Abstract
Patients with Alzheimer’s disease (AD) show a deficit in olfactory threshold sensitivity. The Apolipoprotein E (ApoE) ε4 allele is associated with increased risk of AD and earlier symptom onset. Hormone therapy (HT) may exert neuroprotective effects on brain areas affected by AD. The current study investigated the effect of HT on performance on an olfactory threshold test in ε4 positive and ε4 negative non-hysterectomized, non-demented, elderly females and AD patients. Among the non-demented participants, ε4 positive females who had received HT performed 1) significantly better than those without HT, and 2) at levels similar to those of ε4 negative females. In contrast, those without HT who were ε4 positive performed significantly worse than those who were ε4 negative. HT had no effect on performance in AD patients regardless of ε4 status. These results suggest that HT may offer protection against loss of olfactory function in ε4 positive individuals in preclinical stages of AD. Future research is warranted in order to investigate further the neuroprotective role of HT on sensory and cognitive functions in non-demented aging individuals.
Keywords: olfaction, hormone replacement therapy, APOE, Alzheimer’s disease, aging
Introduction
Alzheimer’s Disease (AD) is an age-associated degenerative brain disorder afflicting over five million Americans. AD is difficult to detect in its early stages because of the gradual progression of cognitive impairment and this difficulty in early detection has placed great value on research focusing on the initial neurodegeneration of the brain and its symptomatic expression in very early AD. Neuropathologically, AD is characterized by the presence of amyloid plaques, neurofibrillary tangles and cell atrophy in susceptible brain regions. These cytoskeletal abnormalities develop initially in brain regions of the medial temporal lobe that are involved in processing olfactory information such as the entorhinal and transentorhinal cortex (Braak et al., 1996). The brain degeneration proceeds from these initial areas to other temporal lobe structures and eventually reaches association areas (Braak et al., 1996). This pattern of brain degeneration suggests that AD patients may show impairments in olfactory-mediated tasks in the early stages of the disease. Damage to the olfactory system would suggest that olfactory sensitivity may be impaired in AD patients and that olfactory thresholds should be poorer in AD patients when compared to non-demented older adults.
If olfactory sensitivity is impaired in AD patients, then an olfactory threshold task may contribute to the diagnosis and the understanding of the disease. Results from psychophysical studies found that olfactory thresholds of probable AD patients and questionable AD patients were significantly elevated (indicating olfactory impairment) relative to non-demented older adults and were significantly correlated with disease severity (Murphy et al., 1990; Nordin & Murphy, 1996). A study analyzing early changes in olfactory functioning due to AD demonstrated significant changes in olfactory threshold the year preceding the change in the patients’ diagnosis from non-demented older adults to AD (Bacon et al., 1998). These results suggest that olfactory impairment can occur early in the disease process (Nordin & Murphy, 1996).
The ε4 allele of the apolipoprotein E (ApoE) gene is the genetic risk factor most robustly associated with sporadic AD as well as a higher rate of incidence in some families (Bretsky et al., 2003; Saunders et al., 1993). The ε4 allele has been associated with increased risk of AD (Corder et al., 1993; Saunders et al., 1993) as well as an earlier age of disease onset (Corder et al., 1993). Some studies have shown an association between the ε4 allele and increased risk of cognitive decline through measurements on a variety of cognitive tests in a normal elderly population (Bretsky et al., 2003; Dik et al., 2001). These findings suggest the involvement of the ε4 allele in the initial neurodegenerating changes associated with AD in individuals at risk for AD. Other studies have shown that presence of the ε4 allele may be associated with olfactory functioning deficits in a normal elderly population. Non-demented ε4 positive participants showed impairments in olfactory functioning such as odor identification when compared to non-demented ε4 negative participants (Murphy et al., 1998). Graves et al., (1999) reported that impaired odor identification in ε4 positives was associated with more rapid cognitive decline over a two year period. Schiffman et al. (2002) suggested that familial risk may be separate from that associated with the apoE ε4 allele and thus, multiple genes are likely responsible for AD and associated neurocognitive symptoms. Handley et al. (2006) observed the greatest deficits in odor identification to be in siblings of AD patients who also carried the ε4 allele relative to siblings without the ε4 allele. It has also been found that individuals with the ε4 allele demonstrated greater odor threshold impairments than individuals without the ε4 allele in the year preceding a change in diagnosis from non-demented to AD (Bacon et al., 1998).
Recent studies have investigated the potential benefits of hormonal replacement therapy (HT) in preventing AD, delaying onset of the disease, and/or minimizing symptoms post onset of AD in females. Estrogen plays a key role in normal brain functioning and estrogen may have a positive effect on cognition in AD (Fillit, 1994; Henderson et al., 1994). Although the findings of research investigating the effects of HT on AD have been disparate, some studies have reported that females who have used HT are significantly less likely to suffer from AD than females who have never used HT, although HT administered after the diagnosis of AD has not been shown to reverse the pathology (Compton, van Amelsvoort & Murphy, 2001; Tang et al., 1996; Zandi et al., 2002).
Whether the ApoE allele type interacts with HT and its potential neuroprotective effects has been of considerable interest and the results have been conflicting. Some studies found that HT’s positive influence on cognition was dependent on the ApoE allele type where only ε4 negative females experienced a beneficial effect from HT treatment while ε4 positive females experienced no benefit (Yaffe et al., 2000). Population studies done by Zandi et al. (2002) failed to find a statistically significant interaction between HT’s neuroprotective effects and ApoE allele type; however, results suggested a trend towards an increase in AD risk reduction associated with HT in women who were ε4 positive. Results from another prospective study also provided evidence of a slightly greater positive effect of HT on women with one ε4 allele (Tang et al., 1996). Wang, Irwin and Brinton (2006) have reported that estrogen receptor alpha (ERα) increases and estrogen receptor beta (ERβ) decreases ApoE expression in the hippocampus in rats. They have argued that the interaction of ε4 allele status and estrogen receptor type may partially explain the complex results in human clinical trials of HT on cognitive function. HT has been shown to increase the risk of AD in those with the ε4 allele and to decrease risk in those with the ε2 or ε3 allele (Kaplitt et al., 1996; Mahley et al., 2006; Saunders et al., 2000). Wang et al., 2006 suggest that an increase of ERα combined with HT will increase ApoE which would selectively increase the risk of cognitive impairment in ApoE 4 positive individuals, in a heterogeneous population in a large clinical trial.
In view of the potential positive effect of estrogen on cognition in AD, the question arises whether or not HT protects against the olfactory impairment associated with AD. To date, research on HT’s effects on olfactory dysfunction has been minimal but it has presented circumstantial evidence suggesting that HT may lessen the degree of olfactory loss associated with aging or AD in women (Deems et al., 1991; Dhong et al., 1999; Sundermann, Gilbert, & Murphy, 2007). In a retrospective study by Deems et al. (1991), only four of 99 postmenopausal women experiencing loss of olfactory functioning were taking estrogen before onset of the problem. This proportion is extremely low in comparison to the proportion of women of similar age and demographic background taking estrogen in the general population (Deems et al., 1991). Another study treated 22 ovariectomized rats with olfactotoxicant 3-methylindole (3-MI), a toxin that decreases behavioral performance on olfactory, but not visual, discrimination tasks (Dhong et al., 1999). Following 3-MI treatment, twelve rats received daily injections of estrogen and it was found that the estrogen treated rats performed better on odor discrimination tasks relative to rats not treated with estrogen (Dhong et al., 1999). The results of these studies suggest that estrogen may offer protection against olfactory dysfunction.
In light of the known olfactory impairments in the early stages of AD coupled with the evidence of HT’s positive effect on cognition and possibly olfaction in the elderly, the current study investigated the effect of HT on performance in an olfactory threshold task in non-demented, elderly females as well as females diagnosed with AD. The discovery of the ApoE-related risk for developing AD and the potential positive effect that HT may exhibit on olfaction has led the current study to further investigate whether the effect of HT on olfactory threshold varies in relation to the ApoE allele type. Comparing ε4 positive and ε4 negative individuals within the HT-user population sample and the HT-non-user population sample will allow new insight to be obtained into how olfactory impairment is differentially affected by HT use and the ApoE ε4 allele.
Methods
Participants
In both Experiments 1 and 2, all participants were volunteers in a longitudinal study at the Alzheimer’s Disease Research Center (ADRC) at the University of California, San Diego (UCSD). All diagnoses were made by two senior staff neurologists at the ADRC according to the criteria for primary degenerative dementia outlined in the Diagnostic and Statistical Manual of Mental Disorders, 3rd Edition Revised (DSM-3R) (American Psychiatric Association, 1987) and according to the criteria for probable AD developed by the National Institute on Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association (Mckhann et al., 1984). Genomic DNA was prepared from blood samples. ApoE genotyping was performed using the polymerase chain reaction with oligonucleotide, followed by digestion with the HhA1 restriction enzyme and polyacrylamide chain gel electrophoresis. Participants were categorized as ε4 positive (having at least one ε4 allele: ε2/4, ε3/4, and ε4/4) or ε4 negative (having no ε4 allele ε2/2, ε2/3, and ε3/3).
All participants were females who had never had a hysterectomy. The most common form of HT is estrogen in combination with a progestin; however, females with a hysterectomy typically use an unopposed ERT (estrogen without a progestin). The study did not include hysterectomized females in order to help control for the differing effects estrogen has demonstrated in recent studies when taken alone versus taken in conjunction with a progestin. Limited information concerning the HT type taken by participants was available; however, it was known that the participants who had used ERT had taken it orally, except for one who received estrogen intramuscularly and three participants who did not report their estrogen intake method. In Experiment 1, participants included 51 females diagnosed as non-demented older adults that were recruited for testing through newspaper advertisements or among the spouses of the questionable or probable Alzheimer’s patients at the ADRC. In addition to meeting the DSM-3R exclusion criteria for dementia, the non-demented participants showed no objective cognitive impairments on the Mini-Mental State Examination and the Dementia Rating Scale and no scores fell into the range indicating possible Mild Cognitive Impairment. Sixteen of the 51 females had a history of post-menopausal HT use. These 16 participants were referred to as the HT comparison group. The total number of years that HT was used ranged from one to 27 with the average number of years falling at 11.9. Within the estrogen comparison group, eight participants were categorized as ε4 positive and the remaining eight were categorized as ε4 negative. Thirty-five of the non-demented older adults identified for the study had never used HT at any point in their life and these 35 participants were referred to as the no HT comparison group. Within the no HT comparison group, eleven participants were ε4 positive while 24 were ε4 negative.
In Experiment 2, participants included 77 females diagnosed with Alzheimer’s disease. Eighteen of the 77 females had used HT at some point post menopause. These 18 participants were referred to as the HT AD group. The total number of years that the HT was used ranged from one to 27 with the average number of years falling at 11.9. Within the HT AD group, 12 participants were categorized as ε4 positive and the remaining six were categorized as ε4 negative. Fifty-nine of the female AD patients had never used HT at any point in their life and these 59 participants were referred to as the no HT AD group. Within the no HT group, 35 participants were ε4 positive while 24 were ε4 negative.
Procedure
Odor Threshold
Odor threshold was assessed for each nostril with a two-alternative, forced-choice, ascending method of limits design with the odorant butanol for each nostril (Cain et al., 1983; modified as in Murphy et al, 1990). The two alternative choices consisted of a blank and the butanol odor stimulus. The butanol odorant was used because it is a potent stimulus for the olfactory nerve at concentrations that have no impact on the trigeminal nerve. A series of 10 aqueous (deionized water) dilution steps was used, ranging from 349 parts per billion (dilution step 9) to 3055 parts per million (dilution step 0). Therefore, dilution step 9 contained the lowest butanol concentration and dilution step 0 contained the highest butanol concentration. Each successive dilution was one-third the concentration of the preceding dilution. The stimuli, vapor phase from 60-ml solutions, were presented via 250 ml squeezable polyethylene bottles with pop-up spouts. The spout was inserted into the nostril of the participant for monorhinic testing. To avoid adaptation, the test began with the lowest concentration (dilution step 9), and progressed toward the higher concentrations. The order in which the odorant and blank were presented was randomized in each trial. In each trial, the participant was instructed to choose which of the two bottles contained the odorant. An incorrect choice (selection of the blank), led to a one-step increase in concentration on the next trail. A correct choice (selection of the odorant) led to presentation of the same concentration to a criterion of five consecutive correct choices. Each trial was separated by at least 90 seconds to avoid adaptation. An odor threshold, which ranged from 0 to 9 was determined for each nostril. The two threshold scores were averaged together for final analysis. The same procedure was used for both Experiment 1 and 2. The research assistant who administered the psychophysical test was blind to the hormonal history and status of the participant.
Results
Table 1 provides a summary (mean ± standard error) of the demographic information and neuropsychological test scores for the non-demented older adults and Alzheimer patients. Both the healthy non-demented older adults and the patients diagnosed with AD showed no statistically significant mean differences in demographic variables between participants in the HT group and the no HT group as well as between participants in the ε4 positive and the ε4 negative group. The demographic variables included age, years of education, MMSE (Mini Mental State Examination) scores and DRS (Dementia Rating Scale). The statistical results were based on a multivariate analysis of variance (MANOVA).
Table 1.
Mean (± SE) demographics, age, education, DRS, and MMSE scores for the older females, expressed as a function of Alzheimer disease status, ε4 status and HT use. No significant differences exist in demographic variables between the HT and no HT groups and the ε4+ and ε4− groups within the non-demented females and the AD patients.
| Non-demented Older Females (n=51) | Alzheimer’s Disease (n=77) | |||||||
|---|---|---|---|---|---|---|---|---|
| ε4 − | ε4 + | ε4 − | ε4 + | |||||
| HT | No HT | HT | No HT | HT | No HT | HT | No HT | |
| n = 8 | n = 24 | n = 8 | n = 11 | n = 6 | n = 24 | n = 12 | n = 35 | |
| Age | 73.18 ± 4.22 |
73.77 ± 2.43 |
70.41 ± 4.22 |
69.70 ± 3.60 |
72.97 ± 3.69 |
75.61 ± 1.89 |
72.94 ± 2.61 |
76.48 ± 1.53 |
| Edu | 14.38 ± 0.97 |
14.67 ± 0.56 |
15.00 ± 0.97 |
15.27 ± 0.83 |
14.83 ± 1.22 |
13.43 ± 0.62 |
13.33 ± 0.86 |
13.80 ± 0.50 |
| MMS | 29.5 ± 0.30 |
29.21 ± 0.17 |
29.62 ± 0.30 |
28.91 ± 0.30 |
18.50 ± 2.83 |
21.04 ± 1.48 |
18.75 ± 2.00 |
20.03 ± 1.17 |
| DRS | 140.12 ± 1.72 |
139.25 ± 1.00 |
140.00 ± 1.72 |
135.91 ± 1.47 |
101.18 ± 12.4 |
108.26 ± 6.36 |
101.92 ± 8.80 |
105.34 ± 5.15 |
Experiment 1
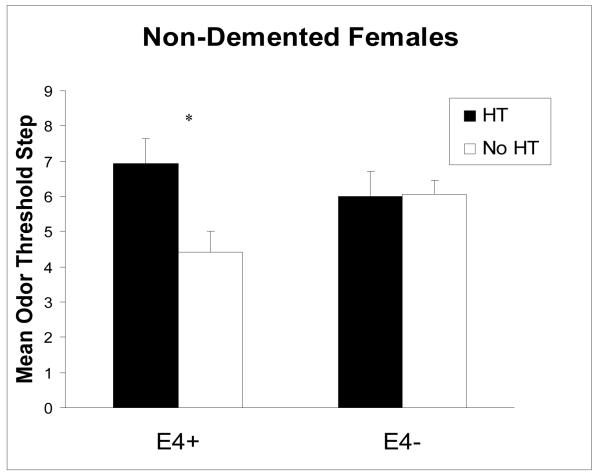
A 2 × 2 ANOVA with a two-factor between-subjects design was used to examine mean odor threshold differences as a function of HT group (2 levels: HT group, no HT group) and ε4 allele status (2 levels: ε4 positive, ε4 negative) for the non-demented older females. Among the non-demented older females, there was a significant main effect for HT where odor threshold levels were significantly better for females in the HT comparison group relative to females in the no HT comparison group. There was no significant main effect for ε4 allele status. Odor threshold levels for females who were ε4 positive did not significantly differ from odor threshold levels for females who were ε4 negative. Finally, there was a significant interaction between the HT group and the presence of the ε4 allele status. Table 2 (left panel) provides a summary (mean ± SE) of odor threshold scores and the results of the ANOVA conducted for non-demented older adults.
Table 2.
Mean (± SE) odor threshold step as a function of HT use and ε4 allele status and significant results from a 2 × 2 ANOVA with ApoE (ε4 positive, ε4 negative) and HT group (HT, no HT) as the between-group variables for non-demented older females (left panel) and AD patients (right panel).
| Non-demented Older Females (N = 51) | ||
|---|---|---|
| HT | No HT | |
| ε4 Positive | 6.94 ± .695* (n = 8) |
4.41 ± .593* (n = 11) |
| ε4 Negative | 6.00 ± .695 (n = 8) |
6.06± .401 (n = 24) |
| ApoE | F(1, 47) = .346 | p = .559 |
| HT Group | F(1, 47) = 4.109 | p < .05 |
| ApoE × HT Group |
F(1, 47) = 4.537 | p < .05 |
| Alzheimer’s Disease (N = 77) | ||
|---|---|---|
| HT | No HT | |
| ε4 Positive | 5.04 ± .673 (n = 12) |
4.31 ± .394 (n = 35) |
| ε4 Negative | 3.67 ± .952 (n = 6) |
4.71 ± .476 (n = 24) |
| ApoE | F(1, 73) = .552 | p = .460 |
| HT Group | F(1, 73) = .057 | p = .812 |
| ApoE × HT Group |
F(1,73)= 1.797 | p = .184 |
Denotes a significant difference in mean odor threshold steps between the ε4 positive and ε4 negative groups at the .05 alpha level. Within non-demented older females that are ε4 positive, HT users had significantly higher mean odor threshold compared to HT non-users.
A one-way analysis of variance was conducted with the non-demented older adults with HT group as the between-subject variable to examine the simple effects of HT use at each level of ε4 allele status. For those who were ε4 positive, the HT comparison group had significantly higher odor threshold sensitivity than the no HT comparison group F(1, 17) = 6.28, p < .05. For those who were ε4 negative, no significant differences were found between the HT comparison group and the no HT comparison group (Figure 1).
Figure 1.
Mean (±SE) odor threshold step, shown as a function of ε4 allele status and HT use for non-demented older females
Next, a one-way analysis of variance was conducted with ε4 allele status as the between-subject factor in order to examine the simple effects of ε4 allele status at each level of HT use. For those in the HT comparison group, no significant differences were found between ε4 positive females and ε4 negative females. For those in the no HT comparison group, females who were ε4 negative had significantly higher odor threshold sensitivity than females who were ε4 positive F(1, 33) = 4.91, p < .05.
Experiment 2
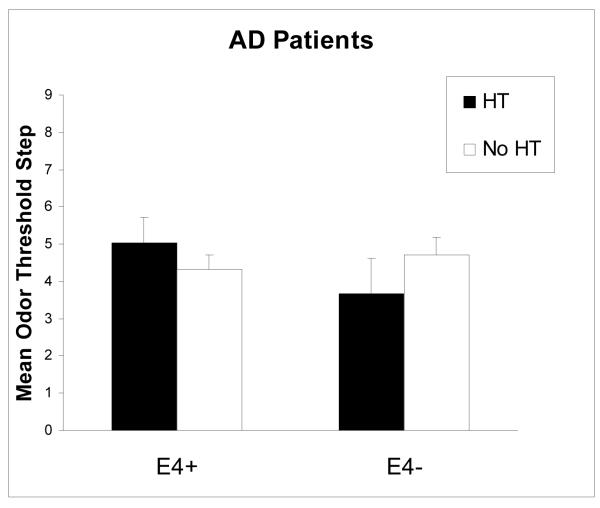
A 2 × 2 ANOVA with a two-factor between-subjects design was used to examine mean odor threshold differences as a function of HT group (2 levels: HT group, no HT group) and ε4 allele status (2 levels: ε4 positive, ε4 negative) for the AD patient group. Among the AD patients, no significant main effects occurred for HT group or ε4 allele status and no significant interaction occurred between HT group and ε4 allele status. Table 2 (right panel) provides a summary (mean ± SE) of odor threshold scores and results of the ANOVA conducted for AD patients.
Discussion
Olfactory impairment occurs in the initial, silent stages of AD when clinical impairments are not detected. It has been suggested that the presence of the ε4 allele could lead to the clinical manifestation of AD through an association with the neurodegenerative changes. Among the non-demented older females, the only group showing olfactory sensitivity impairments was the ε4 positive no HT group. This suggests that preclinical impairments in odor threshold may be emerging in participants at risk for AD due to presence of the ε4 allele. However, the ε4 positive HT group showed no olfactory sensitivity impairments. These results indicate that HT use may offer protection against loss of olfactory function in ε4 positive females who may be in the early stages of AD.
Circumstantial evidence suggests that estrogen deprivation in post-menopausal women could be harmful to brain function due to the key role estrogen plays in maintaining normal neural activity (Simpkins, Singh & Bishop, 1994). Estrogen’s role in healthy brain function could result from a variety of effects that estrogen has on cellular mechanisms in the brain. These neuroprotective effects include increasing dendritic spine density and the stimulation of axon sprouting and connectivity (Gould et al., 1990) as well as nerve growth factor activity (Gibbs, 1998). This effect was found specifically in the entorhinal cortex when estrogen supplements restored synaptic sprouting in entorhinal cortex lesioned mice that were devoid of synaptic sprouting after ovariectomy (Kadish & van Groen, 2002). These findings highlight estrogen’s key role in promoting neuron growth in areas of the brain devastated by AD.
Lastly, evidence suggests that the neuroprotective effects of estrogen are, in part, dependant on Apolipoprotein E, which is a protein responsible for the metabolism and transportation of lipids used in the repair and growth of cell membranes. Studies have shown that, in mice, exogenous 17β-estradiol treatments increased the production and expression of the Apolipoprotein ε (McAsey et al., 2006) particularly in the olfactory bulb and cerebellum (Struble et al., 2003) and in the cortex and hippocampus (Levin-Allerhand et al., 2001; Srivastava et al., 1996). This effect has been demonstrated in brain glial cells as well when high levels of endogenous 17β-estradiol are present (Stone et al.,1997). In addition, Stone et al. (1998) demonstrated that, in the dentate gyrus, estradiol replacement restored decreased levels of synaptic sprouting in an AD mouse model that was estrogen deficient due to ovariectomy. However, this restorative effect of exogenous estradiol on decreased sprouting was absent in ApoE-knock-out mice (Stone et al., 1998). Further demonstrating the interaction between estrogen and ApoE is the finding by Nathan and colleagues (2004) that estradiol increased neuron growth in cultured mouse cortical neurons in neurons expressing the ApoE ε2 or ε3 allele; however, estradiol had no effect on neurons expressing the ε4 allele.
The location of estrogen receptors in the brain as well as the demonstrated effect of HT on medial temporal lobe activity also may help to explain the observed positive effect of HT on olfaction. The neuroprotective action of estrogen is mediated by the estrogen receptors, ERα and ERβ (Ostlund, Keller & Hurd, 2003). Estrogen receptors are located in peripheral regions and central brain regions that are involved in olfaction such as the olfactory epithelium (Barni et al., 1999), the olfactory bulb, and the entorhinal cortex (Dhong et al., 1999; Osterlund et al., 2003). The medial temporal lobe is a brain region involved in olfactory processing that has shown beneficial effects on cognitive activity in long-term HT users during a PET study (Maki & Resnick, 2000; Resnick et al., 1998). Consequently, it is possible for olfactory sensitivity to benefit from neuroprotection that may be provided to brain areas involved in olfaction through the use of HT.
There were no significant differences in odor threshold between the HT AD group and the no HT AD group. In addition, both groups demonstrated mean odor threshold values that signify olfactory sensitivity impairments as indicated by previous odor threshold studies in an older population (Murphy et al., 1990; Nordin & Murphy, 1996; Murphy, 1999). The data suggest that the possible protective effect of HT on olfaction may diminish in the later stages of the disease. This result corroborates with other recent studies investigating differences in olfactory-related tasks between ε4 positive and negative non-demented older adults and AD patient populations (Gilbert & Murphy, 2004; Murphy et al., 1998). Generally, these studies have only found differences between ε4 genotype in AD onset and severity of symptoms in the very early, preclinical stages of AD but these differences seem to wash out in the middle to later stages of AD.
The results of the current study are also in accord with the proposed critical window of time hypothesis. Observational studies, basic science studies as well as a number of early randomized controlled trials have provided evidence of HT exerting a beneficial effect on cognition and risk of AD in women (Henderson, 2006; Maki, 2006; Sherwin, 2006). However, The Women’s Health Initiative Memory Study (WHIMS), a randomized clinical trial investigating hormone therapy and dementia, provided discrepant results where hormone therapy demonstrated a detrimental effect on cognition and risk of dementia (Shumaker et al., 1998). The females in the WHIMS study were all 65 years of age and older with an average age of 70. Therefore, a convincing argument for the discrepant results between the WHIMS study and previous studies is the critical window of time hypothesis, which proposes that HT must be initiated near the menopausal transition time in order to maximize the potential for HT to exert beneficial cognitive effects (Brinton, 2004; Maki, 2006; Sherwin, 2006). Further evidence supporting the critical window of time hypothesis was found in a study by Suzuki and colleagues that demonstrated that estradiol administration immediately following ovariectomy in a mouse stroke model exerts a neuroprotective effect via antiinflammatory action. However, estradiol administration ten weeks post ovariectomy had no neuroprotective effects (Suzuki et al., 2007).
In support of this hypothesis, the ε4 positive healthy older adults experienced a benefit of HT in olfaction. In addition, 10 of the 16 non-demented females that were HT users also reported an age of menopause. Nine of the 10 females who reported an age at menopause had begun HT during the time period from the start of menopause to 3 years post menopause. One female initiated HT 6 years following menopause. Consequently, the majority of the non-demented, HT users are known to have received HT during the purported critical window of time. A large proportion of neurons may have been healthy in this time period; therefore increasing the potential for HT to exert neuroprotective effects and minimize any further development of degeneration in brain areas mediating olfaction.
The present study has several limitations that should be addressed in future investigations. There are a disproportionately high number of ε4+ non-demented older females in the HT group considering that the prevalence rate of the allele in the general population of non-demented individuals is approximately 30% (Corder et al., 1993). It is possible that this higher rate reflects a bias where the study sample of non-demented ε4+ females share a certain characteristic that predisposes them to HT usage. This potential bias should be taken into consideration when drawing conclusions from the study results. The HT and no HT groups were matched on a number of demographic variables, i.e., age, ethnicity, education, MMSE and DRS scores; however, the possibility cannot be excluded that other factors related to HT use may contribute to odor threshold differences between groups. The study relies on self or companion report of HT. Different progestational hormones differ in their interaction with estrogen and their effects on brain behavior (Brinton, 2004). The current sample size and the limited information concerning HT type did not allow for investigating differences in the effects of different hormonal combinations. The results suggest that future large-scale, prospective trials that have access to patients’ long-term medical records are warranted to investigate how the effect of HT on olfactory sensitivity, and other sensory and cognitive measures, may differ as a function of different hormone combinations.
In conclusion, the present study revealed that HT had no effect on olfactory sensitivity in female AD patients regardless of ε4 genotype. In contrast, within the non-demented no HT group, ε4 negative females had a significantly better threshold score than ε4 positive females. Importantly, within the HT comparison group, no significant differences existed in odor threshold scores between the ε4 positive and ε4 negative females. The results suggest that non-demented females who are at risk for AD may be experiencing the early olfactory system deficits associated with AD. HT use may offer protection against olfactory impairment in healthy older females; however, this effect may diminish in the middle to later stages of AD. Future research is warranted in order to investigate the neuroprotective role of HT on other cognitive and sensory functions in non-demented aging individuals.
Figure 2.
Mean (±SE) odor threshold step, shown as a function of HT use and ε4 allele status in AD patients.
Acknowledgments
This research was supported by NIH grant #AG04085 from the National Institute on Aging to Claire Murphy. We gratefully acknowledge Drs. Robert Katzman, the late Leon Thal, David Salmon, the staff, patients, and volunteers at the Alzheimer’s Disease Research Center, University of California, San Diego, and the ADRC NIH grant #P50AG05131.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association . Diagnositc and statistical manual of mental disorders. 3rd ed American Psychiatric Association; Washington DC: 1987. [Google Scholar]
- Bacon A, Bondi M, Salmon D, Murphy C. Very early changes in olfactory functioning due to Alzheimer’s disease and the role of apolipoprotein ε in olfaction. Annals of the New York Academy of Sciences. 1998;855:723–731. doi: 10.1111/j.1749-6632.1998.tb10651.x. [DOI] [PubMed] [Google Scholar]
- Barni T, Maggi M, Fantoni G, Granchi S, Mancina R, Gulisano M, et al. Sex Steroids and Odorants Modulate Gonadotropin-Releasing Hormone Secretion in Primary Cultures of Human Olfactory Cells. The Journal of Clinical Endocrinology and Metabolism. 1999;84(11):4266–4273. doi: 10.1210/jcem.84.11.6150. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, Yilmazer D, de vos R, Jansen E, Bohl J. Pattern of brain destruction in Parkinson’s and Alzheimer’s diseases. Journal of Neural Transmission. 1996;103:455–490. doi: 10.1007/BF01276421. [DOI] [PubMed] [Google Scholar]
- Bretsky P, Guralnik J, Launer L, Albert M, Seeman T. The role of APOE-epsilon4 in longitudinal cognitive decline: Macarthur studies of successful aging. Neurology. 2003;60:1077–1081. doi: 10.1212/01.wnl.0000055875.26908.24. [DOI] [PubMed] [Google Scholar]
- Brinton RD. Impact of estrogen therapy on Alzheimer’s disease. CNS Drugs. 2004;18(7):405–422. doi: 10.2165/00023210-200418070-00001. [DOI] [PubMed] [Google Scholar]
- Cain WS, Gent JF, Catalanotto FA, Goodspeed RB. Clinical evaluation of olfaction. Am. J. Otolaryngol. 1983;4:252–256. doi: 10.1016/s0196-0709(83)80068-4. [DOI] [PubMed] [Google Scholar]
- Compton J, van Amelsvoort T, Murphy D. HT and its effect on normal ageing of the brain and dementia. British Journal of Clinical Pharmacology. 2001;52(6):647–653. doi: 10.1046/j.1365-2125.2001.01492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel ED, Gaskell PC, Jr., Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Deems DA, Doty RL, Settle G, Moore-Gillon V, Shaman P, Mester AF, Kimmelman CP, Brightman VJ, Snow JB., Jr. Smell and taste disorders, a study of 750 patients from the University of Pennsylvania Smell and Taste Center. Archives of Otolaryngology-Head and Neck Surgery. 1991;117:519–528. doi: 10.1001/archotol.1991.01870170065015. [DOI] [PubMed] [Google Scholar]
- Dhong H, Chung S, Doty RL. Estrogen protects against 3-methylindole-induced olfactory loss. Brain Research. 1999;824(2):312–315. doi: 10.1016/s0006-8993(99)01241-x. [DOI] [PubMed] [Google Scholar]
- Dik MG, Jonker C, Bouter LM, Geerlings MI, van Kamp GJ, Deeg DJ. APOE-epsilon4 is associated with memory decline in cognitively impaired elderly. Neurology. 2000;54:1492–1497. doi: 10.1212/wnl.54.7.1492. [DOI] [PubMed] [Google Scholar]
- Doty R. Odor perception in neurodegenerative diseases. In: Doty R, editor. Handbook of Olfaction and Gustation. 2nd ed. Rev. Marcel Dekker, Inc.; New York: 2003. pp. 479–501. [Google Scholar]
- Fillit H, Weinreb H, Cholst I, Luine V, McEwen B, Amador R, Zabriskie J. Observations in a preliminary open trial of estradiol therapy for senile dementia-Alzheimer’s type. Psychoneuroendocrinology. 1986;11:337–345. doi: 10.1016/0306-4530(86)90019-3. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Levels of trkA and BDNF mRNA, but not NGF mRNA, fluctuate across the estrous cycle and increase in response to acute hormone replacement. Brain Research. 1998;787(2):259–268. doi: 10.1016/s0006-8993(97)01511-4. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Murphy C. The effect of the ApoE e4 allele on recognition memory for olfactory and visual stimuli in patients with pathologically confirmed Alzheimer’s disease, probable Alzheimer’s disease and healthy elderly controls. Journal of Clinical and Experimental Neuropsychology. 2004;26(6):779–794. doi: 10.1080/13803390490509439. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal Steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. The Journal of Neuroscience. 1990;10(4):1286–1290. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves AB, Bowen JD, Rajaram L, McCormick WC, McCurry SM, Schellenberg GD, Larson EB. Impaired olfaction as a marker for cognitive decline. Neurology. 1999;53:1480–1487. doi: 10.1212/wnl.53.7.1480. [DOI] [PubMed] [Google Scholar]
- Handley OJ, Morrison CM, Miles C, Bayer AJ. ApoE gene and familial risk of Alzheimer’s disease as predictors of odour identification in older adults. Neurobiol. Aging. 2006;27:1425–1430. doi: 10.1016/j.neurobiolaging.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Harris R, Davidson TM, Murphy C, Gilbert PE, Chen M. Clinical evaluation and symptoms of chemosensory impairment: 1000 consecutive cases from the Nasal Dysfunction Clinic in San Diego. Am. J Rhinology. 2006;20:101–108. [PubMed] [Google Scholar]
- Henderson VW. Estrogen-containing hormone therapy and Alzheimer’s disease risk: understanding discrepant inferences from observational and experimental research. Neuroscience. 2006;138:1031–1039. doi: 10.1016/j.neuroscience.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Henderson VW, Watt L, Buckwalter G. Cognitive skills associated with estrogen replacement in women with Alzheimer’s disease. Psychoneuroendocrinology. 1995;21(4):421–430. doi: 10.1016/0306-4530(95)00060-7. [DOI] [PubMed] [Google Scholar]
- Kadish I, van Groen T. Low levels of estrogen significantly diminish axonal sprouting after entorhinal cortex lesions in the mouse. The Journal of Neuroscience. 2002;22(10):4095–4102. doi: 10.1523/JNEUROSCI.22-10-04095.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplitt M, Gouras GK, Makimura H, Jovanovic J, Sweeney D, Greengard P, et al. Apolipoprotein E, A beta-amyloid, and the molecular pathology of Alzheimer’s disease. Therapeutic implications. Annals of the New York Academy of Sciences. 1996;802:42–49. doi: 10.1111/j.1749-6632.1996.tb32597.x. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: A causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(15):5644–5651. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM. Hormone Therapy and Cognitive Function: is there a critical period For benefit? Neuroscience. 2006;138:1027–1030. doi: 10.1016/j.neuroscience.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Maki PM, Resnick SM. Longitudinal effects of estrogen replacement Therapy on PET cerebral blood flow and cognition. Neurobiology of Aging. 2000;21:373–383. doi: 10.1016/s0197-4580(00)00123-8. [DOI] [PubMed] [Google Scholar]
- McAsey M, Cady C, Jackson L, Li M, Randall S, Nathan B, Struble R. Time course of response to estradiol replacement in ovariextomized mice: brain apolipoprotein E and synaptophysin transiently increase and glial fibrillary acidic protein is suppressed. Experimental Neurology. 2006;197(1):197–205. doi: 10.1016/j.expneurol.2005.09.008. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Murphy C, Gilmore MM, Seery CS, Salmon DP, Lasker BR. Olfactory thresholds are associated with degree of dementia in Alzheimer’s disease. Neurobiology of Aging. 1990;11:465–469. doi: 10.1016/0197-4580(90)90014-q. [DOI] [PubMed] [Google Scholar]
- Murphy C. Loss of olfactory function in dementing disease. Physiology & Behavior. 1999;66:177–182. doi: 10.1016/s0031-9384(98)00262-5. [DOI] [PubMed] [Google Scholar]
- Murphy C, Bacon A, Bondi M, Salmon D. Apolipoprotein E status is associated with odor identification deficits in nondemented older persons. Annals of the New York Academy of Sciences. 1998;855(1):744–750. doi: 10.1111/j.1749-6632.1998.tb10654.x. [DOI] [PubMed] [Google Scholar]
- Nathan B, Barsukova A, Shen F, McAsey M, Struble R. Estrogen facilitates neurite extension via apolipoprotein E in cultured adult mouse cortical neurons. Endocrinology. 2004;145(7):3062–3064. doi: 10.1210/en.2003-1707. [DOI] [PubMed] [Google Scholar]
- Nordin S, Murphy C. Impaired sensory and cognitive olfactory function in Questionable Alzheimer’s disease. Neuropsychology. 1996;10(1):113–119. [Google Scholar]
- Osterlund M, Keller, Hurd Y. Estrogen receptors in the human forebrain and the Relation to neuropsychiatric disorders. Progress in Neurobiology. 2003;64(3):251–267. doi: 10.1016/s0301-0082(00)00059-9. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Maki PM, Golski S, Kraut MA, Zonderman AB. Estrogen effects on PET cerebral blood flow and neuropsychological performance. Hormone Behavior. 1998;34:171–184. doi: 10.1006/hbeh.1998.1476. [DOI] [PubMed] [Google Scholar]
- Saunders A, Strittmatter W, Schmechel D, George-Hyslop P, Pericak-Vance M, Joo S, et al. Association of apolipoprotein ε alleles epsilon 4 with late-onset familial and Sporadic Alzheimer’s disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Saunders A, Trowers M, Shimkets M, Blakemore S, Crowther D, Mansfield T, et al. The role of apolipoprotein E in Alzheimer’s disease: pharmacogenomic target selection. Biochimica et Biophysica Acta. 2000;1502(1):85–94. doi: 10.1016/s0925-4439(00)00035-1. [DOI] [PubMed] [Google Scholar]
- Schiffman SS, Graham BG, Sattely-Miller EA, Zervakis J, Welsh-Bohmer K. Taste, smell and neuropsychological performance of individuals at familial risk for Alzheimer’s disease. Neurobiology of Aging. 2002;23:397–404. doi: 10.1016/s0197-4580(01)00337-2. [DOI] [PubMed] [Google Scholar]
- Shumaker S, Reboussin B, Espeland M, Rapp S, McBee W, Dailey M, et al. The women’s health initiative memory study: A trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Controlled Clinical Trials. 1998;19(6):604–621. doi: 10.1016/s0197-2456(98)00038-5. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive aging in women. Neuroscience. 2006;138:1021–1026. doi: 10.1016/j.neuroscience.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Singh M, Bishop J. The potential role for estrogen replacement therapy in the treatment of the cognitive decline and neurodegeneration associated with Alzheimer’s Disease. Neurobiology of Aging. 1994;15(2):S195–S197. doi: 10.1016/0197-4580(94)90205-4. [DOI] [PubMed] [Google Scholar]
- Srivastava RK, Bhasin N, Srivastava N. Apolipoprotein ε gene expression in various tissues of mouse and regulation by estrogen. Biochemistry and Molecular Biology International. 1996;38(1):91–101. [PubMed] [Google Scholar]
- Stone DJ, Rozovsky I, Morgan TE, Anderson CP, Hajian H, Finch CE. Astrocytes and microglia respond to estrogen with increased apoE mRNA in vivo and in vitro. Exp. Neurol. 1997;143:313–318. doi: 10.1006/exnr.1996.6360. [DOI] [PubMed] [Google Scholar]
- Stone DJ, Rozovsky I, Morgan TE, Anderson CP, Finch CE. Increased synaptic sprouting in response to estrogen via an apolipoprotein E-dependent mechanism: implications for Alzheimer’s disease. J. Neurosci. 1998;18:3180–3185. doi: 10.1523/JNEUROSCI.18-09-03180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struble RG, Rosario ER, Kircher ML, Ludwig SM, McAdamis PJ, Watabe K, McAsey ME, Cady C, Nathan BP. Regionally specific modulation of brain apolipoprotein E in the mouse during the estrous cycle and by exogenous 17β estradiol. Exp. Neurol. 2003;183:638–644. doi: 10.1016/s0014-4886(03)00215-2. (2003) [DOI] [PubMed] [Google Scholar]
- Sundermann E, Gilbert PE, Murphy C. Estrogen and performance in recognition memory for olfactory and visual stimuli in women diagnosed with Alzheimer’s disease. Journal of International Neuropsychological Society. 2006;12:400–404. doi: 10.1017/s1355617706060474. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Brown C, Dela Cruz C, Yang E, Bridwell D, Wise P. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(14):6013–6018. doi: 10.1073/pnas.0610394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B. Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. The Lancet. 1996;348(9025):429–432. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- Wang JM, Irwin RW, Brinton RD. Activation of estrogen receptor α increases and estrogen receptor β decreases apolipoprotein E expression in hippocampus in vitro and in vivo. PNAS. 2006;103:16983–16988. doi: 10.1073/pnas.0608128103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Arnold SE, Tang Y, Boyle PA, Bennett CA. Olfactory identification and incidence of mild cognitive impairment in older age. Arch. Gen Psychiatry. 2007;64(7):802–808. doi: 10.1001/archpsyc.64.7.802. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Haan M, Byers B, Tangen C, Kuller L. Estrogen use, APOE, and cognitive decline. The American Academy of Neurology. 2000;54:1949–1953. doi: 10.1212/wnl.54.10.1949. [DOI] [PubMed] [Google Scholar]
- Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC. Hormone replacement therapy and incidence of Alzheimer Disease in older women. The Journal of the American Medical Association. 2002;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]