Abstract
Study Objectives:
The objective of this study was to determine if primary care providers (PCPs) screen for sleep disorders during clinical evaluation of new patients, and to compare this to likely sleep diagnoses as assessed by validated questionnaires.
Methods:
Adult patients evaluated as new patients in a primary care clinic at a tertiary care center were included in a prospective study. Following their appointment, patients completed the Cleveland Sleep Habits questionnaire (CSHQ), Berlin questionnaire, Epworth Sleepiness Scale (ESS), and STOP questionnaire. The encounters were subsequently reviewed for elements of a sleep history, sleep review of systems, and/or sleep workup.
Results:
101 patients participated in the study. Demographics: 58 (52%) females, mean age 38 ± 12.9 years, body mass index (BMI) 29.5 ± 8.3 kg/m2 (BMI > 30 kg/m2 in 44%), 46% Caucasian, 38% African American, 11% Hispanic, and 5% other. House staff evaluated 57.4%; faculty evaluated the remainder. The ESS was > 10 in 28% of subjects. High risk for obstructive sleep apnea (OSA) risk was found in 33% (Berlin) and 34% (STOP) (24.8% by both). The CSHQ suggested possible diagnoses of insomnia in 30% and restless legs syndrome in 22%. In the clinic encounters, a limited sleep history was found in 24.8%, documentation of a sleep disorder in 8.9%, referral to sleep clinic in 2%, and referral to psychiatry clinic in 6.9%.
Conclusion:
Symptoms suggestive of sleep disorders are common but are not routinely screened for in the primary care setting. Validated questionnaires can efficiently identify patients at risk for common sleep disorders in this setting.
Citation:
Senthilvel E; Auckley D; Dasarathy J. Evaluation of sleep disorders in the primary care setting: history taking compared to questionnaires. J Clin Sleep Med 2011;7(1):41-48.
Keywords: Sleep disorders, primary care, questionnaires, screening
Sleep disorders are highly prevalent in modern society. The majority of the patients with sleep disorders remain undiagnosed and untreated.1 A 2006 report from the Institute of Medicine revealed that 50 to 70 million American chronically suffer from a disorder of sleep and wakefulness, hindering daily functioning and adversely affecting their health and longevity.1 The most common sleep disorders seen in primary care settings are insomnia, obstructive sleep apnea (OSA), and restless legs syndrome (RLS). In the general population, symptoms of insomnia are present in up to 33% of individuals,2 OSA has a prevalence of about 5% (though 26% to 32% have symptoms suggesting they are at risk for OSA),3–5 and RLS is present in 5% to 15% of the population.6,7 Sleep disorders have also been associated with motor vehicle accidents, impaired cognition, metabolic syndrome, impaired immune function, altered mood, decreased quality of life, and increased mortality.8–16
Despite the high prevalence of sleep disorders and their significant consequences, sleep complaints are often not addressed by primary care physicians.17 To date, the United States Preventive Services Task Forces, the American Academy of Family Physicians and the Center for Disease Control have not recommended routine screening for sleep disorders.18 In addition, limited time, lack of reimbursement, and high demand may be factors that hinder the provision of preventive health services by primary care physicians (PCP).19–21
BRIEF SUMMARY
Current Knowledge/Study Rationale: Past studies have suggested that primary care providers infrequently screen for sleep disorders. This study sought to determine if screening questionnaires would identify more patient's at risk for common sleep disorders than current practice patterns of primary care providers during new patient encounters.
Study Impact: Symptoms of common sleep disorders were found to be highly prevalent in the study population, though were infrequently identified by the primary care providers. Utilizing screening questionnaires during new patient encounters would likely increase detection of significant and common sleep disorders.
The aim of the present study is to determine if PCPs obtain a sleep history or systematically review complaints of sleep disorders as part of their standard new patient evaluations and to compare this with the prevalence of likely sleep diagnoses in the same patients as established by validated questionnaires (Cleveland Sleep Habits Questionnaire [CSHQ]22 and STOP questionnaire23). We also examined the time required to administer the CSHQ and STOP questionnaires in the primary care setting. We hypothesized that PCPs do not routinely evaluate for sleep disorders in new patient evaluations and that screening questionnaires (CSHQ and STOP) could efficiently identify individuals who need further sleep evaluation.
METHODS
General Study Design
A prospective survey study was performed at MetroHealth Medical Center (MHMC), an urban academic medical center that serves as the county hospital for Cuyahoga County (Cleveland), Ohio, and is affiliated with Case Western Reserve University. The study was approved by the institutional review board at MHMC.
Consecutive adult patients (18 to 65 years old) presenting for new patient evaluations were recruited from a primary care (family practice) clinic. Pregnant women, acutely ill patients, mentally incompetent patients, and non-English speaking patients were excluded. Patients were approached during their check-out process (after completing their new patient visit with the physician); the study was explained in detail to them, and informed consent was obtained. Subjects then completed the CSHQ and STOP questionnaires (see below for the details of the questionnaires) while on site and were timed with a stopwatch while doing so.
Patients being evaluated by attending physicians and house staff (second and third year residents only, with an attending physician as preceptor) were included. The faculty and house staff whose patients participated in the study were not part of the research group. All faculty and house staff were masked to their patients' participation in the study. While the faculty and house staff were aware of a sleep survey study being performed at the institution, they were unaware of which patients might be participating, as this was a convenience sample taken after the patient's encounter with the physician.
Questionnaires
Cleveland Sleep Habits Questionnaire (CSHQ)
The CSHQ is a patient self-reported instrument that consists of 2 pages of sleep related questions, the Epworth Sleepiness Scale (ESS) and one page of self-reported comorbidities. Embedded within the sleep related questions section of the CSHQ is the Berlin questionnaire (Appendix A), a 10-item questionnaire that stratifies patients into a high or low risk category for OSA. The Berlin questionnaire has been validated in the primary care setting with a positive predictive value of 0.89 for detecting OSA (defined by an apnea-hypopnea index > 5 with associated symptoms).24 The Berlin questionnaire specifically evaluates snoring history and witnessed episodes of apnea (category 1; 5 questions), tiredness and sleepiness (category 2; 4 questions), and a history of high blood pressure and/or body mass index (BMI) > 30 kg/m2 (category 3). Patients must score positive in 2 of the 3 domains to be considered high risk for OSA. Additional questions in the CSHQ probe for symptoms of insomnia (3 questions), the use of drugs or alcohol to promote sleep (one question), leg jerks during sleep (one question), strange sensations in the leg while awake (one question), and drop attacks or sudden weakness with surprise, anger, or laughter (one question).22 The scoring for these questions with regards to high pretest probability for a disorder is based upon the persistent presence of these problem as defined by the patient having checked off “ > 3-4 days a week” or “every day (night).” A high pretest probability for insomnia is based on persistent symptoms in 2 of the 3 questions; a high test probability for restless legs syndrome (RLS) is based on the report of persistent symptoms of both leg jerks during sleep and awake leg sensations as well as a positive score in category 2 (sleepiness) from the Berlin questionnaire; and a high pretest probability for narcolepsy is based upon the report of drop attacks and a positive score in category 2 (sleepiness) from the Berlin questionnaire.
The ESS is part of the CSHQ. The ESS contains 8 items that ask for the self-perceived expectation of dozing in a variety of situations. A score > 10 is generally considered suggestive of significant sleepiness.25 See Appendix B for details of the ESS.
STOP Questionnaire (Appendix C)
The STOP questionnaire was developed as a rapid screening tool for OSA in the preoperative setting.23 In that setting, its testing characteristics (sensitivity and specificity) have been found to be similar to that of the Berlin questionnaire.26 The STOP questionnaire includes 4 questions related to snoring, tiredness during daytime, observed apneas, and the presence of high blood pressure. A score ≥ 2 places an individual into a high-risk category for OSA.
Data Collection
All new patient encounters were entered into the electronic medical record (EMR) by the physician who evaluated the patient. All new patient encounters included a history of present illness (HPI), medication list, past medical history, social history, family history, review of systems (ROS), examination, data section, and assessment and plan. Demographic data abstracted from each new patient evaluation in the EMR included the following: age, gender, ethnicity, weight, height, BMI, comorbidities (hypertension, diabetes mellitus, cardiovascular diseases, hypothyroidism, depression, and any listed known history of sleep disorders in the past medical history), and current medications. The HPI and ROS for each encounter were reviewed for any documentation of snoring, apnea/gasping/choking during sleep, excessive daytime sleepiness, unrefreshing sleep, insomnia symptoms, restless legs symptoms, and symptoms suggestive of cataplexy. The symptom evaluation was recorded as “yes” if there was any mention of the symptom or “no” if there was no mention. Documented physical examinations were reviewed for evidence of oropharyngeal Mallampati score, tonsillar size, nasal passage patency, and examination of the neck, lungs, heart, extremities, and neurologic system. The assessment and plan section of the encounters as well as orders placed during the encounter (all orders are required to be placed in the EMR by the PCP) were reviewed for sleep diagnoses and sleep-relevant orders (order for polysomnogram [PSG], thyroid stimulating hormone [TSH] level, serum ferritin level) and referral to the sleep clinic or allied specialties, including pulmonary, psychiatry, neurology, or otolaryngology clinics.
Statistical Analysis
Continuous variables are reported in means and standard deviations. The categorical variables of sleep symptoms and suspected sleep disorders are reported as percentages of the total population. McNemar's test and κ coefficient were used to determine the degree of agreement between tests. A κ > 0.80 was considered to represent excellent agreement beyond chance; κ between 0.60 and 0.80, substantial to moderate; κ between 0.21 and 0.40, fair; and κ < 0.21 represented poor agreement.27 All data were analyzed using SAS (SAS Inc, Cary, NC).
RESULTS
Demographic and Other Health Characteristics
Of 111 patients approached, 101 (91.8%) agreed to participate in the study. Of the 10 patients who refused, 3 cited lack of time and 7 were not interested in participating. The clinical and demographic characteristics of the study population are shown in Table 1. There was a slight female preponderance in the subjects evaluated, most of whom were Caucasians. There were 39 (38.6%) subjects who were obese (BMI ≥ 30 kg/m2).
Table 1.
Demographic and health characteristics of patients (N = 101)
| Gender (% male) | 42.6 |
| Age (years) | 38.2 ± 12.8 |
| Ethnicity (%) | |
| Caucasian | 45.5 |
| African American | 37.6 |
| Hispanic | 10.9 |
| Other | 5.0 |
| BMI (kg/m2) | 29.5 ± 8.3 |
| (Range) | (18.2 to 64.7) |
| Comorbidities (%) | |
| Hypertension | 22.8 |
| Diabetes | 8.9 |
| Hypothyroidism | 3 |
| Coronary artery disease | 0 |
| Heart failure | 0 |
| Arrhythmias | 0 |
| Depression | 21.8 |
Prevalence of Sleep Symptoms: PCPs vs. CSHQ
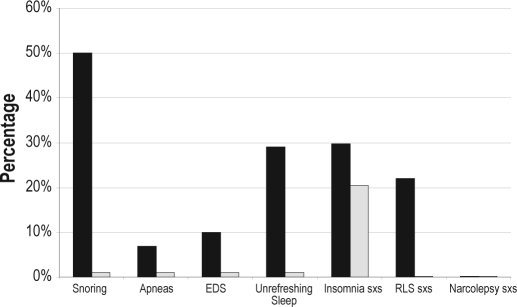
At least one sleep related symptom was documented by the PCP in 24.8% of the patients (Figure 1). Questions pertaining to insomnia were the most commonly documented symptoms, with 20% of encounters having evidence of screening for insomnia (4% in the HPI, 16% in the ROS). Among these patients, positive symptoms of insomnia were found in 7 (6.9% of the total sample), with the remainder being negative. Conversely, 30 (29.7%) patients reported some insomnia symptoms in the CSHQ. One patient was screened for snoring (HPI) in the PCP encounters (screen positive, BMI 64 kg/m2), while 51 (50.5%) reported snoring in the CSHQ. Excessive daytime sleepiness was documented in one patient (HPI); CSHQ identified 10 (9.9%) patients as having excessive daytime sleepiness, and 29 (28.7%) reported ESS > 10 (suggesting pathological sleepiness). The mean ESS score for the entire group was 7.1 ± 4.9 (range 0-23). Unrefreshing sleep was also noted in one patient (HPI, same patient who noted excessive daytime sleepiness) and an additional patient was documented to have witnessed episodes of apnea (HPI). No patient encounters documented symptoms of or screening for RLS or cataplexy. The CSHQ revealed that 29 (28.7%) patients had unrefreshing sleep, 7 (6.9%) reported witnessed episodes of apnea, 22 (21.7%) had symptoms suggestive of RLS, and none had symptoms of cataplexy. The degree of agreement of all symptoms between the clinical documentation by the PCP and the questionnaires revealed a κ of < 0.21, suggesting poor agreement (Table 2).
Figure 1.
Prevalence of sleep symptoms as determined by the CSHQ vs. PCPs
■ CSHQ,  PCP. EDS, excessive daytime sleepiness; RLS, restless legs syndrome; sxs, symptoms.
PCP. EDS, excessive daytime sleepiness; RLS, restless legs syndrome; sxs, symptoms.
Table 2.
Correlation between screening instruments and clinical symptoms by PCP
| CSHQ vs. PCP | McNemar's | κ Coefficient |
|---|---|---|
| Insomnia | 2.78 | 0.101 |
| (−0.1) | ||
| Snoring | 50 | 0.019 |
| (< 0.0001) | ||
| Apnea | 4.5 | −0.018 |
| (−0.03) | ||
| EDS | 7.36 | −0.018 |
| (−0.006) | ||
| Unrefreshing Sleep | 26.1 | −0.019 |
| (< 0.001) |
CSHQ, Cleveland Sleep Habits Questionnaire; PCP, Primary Care Provider
Sleep Related Clinical Examination Findings by PCP
Just over a third (34.7%) of the encounters commented on tonsils in their examination finding, all of them stating the tonsils were normal without grading of tonsillar size. Similarly, 41 (40.6%) encounters had evidence of a nasal examination, while documentation of the Mallampati grading of the oropharynx or a neck circumference were not reported in any of the physician encounters. All of the encounters documented heart, lung, and extremity examinations, and 79 (78.2%) of the clinical evaluations documented neurologic examinations.
Sleep Diagnoses as Listed by the PCP
A diagnosis of OSA or suspected OSA was made by the PCPs in 2 subjects—one was newly suspected, and the other was a patient with an established diagnosis of OSA. Seven patients (6.9%) had a diagnosis of insomnia listed as a problem by the PCP, and no patient was suspected of having RLS or narcolepsy.
Sleep Related Evaluation Ordered by the PCP
One patient was referred for PSG as well as a sleep clinic appointment for suspected OSA. An additional patient was sent to the sleep clinic for further management of known OSA. Referrals for psychiatric evaluation were placed in 7 (6.9%) patients, mainly for depression with associated symptoms of insomnia. No referrals were placed for pulmonary, neurology, or otolaryngology clinics. One patient had a TSH level ordered for the sleep related complaint of excessive daytime sleepiness and tiredness. In addition, 6 patients had TSH levels ordered for non–sleep related issues. A ferritin level was ordered in 2 patients, but not for the evaluation of RLS.
Sleep Evaluation: House Staff vs. Faculty
There were no differences in documentation of sleep symptoms, sleep related clinical examination, sleep diagnoses, or sleep related orders between house staff and faculty encounters (data not shown).
Suspected Sleep Disorders by Questionnaires
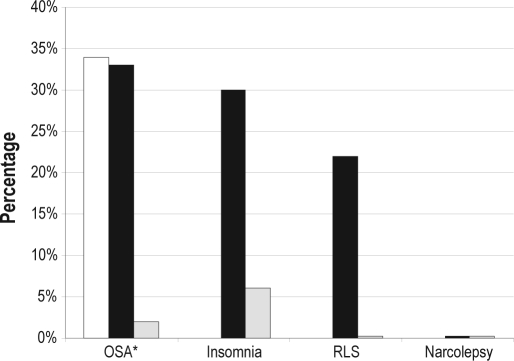
The questionnaires identified patients as being at-risk or having symptoms suggestive of sleep disorders at a greater frequency than the PCPs. The Berlin questionnaire (embedded in the CSHQ) suggested that 33 (32.7%) patients were at high risk for OSA, while the STOP questionnaire found that 34 (33.7%) patients were at high risk. Twenty-five patients (24.8%) were found to be categorized as high risk for OSA by both the Berlin and STOP questionnaires. Further analysis of the specific questionnaire answers in those scoring high risk for OSA was also performed. For those scoring as high risk for OSA on the Berlin questionnaire, 85% scored positive on category 1 (snoring and witnessed apneas), 70% scored positive in category 2 (tiredness and sleepiness), and 79% scored positive in category 3 (hypertension and/or elevated BMI). The most common category combinations resulting in high-risk stratification were scoring positive in all 3 categories (33%), followed by positives in categories 2 and 3 (30%). Regarding the STOP questionnaire, those considered to be at high risk for OSA scored positive on each of the questions as follows: first question (snoring) 64% of the time; second question (tiredness) 82%; third question (observed apneas) 39%; and the fourth question (hypertension) 55%. The most common combinations of questions resulting in high-risk scoring on the STOP questionnaire were scoring positive on questions 1 and 2 (24%) and questions 2 and 4 (24%). Clinical assessment for OSA by the PCP correlated poorly with the identification of patients at risk for sleep apnea by either of the questionnaires (Table 3). The CSHQ suggested patients had symptoms consistent with insomnia in 32 (31.7%) and RLS in 22 (21.8%) of patients. None of the subjects evaluated had symptoms suggestive of narcolepsy (Figure 2).
Table 3.
Correlation between diagnosis of OSA by screening instruments and diagnosis by PCP
| Diagnosis of OSA | McNemar's | κ Coefficient |
|---|---|---|
| STOP vs. PCP | 33 | 0.04 |
| (< 0.0001) | ||
| Berlin vs. PCP | 32 | 0.04 |
| (< 0.0001) |
STOP, STOP questionnaire; PCP, Primary Care Provider; Berlin, Berlin questionnaire; OSA, Obstructive sleep apnea
Figure 2.
Percentage of patients at risk for specific sleep diagnoses as determined by the STOP questionnaire (OSA only), CSHQ, and PCPs
□ STOP questionnaire, ■ CSHQ,  PCPs. RLS, restless legs syndrome.
PCPs. RLS, restless legs syndrome.
Time to Complete the Questionnaires
It took patients an average of 302 ± 97 sec to complete the CSHQ. Time to complete each separate section of the CSHQ (i.e., the Berlin questionnaire vs. the rest of the questionnaire) was not recorded. Patients completed the STOP questionnaire in an average of 24 ± 12 sec.
DISCUSSION
The present prospective study confirms previous data that PCPs do not routinely screen patients for sleep disorders. The observations in this study suggest an efficient and relatively simple mechanism to identify patients at risk for significant sleep disorders in the primary care setting. In the present study population, the prevalence rates of patients with symptoms suggestive of OSA, insomnia, and RLS are consistent with those reported in larger epidemiologic studies1,2,4–7 and confirm that these sleep disorders are likely underdiagnosed. This is reiterated by our observation of the low concordance between the clinical assessment for sleep disorders and questionnaire risk stratification for common sleep disorders.
Data from the 1990s suggested that PCPs were not routinely obtaining sleep histories in their outpatient encounters.28,29 This specific issue has not been readdressed recently, though one might believe that as a result of the dramatic rise in publications regarding the prevalence and consequence of sleep disorders, as well as educational efforts linked to sleep medicine, that improved screening rates would be realized. Our observations, however, suggest that screening for sleep disorders continues to be infrequent in the primary care setting, and that this is not related to either of the level of physician training or the frequency of the sleep disorder. This observation is unlikely to be related specifically to the institution where the study was performed as others have reported similar findings in different clinical settings.28–30 In addition, the present study was performed in an academic center with both a Family Medicine training program and a Sleep Medicine Fellowship program. The Sleep Center at our institution is highly visible and located on the same site as the PCP clinics. A recent national survey of 14 distinct database batteries utilized by Family Medicine Clinics found that only 6 of the 14 contained any questions related to sleep symptoms,18 suggesting the lack of screening for sleep disorders is widespread. At present, the United States Preventive Services Task Forces, the American Academy of Family Physicians and the Center for Disease Control have not recommended screening for sleep disorders as a part of routine preventive clinical services.18 Taken together, these findings suggest that lack of screening for sleep disorders is a pervasive problem.
The reason for the infrequent screening for common sleep disorders by PCPs is unclear, and needs to be examined in prospective studies. One potential reason is a lack of awareness amongst PCPs regarding the importance of recognizing and treating common sleep disorders. This is supported by a relatively recent study that showed that PCPs demonstrated a lack of awareness regarding the importance of screening for OSA during unstructured standardized patient examinations.30 Additional studies have revealed limited awareness of common sleep disorders among PCPs, even though they recognized the clinical importance of the same.31–33 In contrast to this, a survey regarding the knowledge and attitudes of PCPs about OSA performed at a single institution suggested that PCPs were reasonably knowledgeable about OSA, and similarly appeared to show concern that this was an important condition to diagnose and treat.34 A retrospective review of patients referred for polysomnography by PCPs revealed that in most instances (96%), an appropriate referral was made by the PCP when patients presented with classic symptoms of OSA, even though the referred patients represented only 0.13% of the primary care patient population.35 Thus, increasing awareness and education regarding the clinical presentation of undiagnosed patients may improve identification of OSA and other sleep disorders in the primary care setting.
Additional reasons for PCPs not screening for sleep disorders may be related to the limited time allocated for evaluation in the outpatient clinical setting,19,20 lack of reimbursement,21 and high demand21 for their services with pressure to address the patients' most immediate concerns. For example, identification of insomnia during an outpatient evaluation may lead to an extensive investment of time on the insomnia history at the cost of reduced time for the primary complaint. Availability of sleep consultation services to aid in the evaluation and management of common sleep disorders may also affect willingness to screen for these conditions. In the present study, however, there was a low screening rate for sleep abnormalities by the PCPs despite the availability of a sleep center.
One of questions raised by the present study is how to improve screening rates for sleep disorders in the primary care setting. Raising awareness through improved communication and educational measures has not been well studied, and its impact on physician behavior in general is unclear.36 Use of chart reminders has been attempted in a number of clinical conditions with variable success in altering physician's actions.37,38 The impact of educational maneuvers on the evaluation and management specifically of sleep disorders in the primary care setting has been examined in some studies, though further work is needed. A 1999 study looked at the use of chart reminders to improve the frequency of PCPs obtaining sleep histories.28 The authors found that the use of chart reminders increased the frequency of PCPs obtaining a sleep history as part of the clinical evaluation, though the overall rate remained low at 29%, comparable to 25% in the present study. Using an intensive approach of sleep education, sleep equipment support, and sleep physician support, the Walla Walla project found an 8-fold increase in referrals for sleep studies from 0.27% prior to the intervention to 2.1% following the intervention.39 Taken together, these studies suggest that chart reminders, education, and support can improve screening for sleep disorders in primary care, though the rate of screening remains low.
Another strategy is to implement standardized protocols that utilize patient completed screening instruments or questionnaires before physician evaluation. This reduces time spent by the physician gathering data and keeps the physician in a decision-making capacity. This approach has proven effective at improving patient care in other clinical settings.40,41 The questionnaires utilized in this study have been shown to accurately identify patients at risk for the more common sleep disorders encountered in primary care.22 The CSHQ has the benefit of risk stratifying patients for OSA, insomnia, RLS, and narcolepsy. However, the 5-minute average completion time, coupled with time required to score the various domains, would necessitate that patients complete this survey prior to their visit with the physician. The STOP questionnaire is quickly completed and easy to score, but only risk stratifies patients for OSA and still needs to be validated in the primary care setting. Nonetheless, a protocol in which patients are prescreened and identified as at risk for sleep disorders may encourage PCPs to act to upon this information and conceivably affect patient care.
The results of this study need to be placed in the context of certain limitations. Only a small number of patients were surveyed, and larger studies are needed to confirm these findings. The present study was performed at a single tertiary care institution, and its implications for the general population may be limited. Finally, the STOP questionnaire has not been validated in the primary care setting, and this needs to be performed prior to large-scale use of this instrument in clinical practice.
In summary, symptoms of sleep disorders appear common but are not routinely screened for in the primary care setting. The use of validated questionnaires may be able to efficiently identify patients at risk for common sleep disorders in primary care, though further study is required.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Colten HR, Altevogt BM. Washington, DC: The National Academies Press; 2006. Sleep disorder and sleep deprivation: An unmet public health problem. [PubMed] [Google Scholar]
- 2.Ohayon NM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 3.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 4.Netzer NC, Hoegel JJ, Loube D, Netzer CM. Prevalence of symptoms and risk of sleep apnea in primary care. Chest. 2003;124:1406–14. doi: 10.1378/chest.124.4.1406. [DOI] [PubMed] [Google Scholar]
- 5.Hiestand DM, Britz P, Goldman M, et al. Prevalence of symptoms and risk of sleep apnea in the US population: Results from the National Sleep Foundation Sleep in America 2005 poll. Chest. 2006;130:780–6. doi: 10.1378/chest.130.3.780. [DOI] [PubMed] [Google Scholar]
- 6.Lavigne GJ, Montplaisir JY. Restless legs syndrome and sleep bruxism: prevalence and association among Canadians. Sleep. 1994;17:739–43. [PubMed] [Google Scholar]
- 7.Hening W, Walter AS, Allen RP, et al. Impact, diagnosis, and treatment of restless legs syndrome (RLS) in a primary care population: the REST (RLS, Epidemiology, Symptoms, and Treatment) Primary Care Study. Sleep Med. 2004;5:237–46. doi: 10.1016/j.sleep.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Connor J, Norton R, Ameratunga S, et al. Driver sleepiness and risk of serious injury to car occupants: population-based case control study. BMJ. 2002;324:1125. doi: 10.1136/bmj.324.7346.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25:117–29. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- 10.Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165:863–7. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- 11.Babu AR, Herdegen J, Fogelfeld L, et al. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch Intern Med. 2005;165:447–52. doi: 10.1001/archinte.165.4.447. [DOI] [PubMed] [Google Scholar]
- 12.Vgontzas AN, Papnicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP. Elevation of plasma cytokine in disorders of excessive daytime sleepiness: role of sleep disturbances and obesity. J Clin Endocrinol Metab. 1997;82:1313–6. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 13.Baldwin DC, Jr, Daugherty SR. Sleep deprivation and fatigue in residency training: results of a national survey of first and second year residents. Sleep. 2004;27:217–23. doi: 10.1093/sleep/27.2.217. [DOI] [PubMed] [Google Scholar]
- 14.Baldwin CM, Griffith KA, Nieto FJ, O'Connor GT, Walsleben JA, Redline S. The association of sleep disordered breathing and sleep symptoms with quality of life in the Sleep Heart Health Study. Sleep. 2001;24:96–105. doi: 10.1093/sleep/24.1.96. [DOI] [PubMed] [Google Scholar]
- 15.Haack G, Mullington JM. Sustained sleep restriction reduces emotional and physical well-being. Pain. 2005;119:56–64. doi: 10.1016/j.pain.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Kripe DF, Garfinkel L, Winggard DL, Kluaber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 17. http//www.sleepfoundation.org/sites/default/files/2005_summary_of_findings.pdf NSF Sleep in America Poll 2005. Page 34.
- 18.Sorscher AJ. How is your sleep: A neglected topic for health care screening. JABFM. 2008;21:141–8. doi: 10.3122/jabfm.2008.02.070167. [DOI] [PubMed] [Google Scholar]
- 19.Yarnall KS, Pollak KI, Ostbye T, Krause KM, Michener JL. Primary care: is there enough time for prevention? Am J Public Health. 2003;93:635–41. doi: 10.2105/ajph.93.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stange KC, Flocke SA, Goodwin MA. Opportunistic preventive services delivery. Are time limitations and patient satisfaction barriers? J Fam Pract. 1998;46:419–24. [PubMed] [Google Scholar]
- 21.Jaen CR, Stange KC, Nutting PA. Competing demands of primary care: a model for the delivery of clinical preventive services. J Fam Pract. 1994;38:166–71. [PubMed] [Google Scholar]
- 22.Mustafa M, Erokwu N, Ebose I, Strohl K. Sleep problems and the risk for sleep disorders in an outpatient veteran population. Sleep Breath. 2005;9:57–63. doi: 10.1007/s11325-005-0016-z. [DOI] [PubMed] [Google Scholar]
- 23.Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire. A tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812–21. doi: 10.1097/ALN.0b013e31816d83e4. [DOI] [PubMed] [Google Scholar]
- 24.Netzer NC, Stoohs RA, Netzer CM, et al. Using the Berlin Questionnaire to identify patients at risk for sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 25.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 26.Chung F, Yegneswaran B, Liao P, et al. Validation of the Berlin Questionnaire and American Society of Anesthesiologists checklist as screening tools for obstructive sleep apnea in surgical patients. Anesthesiology. 2008;108:822–30. doi: 10.1097/ALN.0b013e31816d91b5. [DOI] [PubMed] [Google Scholar]
- 27.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 28.Namen AM, Wymer A, Case D, Haponik EF. Performance of sleep histories in an ambulatory medicine clinic: impact of simple chart reminders. Chest. 1999;116:1558–63. doi: 10.1378/chest.116.6.1558. [DOI] [PubMed] [Google Scholar]
- 29.Haponik EF, Frye AW, Richards B, et al. Sleep history is neglected diagnostic information. Challenge for primary care physicians. J Gen Intern Med. 1996;11:759–61. doi: 10.1007/BF02598994. [DOI] [PubMed] [Google Scholar]
- 30.Reuveni H, Tarasiuk A, Wainstock T, et al. Awareness level of obstructive sleep apnea syndrome during unstructured interviews of a standardized patient by primary care physicians. Sleep. 2004;8:1518–25. doi: 10.1093/sleep/27.8.1518. [DOI] [PubMed] [Google Scholar]
- 31.Papp KK, Penrod CE, Strohl KP. Knowledge and attitudes of primary care physicians towards sleep and sleep disorders. Sleep Breath. 2002;6:103–09. doi: 10.1007/s11325-002-0103-3. [DOI] [PubMed] [Google Scholar]
- 32.Hussain SF, Zahid S, Haqqee R, et al. General physician's perspective of sleep apnea from a developing country. Southeast Asia J Trop Med Public Health. 2003;34:420–3. [PubMed] [Google Scholar]
- 33.Bahammam AS. Knowledge and attitude of primary health care providers towards sleep disorders. Saudi Med J. 2000;21:1164–7. [PubMed] [Google Scholar]
- 34.Schotland M, Jeffe DB. Development of the obstructive sleep apnea knowledge and attitudes (OSAKA) questionnaire. Sleep Med. 2003;4:443–50. doi: 10.1016/s1389-9457(03)00073-x. [DOI] [PubMed] [Google Scholar]
- 35.Kramer NR, Cook TE, Carlisle CC, et al. The role of the primary care physician in recognizing obstructive sleep apnea. Arch Intern Med. 1999;159:965–8. doi: 10.1001/archinte.159.9.965. [DOI] [PubMed] [Google Scholar]
- 36.Lowe MM, Bennett N, Aparicio A. American College of Chest Physicians Health and Science Policy Committee. The role of audience characteristics and external factors in continuing medical education and physician change: effectiveness of continuing medical education: American College of Chest Physicians Evidence-Based Educational Guidelines. Chest. 2009;135(3 Suppl):56S–61S. doi: 10.1378/chest.08-2519. [DOI] [PubMed] [Google Scholar]
- 37.Bloomfield HE, Nelson DB, van Ryn M, et al. A trial of education, prompts, and opinion leaders to improve prescription of lipid modifying therapy by primary care physicians for patients with ischemic heart disease. Qual Saf Health Care. 2005;14:258–63. doi: 10.1136/qshc.2004.012617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rogovik AL, Rostami M, Hussain S, Goldman RD. Physician pain reminder as an intervention to enhance analgesia for extremity and clavicle injuries in pediatric emergency. J Pain. 2007;8:26–32. doi: 10.1016/j.jpain.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 39.Ball EM, Simon RD, Jr, Tall AA, et al. Diagnosis and treatment of sleep apnea within the community. The Wall Walla Project. Arch Intern Med. 1997;157:419–24. [PubMed] [Google Scholar]
- 40.Lellouche F, Mancebo J, Jolliet P, et al. A multicenter randomized trial of computer-driven protocolized weaning from mechanical ventilation. Am J Respir Crit Care Med. 2006;174:894–900. doi: 10.1164/rccm.200511-1780OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bair H, Ivascu F, Janczyk R, Nittis T, Bendick P, Howells G. Nurse driven protocol for head injured patients on warfarin. J Trauma Nurs. 2005;12:120–6. [PubMed] [Google Scholar]