of those with apnea would be further confounded by the observation of Krakow and colleagues42 that treatment of OSA alone resulted in subjective improvements in sleep, PTSD symptoms, and nightmares. However, since we did not assess participants with PSG, we cannot rule out the possibility that veterans with occult apnea were enrolled in our study. To assure the generalizability of SIP to the larger population of veterans with PTSD, a larger trial of SIP should include veterans with sleep apnea.
Future Research
All of those dropping out of the study were OEF/OIF veterans, and these veterans are also less likely to utilize VA clinical services.43 In future research, SIP will be tailored to assure that it is acceptable to and addresses the needs of our recently deployed veterans. With many younger veterans returning from deployment with trauma-related sleep disturbance, it will be critical to identify sleep interventions that are not only effective, but accessible. Sleep and trauma researchers should also attempt to determine the optimal sequence of treatment for those with PTSD. Should sleep disturbance be addressed before, after, or concurrent with PTSD treatment, and what are the characteristics of those who remit from PTSD following a trauma-focused sleep intervention versus those who do not remit?
In spite of the limitations of our study, the findings demonstrate that an intervention targeting both insomnia and nightmares produces large short-term effects. The collective findings of our research along with those of other sleep and trauma researchers suggest that the treatment of sleep disturbance produces significant reductions in PTSD symptoms, in addition to the amelioration of sleep disturbance. Thus, the field of behavioral sleep medicine is poised to extend the reach of our trauma-specific interventions beyond sleep clinics and into mental health clinics through the provision of clinical services and mental health provider training.
DISCLOSURE STATEMENT
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States Government. This was not an industry supported study. Dr. Edinger has received research support from Philips Respironics and has consulted for Philips Respironics and Kingsdown, Inc. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The first author was funded by a Department of Veterans Affairs HSR&D Career Development Award CDA 09-218. The authors would like to thank Maren Olsen, Ph.D., and Daniel Almirall, Ph.D., for their statistical support in preparing this manuscript. This material is based upon work performed at the Durham VA Medical Center and supported by the Institute for Medical Research and the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development.
REFERENCES
- 1.Seal KH, Maguen S, Cohen B, et al. VA mental health services utilization in Iraq and Afghanistan veterans in the first year of receiving new mental health diagnoses. J Trauma Stress. 2010;23:5–16. doi: 10.1002/jts.20493. [DOI] [PubMed] [Google Scholar]
- 2.Maher MJ, Rego SA, Asnis GM. Sleep disturbances in patients with post-traumatic stress disorder: epidemiology, impact and approaches to management. CNS Drugs. 2006;20:567–90. doi: 10.2165/00023210-200620070-00003. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi I, Boarts JM, Delahanty DL. Polysomnographically measured sleep abnormalities in PTSD: a meta-analytic review. Psychophysiology. 2007;44:660–9. doi: 10.1111/j.1469-8986.2007.537.x. [DOI] [PubMed] [Google Scholar]
- 4.Calhoun PS, Wiley M, Dennis MF, Means MK, Edinger JD, Beckham JC. Objective evidence of sleep disturbance in women with posttraumatic stress disorder. J Trauma Stress. 2007;20:1009–18. doi: 10.1002/jts.20255. [DOI] [PubMed] [Google Scholar]
- 5.Galovski T, Monson C, Bruce SE, Resick PA. Does cognitive-behavioral therapy for PTSD improve perceived health and sleep impairment? J Trauma Stress. 2009;22:197–204. doi: 10.1002/jts.20418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zayfert C, DeViva JC. Residual insomnia following cognitive behavioral therapy for PTSD. J Trauma Stress. 2004;17:69–73. doi: 10.1023/B:JOTS.0000014679.31799.e7. [DOI] [PubMed] [Google Scholar]
- 7.Belleville G, Guay S, Marchand A. Impact of sleep disturbances on PTSD symptoms and perceived health. J Nerv Ment Dis. 2009;197:126–32. doi: 10.1097/NMD.0b013e3181961d8e. [DOI] [PubMed] [Google Scholar]
- 8.Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE. Cognitive behavioral therapy for treatment of chronic primary insomnia: A randomized controlled trial. JAMA. 2001;285:1856–64. doi: 10.1001/jama.285.14.1856. [DOI] [PubMed] [Google Scholar]
- 9.Morin C, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004) Sleep. 2006;29:1398–414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 10.Ulmer C, Edinger J, Means MK, et al. Orlando, FL: Annual meeting of the Association for Behavioral and Cognitive Therapies; 2008. Cognitive behavioral therapy for insomnia in veterans with PTSD. [Google Scholar]
- 11.Kellner R, Singh G, Irigoyen-Rascon F. Rehearsal in the treatment of recurring nightmares in post-traumatic stress disorders and panic disorder: case histories. Ann Clin Psychiatry. 1991;3:67–71. [Google Scholar]
- 12.Kellner R, Neidhardt J, Krakow B, Pathak D. Changes in chronic nightmares after one session of desensitization or rehearsal instructions. Am J Psychiatry. 1992;149:659–63. doi: 10.1176/ajp.149.5.659. [DOI] [PubMed] [Google Scholar]
- 13.Krakow B, Zadra A. Clinical management of chronic nightmares: Imagery rehearsal therapy. Behav Sleep Med. 2006;4:45–70. doi: 10.1207/s15402010bsm0401_4. [DOI] [PubMed] [Google Scholar]
- 14.Krakow B, Hollifield M, Johnston L, et al. Imagery rehearsal therapy for chronic nightmares in sexual assault survivors with posttraumatic stress disorder: A randomized controlled trial. JAMA. 2001;286:537–45. doi: 10.1001/jama.286.5.537. [DOI] [PubMed] [Google Scholar]
- 15.Moore B, Krakow B. Imagery rehearsal therapy for acute posttraumatic nightmares among combat soldiers in Iraq. Am J Psychiatry. 2007:164. doi: 10.1176/ajp.2007.164.4.683. [DOI] [PubMed] [Google Scholar]
- 16.Krakow B, Johnston L, Melendrez D, et al. An open-label trial of evidenced-based cognitive behavior therapy for nightmares and insomnia in crime victims with PTSD. Am J Psychiatry. 2001;158:2043–7. doi: 10.1176/appi.ajp.158.12.2043. [DOI] [PubMed] [Google Scholar]
- 17.Germain A, Shear MK, Hall M, Buysse DJ. Effects of a brief behavioral treatment for PTSD-related sleep disturbances: a pilot study. Behav Res Ther. 2007;45:627–32. doi: 10.1016/j.brat.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Nappi CM, Drummond SPA, Thorp SR, McQuaid JR. Effectiveness of imagery rehearsal therapy for the treatment of combat-related nightmares in veterans. Behav Ther. 2010;41:237–44. doi: 10.1016/j.beth.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Davis J, Wright DC. Randomized clinical trial for treatment of chronic nightmares in trauma-exposed adults. J Trauma Stress. 2007;20:123–33. doi: 10.1002/jts.20199. [DOI] [PubMed] [Google Scholar]
- 20.Swanson LM, Favorite TK, Horin E, Arnedt JT. A combined group treatment for nightmares and insomnia in combat veterans: a pilot study. J Trauma Stress. 2009;22:639–42. doi: 10.1002/jts.20468. [DOI] [PubMed] [Google Scholar]
- 21.Blake DD, Weathers FW, Nagy LM, et al. The development of a clinician-administered PTSD scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 22.Edinger JD, Olsen MK, Stechuchak KM, et al. Cognitive behavioral therapy for patients with primary insomnia or insomnia associated predominantly with mixed psychiatric disorders: a randomized clinical trial. Sleep. 2009;32:499–510. doi: 10.1093/sleep/32.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folstein M, Folstein SE, McHugh PR. Mini Mental State: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 24.Morin CM. Insomnia: Psychological assessment and management. New York: Guilford Press; 1993. [Google Scholar]
- 25.Bastien C, Vallieres A, Morin C. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 26.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 27.Germain A, Hall M, Krakow B, Shear MK, Buysse DJ. A brief sleep scale for Posttraumatic Stress Disorder: Pittsburgh Sleep Quality Index Addendum for PTSD. J Anxiety Disord. 2005;19:233–44. doi: 10.1016/j.janxdis.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Weathers F, Huska J, Keane TM. The PTSD Checklist Military Version (PCL-M) Boston, MA: National Center for PTSD; 1991. [Google Scholar]
- 29.Weathers F, Litz B, Herman D, Huska J, Keane T. The PTSD Checklist (PCL): Reliability, validity, and diagnostic utility. San Antonio, TX: In: Annual Convention of the International Society for Traumatic Stress Studies; 1993. [Google Scholar]
- 30.Arroll B, Crengle S, Kerse N, Falloon K. Validation of PHQ-2 and PHQ-9 to screen for major depression in the primary care population. Ann Fam Med. 2010;8:348–53. doi: 10.1370/afm.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diggle PJ, Kenward MG. Informative dropout in longitudinal data analysis. Appl Stat. 1994;43:49–94. [Google Scholar]
- 32.Morin CM, Vallières A, Guay B, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA. 2009;301:2005–15. doi: 10.1001/jama.2009.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 34.Mellman TA, Bustamante V, Fins A, Pigeon WR, Nolan B. REM sleep and the early development of posttraumatic stress disorder. Am J Psychiatry. 2002;159:1696–701. doi: 10.1176/appi.ajp.159.10.1696. [DOI] [PubMed] [Google Scholar]
- 35.Mellman TA, Pigeon WR, Nowell PD, Nolan B. Relationships between REM sleep findings and PTSD symptoms during the early aftermath of trauma. J Trauma Stress. 2007:893–901. doi: 10.1002/jts.20246. [DOI] [PubMed] [Google Scholar]
- 36.Mellman TA, Knorr BR, Pigeon WR, Leiter JC, Akay M. Heart rate variability during sleep and the early development of posttraumatic stress disorder. Biol Psychiatry. 2004;55:953–6. doi: 10.1016/j.biopsych.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 37.Koren D, Arnon I, Lavie P, Klein E. Sleep complaints as early predictors of posttraumatic stress disorder: A 1-year prospective study of injured survivors of motor vehicle accidents. Am J Psychiatry. 2002;159:855–7. doi: 10.1176/appi.ajp.159.5.855. [DOI] [PubMed] [Google Scholar]
- 38.Bryant RA, Creamer M, O’Donnell M, Silove D, McFarlane AC. Sleep disturbance immediately prior to trauma predicts subsequent psychiatric disorder. Sleep. 2010;33:69–74. doi: 10.1093/sleep/33.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krakow B, Schrader R, Tandberg D, et al. Nightmare frequency in sexual assault survivors with PTSD. J Anxiety Disord. 2002;16:175–90. doi: 10.1016/s0887-6185(02)00093-2. [DOI] [PubMed] [Google Scholar]
- 40.Teasdale JD. Metacognition, mindfulness and the modification of mood disorders. Clin Psychol Psychother. 1999;6:146–55. [Google Scholar]
- 41.Herwig U, Kaffenberger T, Jäncke L, Brühl AB. Self-related awareness and emotion regulation. Neuroimage. 2010;50:734–41. doi: 10.1016/j.neuroimage.2009.12.089. [DOI] [PubMed] [Google Scholar]
- 42.Krakow B, Lowry C, Germaine A, et al. A retrospective study on improvements in nightmares and post-traumatic stress disorder following treatment for co-morbid sleep-disordered breathing. J Psychosom Res. 2000;49:291–8. doi: 10.1016/s0022-3999(00)00147-1. [DOI] [PubMed] [Google Scholar]
- 43.Pietrzak RH, Johnson DC, Goldstein MB, Malley JC, Southwick SM. Perceived stigma and barriers to mental health care utilization among OEF-OIF veterans. Psychiatr Serv. 2009;60:1118–22. doi: 10.1176/ps.2009.60.8.1118. [DOI] [PubMed] [Google Scholar]