Abstract
Context
Muscle aches, numbness in the feet/toes (neuropathy), and fatigue are often reported concurrently and are among the symptoms most frequently reported by individuals with HIV/AIDS, whether or not they are taking antiretroviral medications (ARVs).
Objectives
This study used a longitudinal analytical methodology to analyze these symptoms together to determine whether symptom clusters are maintained over time and to determine whether there is a temporal relationship between fatigue and reports of neuropathic pain and muscle aches.
Methods
This was a secondary analysis of a subset of data from a six-month, longitudinal, randomized, controlled trial of 243 HIV-positive individuals taking ARVs. Self-reported symptom frequency and intensity were recorded using the Revised Sign and Symptom Checklist for Persons with HIV Disease at baseline (month 0), one month, three months, and six months. Multilevel, logistic regression models were used to analyze time-lagged effects of muscle aches, numbness of the feet/toes, and fatigue to estimate any predictive and interactive effects that the symptoms have upon one another.
Results
A significant relationship between muscle aches and fatigue intercepts was noted (odds ratio [OR] =1.80, P ≤ 0.05). Significant relationships between numbness and fatigue were also noted for the entire measurement period (OR=2.70, P ≤ 0.05). Time-lagged models showed persons reporting neuropathic-related numbness in one period were nearly twice as likely to report fatigue in subsequent periods (OR=1.89, P ≤ 0.05). The final model revealed that the addition of muscle aches and numbness explained 28% of the random variance in the occurrence of fatigue. Between-person descriptive variables including years living with HIV, age, having an AIDS diagnosis, ethnicity, and nucleoside reverse transcriptase inhibitor (NRTI) treatment regimens with stavudine, zalactabine, or didanosine did not significantly explain any additional model variation.
Conclusion
These findings are consistent with physiological research and provide evidence that analyzing multiple symptom change over time can provide a more accurate representation of an individual’s symptom experience. When evaluating patients with muscle aches or numbness, particularly when both symptoms are present, an evaluation of fatigue should be considered. Similarly, if fatigue is reported, underlying physiological assessments for neuropathic symptoms and muscle aches may be considered.
Keywords: Multilevel modeling, growth curve, symptom experience, HIV/AIDS, fatigue, neuropathy, muscle ache
Introduction
Symptoms associated with HIV illness and with the antiretroviral (ARV) regimens used to treat it have been associated with both decreased HIV medication adherence (1–5) and decreased quality of life (6–9). In descriptive and cross-sectional studies, muscle aches, numbness in the feet/toes (neuropathy) and fatigue are commonly reported concurrently and are often among the symptoms most frequently reported by individuals with HIV/AIDS, both those taking or not taking antiretroviral medications (ARVs).
Fatigue in clinical conditions is generally assessed subjectively and is often described as a whole body feeling of muscle fatigue and loss of energy. Fatigue is frequently reported in individuals with HIV both taking and not taking ARVs. In addition to HIV, fatigue is documented as a frequently occurring symptom in several other chronic conditions, including fibromyalgia, osteoarthritis, and rheumatoid arthritis. As many as 76% of people with chronic widespread musculoskeletal pain report fatigue, and as many as 94% of people with chronic fatigue syndromes report muscle pain (10). Recent animal studies in mice by Burnes and colleagues (10) suggest a biological link between testosterone and the pathways relating pain and fatigue in female and male mice that may help explain why more women than men are diagnosed with chronic pain and fatigue conditions like fibromyalgia and chronic fatigue syndrome. The findings suggest that testosterone works in conjunction with a specific acid-sensing ion channel (ASIC3) involved in muscle pain to protect against muscle fatigue.
Mitochondrial Toxicity, Neuropathies and Fatigue
Physiological research shows that in patients living with HIV/AIDS and receiving ARVs, acute fatigue is a lead indicator of cellular mitochondrial dysfunction and, as a result, acute fatigue onset and chronic fatigue may have differing physiological and psychological origins (11). Sensory neuropathy occurring in the context of HIV/AIDS is also a frequently reported symptom, particularly in patients who received the nucleoside reverse transcriptase inhibitor (NRTI) antiretrovirals zalcitabine (ddC, Hivid®), stavudine (d4T, Zerit®) and didanosine (ddI, Videx®) as part of their regimen. Although these NRTIs are recommended for cessation by the World Health Organization for use internationally, the long-term and irreversible neuropathic effects of stavudine are still likely. Only in April 2010 was stavudine removed from first-line treatment in current HIV treatment guidelines in South Africa (12).
NRTI-sensory neuropathy presents as a length-dependent sensory neuropathy (i.e., feet affected first) manifested by pain in the soles of the feet in over 60% of individuals and by paresthesias (numbness) in 40%. Sensory neuropathy not only affects quality of life, but also is frequently undertreated, even by expert HIV providers. Both the development of neuropathic symptoms and the fear of sensory neuropathy may reduce adherence to ARVs (13). Finally, peripheral neuropathy may actually be an early marker of mitochondrial dysfunction, which is now believed to contribute to the development of lipodystrophy/fat redistribution, lactic acidosis, and other toxicities.
Symptom Cluster Research
Developments in symptom research in cancer and cancer treatment have produced results that are applicable to HIV symptom management research. In their seminal research of symptom occurrence in cancer, Dodd, Miaskowski and Paul (14) found that symptoms do not occur alone but in groups, illustrating an adverse synergism of multi-symptom occurrences in the symptom experience. These researchers identified a group of three or more concurrent symptoms as being an association of symptoms they termed symptom clusters (14).
The concept of symptom clusters has long been the basis for disease classification and diagnosis of psychological disorders and some medical disorders (15). Amdur and Liberzon (16) proposed that symptom clusters provide the basis for diagnosis and classification and that in combination, well-defined multiple symptom clusters may better conceptualize illness (16).
To date, symptom research has not explicitly taken into account the symptom experience over multiple time periods to quantitatively assess any additive effects in symptom frequency and/or intensity caused by the presence of multiple symptoms. Further, investigators have not applied an adequate longitudinal analytical methodology to analyze these symptoms together to ascertain that the symptom cluster is maintained over time in the same individuals. These prior research findings prompted the secondary analysis of a 2004 dataset of 243 HIV-positive males and females taking a variety of ARV regimens, to assess the possibility of a temporal relationship between reports of neuropathic pain and muscle aches to fatigue.
Methods
This analysis sought to determine whether the presence or absence of additional symptoms changes the proportion of fatigue occurrence, and to quantify the likelihood of change in the occurrence of fatigue. Longitudinal, repeated measures data are used to describe changes in fatigue over time, to examine predictors of fatigue, and to assess the impact of other symptoms on fatigue.
Design
Analysis was conducted on a subset of data from a Texas-based, six-month longitudinal randomized controlled trial of 243 HIV-positive individuals taking ARVs. The aim of the parent study was to determine the effectiveness of a tailored nursing intervention on increasing adherence to ARVs (17). The parent study showed no significant difference in adherence between the treatment and control groups. This subset analysis is a review of symptoms and symptom occurrence over time, for which there was no intervention in the design of the study. Self-reported symptom frequency and intensity were recorded at baseline (month 0), one month, three months, and six months. Multi-level growth mixture models using Mplus (18) were used to assess single and multiple symptom models for self-reported muscle aches, numbness, and fatigue and demographic variables associated with HIV illness and the side effects of ARVs.
Instruments Used in This Substudy
Two instruments from the main study were used in this subset analysis of symptoms: the Demographic Questionnaire and the Revised Sign and Symptom Checklist for Persons with HIV Disease (SSC-HIVrev) (19). These instruments were completed by the participant at baseline (at time of randomization into the study), and at one-month, three-month, and six-month intervals thereafter. The Demographic Questionnaire was used to obtain demographic data from the participants. The SSC-HIVrev consists of 72 items that measure symptom frequency and intensity. Respondents select only those symptoms that they have experienced in the past 24 hours, and record whether the intensity has been “mild,” “moderate,” or “severe.” The symptoms not experienced are left blank and are coded 0 “no symptom.” The SSC-HIVrev has three parts. Part I consists of 45 HIV-related physical and psychological symptoms, clustered into eleven factor scores, along with a total score, with Cronbach’s internal consistency reliability estimates ranging from 0.76–0.91. Part II consists of 19 HIV-related symptoms that did not cluster into factor scores but that may be of interest to a care provider. Part III consists of eight items related to gynecological symptoms for women. In this analysis, the Cronbach’s internal consistency reliability was 0.97 for the overall instrument.
Each symptom variable had a preponderance of zeros (no symptom), and symptom intensities were rated mild or moderate in nearly 75% of the cases. Because of the non-normal distributions, these were recoded into dichotomous variables with binomial distributions. To achieve this, the response choices of 1, 2, and 3 (mild, moderate, severe) were all recoded as 1 “yes.” The zero was retained as a “no” response. These dichotomous variables were analyzed using multi-level logistic regression.
Choosing Multi-Level Growth Modeling Over Other Methods
To examine individual symptom change over time, a two-level growth modeling method was used. At level 1, each person’s successive symptom measurement is noted by an individual growth trajectory and random error. Level 2 trajectory differences allow for the assessment of between-individual growth differences. The method does not require equal waves of data as in repeated measures analysis of variance, making it appealing for longitudinal analyses, where participant attrition is so often a problem, as participants often drop out and return for later follow-up or drop out entirely. It is, therefore, useful in retaining power in statistical analyses. An assumption for using mixed modeling methods is that the cases missing to subsequent follow-up assessments are missing at random. It is assumed that there is correlation between the reports of symptoms as these reports are measured from the same subject repeatedly over time. To account for this correlation, repeated measures statistical analysis tools may be used to make inferences.
The multi-level model using dichotomous data is modified from the traditional (continuous data) format (20). The logistic regression method provides an odds estimation of the probability of a certain event occurring. In all models, the assessment of the level of influence that each symptom had on another was assessed by comparison of the fit between a model that held the parameter intercepts constant (constrained model) and a model that allowed the intercepts to be random (unconstrained model). The constrained model limited the intercept to a single averaged value of the intercepts for each parameter. The model fit statistics -- log likelihood, Akaike information criterion (AIC) statistic and Bayesian information criterion (BIC) statistic -- were compared for differences. If the two models did not greatly differ in their fit statistics, then the modeled parameter estimations of the constrained models reflected the changes in the fatigue outcome resulting only from the effects of the independent symptom variables. If the models differed in their fit statistics, then there were other non-measured influences on the fatigue outcome.
Assessment for Temporal Symptom Occurrence
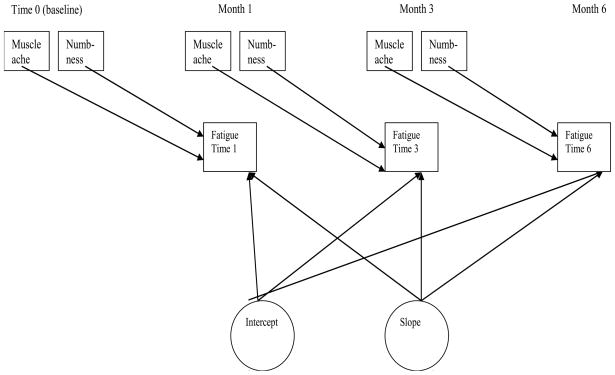
Time-lagged effects linear growth models were assessed to determine whether a temporal relationship exists between muscle aches, numbness, and fatigue (Figure 1). These models assessed fatigue occurrence as the outcome variable dependent upon the presence or absence of muscle aches and/or numbness in prior time periods. The models measured the individual and average intercepts and slope changes. The models were evaluated for correlation among the observed variables and the unobserved (latent) variables.
Figure 1.
Diagram of the Symptom Change Lagged effects Model
Results
Of the original 243 cases, three were excluded because of no symptom data at baseline, leaving a total sample size of 240 individuals with the potential for four assessment observations each -- at baseline, one month, three months, and six months. The multi-level analysis is based on a total of 825 observations. The demographic variables revealed that the sample was 65% (n=159) male, 87% (n=212) non-white (72.8% African American, 10.8% Hispanic), mean age of 41.83 years (SD=7.6, range 22 to 68), HIV-positive for a mean of 8.7 years (SD=4.7, range <1 to 23), with 51.4% (n=125) having an AIDS diagnosis, and 26% (n=64) having a medical diagnosis of depression at enrollment (Table 1). Of the 243 cases, 176 cases had complete pharmaceutical information. Of these, 65% (n=113) were prescribed a protease inhibitor (PI)-based regimen and 27% (n=47) were prescribed a non-nucleoside reverse transcriptase inhibitor (NNRTI)-based regimen. NRTI medications that included stavudine (d4T), zalcitabine (ddC), or didanosine (ddI) were prescribed for 62.5% (n=110) individuals. These individuals had no difference in mean adherence rates than those who received other NRTI medications. No differences in adherence rates were noted in the PI-based and NNRTI-based regimens, eliminating adherence as a potential source of bias in the symptom occurrence analyses. Additionally, there were no differences in the reports of numbness of the feet and toes in individuals taking either d4t, ddc, or ddI in their regimen and those taking other NRTIs. The mean intensities of muscle aches, numbness of the feet/toes, and fatigue ranged from 1.7 to 2.1 for all the measurement periods on a scale of one to three. For all three symptoms, reports of severe symptom intensity were highest at baseline, decreased at the one- and three-month measurement periods then increased slightly at six months. Over time, there was a decrease in the frequency of the reports of fatigue and numbness, and a slight increase in reports of muscle aches (Table 1).
Table 1.
Demographic and Symptom Characteristics (n=240)
| Variables | Mean | SD | Range | |
|---|---|---|---|---|
| Age | 41.83 | 7.60 | 22–68 | |
| Years of Formal Education | 11.57 | 2.53 | 0–19 | |
| Years living with HIV | 8.67 | 4.71 | 0 – 23 | |
| Gender | Percent | Frequency | ||
| Female | 30.5% | 74 | ||
| Male | 65.4% | 159 | ||
| Transgender | 2.9% | 7 | ||
| Ethnicity | ||||
| African American | 72.8% | 176 | ||
| White/Anglo | 12.9% | 31 | ||
| Hispanic/Latin | 10.8% | 26 | ||
| Native American Indian | 1.5% | 3 | ||
| Asian Pacific Islander | 0.5% | 1 | ||
| Other | 1.5% | 3 | ||
| Highest Education Qualification | ||||
| Grade School | 23.9% | 58 | ||
| High School | 42.0% | 102 | ||
| Technical/Vocational | 12.3% | 30 | ||
| College/Post-graduate | 14.0% | 34 | ||
| Other | 7.8% | 19 | ||
| Have an AIDS diagnosis = Yes | 51.4% | 125 | ||
| Depression diagnosis at enrollment = Yes | 26.0% | 64 | ||
| ARV Medications* | ||||
| PI-based medication regimen = Yes | 65.0% | 113 | ||
| NNRTI-based medication regimen = Yes | 27.0% | 47 | ||
| Stavudine (d4T), or zalcitabine (ddC), or didanosine (ddI) | 62.5% | 110 | ||
|
Symptom Variables | ||||
| Month 0 | Month 1 | Month 3 | Month 6 | |
| Muscle ache no | 58% | 66% | 63% | 54% |
| Muscle ache yes | 42% | 34% | 37% | 46% |
| Numbness feet/toes no | 71% | 78% | 78% | 80% |
| Numbness feet/toes yes | 28% | 21% | 22% | 20% |
| Fatigue no | 61% | 66% | 68% | 64% |
| Fatigue yes | 39% | 34% | 32% | 36% |
|
Symptom Intensity (range = 1 to 3) Mean (SD) | ||||
| Muscle ache | 1.8 (0.80) | 1.8 (0.76) | 1.8 (0.80) | 2.0 (0.70) |
| Numbness feet/toes | 2.1 (0.83) | 2.0 (0.83)` | 1.8 (0.71) | 1.7 (0.82) |
| Fatigue | 1.8 (0.79) | 1.8 (0.70) | 1.7 (0.73) | 1.8 (0.68) |
SD = standard deviation; ARV = antiretroviral; PI = protease inhibitor; NNRTI = non-nucleoside reverse transcriptase inhibitor.
There were significant correlations between symptoms at all four data collection periods. These correlations ranged from 0.44 to 0.60 for fatigue with muscle aches; 0.20 to 0.42 for fatigue with numbness; and 0.21 to 0.34 for muscle aches with numbness (Table 2).
Table 2.
Correlations Among Symptoms for Each Time Period
| Baseline | Month 1 | Month 3 | Month 6 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fat | MA | Nb | Fat | MA | Nb | Fat | MA | Nb | Fat | MA | Nb | |
| Fatigue | 1 | 1 | 1 | 1 | ||||||||
| MA | 0.54 | 1 | 0.44 | 1 | 0.60 | 1 | 0.50 | 1 | ||||
| Nb | 0.21 | 0.27 | 1 | 0.35 | 0.28 | 1 | 0.42 | 0.34 | 1 | 0.20 | 0.21 | 1 |
Fat = fatigue; MA = muscle aches; Nb = numbness of the feet/toes.
The Single Symptom Growth Model
The initial model to assess symptom change looked at the initial estimates of fatigue and fatigue change over time without any additional variable influence (Table 2, Model A). The fatigue intercept for this model revealed that the odds of reporting fatigue was 0.80, translating into a relative risk of about 45% to report fatigue. This estimate validates that most cases at baseline reported not having fatigue as seen in Table 1. The slope of fatigue was generally flat (OR=0.97), indicating very little change over time. This finding suggests that there is consistency in the proportions of individuals reporting fatigue initially, and while the small negative slope value indicates a decrease in fatigue report over time, the proportions remained constant.
Time-Lagged Effects Change Model
To assess the lagged effect of symptom occurrence over time, and any additive effects of additional symptoms, individual models were first developed to estimate the effect of muscle aches on fatigue followed by a model estimating the effects of numbness of the feet/toes on fatigue. This was followed by the development of a combined model that estimated the effect of both symptoms simultaneously on the outcome of fatigue. In all three models, there were no significant differences in the fit indices between the constrained and unconstrained models, indicating that the constrained model represents the data well in estimating the effects of the symptoms on fatigue.
A significant relationship between muscle aches and fatigue intercepts was noted (OR=1.80, P ≤ 0.05). Significant relationships between numbness and fatigue also were noted for the entire measurement period (OR=2.70, P ≤ 0.05). In the combined model (Table 2, model B), the relationship between muscle aches and fatigue is still elevated but no longer statistically significant (OR=1.21, P > 0.05). This model also showed a continued significant effect of numbness on fatigue (OR=1.89, P ≤ 0.05). This indicated a temporal relationship between numbness and the report of fatigue in this sample over the six-month time period. No significant interactions between symptoms and time were noted, indicating that in the time-lagged symptom models, the presence of fatigue did not change as a result of the effect of time alone. While there was a negative slope change for fatigue in the combined symptoms model, the change was not significant indicating those initially reporting fatigue generally continued to do so over the six month period.
Descriptive Characteristics
Additional personal descriptive variables including years living with HIV, age, gender, having an AIDS diagnosis, race (defined as white/nonwhite), and treatment regimen with stavudine, zalactabine, or didanosine were added to the combined symptom model to assess any between-person differences that may exist either initially or over time. None of these variables were significant in explaining any additional model variation.
The multi-symptom lagged symptom model (Model B) showed significantly improved intercept variance parameters from the fatigue only symptom model (Model A) with the addition of the time-varying covariates of muscle aches and numbness of the feet and toes. The final combined model explained 28% of the variance in individual fatigue intercepts compared to the fatigue only model, indicating that the presence of the additional symptoms increases the likelihood to report fatigue. There was a change in the fit statistics of the log likelihood, AIC statistic and BIC statistic. The fit statistics of both models improved (values closer to zero) with the addition of symptoms.
Discussion
This analysis provides evidence that the multiple symptoms of muscle aches, numbness, and fatigue do affect one another in a temporal manner. The level of effect is at the minimum elevated and is generally significantly predictive for fatigue. The significant between-symptom correlations at each measurement period confirm that these symptoms are commonly reported together.
A review of adherence rates in the sample showed no differences in the PI-based and NNRTI-based regimens, eliminating adherence as a potential source of bias in the symptom occurrence analyses. Additionally, there was no difference in the reports of numbness of the feet and toes in individuals taking either d4t, ddc, or ddI in their regimen and those taking other NRTIs. While stavudine is no longer widely prescribed because of its neurotoxic effects, our analysis, using a longitudinal dataset from 2004, provided a reasonable source of data from which we could assess the role of neuropathy and its additive effects with muscle aches in fatigue occurrence. One limitation of the study was that this was a subset analysis reviewing symptoms and symptom occurrence over time for which there was no intervention to ameliorate symptoms in the design of the parent study. However, this study methodology is useful for quantitatively characterizing simultaneous symptom occurrence and its change over time.
This analysis does not fully quantify the role of a current symptom status of fatigue in predicting ongoing fatigue. In this dataset, all three symptoms reports were most frequent at baseline, decreased to the three-month measurement period then increased slightly at six months. Over time, there was a decrease in the reports of fatigue and numbness, and a slight increase in reports of muscle aches. It was determined that, once observed, symptom presence changed very little over the six-month evaluation period.
Decreased Testosterone and Associated Muscle Aches, Pain and Fatigue
As noted earlier, the physiological role of testosterone in animal studies in mice further suggest a biological link between testosterone and the pathways relating pain and fatigue in female and male mice that may help explain why more women than men are diagnosed with chronic pain and fatigue conditions like fibromyalgia and chronic fatigue syndrome. The physiological role of testosterone in the presence of HIV-related neuropathies, muscle aches, and fatigue is plausible, as there is a high prevalence of decreased testosterone levels in HIV-infected men and women. Prior studies present that 25% to 60% of HIV-infected men have serum testosterone levels in the hypogonadal range (20, 21). Earlier research has shown that HIV-positive women frequently have hormone deficiencies, including low levels of testosterone and report increased fatigue (23). Recent studies suggest benefits by use of testosterone in women. (24). Barroso and colleagues (25) 24showed a bivariate association between testosterone level and fatigue outcomes, but found no multivariate associations between physiological variables including testosterone and the outcome of fatigue. They concluded that fatigue suffered by HIV seropositive people is better predicted by other variables to successfully reduce HIV-related fatigue symptoms (25).
Implications for Practice
The results from longitudinal analyses of symptom occurrence and change over time suggest that there is evidence of symptom synergism in individuals with HIV/AIDS. These symptoms include both the symptom of fatigue and the physical symptoms of muscle aches and numbness. These findings suggest that a patient who exhibits symptoms of neuropathy/numbness and/or muscle aches will very likely report fatigue. The health care provider may want to consider strategies with the patient to reduce fatigue. Similarly, a patient reporting fatigue may also report numbness and pain or muscle aches upon further inquiry by the provider for which a plan of ongoing treatment can be developed.
The understanding of individual differences in reported symptom frequencies and intensity is important for health care providers in helping their patients manage symptoms, improve medication adherence, and other desirable outcomes. The information obtained from studies as this may help clinicians and researchers to better understand how symptoms interact upon one another. Ongoing research that identifies and describes groupings of symptoms in individuals with HIV/AIDS by determining the effects of these symptom clusters on the individual, and developing interventions to manage multiple simultaneous symptoms is needed.
Table 3.
Lagged Symptom Models
Log Odds Values for Symptom Intercept and Change Over Time (Constrained Parameter Models)
| Dependent Variable = Fatigue | Fatigue Only Model | Fatigue, Numbness, Muscle Ache Model | Guide to Coefficients in Model B |
|---|---|---|---|
| Model Parameter | A. | B. | |
| fatigue outcome Intercept (I) (Odds Ratio) | 0.80 | 0.30 | Significant variation in individual initial fatigue occurrence |
| {logit} | {−0.218} | {−1.188} | |
| (SE) | (0.194) | (0.300) | |
| [z-score] | [−1.126] | [−3.964] | |
| fatigue outcome Slope (S) (Odds Ratio) | 0.97 | 1.0 | There is a non-significant decrease (nearly 0) in the report of fatigue over the 6-month period |
| {logit} | {−0.037} | {−0.046} | |
| (SE) | (0.060) | (0.080) | |
| [z-score] | [−0.619] | [−0.576] | |
| numbness Intercept (Odds Ratio) | 1.89 | There is a significant effect of numbness on fatigue. Increasing the likelihood of reporting fatigue by 1.89 times. | |
| {logit} | {0.638} | ||
| (SE) | (0.278) | ||
| [z-score] | [2.298] | ||
| muscle ache Intercept (Odds Ratio) | 1.21 | There is a non-significant effect of muscle aches on fatigue. Increasing the likelihood of reporting fatigue by 1.21 times. | |
| {logit} | {0.189} | ||
| (SE) | (0.244) | ||
| [z-score] | [0.774] | ||
| Covariance Intercept with Slope (Odds Ratio) | 1.0 | 1.1 | An individual’s report of fatigue stayed constant over time |
| {logit} | {−0.020} | {−0.088} | |
| (SE) | (0.200) | (0.371) | |
| [z-score] | [−.102] | [−0.237] | |
| Slope (linear) | There is little variation remaining in the change patterns of individuals reporting fatigue | ||
| {logit} | {0.012} | {0.075} | |
| (SE) | (0.049) | (0.123) | |
| [z-score] | [0.242] | [0.612] | |
| Intercept | The addition of the muscle aches and numbness of the feet and toes symptoms in the model reduced the remaining variation in the initial report of fatigue to a non-significant level. The reduction explains 28% of the variation in the final model. | ||
| {logit} | {3.680} | {2.64} | |
| (SE) | (1.353) | (2.425) | |
| [z-score] | [2.717] | [1.089] | |
| Log Likelihood | −1478.58 | −1277.23 | The log likelihood fit statistic improved (values closer to zero) with the addition of symptoms. |
| AIC | 2985.14 | 2570.41 | The AIC fit statistic improved (values closer to zero) with the addition of symptoms. |
| BIC | 3033.87 | 2614.89 | The BIC fit statistics improved (values closer to zero) with the addition of symptoms. |
z score value ≥ 1.96, P≤ 0.05. logit=log odds parameter estimate; OR=odds ratio; SE=standard error; AIC = Akaike information criterion statistic; BIC = Bayesian information criterion statistic.
Acknowledgments
This data set was provided by a study funded by the National Institute of Nursing Research (R01 NR004849, W. Holzemer, Principal Investigator). Preparation of the manuscript was partially supported by P30BR010677-03, Videopodcasts for Symptom Management in People Living with HIV (D. Wantland, Principal Investigator) from the Center for Evidence- Based Practice in the Underserved of the Columbia University School of Nursing.
Footnotes
Disclosures
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ammassari A, Antinori A, Aloisi MS, et al. Depressive symptoms, neurocognitive impairment, and adherence to highly active antiretroviral therapy among HIV-infected persons. Psychosomatics. 2004;45(5):394–402. doi: 10.1176/appi.psy.45.5.394. [DOI] [PubMed] [Google Scholar]
- 2.Bonfanti P, Ricci E, Landonio S, et al. Predictors of protease inhibitor-associated adverse events. Biomed Pharmacother. 2001;55(6):321–323. doi: 10.1016/s0753-3322(01)00070-1. [DOI] [PubMed] [Google Scholar]
- 3.Catz SL, Kelly JA, Bogart LM, Benotsch EG, McAuliffe TL. Patterns, correlates, and barriers to medication adherence among persons prescribed new treatments for HIV disease. Health Psychol. 200;19(2):124–133. [PubMed] [Google Scholar]
- 4.Holzemer WL. HIV and AIDS: the symptom experience. What cell counts and viral loads won’t tell you. Am J Nurs. 2002;102(4):48–52. doi: 10.1097/00000446-200204000-00023. [DOI] [PubMed] [Google Scholar]
- 5.Vogl D, Rosenfeld B, Breitbart W, et al. Symptom prevalence, characteristics, and distress in AIDS outpatients. J Pain Symptom Manage. 1999;18(4):253–262. doi: 10.1016/s0885-3924(99)00066-4. [DOI] [PubMed] [Google Scholar]
- 6.Burgoyne RW, Rourke SB, Behrens DM, Salit IE. Long-term quality-of-life outcomes among adults living with HIV in the HAART era: the interplay of changes in clinical factors and symptom profile. AIDS Behav. 2004;8(2):151–163. doi: 10.1023/B:AIBE.0000030246.19577.fb. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham WE, Hays RD, Ettl MK, et al. The prospective effect of access to medical care on health-related quality-of-life outcomes in patients with symptomatic HIV disease. Med Care. 1998;36(3):295–306. doi: 10.1097/00005650-199803000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Davis S. Clinical sequelae affecting quality of life in the HIV-infected patient. J Assoc Nurses AIDS Care. 2004;15(5 Suppl):28S–33S. doi: 10.1177/1055329004269478. [DOI] [PubMed] [Google Scholar]
- 9.Hudson A, Kirksey K, Holzemer W. The influence of symptoms on quality of life among HIV-infected women. West J Nurs Res. 2004;26(1):9–23. doi: 10.1177/0193945903259221. discussion 24–30. [DOI] [PubMed] [Google Scholar]
- 10.Burnes LA, Kolker SJ, Danielson JF, Walder RY, Sluka KA. Enhanced muscle fatigue occurs in male but not female ASIC3 mice. Am J Physiol Regul Integr Comp Physiol. 2008;294(4):R1347–1355. doi: 10.1152/ajpregu.00687.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voss JG. Predictors and correlates of fatigue in HIV/AIDS. J Pain Symptom Manage. 2005;29(2):173–184. doi: 10.1016/j.jpainsymman.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Department of Health: Republic of South Africa. [Accessed April 2010];The South African Antiretroviral Treatment Guidelines. 2010 March 30; Available from: http://www.sanac.org.za/documents/2010%20ART%20Guideline-Short.pdf.
- 13.MacArthur 2001 –
- 14.Dodd MJ, Miaskowski C, Paul SM. Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum. 2001;28(3):465–470. [PubMed] [Google Scholar]
- 15.Ringel Y, Drossman DA. Toward a positive and comprehensive diagnosis of irritable bowel syndrome. Medscape Gastroenterology. 2000;6(2) [Google Scholar]
- 16.Amdur RL, Liberzon I. The structure of posttraumatic stress disorder symptoms in combat veterans: a confirmatory factor analysis of the impact of event scale. J Anxiety Disord. 2001;15(4):345–357. doi: 10.1016/s0887-6185(01)00068-8. [DOI] [PubMed] [Google Scholar]
- 17.Holzemer WL, Bakken S, Portillo CJ, et al. Testing a nurse-tailored HIV medication adherence intervention. Nurs Res. 2006;55(3):189–197. doi: 10.1097/00006199-200605000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Muthen L, Muthen B. Mplus user’s guide. 3. Los Angeles, CA: Muthen & Muthen; 2009. [Google Scholar]
- 19.Holzemer WL, Hudson A, Kirksey KM, Hamilton MJ, Bakken S. The revised Sign and Symptom Check-List for HIV (SSC-HIVrev) J Assoc Nurses AIDS Care. 2001;12(5):60–70. doi: 10.1016/s1055-3290(06)60263-x. [DOI] [PubMed] [Google Scholar]
- 20.Snijders T, Bosker R. Multilevel analysis: An introduction to basic and advanced multilevel modeling. Thousand Oaks, California: Sage; 1999. [Google Scholar]
- 21.Bhasin S, Bremner WJ. Emerging issues in androgen replacement therapy. J Clin Endocrinol Metab. 1997;82:3–8. doi: 10.1210/jcem.82.1.3640. [DOI] [PubMed] [Google Scholar]
- 22.Rabkin JG, Wagner GJ, Rabkin R. A double-blind, placebo-controlled trial of testosterone therapy for hiv-positive men with hypogonadal symptoms. Arch Gen Psychiatry. 2000;57(2):141–147. doi: 10.1001/archpsyc.57.2.141. [DOI] [PubMed] [Google Scholar]
- 23.Miller K, Corcoran C, Armstrong C, et al. Transdermal testosterone administration in women with acquired immunodeficiency syndrome wasting: a pilot study. The Journal of Clinical Endocrinology & Metabolism. 1998;83(8) doi: 10.1210/jcem.83.8.5051. [DOI] [PubMed] [Google Scholar]
- 24.Dolan-Looby SE, Collins M, Lee H, Grinspoon S. Effects of long-term testosterone administration in HIV-infected women: a randomized, placebo-controlled trial. AIDS. 2009;23:951–959. doi: 10.1097/QAD.0b013e3283299145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barroso J, Pence BW, Salahuddin N, Harmon JL, Leserman J. Physiological correlates of HIV-related fatigue. Clin Nurs Res. 2008;17(1):5–19. doi: 10.1177/1054773807311382. [DOI] [PMC free article] [PubMed] [Google Scholar]