Abstract
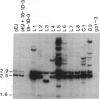
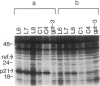
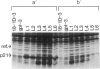
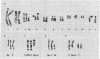
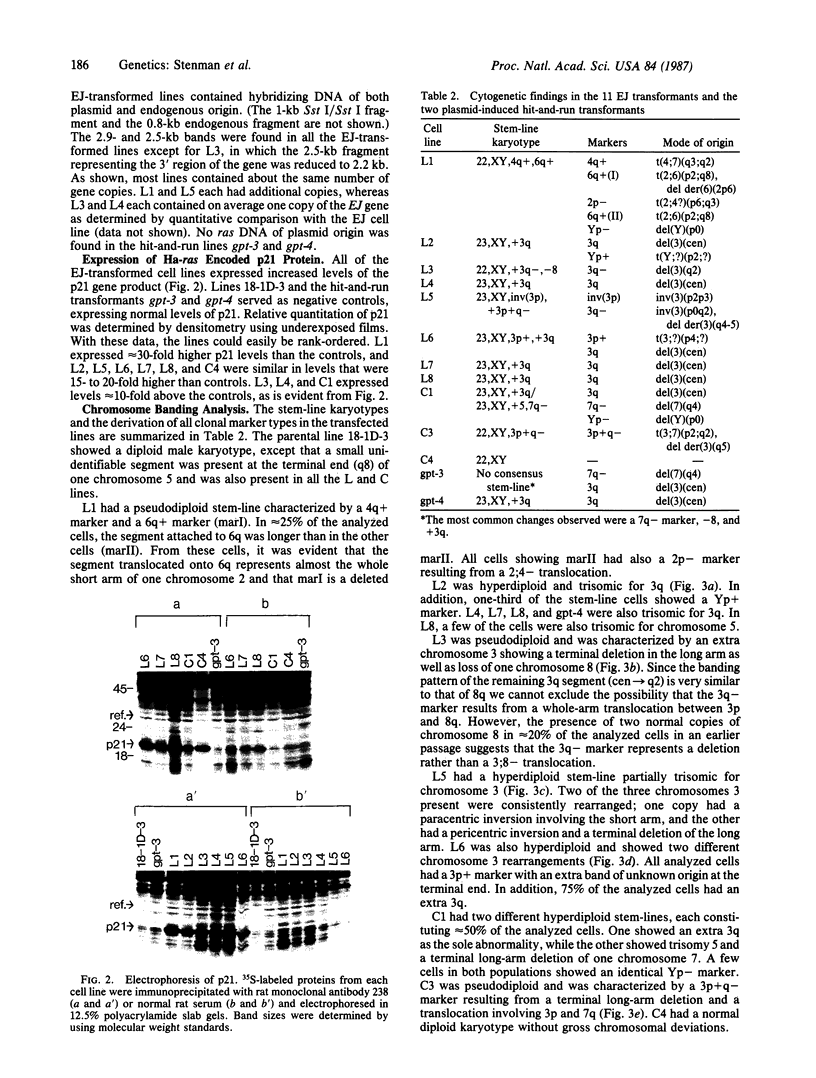
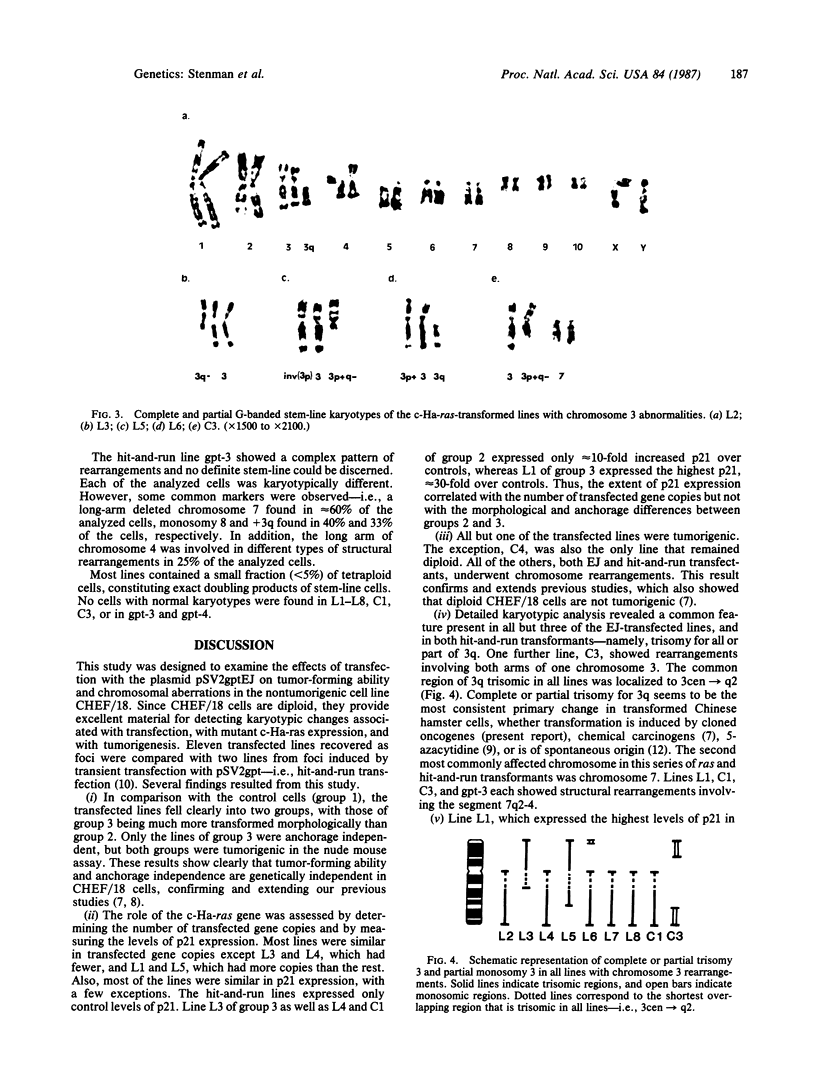
In previous cytogenetic studies, trisomy for 3q was found to be the most frequent chromosome change associated with induced tumorigenicity by a variety of agents in Chinese hamster cells. Here we describe similar chromosome changes in 11 lines of CHEF/18 cells transfected with the mutant c-Ha-ras containing plasmid pSV2gptEJ. All 11 lines contained the transfected EJ gene and expressed increased levels of p21, the EJ gene product. Ten of the 11 lines were tumorigenic and all but 2 of these were trisomic for all or part of 3q. One line remained diploid and was nontumorigenic despite expressing elevated p21. Two tumorigenic lines from "hit-and-run" transfection with pSV2gpt were shown to express only control levels of p21, but they were trisomic for 3q. These results show that increased p21 expression is neither necessary nor sufficient for inducing tumorigenicity of CHEF cells. We propose that tumorigenicity in the transfected CHEF/18 cells of this study was induced by chromosome rearrangements, especially trisomy for 3q, that occurred at increased frequencies following transfection with pSV2gptEJ.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Furth M. E., Davis L. J., Fleurdelys B., Scolnick E. M. Monoclonal antibodies to the p21 products of the transforming gene of Harvey murine sarcoma virus and of the cellular ras gene family. J Virol. 1982 Jul;43(1):294–304. doi: 10.1128/jvi.43.1.294-304.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadi I. K., Harrison J. J., Sager R. Genetic analysis of tumorigenesis: XVI. Chromosome changes in azacytidine- and insulin-induced tumorigenesis. Somat Cell Mol Genet. 1984 Sep;10(5):521–529. doi: 10.1007/BF01534856. [DOI] [PubMed] [Google Scholar]
- Harrison J. J., Anisowicz A., Gadi I. K., Raffeld M., Sager R. Azacytidine-induced tumorigenesis of CHEF/18 cells: correlated DNA methylation and chromosome changes. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6606–6610. doi: 10.1073/pnas.80.21.6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakati S., Sinha A. K. Banding patterns of Chinese hamster chromosomes. Genetics. 1972 Oct;72(2):357–362. doi: 10.1093/genetics/72.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchin R. M., Gadi I. K., Smith B. L., Sager R. Genetic analysis of tumorigenesis: X. Chromosome studies of transformed mutants and tumor-derived CHEF/18 cells. Somatic Cell Genet. 1982 Sep;8(5):677–689. doi: 10.1007/BF01542860. [DOI] [PubMed] [Google Scholar]
- Kitchin R. M., Sager R. Genetic analysis of tumorigenesis: VI. Chromosome rearrangements in tumors derived from diploid premalignant Chinese hamster cells in nude mice. Somatic Cell Genet. 1980 Sep;6(5):615–630. doi: 10.1007/BF01538641. [DOI] [PubMed] [Google Scholar]
- Klinger H. P., Shows T. B. Suppression of tumorigenicity in somatic cell hybrids. II. Human chromosomes implicated as suppressors of tumorigenicity in hybrids with Chinese hamster ovary cells. J Natl Cancer Inst. 1983 Sep;71(3):559–569. [PubMed] [Google Scholar]
- Lau C. C., Gadi I. K., Kalvonjian S., Anisowicz A., Sager R. Plasmid-induced "hit-and-run" tumorigenesis in Chinese hamster embryo fibroblast (CHEF) cells. Proc Natl Acad Sci U S A. 1985 May;82(9):2839–2843. doi: 10.1073/pnas.82.9.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall C. J., Kitchin R. M., Sager R. Genetic analysis of tumorigenesis: XII. Genetic control of the anchorage requirement in CHEF cells. Somatic Cell Genet. 1982 Nov;8(6):709–721. doi: 10.1007/BF01543013. [DOI] [PubMed] [Google Scholar]
- Oshimura M., Gilmer T. M., Barrett J. C. Nonrandom loss of chromosome 15 in Syrian hamster tumours induced by v-Ha-ras plus v-myc oncogenes. Nature. 1985 Aug 15;316(6029):636–639. doi: 10.1038/316636a0. [DOI] [PubMed] [Google Scholar]
- Ray F. A., Bartholdi M. F., Kraemer P. M., Cram L. S. Spontaneous in vitro neoplastic evolution: recurrent chromosome changes of newly immortalized Chinese hamster cells. Cancer Genet Cytogenet. 1986 Mar 1;21(1):35–51. doi: 10.1016/0165-4608(86)90199-8. [DOI] [PubMed] [Google Scholar]
- Sager R., Kovac P. E. Genetic analysis of tumorigenesis: I. Expression of tumor-forming ability in hamster hybrid cell lines. Somatic Cell Genet. 1978 May;4(3):375–392. doi: 10.1007/BF01542849. [DOI] [PubMed] [Google Scholar]
- Sager R., Tanaka K., Lau C. C., Ebina Y., Anisowicz A. Resistance of human cells to tumorigenesis induced by cloned transforming genes. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7601–7605. doi: 10.1073/pnas.80.24.7601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C., Weinberg R. A. Isolation of a transforming sequence from a human bladder carcinoma cell line. Cell. 1982 May;29(1):161–169. doi: 10.1016/0092-8674(82)90100-3. [DOI] [PubMed] [Google Scholar]
- Stanbridge E. J., Flandermeyer R. R., Daniels D. W., Nelson-Rees W. A. Specific chromosome loss associated with the expression of tumorigenicity in human cell hybrids. Somatic Cell Genet. 1981 Nov;7(6):699–712. doi: 10.1007/BF01538758. [DOI] [PubMed] [Google Scholar]
- Stenman G., Mark J. Loss of the Y chromosome in a cultured human salivary-gland adenocarcinoma. J Oral Pathol. 1983 Dec;12(6):458–464. doi: 10.1111/j.1600-0714.1983.tb00357.x. [DOI] [PubMed] [Google Scholar]
- Varmus H. E. The molecular genetics of cellular oncogenes. Annu Rev Genet. 1984;18:553–612. doi: 10.1146/annurev.ge.18.120184.003005. [DOI] [PubMed] [Google Scholar]