Summary
Objective
A relationship between T1ρ relaxation time and glycosaminoglycan (GAG) content has been demonstrated in chemically degraded bovine cartilage, but has not been demonstrated with quantitative biochemistry in human cartilage. A relationship has also been established between T2 relaxation time in cartilage and osteoarthritis severity. We hypothesized that T1ρ relaxation time would be associated with GAG content in human cartilage with normal T2 relaxation times.
Methods
T2 relaxation time, T1ρ relaxation time, and glycosaminoglycan as a percentage of wet weight (sGAG) were measured for top and bottom regions at seven anatomical locations in twenty-one human cadaver patellae. For our analysis, T2 relaxation time was classified as normal or elevated based on a threshold defined by the mean plus one standard deviation of the T2 relaxation time for all samples.
Results
In the normal T2 relaxation time subset, T1ρ relaxation time correlated with sGAG in the full-thickness and bottom regions, but only marginally in the top region alone. sGAG decreased significantly with age in all regions.
Conclusion
In the subset of cartilage specimens with normal T2 relaxation time, T1ρ relaxation time was inversely associated with sGAG content, as hypothesized. A predictive model, which accounts for T2 relaxation time and the effects of age, might be able to determine longitudinal trends in GAG content in the same person based on T1ρ relaxation time maps.
Keywords: cartilage, glycosaminoglycan, T1ρ MRI, T2 MRI
Introduction
Knee osteoarthritis (OA) is a debilitating disease that causes pain and limits mobility; cartilage degeneration is a major aspect of the disease process. At least 12% of U.S. adults over age 60 have symptomatic knee OA [1], and this percentage is growing due to an aging baby boomer generation, increased life expectancy and rising rates of obesity [2, 3]. Cartilage has a zonal architecture with collagen orientation and content and glycosaminoglycan (GAG) content changing through the depth. Early in the OA disease process, GAG concentration decreases, especially in the superficial layer, and as OA progresses, collagen orientation changes and collagen content decreases [4–7]. GAG content in cartilage decreases with increasing age [8], and the incidence of OA increases with increasing age [9]. The development of non-invasive early detection methods is critical for assessing knee OA progression and monitoring prevention and treatment strategies.
Magnetic Resonance Imaging (MRI) can document age-related changes in knee joints and has promise as a non-invasive modality for the detection of early OA [10]. MRI can show changes in all of the joint tissues affected by OA, including osteophytes and bone marrow edema [11]. Many MRI methods have been proposed for early detection of changes in cartilage macromolecular content due to osteoarthritis, including dGEMRIC, sodium, T2 and T1ρ MRI [12–15].
T2 relaxation time is used to identify joint changes associated with OA. T2 relaxation time has been shown to increase focally in the radial zone of cartilage in patients with symptomatic OA [16]. In ex vivo cartilage specimens, T2 relaxation time was increased significantly with cartilage degeneration, and T2 relaxation time, in cartilage classified as moderate OA, was greater than T2 relaxation time in healthy cartilage [17, 18]. T2-weighted signal has also been shown to indicate osteoarthritis severity [12, 19], and T2 relaxation time to distinguish between radiographically healthy and OA knee joints [20].
When measuring T2 relaxation time in cartilage, care needs to be taken to account for the magic angle effect. The magic angle effect occurs when imaging structures with highly aligned constituents, such as collagen fibrils in cartilage. MR signal strength and T2 relaxation time change depending on the orientation of the aligned collagen fibrils with respect to the main magnetic field (B0) [21, 22]. In a study using MRI and polarized light microscopy, approximately 40% of depth-wise variation in T2 relaxation time was attributed to collagen fiber anisotropy [23]. Fibrillation in the radial zone, a decrease in anisotropy, has been shown to cause T2 relaxation time elevation [24].
T1ρ relaxation time is sensitive to protons on large macromolecules such as GAG; thus a direct relationship between T1ρ relaxation time and GAG concentration is expected, but has not been shown in human cartilage. Duvvuri et al. hypothesized that as fewer GAGs interact with fewer free water protons, T1ρ relaxation time would increase [13]. As expected, T1ρ relaxation time increased with decreasing GAG content in bovine cartilage following enzymatic degradation [13, 25–27].
Previous human cartilage studies using specimens from total knee replacement patients found no correlation between GAG content (measured using histology) and T1ρ relaxation time [28–29]. T1ρ relaxation time could distinguish early OA from moderate and severe OA better than T2 relaxation time in ex vivo cartilage from total knee replacements, but T1ρ was not compared to GAG content using a quantitative biochemical technique [30]. Cartilage obtained from total knee replacements may be at a late stage of the OA disease process and therefore may not have the expected inverse correlation between T1ρ relaxation time and GAG content.
The relationship between T1ρ relaxation time and GAG content in human cartilage may be more accurately assessed with quantitative cartilage biochemistry. Recent editorials call for a thorough study of the T1ρ method and GAG content in human cartilage [31, 32] similar to the dGEMRIC method study by Bashir et al., which used biochemistry to measure GAG content [14]. If T1ρ relaxation time is correlated with GAG content in human cartilage, early detection of OA through a non-invasive, non-contrast-agent method may be possible.
The purpose of this study was to quantitatively compare T1ρ relaxation time and GAG content, considering macromolecular changes through the cartilage depth, while accounting for subject age and T2 relaxation time. Elevated T2 relaxation time has been shown to be a marker for OA changes; however, we wanted to test whether T1ρ relaxation time could detect GAG content changes in cartilage with normal T2 relaxation time values. We hypothesized that T1ρ relaxation time would be associated with GAG content in human cartilage with normal T2 relaxation times.
Methods
Specimen Preparation
Human cadaver fresh-frozen knee joints (mid-femur to mid-tibia) were obtained from the National Disease Research Interchange (Philadelphia, PA), Anatomy Gifts Registry (Glen Burnie, MD) and the University of California San Francisco Willed Body Program (San Francisco, CA). Twenty-one patellae were obtained from thirteen males and eight females ranging in age from 20 to 90 years old (median age 66 years old). Eleven specimens were from left knees and ten from right knees. Healthy patellae and patellae with varied degrees of degeneration were accepted for the study; any patellae regions with full-thickness defects were excluded from the study.
The patellae were dissected from the knee joint and some of the bone was removed with a bandsaw to create a flat subchondral-bone surface that was fixed to an acrylic plate using ethyl-2-cyanoacrylate adhesive (Krazy Glue, New York, NY). The acrylic plate was 5 cm square with two intersecting channels machined into the base. Between MRI studies and biochemical analysis, the specimens were stored on the plates surrounded by gauze soaked in PBS and protease inhibitors at −20°C. Specimens were brought to room temperature prior to MRI and biochemical analysis.
MR Imaging
For the MRI studies, the plate-mounted specimen was placed in a secondary container, which was filled with phosphate buffered saline (PBS) containing protease inhibitors [33] (Fig. 1). The channels filled with PBS were bright in MR images due to the long T2 relaxation time of PBS and served as reference markers for image registration.
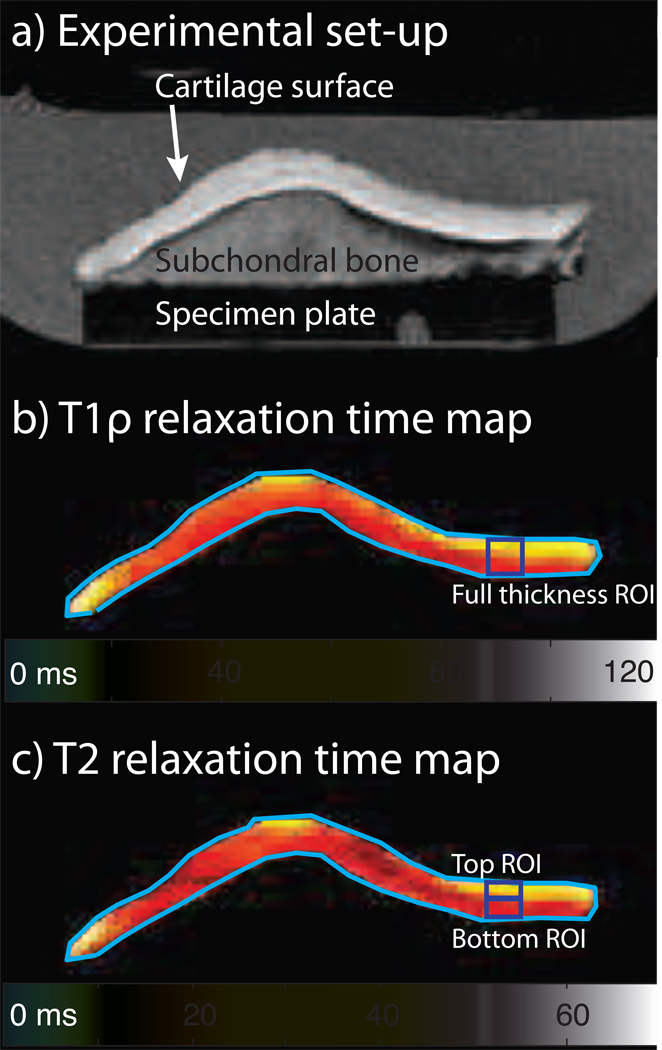
Figure 1.
Patella specimens were imaged at 3 T using multi-slice, multi-echo 2D spiral sequences, and T1ρ and T2 relaxation time maps were computed using OsiriX. An axial image from one patella specimen mounted on the acrylic plate in the PBS bath is shown (1a); the main magnetic field, B0, is out of the image plane. Representative T1ρ and T2 relaxation time maps are shown (1b, 1c).
MR imaging at 3 T was performed using a GE HDx system (GE Healthcare, Milwaukee, WI) with a transmit/receive quadrature wrist coil (Mayo Clinic Medical Devices, Rochester, MN). The patella was oriented with the normal to the subchondral bone surface perpendicular to B0; subchondral bone was used as a surrogate for collagen fiber orientation, which was not measured in this study. The image plane was also oriented perpendicular to B0. A multi-slice, multi-echo spiral 2D sequence was used to acquire T1ρ (spin locking frequency 500 Hz) [34] and T2 images [35] with 3.0 mm slice thickness, 0 mm slice spacing, 10 cm field of view, 0.3 mm in-plane pixel size, a 2 s TR and five echo/spin-lock times: 7, 21, 36, 65 and 124 ms. To determine the T2 relaxation time, the fifth echo was not used because the signal in the cartilage was not significantly different from the noise (T2 sequence fifth echo average SNR = 1.4). T1ρ and T2 relaxation times were obtained using OsiriX [36].
Biochemistry
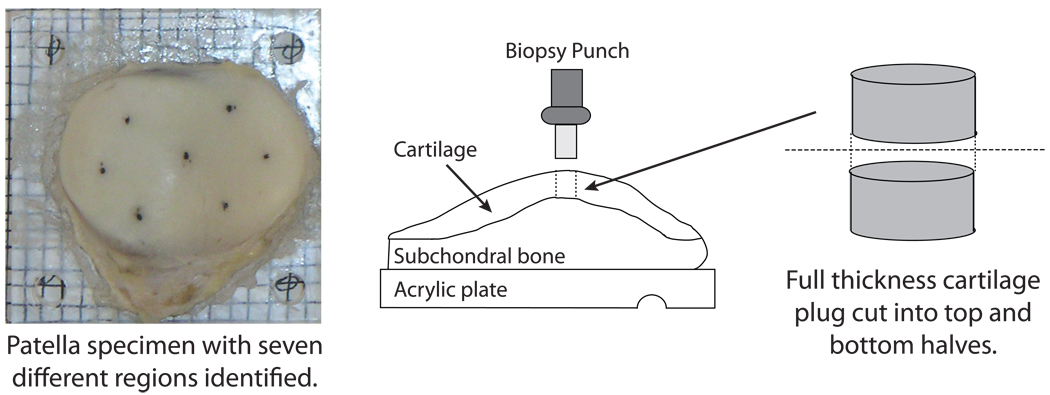
For biochemical analyses, the specimen mounted on the acrylic plate was placed on a 3 mm × 3 mm grid that matched the MR slice locations. Full-thickness plugs (3 mm diameter) of cartilage were removed from seven locations across the surface of the patella (Fig. 2); if any subchondral bone was present on the bottom of the plug, it was removed with a scalpel. The plugs were then cut in half to allow for an examination of differences between top and bottom regions. Note that the top and bottom samples do not correspond exactly to the superficial, intermediate and deep zones of cartilage. Results are reported for the top, bottom, and full-thickness (weighted-average of top and bottom into a single value) regions.
Figure 2.
Glycosaminoglycan content per wet weight (sGAG content) was measured biochemically for top and bottom half regions of human cadaver patellae and correlated with T1ρ relaxation time and age. The top view of a patella specimen mounted on an acrylic plate (left) and side view of the patellae and schematic of the plug used for biochemistry (right).
Each sample was weighed, dried at 50°C for 12 h and weighed again to obtain wet and dry weights. Each sample was digested in 1 ml papain solution overnight at 63°C and stored at 4°C. Total GAG content was quantified using the dimethylmethylene blue assay, which measures sulfated GAG using chondroitin sulfate as a standard [37]. Sulfated glycosaminoglycan content as a percentage of wet weight (sGAG) was calculated.
Data Collection and Analysis
Seven regions across the surface of each patella were examined: center, lateral center, lateral inferior, lateral superior, medial center, medial inferior and medial superior. These were similar to the regions used by Lammentausta et al. [18] with the addition of the central region. Seven regions on 21 patellae resulted in 147 potential data points.
MRI regions of interest (ROI) were determined at the location corresponding to the biochemical measurement. In the plane of the 3.0 mm MRI slice, the ROI was approximately 3 mm wide and a minimum of 0.6 mm (2 pixels) tall; the height of the ROI varied with specimen thickness.
Data points were excluded due to imaging artifact, full-thickness defect or thin cartilage (fewer than four pixels through the depth of the cartilage), and potential magic angle interference [21, 22]. Thirty-four data points were excluded due to a bubble or other image artifact, full-thickness defect or thin cartilage. Two patellae (14 data points) and nine additional data points were excluded due to potential magic angle interference. When these patellae were viewed in the sagittal plane, the plane parallel to B0, large curvature of the subchondral bone surface resulted in an orientation angle greater than 10° from perpendicular to B0 and possibly introduced magic angle interference. Of the original, possible 147 data points, 95 data points for the top region, 93 data points for the bottom region, and 90 data points for the full-thickness were included in the data analysis.
Analyses were performed on all specimens and on the subset of specimens with normal T2 relaxation times. The mean T2 relaxation time plus one standard deviation, specific to each region, was used as a threshold to define specimens with normal and elevated T2 relaxation times. The T2 relaxation time threshold was 36 ms for the bottom, 43 ms for the top and 38 ms for the full-thickness regions. Bottom, top and full-thickness data points were analyzed separately; then, top and bottom data points were combined for analysis.
The effects of T1ρ and T2 relaxation times and age on sGAG were assessed by univariate and multivariate mixed effects regression, with knee nested within cadaver as random effects. Regression R2 was calculated by squaring the linear correlation between the model predictions and observed values. Correlations among T1ρ and T2 relaxation times and age were adjusted for clustering within knee. A p-value less than 0.05 was taken to be statistically significant. Statistical analyses were performed using Stata Release 9.2 (StataCorp LP, College Station, TX) and the statistical package R for Mac 2.8.0 (r-project.org).
Results
Correlations of T1ρ and T2 relaxation times, and Age with sGAG
In normal T2 relaxation time specimens, with or without adjusting for the effects of age, T1ρ relaxation time (ms) correlated with sGAG content (% wet weight) in the full-thickness and bottom regions, but only marginally in the top region alone (Table 1 and Fig. 3). The data varied from specimen to specimen, but the same trend was found across all specimens (Fig. 4). sGAG content decreased significantly with age in all regions.
Table 1.
Univariate and multivariate mixed effects regression of glycosaminoglycan content as a percentage of wet weight (sGAG) with T1ρ relaxation time and age for the normal T2 relaxation time subset. Normal T2 relaxation time is defined to be T2 relaxation time less than the mean plus one standard deviation of the T2 relaxation time for all samples. The regression coefficients are given; the p-value for each regression coefficient is given in the footnote. Regression R2 was calculated by squaring the linear correlation between model predictions and observed values.
| Table 1. Regression Coefficients on sGAG for Normal T2 Specimens | ||||||||
|---|---|---|---|---|---|---|---|---|
| Samples (N) | Threshold T2 value (ms) |
Univariate sGAG analysis | Multivariate sGAG analysis | |||||
| Age | R2 | T1ρ | R2 | Age | T1ρ | R2 | ||
| Bottom region (69) | 36 | −0.03** | 0.57 | −0.04*** | 0.68 | −0.03** | −0.04*** | 0.66 |
| Top region (73) | 43 | −0.03** | 0.67 | −0.02* | 0.71 | −0.03** | −0.01^ | 0.70 |
| Full thickness (67) | 38 | −0.03*** | 0.69 | −0.03*** | 0.83 | −0.02** | −0.03*** | 0.83 |
| Top & Bottom (142) | -- | −0.03*** | 0.41 | −0.05*** | 0.64 | −0.03** | −0.05*** | 0.63 |
p<0.05
p<0.01
p<0.001
p=0.063
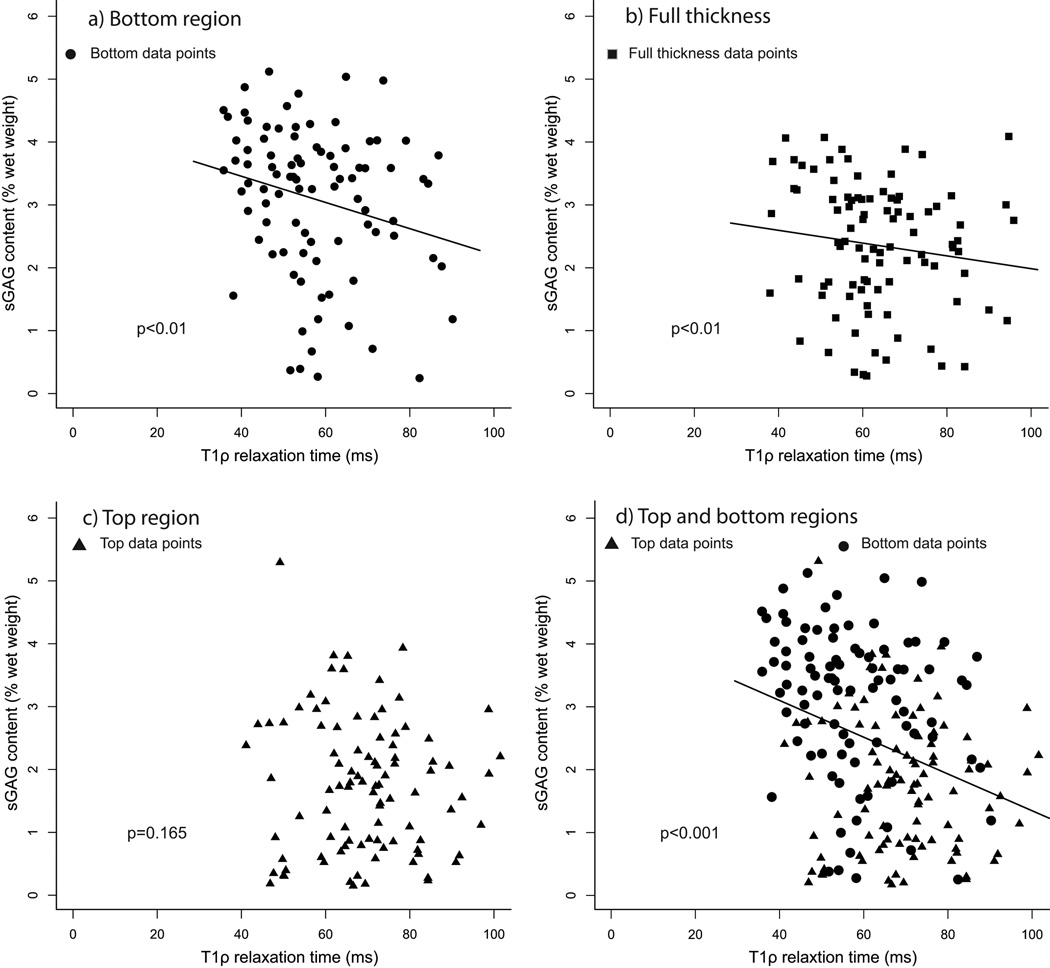
Figure 3.
Glycosaminoglycan content as a percentage of wet weight (sGAG) vs T1ρ relaxation time plots for the normal T2 relaxation time subset: Normal T2 relaxation time is defined to be T2 relaxation time less than the mean plus one standard deviation of the T2 relaxation time for all samples. The reported p-values are from the mixed effects model. In the bottom region (a), there is a relationship between sGAG content and T1ρ relaxation time. When bottom and top region data points are aggregated to obtain full-thickness values (b), there is a relationship between sGAG content and T1ρ relaxation time. In the top region (c), there is a moderate relationship between sGAG content and T1ρ relaxation time. If top & bottom region data points are analyzed together (d), there is a correlation between sGAG content and T1ρ relaxation time.
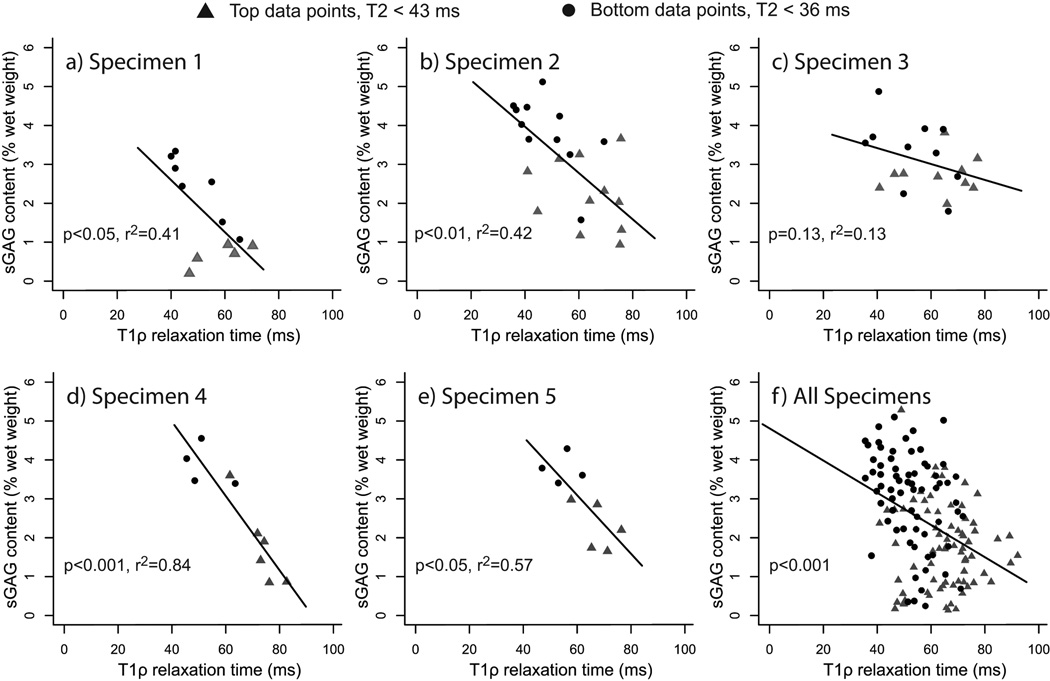
Figure 4.
Glycosaminoglycan content as a percentage of wet weight (sGAG) vs T1ρ relaxation time plots for the top and bottom samples of the normal T2 relaxation time subset: Normal T2 relaxation time is defined to be T2 relaxation time less than the mean plus one standard deviation of the T2 relaxation time for all samples. The data for five specimens, representative of the whole data set, are shown (a–e) along with the data for all normal T2 relaxation time specimens (f). The p-values and regression coefficients are determined for each specimen (a–e). For the entire normal T2 relaxation time data set (f), the p-value is determined from the mixed effects model. The specimen plots illustrate the variability in the data; four specimens have significant correlations (a,b,d,e) and the other follows the same trend (c).
Similar patterns were found in all specimens (normal T2 relaxation time plus elevated T2 relaxation time; Fig. 5), with one exception (Table 2). For all specimens, T1ρ relaxation time was not correlated with sGAG content in the top region.
Figure 5.
Glycosaminoglycan content as a percentage of wet weight (sGAG) vs T1ρ relaxation time plots for all data points: The reported p-values are from the mixed effects model. When all bottom data points are included (a), there is a moderate correlation between sGAG content and T1ρ relaxation time. When bottom and top data points are aggregated to obtain full-thickness values (b), there is a relationship between sGAG content and T1ρ relaxation time. For the top region, there is no relationship between sGAG content and T1ρ relaxation time for all data points (c). If top & bottom half data points are analyzed together (d), there is a correlation between sGAG content and T1ρ relaxation time.
Table 2.
Univariate and multivariate mixed effects regression of glycosaminoglycan content as a percentage of wet weight (sGAG) with T1ρ relaxation time and age for all data points. The regression coefficients are given; the p-value for each regression coefficient is given in the footnote. Regression R2 was calculated by squaring the linear correlation between model predictions and observed values.
| Table 2. Regression Coefficients on sGAG for All Specimens | |||||||
|---|---|---|---|---|---|---|---|
| Samples (N) | Univariate sGAG analysis | Multivariate sGAG analysis | |||||
| Age | R2 | T1ρ | R2 | Age | T1ρ | R2 | |
| Bottom region (93) | −0.02*** | 0.39 | −0.02** | 0.50 | −0.02** | −0.02** | 0.47 |
| Top region (95) | −0.03*** | 0.64 | −0.01# | 0.66 | −0.03*** | −0.01^ | 0.65 |
| Full thickness (90) | −0.02*** | 0.59 | −0.02*** | 0.68 | −0.02** | −0.02** | 0.67 |
| Top & Bottom (188) | −0.03*** | 0.37 | −0.03*** | 0.53 | −0.02** | −0.04*** | 0.52 |
p<0.01
p<0.001
p=0.165
p=0.268
In normal T2 relaxation time specimens, with or without adjusting for the effects of age, T2 relaxation time (ms) did not significantly correlate with sGAG content except when both top and bottom regions were pooled (Table 3). This effect was due to a difference in ranges of T2 relaxation time values in the top and bottom regions, as illustrated in Fig. 6 for all specimens. A similar pattern was found when analyzing all specimens together (normal T2 relaxation time plus elevated T2 relaxation time; Table 4).
Table 3.
Univariate and multivariate mixed effects regression of glycosaminoglycan content as a percenter of wet weight (sGAG) with T2 relaxation time and age for the normal T2 relaxation time subset. Normal T2 relaxation time is defined to be T2 relaxation time less than the mean plus one standard deviation of the T2 relaxation time for all samples. The regression coefficients are given; the p-value for each regression coefficient is given in the footnote. Regression R2 was calculated by squaring the linear correlation between model predictions and observed values.
| Table 3. Regression Coefficients on sGAG for Normal T2 Specimens | ||||||||
|---|---|---|---|---|---|---|---|---|
| Samples (N) | Threshold T2 value (ms) |
Univariate sGAG analysis | Multivariate sGAG analysis | |||||
| Age | R2 | T2 | R2 | Age | T2 | R2 | ||
| Bottom region (69) | 36 | −0.03*** | 0.57 | −0.03^ | - | −0.03** | −0.03^ | 0.58 |
| Top region (73) | 43 | −0.03*** | 0.67 | −0.03# | - | −0.03** | −0.02# | 0.68 |
| Full thickness (67) | 38 | −0.03*** | 0.69 | −0.02^ | - | −0.03*** | −0.02^ | 0.70 |
| Top & Bottom (142) | -- | −0.03*** | 0.41 | −0.08*** | 0.58 | −0.03** | −0.08*** | 0.58 |
p<0.01
p<0.001
p>0.10
p>0.20
Figure 6.
Glycosaminoglycan content as a percentage of wet weight (sGAG) vs T2 relaxation time linear regression plot for all top and bottom region data points: When only the top region data points are analyzed, there is no relationship between sGAG content and T2 (grey triangles). When only the bottom region data points are analyzed, there is no relationship between sGAG content and T2 (black circles). When the top and bottom region data points are analyzed together, there is a correlation between sGAG content and T2 due to the difference in ranges of values in the two subgroups.
Table 4.
Univariate and multivariate mixed effects regression of glycosaminoglycan content as a percentage of wet weight (sGAG) with T2 relaxation time and age for all data points. The regression coefficients are given; the p-value for each regression coefficient is given in the footnote. Regression R2 was calculated by squaring the linear correlation between model predictions and observed values.
| Table 4. Regression Coefficients on sGAG for All Specimens | |||||||
|---|---|---|---|---|---|---|---|
| Samples (N) | Univariate sGAG analysis | Multivariate sGAG analysis | |||||
| Age | R2 | T2 | R2 | Age | T2 | R2 | |
| Bottom region (93) | −0.02*** | 0.39 | −0.00# | - | −0.02*** | 0.00# | 0.39 |
| Top region (95) | −0.03*** | 0.64 | 0.00# | - | −0.03*** | 0.00# | 0.64 |
| Full thickness (90) | −0.02*** | 0.59 | 0.00# | - | −0.02*** | −0.00# | 0.59 |
| Top & Bottom (188) | −0.03*** | 0.37 | −0.04*** | 0.44 | −0.03** | −0.03*** | 0.43 |
p<0.60
p<0.01
p<0.001
Correlations among T1ρ and T2 relaxation times, and Age
T1ρ and T2 relaxation times were moderately correlated for all included data points, but neither was correlated with age (Tables 5 and 6).
Table 5.
Linear regressions between T1ρ and T2, T1ρ and age and T2 and age for the normal T2 relaxation time subset. Normal T2 relaxation time is defined to be T2 relaxation time less than the mean plus one standard deviation of the T2 relaxation time for all samples. The correlation coefficient (R2) and p-value are given.
| Table 5. Intercorrelations among T1ρ, T2 and Age for Normal T2 Specimens | |||||||
|---|---|---|---|---|---|---|---|
| Samples (N) | Threshold T2 value (ms) |
T1ρ – T2 Correlation | Age – T1ρ Correlation | Age – T2 Correlation | |||
| R2 | p-value | R2 | p-value | R2 | p-value | ||
| Bottom region (69) | 36 | 0.33 | <0.0001 | 0.01 | 0.39 | 0.01 | 0.22 |
| Top region (73) | 43 | 0.24 | 0.0005 | 0.00 | 0.91 | 0.00 | 0.92 |
| Full thickness (67) | 38 | 0.28 | 0.0014 | 0.01 | 0.31 | 0.01 | 0.38 |
| Top & Bottom (142) | -- | 0.44 | <0.0001 | 0.00 | 0.78 | 0.00 | 0.78 |
Table 6.
Linear regressions between T1ρ and T2, T1ρ and age and T2 and age for all data points. The correlation coefficient (R2) and p-value are given.
| Table 6. Intercorrelations among T1ρ, T2 and Age for All Specimens | ||||||
|---|---|---|---|---|---|---|
| Samples (N) | T1ρ – T2 Correlation | Age – T1ρ Correlation | Age – T2 Correlation | |||
| R2 | p-value | R2 | p-value | R2 | p-value | |
| Bottom region (93) | 0.57 | <0.0001 | 0.01 | 0.39 | 0.00 | 0.53 |
| Top region (95) | 0.50 | <0.0001 | 0.02 | 0.18 | 0.01 | 0.24 |
| Full thickness (90) | 0.54 | <0.0001 | 0.02 | 0.26 | 0.02 | 0.15 |
| Top & Bottom (188) | 0.60 | <0.0001 | 0.01 | 0.24 | 0.01 | 0.25 |
Discussion
We hypothesized that T1ρ relaxation time would be associated with glycosaminoglycan (sGAG) content in human cartilage that had normal T2 relaxation times. We found an increase in T1ρ relaxation time with decreasing sGAG content in our specimens with normal T2 relaxation times, supporting our hypothesis. Except for the top region, T1ρ relaxation time also increased with decreasing sGAG content in specimens with all T2 relaxation times. The relationship between T1ρ relaxation time and sGAG content when all T2 relaxation times were included was similar to that for the normal T2 relaxation time subset; which is likely due to the similar ranges of T1ρ relaxation time and sGAG content in the two groups. Given the variability of the data, one can draw conclusions only from the trends of the data. sGAG content decreased with age in both the normal T2 relaxation time subset and all T2 relaxation times data set in agreement with previous results [8]. Additionally, T2 and T1ρ relaxation times were moderately correlated in this study (Tables 5 and 6), in agreement with the results of Taylor et al. [38].
In our study, the T1ρ relaxation time interaction with sGAG content differed between the top and bottom regions of the cartilage. The relationship between sGAG content and T1ρ relaxation time in the top region was moderate in normal T2 relaxation time specimens, but did not exist for all T2 relaxation time specimens. In both the normal T2 relaxation time and all T2 relaxation time data sets, there was a statistically significant correlation in the bottom region. Without separating the cartilage into top and bottom regions, the depth-wise variation of the relationship between T1ρ relaxation time and sGAG content would not have been identified.
Biochemical analysis of sGAG requires aggregation of the sGAG content into discrete points through the depth, but histology, where the observation is continuous, can be compared to MRI through the depth. We were able to separate information through the depth into two discrete sections, but we were limited to only two cartilage sections by both the biochemistry and MR image resolution. A quantitative histology method, such as normalized carbohydrate region absorption could overcome this limitation [39]. In the biochemical method, wet sample mass equivalent to 4 mg or greater was required to have confidence in the measurement; we could not guarantee a sample mass greater than 4 mg with more than two sections through the thickness. In the image analysis, to avoid partial-volume averaging at the cartilage surface or the subchondral boundary, we could not guarantee more than two pixels through the thickness in each region with more than two sections. Given this limitation, we found the relationship between sGAG content and T1ρ relaxation time varied for top and bottom regions.
T2 and T1ρ relaxation time did not increase with age as reported previously [16], which may be a result of the age distribution of our samples. Most of our knee specimens were either young (< 30 years old, n=7) or old (> 60 years old, n=11), with few specimens (n=3) within the 30 to 60 year age range. Our specimen pool does not necessarily represent the 30 to 60 year age range population; this could be a limitation in understanding changes in T2 and T1ρ relaxation times with age.
Our model used T2 and T1ρ relaxation times, both of which can be altered by the magic angle effect. In this study, care was taken to exclude all samples possibly affected by the magic angle effect. The magic angle effect would increase T2 relaxation time, which could incorrectly cause a data point to be evaluated in the all T2 relaxation time dataset.
Unfortunately, specimen history of joint disease, joint pain, or clinical measures of OA was not available; such information might have aided in our interpretation of the results. Instead, T2 relaxation time was used as a surrogate for classifying the degenerative state associated with OA. A direct, clinical measure of OA progression could add confidence to our T2 relaxation time classification. The mean T2 relaxation times for the normal T2 relaxation time subset was 27 ms for the bottom, 35 ms for the top and 32 ms for the full-thickness regions. These T2 relaxation times were similar to the mean T2 relaxation times for healthy cartilage (35 ms) reported for human cadaver specimens [17].
T1ρ and T2 relaxation times are promising indicators for non-invasive clinical measures of osteoarthritis. In vivo T2 relaxation time increases with OA severity [20], and in vivo T2 relaxation time is substantially less affected by cartilage orientation compared to ex vivo studies [40]. This study shows that, in cartilage regions with normal T2 relaxation time, T1ρ relaxation time is inversely proportional to sGAG content. Although statistically significant, these findings alone do not indicate practical application of T1ρ relaxation time to predict GAG content in a clinical setting. Adjusting for T2 relaxation time and the effects of age, a predictive model might be able to determine longitudinal trends in GAG content in the same person based on T1ρ relaxation time maps. With such a model, T1ρ relaxation time maps might be able to evaluate changes in GAG content in vivo prior to the development of OA.
Acknowledgements
The authors wish to acknowledge Derek Lindsey (Department of Veterans Affairs, Rehabilitation R&D Center, Palo Alto, CA) and Dr. Weitian Chen (Global Applied Science Laboratory, Menlo Park, CA) for technical support. The authors acknowledge financial support from the following sources: NIH EB002524; NIH EB005790; GE Heathcare; Bio-X Fellowship; Department of Veterans Affairs, Rehabilitation R&D Service grant #A2592R and equipment resources from GE Healthcare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement:
Drs. Gold and Pauly receive research support from GE Healthcare. Mr. Han is an employee of GE Healthcare.
Author Contributions:
Drs. Besier, Delp and Beaupre contributed to the conception and design and obtaining of funds for this project. Drs. Pauly and Smith, along with Mr. Han, contributed technical support for the acquisition and analysis of data. Dr. Rosenberg contributed statistical expertise for the interpretation of data. Dr. Gold and Ms. Keenan made significant contributions from conception and design through drafting, critical revision and final approval of the manuscript. All authors critically revised the article and approved the final version to be submitted. Dr. Gold and Ms. Keenan take responsibility for the integrity of the work and can be contacted at gold@stanford.edu and kek@stanford.edu respectively.
References
- 1.Dillon CF, Rasch EK, Gu QP, Hirsch R. Prevalence of knee osteoarthritis in the United States: Arthritis data from the Third National Health and Nutrition Examination Survey 1991–94. Journal of Rheumatology. 2006;33:2271–2279. [PubMed] [Google Scholar]
- 2.Clarfield AM. Teaching public health related to the elderly. Public Health Reviews. 2002;30:271–276. [PubMed] [Google Scholar]
- 3.Elders MJ. The increasing impact of arthritis on public health. Journal of Rheumatology. 2000;27:6–8. [PubMed] [Google Scholar]
- 4.Guilak F, Ratcliffe A, Lane N, Rosenwasser MP, Mow VC. Mechanical and biochemical changes in the superficial zone of articular cartilage in canine experimental osteoarthritis. Journal of Orthopaedic Research. 1994;12:474–484. doi: 10.1002/jor.1100120404. [DOI] [PubMed] [Google Scholar]
- 5.Arokoski JP, Jurvelin JS, Väätäinen U, Helminen HJ. Normal and pathological adaptations of articular cartilage to joint loading. Scandinavian Journal of Medicine & Science in Sports. 2000;10:186–198. doi: 10.1034/j.1600-0838.2000.010004186.x. [DOI] [PubMed] [Google Scholar]
- 6.Bi X, Yang X, Bostrom MP, Camacho NP. Fourier transform infrared imaging spectroscopy investigations in the pathogenesis and repair of cartilage. Biochimica et Biophysica Acta. 2006;1758:934–941. doi: 10.1016/j.bbamem.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Buckwalter JA, Mankin HJ. Articular cartilage .2. Degeneration and osteoarthrosis, repair, regeneration, and transplantation. Journal of Bone and Joint Surgery-American Volume. 1997;79A:612–632. [Google Scholar]
- 8.Elliott RJ, Gardner DL. Changes with age in the glycosaminoglycans of human articular cartilage. Annals of the Rheumatic Diseases. 1979;38:371–377. doi: 10.1136/ard.38.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felson DT, Naimark A, Anderson J, Kazis L, Castelli W, Meenan RF. The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis & Rheumatism. 1987;30:914–918. doi: 10.1002/art.1780300811. [DOI] [PubMed] [Google Scholar]
- 10.Burstein D, Hunter DJ. "Why aren't we there yet?" Re-examining standard paradigms in imaging of OA: summary of the 2nd annual workshop on imaging based measures of osteoarthritis. Osteoarthritis & Cartilage. 2009;17:571–578. doi: 10.1016/j.joca.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Felson DT, McLaughlin S, Goggins J, LaValley MP, Gale ME, Totterman S, et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med. 2003;139:330–336. doi: 10.7326/0003-4819-139-5_part_1-200309020-00008. [DOI] [PubMed] [Google Scholar]
- 12.De Smet AA, Monu JU, Fisher DR, Keene JS, Graf BK. Signs of patellar chondromalacia on sagittal T2-weighted magnetic resonance imaging. Skeletal Radiology. 1992;21:103–105. doi: 10.1007/BF00241832. [DOI] [PubMed] [Google Scholar]
- 13.Duvvuri U, Reddy R, Patel SD, Kaufman JH, Kneeland JB, Leigh JS. T1rho-relaxation in articular cartilage: effects of enzymatic degradation. Magnetic Resonance in Medicine. 1997;38:863–867. doi: 10.1002/mrm.1910380602. [DOI] [PubMed] [Google Scholar]
- 14.Bashir A, Gray ML, Hartke J, Burstein D. Nondestructive imaging of human cartilage glycosaminoglycan concentration by MRI. Magnetic Resonance in Medicine. 1999;41:857–865. doi: 10.1002/(sici)1522-2594(199905)41:5<857::aid-mrm1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 15.Shapiro EM, Borthakur A, Dandora R, Kriss A, Leigh JS, Reddy R. Sodium visibility and quantitation in intact bovine articular cartilage using high field (23)Na MRI and MRS. Journal of Magnetic Resonance. 2000;142:24–31. doi: 10.1006/jmre.1999.1932. [DOI] [PubMed] [Google Scholar]
- 16.Mosher TJ, Dardzinski BJ, Smith MB. Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2--preliminary findings at 3 T. Radiology. 2000;214:259–266. doi: 10.1148/radiology.214.1.r00ja15259. [DOI] [PubMed] [Google Scholar]
- 17.David-Vaudey E, Ghosh S, Ries M, Majumdar S. T2 relaxation time measurements in osteoarthritis. Magnetic Resonance Imaging. 2004;22:673–682. doi: 10.1016/j.mri.2004.01.071. [DOI] [PubMed] [Google Scholar]
- 18.Lammentausta E, Kiviranta P, Töyräs J, Hyttinen MM, Kiviranta I, Nieminen MT, et al. Quantitative MRI of parallel changes of articular cartilage and underlying trabecular bone in degeneration. Osteoarthritis & Cartilage. 2007;15:1149–1157. doi: 10.1016/j.joca.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Brown TR, Quinn SF. Evaluation of chondromalacia of the patellofemoral compartment with axial magnetic resonance imaging. Skeletal Radiology. 1993;22:325–328. doi: 10.1007/BF00198391. [DOI] [PubMed] [Google Scholar]
- 20.Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology. 2004;232:592–598. doi: 10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gründer W, Wagner M, Werner A. MR-microscopic visualization of anisotropic internal cartilage structures using the magic angle technique. Magnetic Resonance in Medicine. 1998;39:376–382. doi: 10.1002/mrm.1910390307. [DOI] [PubMed] [Google Scholar]
- 22.Xia Y, Moody JB, Burton-Wurster N, Lust G. Quantitative in situ correlation between microscopic MRI and polarized light microscopy studies of articular cartilage. Osteoarthritis & Cartilage. 2001;9:393–406. doi: 10.1053/joca.2000.0405. [DOI] [PubMed] [Google Scholar]
- 23.Nissi MJ, Rieppo J, Töyräs J, Laasanen MS, Kiviranta I, Jurvelin JS, et al. T2 relaxation time mapping reveals age- and species-related diversity of collagen network architecture in articular cartilage. Osteoarthritis & Cartilage. 2006;14:1265–1271. doi: 10.1016/j.joca.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Alhadlaq HA, Xia Y. The structural adaptations in compressed articular cartilage by microscopic MRI (microMRI) T(2) anisotropy. Osteoarthritis Cartilage. 2004;12:887–894. doi: 10.1016/j.joca.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Akella SV, Regatte RR, Gougoutas AJ, Borthakur A, Shapiro EM, Kneeland JB, et al. Proteoglycan-induced changes in T1rho-relaxation of articular cartilage at 4T. Magnetic Resonance in Medicine. 2001;46:419–423. doi: 10.1002/mrm.1208. [DOI] [PubMed] [Google Scholar]
- 26.Wheaton AJ, Dodge GR, Elliott DM, Nicoll SB, Reddy R. Quantification of cartilage biomechanical and biochemical properties via T1rho magnetic resonance imaging. Magnetic Resonance in Medicine. 2005;54:1087–1093. doi: 10.1002/mrm.20678. [DOI] [PubMed] [Google Scholar]
- 27.Regatte RR, Akella SV, Borthakur A, Reddy R. Proton spin-lock ratio imaging for quantitation of glycosaminoglycans in articular cartilage. Journal of Magnetic Resonance Imaging. 2003;17:114–121. doi: 10.1002/jmri.10228. [DOI] [PubMed] [Google Scholar]
- 28.Menezes NM, Gray ML, Hartke JR, Burstein D. T2 and T1rho MRI in articular cartilage systems. Magnetic Resonance in Medicine. 2004;51:503–509. doi: 10.1002/mrm.10710. [DOI] [PubMed] [Google Scholar]
- 29.Mlynarik V, Trattnig S, Huber M, Zembsch A, Imhof H. The role of relaxation times in monitoring proteoglycan depletion in articular cartilage. Journal of Magnetic Resonance Imaging. 1999;10:497–502. doi: 10.1002/(sici)1522-2586(199910)10:4<497::aid-jmri1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 30.Regatte RR, Akella SV, Lonner JH, Kneeland JB, Reddy R. T1rho relaxation mapping in human osteoarthritis (OA) cartilage: comparison of T1rho with T2. Journal of Magnetic Resonance Imaging. 2006;23:547–553. doi: 10.1002/jmri.20536. [DOI] [PubMed] [Google Scholar]
- 31.Burstein D, Gray ML. Is MRI fulfilling its promise for molecular imaging of cartilage in arthritis? Osteoarthritis & Cartilage. 2006;14:1087–1090. doi: 10.1016/j.joca.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Gray ML. Toward imaging biomarkers for glycosaminoglycans. Journal of Bone & Joint Surgery - American Volume. 2009;91 Suppl 1:44–49. doi: 10.2106/JBJS.H.01498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fishbein KW, Canuto HC, Bajaj P, Camacho NP, Spencer RG. Optimal methods for the preservation of cartilage samples in MRI and correlative biochemical studies. Magn Reson Med. 2007;57:866–873. doi: 10.1002/mrm.21189. [DOI] [PubMed] [Google Scholar]
- 34.Li X, Han ET, Ma CB, Link TM, Newitt DC, Majumdar S. In vivo 3T spiral imaging based multi-slice T(1rho) mapping of knee cartilage in osteoarthritis. Magnetic Resonance in Medicine. 2005;54:929–936. doi: 10.1002/mrm.20609. [DOI] [PubMed] [Google Scholar]
- 35.Foltz WD, Al-Kwifi O, Sussman MS, Stainsby JA, Wright GA. Optimized spiral imaging for measurement of myocardial T2 relaxation. Magnetic Resonance in Medicine. 2003;49:1089–1097. doi: 10.1002/mrm.10467. [DOI] [PubMed] [Google Scholar]
- 36.Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. Journal of Digital Imaging. 2004;17:205–216. doi: 10.1007/s10278-004-1014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connective Tissue Research. 1982;9:247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 38.Taylor C, Carballido-Gamio J, Majumdar S, Li X. Comparison of quantitative imaging of cartilage for osteoarthritis: T2, T1rho, dGEMRIC and contrast-enhanced computed tomography. Magnetic Resonance Imaging. 2009;27:779–784. doi: 10.1016/j.mri.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saarakkala S, Julkunen P, Kiviranta P, Mäkitalo J, Jurvelin JS, Korhonen RK. Depth-wise progression of osteoarthritis in human articular cartilage: investigation of composition, structure and biomechanics. Osteoarthritis & Cartilage. 2010;18:73–81. doi: 10.1016/j.joca.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Mosher TJ, Smith H, Dardzinski BJ, Schmithorst VJ, Smith MB. MR imaging and T2 mapping of femoral cartilage: in vivo determination of the magic angle effect. AJR. American Journal of Roentgenology. 2001;177:665–669. doi: 10.2214/ajr.177.3.1770665. [DOI] [PubMed] [Google Scholar]