Abstract
Study Objectives:
Since on CPAP, the nose is the primary determinant of upper airway resistance, we assess utility of noninvasive measures of nasal resistance during wakefulness as a predictor of directly assessed upper airway resistance on CPAP during sleep in patients with obstructive sleep apnea/hypopnea syndrome.
Methods:
Patients with complaints of snoring and excessive daytime sleepiness were recruited. 14 subjects underwent daytime evaluations including clinical assessment, subjective questionnaires to assess nasal symptoms and evaluation of nasal resistance with acoustic rhinometry (AR) and active anterior rhinomanometry (RM) in the sitting and supine positions. Patients underwent nocturnal polysomnography on optimal CPAP with measurements of supraglottic pressure to evaluate upper airway resistance. Comparisons were made between nasal resistance using AR and RM during wakefulness, and between AR and RM awake and upper airway resistance during sleep.
Results:
Our study shows that measures of awake nasal resistance using AR and RM had little or no correlation to each other in the sitting position, whereas there was significant but weak correlation in the supine position. Upper airway resistance measured while on CPAP during sleep did not show significant relationships to any of the awake measures of nasal resistance (AR or RM).
Conclusion:
Awake measurements of nasal resistance do not seem to be predictive of upper airway resistance during sleep on CPAP.
Citation:
Masdeu MJ; Seelall V; Patel AV; Ayappa I; Rapoport DM. Awake Measures of Nasal Resistance and Upper Airway Resistance on CPAP during Sleep. J Clin Sleep Med 2011;7(1):31-40.
Keywords: Obstructive sleep apnea/hypopnea syndrome, CPAP, acoustic rhinometry, rhinomanometry, supraglottic catheter, nasal resistance, nasal cross-sectional area, upper airway resistance
Continuous positive airway pressure (CPAP) is the primary treatment for obstructive sleep apnea/hypopnea syndrome (OSAHS),1,2 and has been shown to normalize sleep architecture,3 reduce daytime sleepiness,4 enhance daytime function,5,6 reduce automobile accidents,7 improve hypertension8,9 and decrease cardiovascular events10,11 in a dose-related fashion.12
Despite the efficacy of CPAP treatment, 29% to 83% of patients use CPAP less than 4 hours per night13,14 with the most common complaint of patients relating to problems with the mask.15,16 However, nasal symptoms may account for 30% to 50% of CPAP intolerance13 and the otolaryngology literature suggests that, unrelated to sleep and to CPAP, a relationship exists between nasal symptoms and an elevated nasal resistance.17,18 Although some authors attribute only a minor role of nasal symptoms on CPAP compliance,19,20 “difficulty exhaling” against positive pressure is frequently cited by patients on CPAP, and may be increased by elevated nasal resistance. Data directly addressing the relationship of assessments of nasal resistance measured noninvasively and CPAP use remain inconclusive. Several small studies have suggested that initial rejection of CPAP treatment correlates with measures of increased nasal resistance,21,22 while others have failed to show any correlation.23 At least one study shows that reducing nasal resistance by surgery improves CPAP use.24
BRIEF SUMMARY
Current Knowledge/Study Rationale: The role of nasal resistance on CPAP use is not completely established. The aim of this study was to identify a technique to measure the relevant nasal resistance during daytime that could predict the upper airway resistance during sleep and subsequently to test whether this could be used as a predictor of CPAP compliance.
Study Impact: Neither of the awake measurements of nasal resistance was predictive of upper airway resistance during sleep on CPAP, suggesting that differences in upper airway pathophysiology in patients with OSAHS may affect awake and sleep nasal resistances in complex ways.
While the expiratory pressure of CPAP may contribute to “difficulty exhaling,” it also dilates the velopharynx, reducing the contribution of this area to overall upper airway resistance, leaving the nose and related structures as the predominant determinants of resistance.25 Unlike the velopharyngeal resistance, nasal resistance has been shown to be unaffected by sleep state,26,27 and CPAP has been shown to produce only a 15% to 25% drop in nasal resistance.25,28 There has been no comparison of awake noninvasive measures of nasal resistance and total upper airway resistance on CPAP (which, as pointed out above, is assumed to reflect primarily nasal factors).
Two potential noninvasive techniques for measuring awake physiology of the nasal cavity are rhinomanometry (RM), which directly assesses resistance of the nose,29 and acoustic rhinometry (AR),30 which measures cross-sectional area (CSA). It is generally assumed that the minimal cross-sectional area (mCSA) bears a monotonic relationship to the resistance of the upper airway (UA).
Prior to studying the relationship of nasal resistance to CPAP use, in the present study we examine the relationship between awake noninvasive measures of nasal resistance (AR and RM) and directly assessed UA resistance while on CPAP during sleep.
METHODS
Twenty-seven adult patients with complaints of snoring and excessive daytime sleepiness, presenting to the New York University Sleep Disorders Center for evaluation of OSAHS were recruited. All patients underwent nocturnal polysomnography (NPSG) to confirm the diagnosis of OSAHS. A nasal cannula pressure transducer system (Protech PTAF2) was used to measure airflow and an oral thermistor to detect mouth breathing and calculate apnea-hypopnea index 4% (AHI 4%) and respiratory disturbance index (RDI) by American Academy of Sleep Medicine criteria.31 If CPAP treatment was clinically indicated, the patients were referred for CPAP titration during which supraglottic pressure (SGP) measurements were performed during the NPSG. Patients were excluded if they had a medically unstable condition (i.e., recent myocardial infarction, congestive heart failure) or if they were unable to sleep with CPAP.
All subjects included in the study underwent daytime evaluation including clinical assessment, subjective questionnaires to assess nasal symptoms and evaluation of NR with AR and anterior RM in the sitting and supine positions. Nighttime tests performed were in-laboratory CPAP titration NPSG with measurements of SGP on optimal CPAP.
Clinical Assessment
We recorded demographic and clinical variables: age, gender, body mass index, medical history, physical examination, and menopausal status. Subjective daytime sleepiness was measured using the Epworth Sleepiness Scale.32
Subjective Questionnaires of Nasal Symptoms
The assessment of subjective nasal symptoms was made with the nasal obstruction symptom evaluation (NOSE) instrument. The NOSE questionnaire is a validated tool in the subjective assessment of nasal obstruction.33,34 It consists of 5 assessments of nasal obstruction-related symptoms scored using a 5-point Likert scale (not a problem, very mild problem, moderate problem, fairly bad problem, severe problem). Patients are asked to rate their symptoms as perceived over the past month. Higher scoring on the test implies more severe nasal obstruction.
Acoustic Rhinometry
AR measures nasal CSA at different distances from the nasal inlet using acoustic reflections. It has been validated as reproducible, accurate, and noninvasive method.35 Three areas of constriction are identified: CSA1 represents the internal nasal valve at the junction of the upper lateral cartilage and septum (relatively constant in a given patients, independent of congestion); CSA2 represents the head of the inferior turbinate; and CSA3 is bounded by the head of the middle turbinate and the anterior portion of the inferior turbinate. CSA2 and CSA3 are highly variable due to erectile mucovascular tissue. Measurements were performed using the RhinoScan instrument (Rhinometrics A/S, Lynge, Denmark) using standard techniques.29,36,37 This AR device displays the mCSA in 2 sections of the nose, CSA1 with distance range 0-2.20 cm and CSA2 with distance range 2.20-5.40 cm. Before each use the AR device was calibrated using a standardized probe. Sterile surgical lubricant was applied to the nosepiece to create an acoustic seal. The wand was held to each nostril without causing any distortion of the anatomy, and the patient was asked to hold his breath until a stable reading emerged. Three measurements were obtained at each nostril and accepted as normal when they had a coefficient of variation < 2%. We collected daytime data in the sitting position after 30 min of acclimatization to the laboratory environment and in supine position after 15 min of recumbency. Measurements were repeated in a separate session on the night of the CPAP titration NPSG study prior to sleep. From the awake daytime and night measurements, mean CSA1 and CSA2 were calculated for each visit and position by pooling the data from left and right nostrils. Minimal CSA for each patient was defined as the lowest of CSA1 and CSA2. In order to obtain a value proportional to NR, we assumed resistance (NR) was proportional to 1/CSA2, where CSA was the minimum of CSA1 and CSA2 for each nostril, and that the 2 resistances acted in parallel during normal breathing 1/total NR = 1/NRleft + 1/NRright.
Rhinomanometry
Rhinomanometry assesses the nasal airway by simultaneously recording transnasal pressure and airflow during occlusion of one nostril. Measurements were performed using a commercialized rhinomanometer instrument (RhinoStream, Rhinometrics A/S, Lynge, Denmark). We obtained direct measurement of NR by the active anterior technique in accordance with the standard set by the International Committee on Standardization of rhinomanometry.29 The RM was performed during wakefulness in both sitting and supine positions on 2 occasions, on the day of the recruitment and again at night prior to the CPAP titration NPSG.
For each nostril, flow resistance for inspiration and expiration was separately measured at 75 Pa of pressure using the average of three measurements with a maximum deviation between measurements of 10%. Total NR was calculated separately in inspiration and expiration by combining the parallel NR from the 2 nostrils using the formula: 1/total NR = 1/NRleft + 1/NRright.
Nocturnal Polysomnography
The diagnostic and CPAP titration NPSGs were performed in the New York University Sleep Disorders Center as per American Academy of Sleep Medicine recommended clinical guidelines.38 Pressure was directly measured at the CPAP mask using a pressure transducer (Ultima Dual Airflow Pressure Sensor, Braebon 0585, Ontario, Canada). Airflow to the mask was recorded from the output of a Respironics BiPAP Auto M Series device in CPAP mode. CPAP was titrated manually during the first hour of the study to a level that eliminated all sleep disordered breathing events including obstructive apneas, hypopneas, and runs of flow limitation. The optimal pressure was defined as the minimum pressure at which flow limitation disappeared. The minimal therapeutic pressure was confirmed by performing step-down measures dropping the pressure every 2 min by 1 cm H2O until the appearance of flow limitation; this established the minimum therapeutic pressure.
In addition to standard monitoring, SGP was measured using a pressure transducer-tipped catheter (Millar MPC 500, Millar Instruments, Houston, TX, USA). The nose was anesthetized using atomized lidocaine 5% and lidocaine 2% jelly for the throat. The Millar catheter was introduced transnasally, and the tip of the catheter was placed just below the uvula. The catheter position was confirmed visually through the mouth. The catheter was taped to the nose to secure its position throughout the study. The nasal CPAP mask was then applied and leak at the exit site of the catheter was minimized. The output of the Millar catheter was amplified and recorded at 64 Hz.
To verify that the supraglottic catheter tip was placed just below the collapsible segment of the upper airway, the behavior of difference between the supraglottic and CPAP inspiratory pressures after the patient fell asleep was inspected during a brief “step-down” of CPAP pressure. Correct positioning of the catheter tip required that the delta pressure between the mask and the supraglottic area increases substantially during inspiration simultaneously with the appearance of inspiratory flow limitation. If this increase in delta pressure was not observed as CPAP was reduced, it was assumed the catheter position was too high and the catheter was advanced.
We analyzed 5 min segments of pressures recording obtained during at least 2 separate periods of stable stage N2 sleep in the same position for each patient, during which there was no evidence of any sleep disordered breathing event. Mask pressure (MP) and SGP were averaged over 3 breaths. UA resistance was calculated for each inspiration and expiration using the relevant peak flow and the difference between SGP and MP for that breath, then averaged for the 3 breaths.
Measurements of pressure and resistance were repeated both over short periods (< 10 min) and at longer intervals (> 1 h) to assess the stability of the UA resistance across the night. We assessed reproducibility/stability of the UA resistance measurement in three different situations: short-term sleep (in stage N2 sleep and within 10 min), long-term sleep, and long-term awake (measurements in the same position in stage N2 sleep or wake but ≥ 1 h apart). In each of these situations we compared 3 measurements of UA resistance for inspiration and expiration.
All the subjects signed a consent form approved by the Institutional Review Board at the New York University School of Medicine.
STATISTICS
For each variable, comparisons between positions (sitting versus supine) and between daytime, nighttime wake and sleep were made using paired t-test with p < 0.05 as significant. Correlations between variables were evaluated using Pearson correlation coefficient with p < 0.05 as significant.
RESULTS
Of the 27 subjects recruited, 14 patients (10 male/4 female) completed the study. Five subjects dropped out, 8 were excluded due to insufficient sleep (2), excessive mask leak (2), poor supraglottic catheter signal quality (1) and poor AR and RM signal quality (3). The mean age was 47.8 ± 11.7 years, mean body mass index 35.3 ± 10.4 kg/m2, mean AHI 62.8 ± 34.4/h, mean RDI 66.6 ± 33.5/h, mean Epworth Sleepiness Scale 12.7 ± 5.6, mean CPAP level 9.8 ± 3.1 cm H2O. On the NOSE questionnaire, 9 subjects showed no or mild symptoms of nasal obstruction (NOSE scores < 8 of 20) and 5 subjects showed moderate-severe symptoms (NOSE score 11-18). No subject had a NOSE score > 18.
Acoustic Rhinometry
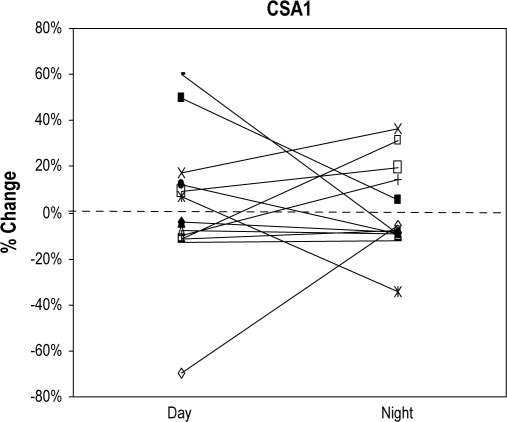
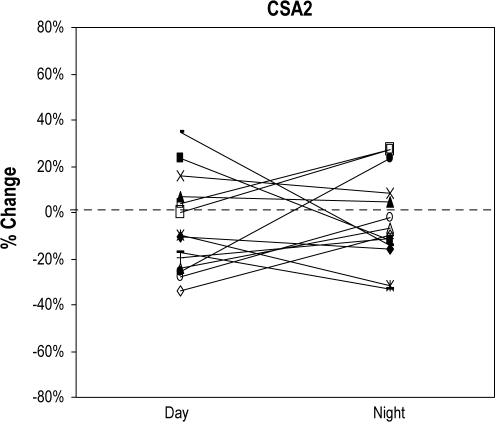
Within each subject and for each position, CSA1 (awake day vs. awake night, p = 0.15 [sitting], p = 0.07 [supine]) and CSA2 (awake day vs. awake night, p = 0.37 [sitting], p = 0.16 [supine]) were reproducible across sessions. CSA1 and CSA2 showed changes from sitting to supine position that tended to stay constant across sessions in each individual. However both increases and decreases in CSA occurred with equal frequency and averaged to zero for the group (Figure 1A, B). Of note, decreases/increases did not always occur in the same subjects for CSA1 and CSA2. Table 1 shows the group mean data for CSA1 and CSA2 by position. In each patient, a single value of CSA1 and CSA2 was calculated using the average of daytime and nighttime awake data.
Figure 1A.
Positional change of CSA1 from sitting to supine position during wakefulness in both sessions, daytime and nighttime
The Y axis shows the percentage of change of CSA1 from sitting to supine position. Each line represents a subject (n = 14) and the first point of the line shows the change of CSA1 from sitting to supine position during the daytime session. Second point of the line represents the change of CSA1 from sitting to supine position during the nighttime session.
Figure 1B.
Positional change of CSA2 from sitting to supine position during wakefulness in both sessions, daytime and nighttime
The Y axis shows the percentage of change of CSA2 from sitting to supine position. Each line represents a subject (n = 14) and the first point of the line shows the change of CSA2 from sitting to supine position during the daytime session. Second point of the line represents the change of CSA2 from sitting to supine position during the nighttime session.
Table 1.
Awake acoustic rhinometry - Values of CSA and nasal resistance (n = 14)*
| Mean (SD) | Range | |
|---|---|---|
| CSA1 (cm2) | ||
| Sitting | 0.58 ± 0.10 | 0.40 – 0.77 |
| Supine | 0.56 ± 0.09 | 0.38 – 0.76 |
| CSA2 (cm2) | ||
| Sitting | 0.53 ± 0.12 | 0.29 – 0.72 |
| Supine | 0.50 ± 0.15 | 0.31 – 0.83 |
| Minimal CSA (cm2)† | ||
| Sitting | 0.50 ± 0.11 | 0.29 – 0.70 |
| Supine | 0.47 ± 0.12 | 0.31 – 0.76 |
| Nasal Resistance (arbitrary units) | ||
| Sitting | 2.46 ± 1.32 | 1.1 – 6.27 |
| Supine | 2.66 ± 1.31 | 0.87 – 5.21 |
CSA refers to cross-sectional area;
Minimal CSA, minimal cross-sectional area between CSA1 and CSA2; SD, standard deviation.
Values for CSA1, CSA2 and nasal resistance were obtained for each patient by averaging daytime and nighttime measurements. Values in the table are the mean values for all subjects.
Similar to the results for CSA itself, NR as calculated from CSA did not show any change across sessions or a consistent position effect for the group (Table 1).
Active Anterior Rhinomanometry
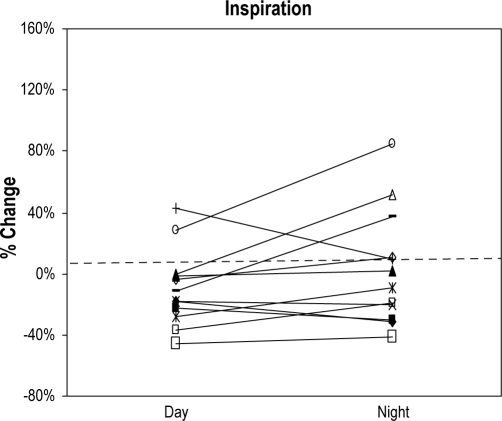
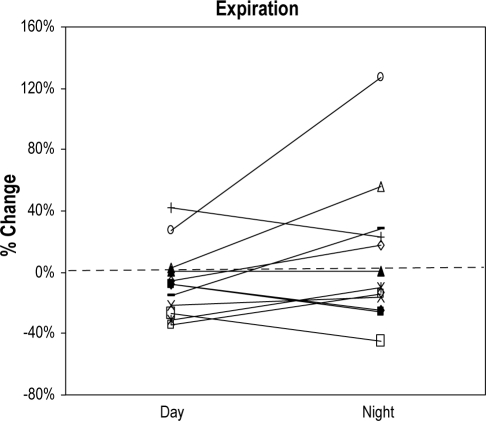
Similar to the data for CSA, measurements of NR by anterior RM did not vary across sessions (day vs. night). Changes of NR from sitting to supine during inspiration and expiration also did not show a statistically significant variation across sessions (Figure 2A, B). In view of this, for each patient a single value of NR was calculated for each position from the average of daytime and nighttime awake measurements and is shown in Table 2.
Figure 2A.
Positional change of inspiratory nasal resistance by rhinomanometry from sitting to supine position during wakefulness in both sessions, daytime and nighttime
The Y axis shows the percentage of change of inspiratory nasal resistance from sitting to supine position. Each line represents a subject (n = 12) and the first point of the line shows the change of inspiratory nasal resistance from sitting to supine position during the daytime session. Second point of the line represents the change of inspiratory nasal resistance from sitting to supine position during the nighttime session.
Figure 2B.
Positional change of expiratory nasal resistance by rhinomanometry from sitting to supine position during wakefulness in both sessions, daytime and nighttime
The Y axis shows the percentage of change of expiratory nasal resistance from sitting to supine position. Each line represents a subject (n = 12), and the first point of the line shows the change of expiratory nasal resistance from sitting to supine position during the daytime session. Second point of the line represents the change of expiratory nasal resistance from sitting to supine position during the nighttime session.
Table 2.
Awake rhinomanometry - Values of nasal resistance (n = 14)*
| Mean (SD) Pa s/cm3 | Range Pa s/cm3 | |
|---|---|---|
| Inspiration | ||
| Sitting | 0.24 ± 0.08 | 0.15 – 0.44 |
| Supine | 0.24 ± 0.09 | 0.13 – 0.42 |
| Expiration | ||
| Sitting | 0.23 ± 0.08 | 0.13 – 0.43 |
| Supine | 0.23 ± 0.07 | 0.14 – 0.43 |
| Mean Nasal Resistance | ||
| Sitting | 0.23 ± 0.07 | 0.14 – 0.44 |
| Supine | 0.23 ± 0.08 | 0.14 – 0.43 |
SD refers to standard deviation.
Values for inspiratory and expiratory nasal resistance are the combined measurements for each patient from daytime and nighttime measurements. Values for mean nasal resistance are the combined data during inspiration and expiration for each position.
Our patients had a wide range of NR by rhinomanometry, with 8 having normal values and 6 having high values. This is similar to other published rhinomanometry data in OSAHS.39 By anterior RM, 6/14 patients showed a sitting NR (average of inspiration and expiration) > 0.25 Pa s/cm3, which has been suggested as the upper limit of normal by Cole et al.40 In the group with NR (8/14) < 0.25 Pa s/cm3, 6 patients showed an increase of NR in supine position; one of these patients had a change > 30%. An increase of 30% of NR with position has been suggested by Altissimi et al.41 as being clinically significant. In the group with high NR by anterior RM, although 4 subjects showed a decrease of NR from sitting to supine position, only one of these patients had a change > 30%.
Upper Airway Resistance
Pressure in the mask remained within 0.5 cm H2O of set pressure at the machine. As expected from the UA resistive behavior, mean SGP fell during inspiration and rose during expiration from that set at the mask/machine. Overall, the mean value of the difference between set pressure and SGP during wakefulness across subjects was 2.63 ± 2.18 cm H2O in inspiration (range from 0.6 to 7.7 cm H2O) and 1.66 ± 1.42 cm H2O in expiration (range from 0.3 to 6.1 cm H2O). During sleep, the mean value of the difference between set pressure and SGP across subjects was 3.02 ± 2.62 cm H2O in inspiration (range from 0.6 to 9.2 cm H2O) and 1.56 ± 1.27 cm H2O in expiration (range from 0.4 to 2.8 cm H2O).
Table 3 shows the results of the resistances calculated for the UA, derived from peak flow and the peak pressure drop from mask to supraglottic area. In 11/14 subjects, inspiratory UA resistance was similar to expiratory UA resistance. However, in 3 subjects inspiratory UA resistance was much higher than expiratory UA resistance, suggesting suboptimal CPAP may have been present. Inspiratory and expiratory resistances were larger during sleep than during wakefulness on CPAP, although the difference did not reach statistical significance.
Table 3.
Sleep upper airway resistance by supraglottic catheter (n = 14)
| Mean (SD) cm H2O/L/min | Range cm H2O/L/min | |
|---|---|---|
| Wakefulness | ||
| Inspiration | 0.09 ± 0.06 | 0.03 – 0.21 |
| Expiration | 0.09 ± 0.06 | 0.04 – 0.22 |
| Sleep | ||
| Inspiration | 0.12 ± 0.08 | 0.02 – 0.27 |
| Expiration | 0.10 ± 0.09 | 0.01 – 0.26 |
| Wakefulness | ||
| (Inspiration & Expiration) | 0.09 ± 0.06 | 0.03 – 0.22 |
| Sleep | ||
| (Inspiration – Expiration) | 0.11 ± 0.08 | 0.01 – 0.26 |
SD refers to standard deviation.
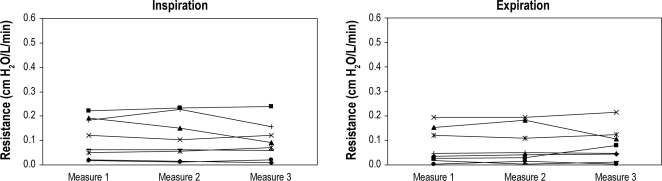
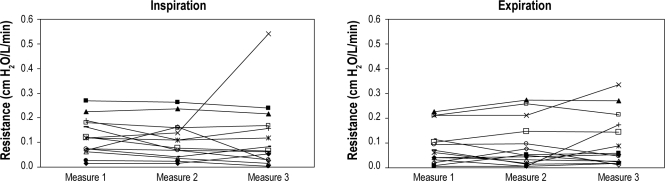
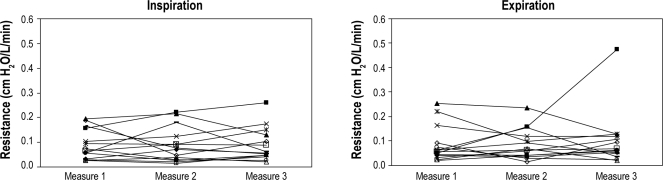
Measurement of UA resistances within a single patient remained stable between repeated measures both short term (within 10 min with multiple measures) and across the night (measurements 1 h apart in the same position in stage 2 sleep or wake) at a statistical significance of 0.05 (Figure 3).
Figure 3A, B.
Reproducibility of the upper airway resistance in short-term sleep (within 10 min of stage N2 sleep)
Panel A represents inspiration. Panel B represents expiration. X axis represents 3 points in time within a period of 10 min of stable stage N2 sleep. Y axis is the value of upper airway resistance measured by supraglottic catheter. Each line represents a subject (n = 8).
Figure 3C, D.
Reproducibility of the upper airway resistance in long-term sleep (measurements at 1 h apart in the same position in stage N2 sleep)
Panel C represents inspiration. Panel D represents expiration. X axis represents 3 points in time across the night of stable stage N2 sleep and separated by ≥ 1 hour. Y axis is the value of upper airway resistance measured by supraglottic catheter. Each line represents a subject (n = 14).
Figure 3E, F.
Reproducibility of the upper airway resistance in long-term awake (measurements at 1 h apart in the same position awake)
Panel E represents inspiration. Panel F represents expiration. X axis represents 3 points in time across the night of stable breathing during wakefulness and separated at least by 1 hour. Y axis is the value of upper airway resistance measured by supraglottic catheter. Each line represents a subject (n = 14).
Relationship Between Techniques
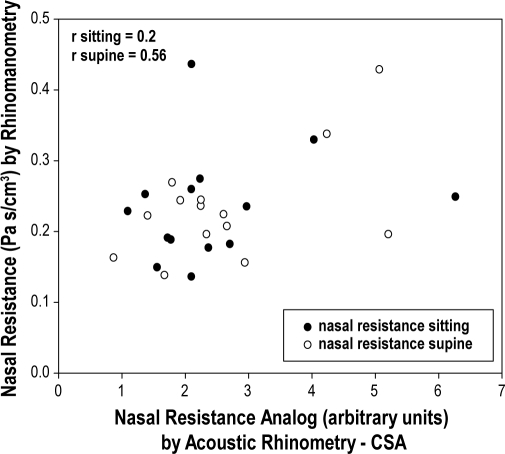
Table 4 shows the correlation coefficients between measurements from the AR and RM. No strong relationships could be shown between the 2 techniques in the sitting position, but there was significant correlation in the supine position. Figure 4 shows the correlation between NR by AR and anterior RM. Table 5 shows the correlation coefficients between UA resistance and NR by AR and RM. Although correlation coefficients were statistically significant they do not seem physiologically plausible, as patients with lower CSA awake have lower UA resistance during sleep on CPAP. In addition, we found no relationship between direct measurement of UA resistance and awake RM.
Table 4.
Correlation coefficients between acoustic rhinometry and rhinomanometry (n = 14)
| Acoustic Rhinometry |
||||
|---|---|---|---|---|
| CSA1 | CSA2 | Minimal CSA | Nasal Resistance | |
| Rhinomanometry | sitting | sitting | sitting | sitting |
| Nasal Resistance (sitting) | ||||
| Inspiration | −0.16 | −0.26 | −0.27 | 0.18 |
| Expiration | −0.17 | −0.34 | −0.32 | 0.23 |
| Mean† | −0.17 | −0.30 | −0.29 | 0.20 |
| CSA1 | CSA2 | Minimal CSA | Nasal Resistance | |
| supine | supine | supine | supine | |
| Nasal Resistance (supine) | ||||
| Inspiration | −0.13 | −0.52 | −0.52 | 0.59* |
| Expiration | −0.08 | −0.44 | −0.41 | 0.47 |
| Mean† | −0.11 | −0.51 | −0.49 | 0.56* |
CSA refers to cross-sectional area.
Values for mean nasal resistance are the combined data during inspiration and expiration for each position.
Statistically significant (p < 0.05)
Figure 4.
Correlation between nasal resistance by acoustic rhinometry (X axis) and nasal resistance by rhinomanometry (Y axis)
Each point represents a subject (n = 14). Black dots represents measures of nasal resistance in sitting position, and open dots are measures of nasal resistance in supine position. Values for mean nasal resistance by rhinomanometry are the combined data during inspiration and expiration for each position.
Table 5.
Correlation coefficients between directly assessed upper airway resistance and nasal resistance by acoustic rhinometry and rhinomanometry (n = 14)
| Directly Assessed Upper Airway Resistance (sleep) |
|||
|---|---|---|---|
| UA Resistance Inspiration | UA Resistance Expiration | UA Resistance Mean | |
| Acoustic Rhinometry (awake) | |||
| CSA1 | |||
| Sitting | −0.15 | 0.45 | −0.54 |
| Supine | 0.37 | 0.54* | 0.50 |
| CSA2 | |||
| Sitting | 0.44 | 0.37 | 0.45 |
| Supine | 0.64* | 0.69* | 0.73* |
| Minimal CSA | |||
| Sitting | 0.14 | 0.20 | 0.19 |
| Supine | 0.59* | 0.64* | 0.68* |
| Nasal Resistance | |||
| Sitting | −0.21 | −0.16 | −0.20 |
| Supine | −0.51 | −0.54* | −0.58 |
| Rhinomanometry (awake) | |||
| Nasal Resistance Inspiration | |||
| Sitting | −0.12 | — | — |
| Supine | −0.47 | — | — |
| Nasal Resistance Expiration | |||
| Sitting | — | 0.22 | — |
| Supine | — | −0.03 | — |
| Mean Nasal Resistance | |||
| Sitting | — | — | 0.64 |
| Supine | — | — | −0.30 |
UA refers to upper airway; CSA, cross-sectional area.
Statistically significant (p < 0.05)
No significant relationships were found between measures of nasal resistance (AR and RM) or UA resistance and RDI, NOSE questionnaire, and CPAP level. The correlation coefficients were all near zero (< 0.12), and p values of these correlations were all > 0.6.
We could not show any association between positional change in RDI from supine to lateral and supine to sitting measurement of resistance in the 7 patients with all measurements. Only 3 patients had positional changes in AHI > 50%.
DISCUSSION
The data in our study show that measures of nasal resistance made in the sitting position while subjects were awake (AR and RM) had little or no correlation to each other. An exception was the significant, if weak, relationship between AR and RM measurements of resistance in the supine position. However, this finding was driven largely by one data point. This lack of agreement between nasal resistance measurements in the sitting and supine positions suggests that the two techniques may measure different aspects of nasal physiology. In addition, as others have previously shown, we did not find a clear relationship between severity of OSAHS42,43 and either reported subjective nasal symptoms44–46 or the measures of awake nasal function (AR and RM). Upper airway resistance measured during sleep did not show significant relationships to any of the awake measures of nasal resistance (AR or RM).
Our measures of the effect of position on awake nasal function merit further comment. First, for both AR and RM, repeated measurements (made on two occasions, daytime and nighttime) were consistent within a single patient, suggesting that the values obtained have physiological meaning. In addition, intra-patient changes in the measurements of both AR and RM from sitting to supine were also consistent on repeat testing. Despite this, across patients we did not find consistent changes in AR or RM with change to the supine position. In healthy subjects a consistent increase in nasal resistance and a decrease of CSA has been has been reported when subjects go from sitting to supine position.47–49 However, similar to our data, studies in patients with OSAHS39,50,51 report variable changes in nasal resistance and CSA with positional change, suggesting that OSAHS patients may respond differently from normal subjects to positional changes. One can speculate that the increased vascular volume frequently associated with obesity, may have caused nasal mucosal edema that saturated mechanisms for postural changes in resistance. However, our data did not include these measures. Other possible mechanisms that could explain the “atypical” response to change in position in patients with OSAHS are altered neurovascular control of the nasal mucosa in supine position, perhaps due to increased sympathetic neurovascular activity with a consequent reduction of the influx of blood through the vessels or to increased levels of inflammatory activity that could affect the nose via circulating adrenalin and noradrenalin or inflammatory cytokines, as these have been reported in OSAHS.52–54
The purpose of the present study was to obtain a daytime/wake noninvasive measurement predictive of nighttime/sleep physiology that might have implications for patients with OSAHS on CPAP. High upstream (nasal) resistance in the Starling resistor model of the upper airway implies that increased UA resistance increases the collapsing force at the (downstream) collapsible segment, but this is not relevant to the condition of sleep on optimum nasal CPAP (titrated to prevent collapse). Thus, on CPAP, behavior of the upper airway should be similar to the awake condition, where there is rigidity of the upper airway at the collapsible area. In contrast to the collapsible behavior of the velopharynx during sleep, nasal behavior is most closely approximated by a single rigid constriction (i.e., a non Starling constant resistance) and is not affected by sleep.26 This conceptualization leads us to predict that high nasal resistance should be perceived by the patient even on CPAP and might contribute to intolerance. Our aim was to identify the best technique to measure the relevant nasal resistance prior to the sleep study (and subsequently to test whether this can be used to anticipate CPAP non-compliance). However, our data do not demonstrate any relationship between awake nasal resistance by AR or RM and upper airway resistance during sleep.
It seems unlikely that the lack of relationship between awake AR and RM with direct measurement of UA resistance during sleep was due to deficiencies in our technique of obtaining AR and RM. We used standard techniques and equipment with multiple measurements, as recommended by standards,29,36,37 and our data show reproducible measurements within a single position and on separate occasions within each patient.
To examine the relationship between AR and RM, we converted both to a form conceptually related to “resistance.” For RM resistance is directly obtained for each measurement and we chose to combine the nostrils as parallel resistors.29 For AR, the measurement is of cross-sectional area, which did not itself show a statistical relationship to RM in our dataset. To use this as a “resistance” analog, we made the simplest assumption that flow was turbulent and proportional to 1/R4 or 1/(cross sectional area)2. While this assumption may be simplistic, one would expect at least a monotonic relationship using this approach, and we did not find this to be present.
The lack of correlation between AR and RM we found is similar to what is reported in the literature. AR assesses a local minimal cross sectional area at a specific site, whereas airflow resistance by RM is a dynamic parameter that assesses all the serial components of the nasal cavity.55–57
There are several limitations in our study. First, lack of correlations may have been due to the small number of unselected patients. However, we studied patients with a wide range of nasal resistances and OSAHS, and this should have maximized our ability to find relationships. A power calculation suggests we can reject the hypothesis of a high correlation (> 0.8) between our variables with a power of 80% to 85% and α of 0.025 with the 14 subjects we studied. While a significant lower correlation between our variables could have existed and become evident with a larger sample size, a lower correlation would not have satisfied the primary goal of our study, which was to find a noninvasive daytime test highly correlated to (and therefore predictive of) the nocturnal directly measured resistance. Second, it can be argued that there was no reason to expect correlations between measurements made during wakefulness and those made during sleep. However, we wished to test this directly as it is generally assumed that sleep does not affect the nose in the same way as it affects the collapsible segment of the nasopharynx responsible for OSAHS.26 In addition, it is difficult to make AR and RM measurements during sleep without disturbing normal sleep. Furthermore, our purpose was to examine potential predictors of nocturnal physiology that could be easily obtained during the daytime. An additional criticism is that we did not obtain a subjective patient report of CPAP “comfort.” However this was not the purpose of the present study, as we felt that the first night of CPAP titration was not the optimal time to assess comfort (as it was the patient's first exposure to CPAP).
CONCLUSION
While acoustic rhinometry and rhinomanometry as often obtained (sitting) were not consistently related to each other they, were correlated in the supine position. However neither of these awake measurements of nasal resistance was predictive of upper airway resistance during sleep on CPAP, suggesting that differences in upper airway pathophysiology in patients with OSAHS may affect awake and sleep nasal resistances in complex ways. It remains possible that we did not find the predicted relationship between awake and sleep measures of nasal resistance because of the small sample size or because patients were not selected specifically for their nasal symptoms.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Dr. R. Lebowitz for his valuable inputs on this article. The work was performed at the Sleep Disorders Center of Pulmonary, Critical Care and Sleep Medicine Division, New York University School of Medicine, New York, NY, USA. This research was supported by grants from ALANY, NCRR M01RR00096, Foundation for Research in Sleep Disorders, Instituto de Salud Carlos III, Fundacio Catalana de Pneumologia, Fundacio Parc Tauli.
ABBREVIATIONS
- CPAP
Continuous positive airway pressure
- OSAHS
Obstructive sleep apnea/hypopnea syndrome
- NR
Nasal resistance
- AR
Acoustic rhinometry
- RM
Rhinomanometry
- CSA
Cross-sectional area
- mCSA
Minimal cross-sectional area
- UA
Upper airway
- NPSG
Nocturnal polysomnography
- AHI
Apnea/hypopnea index
- RDI
Respiratory disturbance index
- SGP
Supraglottic pressure
- NOSE
Nasal obstruction symptom evaluation
- MP
Mask pressure
REFERENCES
- 1.Sanders MH, Montserrat JM, Farre R, Givelber RJ. Positive pressure therapy: a perspective on evidence-based outcomes and methods of application. Proc Am Thorac Soc. 2008;5:161–72. doi: 10.1513/pats.200709-150MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDaid C, Duree KH, Griffin SC, et al. A systematic review of continuous positive airway pressure for obstructive sleep apnoea-hypopnoea syndrome. Sleep Med Rev. 2009;13:427–36. doi: 10.1016/j.smrv.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 3.McArdle N, Douglas NJ. Effect of continuous positive airway pressure on sleep architecture in the sleep apnea-hypopnea syndrome: a randomized controlled trial. Am J Respir Crit Care Med. 2001;164:1459–63. doi: 10.1164/ajrccm.164.8.2008146. [DOI] [PubMed] [Google Scholar]
- 4.Patel SR, White DP, Malhotra A, Stanchina ML, Ayas NT. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med. 2003;163:565–71. doi: 10.1001/archinte.163.5.565. [DOI] [PubMed] [Google Scholar]
- 5.Montserrat JM, Ferrer M, Hernandez L, et al. Effectiveness of CPAP treatment in daytime function in sleep apnea syndrome: a randomized controlled study with an optimized placebo. Am J Respir Crit Care Med. 2001;164:608–13. doi: 10.1164/ajrccm.164.4.2006034. [DOI] [PubMed] [Google Scholar]
- 6.Zimmerman ME, Arnedt JT, Stanchina M, Millman RP, Aloia MS. Normalization of memory performance and positive airway pressure adherence in memory-impaired patients with obstructive sleep apnea. Chest. 2006;130:1772–8. doi: 10.1378/chest.130.6.1772. [DOI] [PubMed] [Google Scholar]
- 7.George CF. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax. 2001;56:508–12. doi: 10.1136/thorax.56.7.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faccenda JF, Mackay TW, Boon NA, Douglas NJ. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med. 2001;163:344–8. doi: 10.1164/ajrccm.163.2.2005037. [DOI] [PubMed] [Google Scholar]
- 9.Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet. 2002;359:204–10. doi: 10.1016/S0140-6736(02)07445-7. [DOI] [PubMed] [Google Scholar]
- 10.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 11.Campos-Rodriguez F, Pena-Grinan N, Reyes-Nunez N, et al. Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure. Chest. 2005;128:624–33. doi: 10.1378/chest.128.2.624. [DOI] [PubMed] [Google Scholar]
- 12.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30:711–9. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grunstein RR. Sleep-related breathing disorders. 5. Nasal continuous positive airway pressure treatment for obstructive sleep apnoea. Thorax. 1995;50:1106–13. doi: 10.1136/thx.50.10.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lofaso F, Coste A, d'Ortho MP, et al. Nasal obstruction as a risk factor for sleep apnoea syndrome. Eur Respir J. 2000;16:639–43. doi: 10.1034/j.1399-3003.2000.16d12.x. [DOI] [PubMed] [Google Scholar]
- 15.Weaver TE. Adherence to positive airway pressure therapy. Curr Opin Pulm Med. 2006;12:409–13. doi: 10.1097/01.mcp.0000245715.97256.32. [DOI] [PubMed] [Google Scholar]
- 16.Kakkar RK, Berry RB. Positive airway pressure treatment for obstructive sleep apnea. Chest. 2007;132:1057–72. doi: 10.1378/chest.06-2432. [DOI] [PubMed] [Google Scholar]
- 17.Tompos T, Garai T, Zemplen B, Gerlinger I. Sensation of nasal patency compared to rhinomanometric results after septoplasty. Eur Arch Otorhinolaryngol. 2010 Jun 11; doi: 10.1007/s00405-010-1278-z. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Andre RF, Vuyk HD, Ahmed A, Graamans K, Nolst Trenite GJ. Correlation between subjective and objective evaluation of the nasal airway. A systematic review of the highest level of evidence. Clin Otolaryngol. 2009;34:518–25. doi: 10.1111/j.1749-4486.2009.02042.x. [DOI] [PubMed] [Google Scholar]
- 19.Pepin JL, Leger P, Veale D, Langevin B, Robert D, Levy P. Side effects of nasal continuous positive airway pressure in sleep apnea syndrome. Study of 193 patients in two French sleep centers. Chest. 1995;107:375–81. doi: 10.1378/chest.107.2.375. [DOI] [PubMed] [Google Scholar]
- 20.McArdle N, Devereux G, Heidarnejad H, Engleman HM, Mackay TW, Douglas NJ. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:1108–14. doi: 10.1164/ajrccm.159.4.9807111. [DOI] [PubMed] [Google Scholar]
- 21.Engleman HM, Wild MR. Improving CPAP use by patients with the sleep apnoea/hypopnoea syndrome (SAHS) Sleep Med Rev. 2003;7:81–99. doi: 10.1053/smrv.2001.0197. [DOI] [PubMed] [Google Scholar]
- 22.Morris LG, Setlur J, Burschtin OE, Steward DL, Jacobs JB, Lee KC. Acoustic rhinometry predicts tolerance of nasal continuous positive airway pressure: a pilot study. Am J Rhinol. 2006;20:133–7. [PubMed] [Google Scholar]
- 23.Tarrega J, Mayos M, Montserrat JR, et al. [Nasal resistance and continuous positive airway pressure treatment for sleep apnea/hypopnea syndrome] Arch Bronconeumol. 2003;39:106–10. doi: 10.1016/s0300-2896(03)75335-6. [DOI] [PubMed] [Google Scholar]
- 24.Nakata S, Noda A, Yagi H, et al. Nasal resistance for determinant factor of nasal surgery in CPAP failure patients with obstructive sleep apnea syndrome. Rhinology. 2005;43:296–9. [PubMed] [Google Scholar]
- 25.Willing S, San Pedro M, Driver HS, Munt P, Fitzpatrick MF. The acute impact of continuous positive airway pressure on nasal resistance: a randomized controlled comparison. J Appl Physiol. 2007;102:1214–9. doi: 10.1152/japplphysiol.00639.2006. [DOI] [PubMed] [Google Scholar]
- 26.Hudgel DW, Robertson DW. Nasal resistance during wakefulness and sleep in normal man. Acta Otolaryngol. 1984;98:130–5. doi: 10.3109/00016488409107544. [DOI] [PubMed] [Google Scholar]
- 27.Miljeteig H, Cole P, Haight JS. Nasal resistance in recumbency and sleep. Rhinology. 1995;33:82–3. [PubMed] [Google Scholar]
- 28.Series F, Cormier Y, Couture J, Desmeules M. Changes in upper airway resistance with lung inflation and positive airway pressure. J Appl Physiol. 1990;68:1075–9. doi: 10.1152/jappl.1990.68.3.1075. [DOI] [PubMed] [Google Scholar]
- 29.Clement PA, Gordts F. Consensus report on acoustic rhinometry and rhinomanometry. Rhinology. 2005;43:169–79. [PubMed] [Google Scholar]
- 30.Lal D, Corey JP. Acoustic rhinometry and its uses in rhinology and diagnosis of nasal obstruction. Facial Plast Surg Clin North Am. 2004;12:397–405. doi: 10.1016/j.fsc.2004.04.002. v. [DOI] [PubMed] [Google Scholar]
- 31.Iber C, Ancoli-Israel S, Chesson A, Quan SF for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 32.Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth Sleepiness Scale. Chest. 1993;103:30–6. doi: 10.1378/chest.103.1.30. [DOI] [PubMed] [Google Scholar]
- 33.Stewart MG, Smith TL, Weaver EM, et al. Outcomes after nasal septoplasty: results from the Nasal Obstruction Septoplasty Effectiveness (NOSE) study. Otolaryngol Head Neck Surg. 2004;130:283–90. doi: 10.1016/j.otohns.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Stewart MG, Witsell DL, Smith TL, Weaver EM, Yueh B, Hannley MT. Development and validation of the Nasal Obstruction Symptom Evaluation (NOSE) scale. Otolaryngol Head Neck Surg. 2004;130:157–63. doi: 10.1016/j.otohns.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 35.Hilberg O. Objective measurement of nasal airway dimensions using acoustic rhinometry: methodological and clinical aspects. Allergy. 2002;57(Suppl 70):5–39. doi: 10.1046/j.0908-665x.2001.all.doc.x. [DOI] [PubMed] [Google Scholar]
- 36.Hilberg O, Pedersen OF. Acoustic rhinometry: recommendations for technical specifications and standard operating procedures. Rhinol Suppl. 2000;16:3–17. [PubMed] [Google Scholar]
- 37.Parvez L, Erasala G, Noronha A. Novel techniques, standardization tools to enhance reliability of acoustic rhinometry measurements. Rhinol Suppl. 2000;16:18–28. [PubMed] [Google Scholar]
- 38.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 39.De Vito A, Berrettini S, Carabelli A, et al. The importance of nasal resistance in obstructive sleep apnea syndrome: a study with positional rhinomanometry. Sleep Breath. 2001;5:3–11. doi: 10.1007/s11325-001-0003-y. [DOI] [PubMed] [Google Scholar]
- 40.Cole P. Nasal airflow resistance: a survey of 2500 assessments. Am J Rhinol. 1997;11:415–20. doi: 10.2500/105065897780914901. [DOI] [PubMed] [Google Scholar]
- 41.Altissimi G, Simoncelli C, Gallucci L. [Postural rhinomanometry in normal subjects] Acta Otorhinolaryngol Ital. 1989;9:555–63. [PubMed] [Google Scholar]
- 42.Metes A, Ohki M, Cole P, Haight JS, Hoffstein V. Snoring, apnea and nasal resistance in men and women. J Otolaryngol. 1991;20:57–61. [PubMed] [Google Scholar]
- 43.Atkins M, Taskar V, Clayton N, Stone P, Woodcock A. Nasal resistance in obstructive sleep apnea. Chest. 1994;105:1133–5. doi: 10.1378/chest.105.4.1133. [DOI] [PubMed] [Google Scholar]
- 44.Roithmann R, Cole P, Chapnik J, Barreto SM, Szalai JP, Zamel N. Acoustic rhinometry, rhinomanometry, and the sensation of nasal patency: a correlative study. J Otolaryngol. 1994;23:454–8. [PubMed] [Google Scholar]
- 45.Lane AP, Zweiman B, Lanza DC, et al. Acoustic rhinometry in the study of the acute nasal allergic response. Ann Otol Rhinol Laryngol. 1996;105:811–8. doi: 10.1177/000348949610501009. [DOI] [PubMed] [Google Scholar]
- 46.Lam DJ, James KT, Weaver EM. Comparison of anatomic, physiological, and subjective measures of the nasal airway. Am J Rhinol. 2006;20:463–70. doi: 10.2500/ajr.2006.20.2940. [DOI] [PubMed] [Google Scholar]
- 47.Rundcrantz H. Postural variations of nasal patency. Acta Otolaryngol. 1969;68:435–43. doi: 10.3109/00016486909121582. [DOI] [PubMed] [Google Scholar]
- 48.O'Flynn P. Posture and nasal geometry. Acta Otolaryngol. 1993;113:530–2. doi: 10.3109/00016489309135858. [DOI] [PubMed] [Google Scholar]
- 49.Roithmann R, Demeneghi P, Faggiano R, Cury A. Effects of posture change on nasal patency. Braz J Otorhinolaryngol. 2005;71:478–84. doi: 10.1016/S1808-8694(15)31203-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Virkkula P, Hurmerinta K, Loytonen M, Salmi T, Malmberg H, Maasilta P. Postural cephalometric analysis and nasal resistance in sleep-disordered breathing. Laryngoscope. 2003;113:1166–74. doi: 10.1097/00005537-200307000-00012. [DOI] [PubMed] [Google Scholar]
- 51.Hellgren J, Yee BJ, Dungan G, Grunstein RR. Altered positional regulation of nasal patency in patients with obstructive sleep apnoea syndrome. Eur Arch Otorhinolaryngol. 2009;266:83–7. doi: 10.1007/s00405-008-0701-1. [DOI] [PubMed] [Google Scholar]
- 52.McNicholas WT. Obstructive sleep apnea and inflammation. Prog Cardiovasc Dis. 2009;51:392–9. doi: 10.1016/j.pcad.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 53.Arnardottir ES, Mackiewicz M, Gislason T, Teff KL, Pack AI. Molecular signatures of obstructive sleep apnea in adults: a review and perspective. Sleep. 2009;32:447–70. doi: 10.1093/sleep/32.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calvin AD, Albuquerque FN, Lopez-Jimenez F, Somers VK. Obstructive sleep apnea, inflammation, and the metabolic syndrome. Metab Syndr Relat Disord. 2009;7:271–8. doi: 10.1089/met.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cole P, Roithmann R, Roth Y, Chapnik JS. Measurement of airway patency. A manual for users of the Toronto systems and others interested in nasal patency measurement. Ann Otol Rhinol Laryngol Suppl. 1997;171:1–23. [PubMed] [Google Scholar]
- 56.Numminen J, Ahtinen M, 3rd, Huhtala H, Laranne J, Rautiainen M. Correlation between rhinometric measurement methods in healthy young adults. Am J Rhinol. 2002;16:203–8. [PubMed] [Google Scholar]
- 57.Naito K, Miyata S, Saito S, Sakurai K, Takeuchi K. Comparison of perceptional nasal obstruction with rhinomanometric and acoustic rhinometric assessment. Eur Arch Otorhinolaryngol. 2001;258:505–8. doi: 10.1007/s004050100360. [DOI] [PubMed] [Google Scholar]