Abstract
Background:
The cognitive neural underpinnings of prototype learning are becoming clear. Evidence points to 2 different neural systems, depending on the learning parameters. A/not-A (AN) prototype learning is mediated by posterior brain regions that are involved in early perceptual learning, whereas A/B (AB) is mediated by frontal and medial temporal lobe regions.
Study Objectives:
To investigate the effects of sleep deprivation on AN and AB prototype learning and to use established prototype models to provide insights into the cognitive-processing locus of sleep-deprivation deficits.
Design:
Participants performed an AN and an AB prototype learning task twice, separated by a 24-hour period, with or without sleep between testing sessions.
Participants:
Eighteen West Point cadets participated in the sleep-deprivation group, and 17 West Point cadets participated in a control group.
Measurements and Results:
Sleep deprivation led to an AN, but not an AB, performance deficit. Prototype model analyses indicated that the AN deficit was due to changes in attentional focus and a decrease in confidence that is reflected in an increased bias to respond non-A.
Conclusions:
The findings suggest that AN, but not AB, prototype learning is affected by sleep deprivation. Prototype model analyses support the notion that the effect of sleep deprivation on AN is consistent with lapses in attentional focus that are more detrimental to AN than to AB. This finding adds to a growing body of work that suggests that different performance changes associated with sleep deprivation can be attributed to a common mechanism of changes in simple attention and vigilance.
Citation:
Maddox WT; Glass BD; Zeithamova D; Savarie ZR; Bowen C; Matthews MD; Schnyer DM. The effects of sleep deprivation on dissociable prototype learning systems. SLEEP 2011;34(3):253-260.
Keywords: Prototype learning, perceptual learning, episodic memory, sleep deprivation, attention
INTRODUCTION
Sleep deprivation is a common state experienced by individuals in many professions, including medical doctors, firefighters, parents, and members of the military.1–4 Because of the critical role these individuals play in society, it is important to have a thorough understanding of the role of sleep deprivation on reasoning and decision making. A common theory is that sleep deprivation leads to a global cognitive slowing, but recent reviews suggest that, whereas some tasks are adversely affected, others are relatively unaffected.5
The focus of this article is to explore the implications of 24 hours of total sleep deprivation on 2 forms of prototype learning that have been shown to be behaviorally and neurally dissociable.6,7 Critically, the tasks differ only in the underlying cognitive and neural processes that are required to solve the task while holding constant the perceptual, motor, and motivational aspects of the task.
Next, we briefly describe the 2 prototype learning tasks and provide evidence for their dissociable neural and cognitive processing. Then we speculate on the effects of sleep deprivation on performance in these tasks and present the results from a 24-hour sleep-deprivation study. We conclude with some general remarks.
Two Prototype Learning Tasks
An important form of learning is prototype learning. Prototypes provide the abstract representation for many natural categories8,9 and form the basis of much categorization in young children.10,11 Prototype theory assumes that individuals presented with examples from specific categories learn to extract the central tendency (e.g., the average or ideal) of each category, referred to as the prototype, and store that prototype in memory as the primary category representation. When presented with a novel item, the individual computes the similarity of that item to each relevant prototype and assigns the item to the category with the most similar prototype. Prototype theory provides an excellent account of many empirical phenomena, and, although competing theories exist in the categorization literature, prototype theory has been very influential.12–15
There is strong empirical evidence from brain-damaged individuals, as well as from function magnetic resonance imaging studies, that 2 types of prototype learning are neurally dissociable.6,7 In a typical prototype learning task, the participant is presented with a series of objects or items that are each drawn from 1 or more structured categories. During this training period, the participants are asked to classify each object into 1 of several categories and receive corrective feedback regarding their responses. Through trial-by-trial feedback, the participant learns to discriminate among the categories. Following training, the participant is generally presented with a series of test items that are used to evaluate the participant's category knowledge. The participant is required to generate a classification response but receives no feedback. These items are also members of the trained categories but are often novel members not presented during training. Both tasks outlined below use this training-test format.
In the A–not-A (AN) prototype learning task, participants are shown members of category A during training and, during testing, are asked to decide whether novel items are in category A or are not in category A. In the AB prototype learning task, participants are shown members of category A and B during training and, during testing, are asked to decide whether novel items are in category A or category B. Critically, in the Zeithamova et al. study7 and in the current study of total sleep deprivation, the same stimuli are used in the test phase for both the AN and AB tasks. Thus, any differences observed in AN and AB performance cannot be attributed to differences between the structures of non-A versus B category, nor to any stimulus-specific differences.
Evidence for Dissociable Neural and Cognitive Processes in AN and AB Prototype Learning
Evidence for dissociable neural and cognitive processes in AN and AB prototype learning comes from the study of brain-damaged patients, as well as through the use of functional magnetic resonance imaging studies. AN prototype learning is intact in patients with Parkinson disease,16 schizophrenia,17 Alzheimer disease,18 and amnesia.19,20 Neuroimaging studies with the AN task report learning-related reductions in occipital/temporal cortex activation to category A exemplars.7,21–23 These data suggest that the perceptual representation system might mediate AN prototype learning.
Unlike the results for AN prototype learning, AB prototype learning is impaired in people with Alzheimer disease24 and amnesia.20,24 Neuroimaging studies with the AB task report learning-related changes in prefrontal and parietal cortexes, along with the occipital/temporal regions seen in AN learning.7,25,26 Taken together, these finding suggest that explicit reasoning and episodic memory processes might mediate AB prototype learning.
Sleep Deprivation and AN Versus AB Prototype Learning
The research summarized above suggests that AN prototype learning is primarily mediated by posterior brain regions that are involved in early perceptual learning, whereas AB prototype learning is primarily mediated by frontal and medial temporal lobe regions—regions also associated with more explicit memory tasks. Although no study to date has examined prototype learning under conditions of sleep deprivation, one reasonable prediction, based on the extant sleep-deprivation literature, is that AB learning should be impaired. This follows because a number of studies suggest that complex decision-making tasks that involve frontal structures are affected by sleep deprivation.27,28 Concrete predictions regarding the AN task are less forthcoming. On the one hand, early perceptual learning processes could be hard hit by sleep deprivation, as has been suggested by some research.29 On the other hand, if sleep deprivation affects primarily frontal brain functioning, it may be the case that AN learning will be unaffected.
Another possibility is that changes in attentional focus associated with sleep deprivation might lead to different predictions30 not considered in the traditional explicit/perceptual dichotomy. For example, during initial AN training, examples from a single category (category A) are presented and are used to extract information about the prototype. On the other hand, during initial AB training, examples from category A and category B are presented and are used to extract information about each prototype (A and B). During the test phase in both cases, examples from both categories are presented and the participant must determine category membership. Because information about only a single category is presented during AN training, any changes in attention could have highly detrimental effects on prototype extraction, as well as, possibly, on critical feature extraction later at test. This follows because information about that single category is all that the participant can rely upon during test. However, because information about both categories is presented during AB training, changes in attention could have smaller effects on both prototype extraction and later during test. This follows because information about both categories is available and can be relied upon during test. Thus a hypothesis from the perspective of changes in attentional focus that have been associated with increased attentional lapsing during conditions of sleep deprivation30 suggests that sleep deprivation might have a larger impact on AN prototype learning than on AB prototype learning.
The current study compared these 2 types of prototype learning under tight experimental control utilizing the same stimuli under equivalent test conditions. In addition we applied a computational model to the data that provides critical insights into the nature of the underlying cognitive-processing deficits.
METHODS
Participants
Eighteen West Point cadets participated in the Sleepless (sleep deprivation) group (15 men, 3 women; mean age, 20.41 years; range, 19-24 years), and 17 West Point cadets participated in a Control group (13 men, 4 women; mean age, 18.82 years; range = 18-23 years). Both groups were volunteers, since the study was funded by the Army and, therefore, additional monetary compensation was not allowed. The 2 samples did not differ in age (P > 0.10) or gender ratio (P > 0.10). Participants in the Sleepless group were tested in 2 sessions separated by 24 hours of total sleep deprivation. Each participant was monitored continuously to ensure that no participant slept. Participants in the Control group were tested in 2 sessions separated by 24 hours and were allowed to engage in a normal night's sleep during the intervening time that was unmonitored. Although Control participants were not monitored, testing took place during the semester when the Cadets' sleep-wake schedule is highly regimented. All participants in both groups were tested between 06:00 and 12:00. All participants had normal or corrected-to-normal vision. The Institutional Review Board of The University of Texas, Austin, and West Point Military Academy approved the study, and informed consent was obtained from all participants.
Stimuli
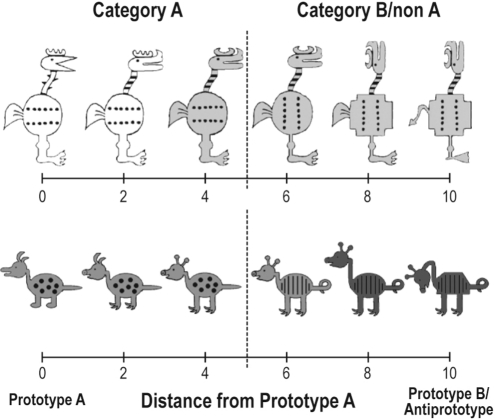
The stimuli were cartoon animals that varied along 10 binary dimensions, such as body shape (round or square), head position (facing forward or upward), tail shape (feathery or pointy), etc., (see Figure 1) that were adapted from a study by Bozoki.31 With 10 binary-valued dimensions, there are 210 (1024) unique stimuli from which to choose. For each run, 1 stimulus served as the A prototype, with all 10 of its feature values being referred to as prototypical features. All other stimuli can be defined relative to the prototype and can differ on 1 to 10 of the prototypical feature values. The stimulus with all 10 nonprototypical features is the B prototype (in the AB task) and the antiprototype (in the AN task). The number of nonprototypical features in each stimulus determines its distance from the prototype (see Figure 1). Category A stimuli were defined as those with a distance of 0 to 4 from the A prototype, and category B (or non-A) stimuli were defined as those with a distance of 6 to 10 from the A prototype. Stimuli equidistant from the 2 prototypes were excluded from the study.
Figure 1.
Example stimuli from both stimulus sets. The left-most stimulus represents the prototype of category A, stimuli to the right of the prototype represent examples of stimuli with increasing distances from the A prototype. The right-most stimulus is the category B prototype. Stimuli with distance 0-4 from prototype A were considered category A members; stimuli with distance 6-10 were considered category B (non-A) members.
A second set of cartoon-animal stimuli with different dimensions were also generated (see Figure 1), and each prototype learning task was tested with both sets of stimuli. Specifically, on Day 1, the AB task was performed with 1 set of stimuli, and the AN task was performed with the other set of stimuli. The same approach was taken on Day 2, except that the task-to-stimulus set pairings were reversed. Note that, in this study, all non-A stimuli in the AN condition were internally consistent and constructed from a fixed prototype. Thus, the only differences between the AN and the AB tasks were in the stimuli presented during training (only A stimuli in the AN, and A and B stimuli in the AB task) and the category labels used during the testing phase. Critically, the same stimuli were used in the testing phase for both the AN and AB tasks. Thus, any differences observed in the effects of total sleep deprivation on AN and AB performance cannot be attributed to differences between the structures of non-A versus B category, nor to any stimulus-specific differences.
Procedure
Day 1
Participants were not allowed to consume alcohol 24 hours prior to the study or to consume caffeine between 12:00 and 06:00 before the first or second day. They were instructed to engage in normal sleep-wake cycles the night before testing, and sleepless participants were peer monitored during this period. Participants in the Control and Sleepless conditions completed the AN and AB task in a counterbalanced order on Day 1.
Training
During training for the AB task, participants were asked to categorize 10 A and 10 B items (presented 1 by 1 in a random order) with corrective feedback. On each trial, 2 seconds after stimulus onset, the participant was prompted to give an A or B response. After each response, the participant was informed whether the response was correct or wrong. Within each category, 2 training stimuli differed from the category prototype on 1 feature, 3 differed on 2 features, 3 differed on 3 features, and 2 differed on 4 features. Across all 10 stimuli within each category, the category typical features were presented 7 or 8 times, and the opposite category typical features were presented 2 or 3 times. Neither prototype was presented. The training stimuli were presented in a random order.
Prior to AN training, participants were informed that they would need to learn to discriminate members of category A from nonmembers (non-A). During AN training, participants were shown stimuli from category A only. Twenty training stimuli from category A were passively viewed 1 by 1 for a minimum of 2 seconds, after which a prompt asked a participant to press any button to proceed to a next example of a category member. There were 5 training stimuli that differed from the A prototype on 1 feature, 5 differed on 2 features, 5 differed on 3 features, and 5 differed on 4 features. Across all 20 stimuli, the prototypical value on each dimension was presented 15 times, and the nonprototypical value on each dimension was presented 5 times.
Testing
The testing phase was identical for both tasks, with only the label of the second category (B versus non-A) differing between the tasks. Participants were presented with 42 stimuli, 1 at a time, that included both prototypes and 5 stimuli selected from each distance from the prototype (except distance 5—ambiguous stimuli). None of the stimuli were previously used in the training phase. No feedback was provided. A fixation cross was presented between each stimulus onset lasting 2.5 seconds.
After testing, participants in the Sleepless group were accompanied by a monitor at all times. During the evening and night, the particpants ate a meal and engaged in both physical and mental activities, such as walking, bowling, and playing video or board games to stay awake. After testing, participants in the Control group were told to engage in normal sleep that evening. Testing was conducted over a weekend when West Point cadets generally engage in sports activities (e.g., rugby).
Day 2
Both groups of participants ate breakfast, and, 24 hours after initial testing, participants in both groups completed the AN and AB task in a counterbalanced order on Day 2, with the stimulus-to-task assignments reversed from Day 1.
RESULTS
The data analyses focus exclusively on the test phase, since the testing procedure was identical across the AN and AB conditions. In addition, since AN training involved only a passive viewing of the stimuli, no training data are available for analysis.
Day 1 Performance
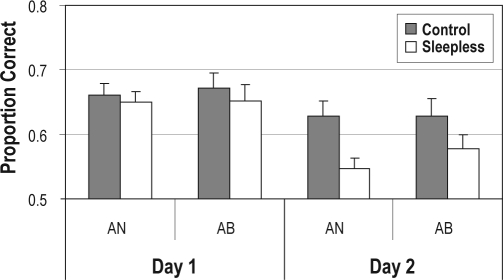
Figure 2 displays Day 1 and Day 2 test performance for Sleepless and Control groups separately for the AN and AB tasks. We began by determining whether performance was at a level better than chance alone. In all 4 cases (Control/AN, Sleepless/AN, Control/AB, and Sleepless/AB), performance was significantly above chance (all P values < 0.01). Next we compared Day 1 AN and AB test performance across Sleepless and Control groups to determine whether there were any differences across tasks and whether these differed across groups. A 2-Group (Sleepless vs Control) × 2-Task (AN vs AB) mixed-design analysis of variance was conducted on average test performance. The main effects of Group (F1,33 = 0.64, NS, η2 = 0.019), Task (F1,33 = 0.07, NS, η2 = 0.002), and the interaction (F1,33 = 0.06, NS, η2 = 0.002) were all nonsignificant. Thus, before we introduced the total sleep-deprivation manipulation, both participant groups showed the same level of AN and AB test performance, and AN and AB test performance did not differ.
Figure 2.
Test phase proportion correct on Day 1 and Day 2 for Control (gray bars) and Sleepless (white bars) participants in the AN and AB tasks. Error bars denote SEM.
As did Zeithamova et al.,7 we examined performance correlations between the AN and AB tasks. Given the neural dissociation observed in previous work, we predicted nonsignificant performance correlations. As predicted, the performance correlation was nonsignificant in the Control (r15 = 0.02, NS) and Sleepless (r16 = −0.22, NS) groups.
Day 2 Performance
Again, we examined whether performance occurred at a level greater than change alone. In all 4 cases (Control/AN, Sleepless/AN, Control/AB, and Sleepless/AB) performance was significantly above chance (all P values < 0.05). Next, we conducted a 2-Group (Sleepless vs Control) × 2-Task (AN vs AB) mixed-design analysis of variance on average Day 2 test performance. The main effects of Group (F1,33 = 7.32, P < 0.005, η2 = 0.182) were significant and suggested worse overall Day 2 performance for the Sleepless group relative to the Control group. The Task (F1,33 = 0.54, NS, η2 = 0.016) and interaction effects (F1,33 = = 0.60, NS, η2 = 0.018) were nonsignificant. Thus, performance in both tasks was worse for the Sleepless relative to the Control group.
Day 2 Proportion Change in Performance from Day 1
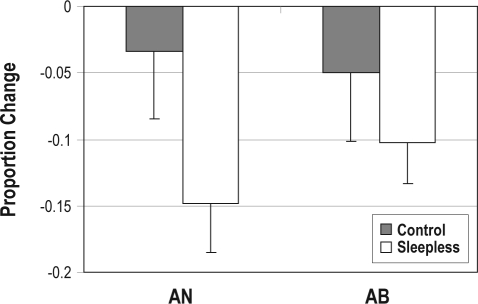
To determine how performance changed from Day 1 to Day 2, we subtracted Day 1 performance from Day 2 performance and then normalized this difference by Day 1 performance. We refer to this as the proportion change, and it is displayed in Figure 3.
Figure 3.
Proportion change defined as Day 2 – Day 1 performance normalized by Day 1 performance for Control (gray bars) and Sleepless (white bars) participants in the AN and AB tasks. Error bars denote SEM.
Because the neural and cognitive dissociation evidence reviewed above suggests that sleep deprivation might affect 1 condition and not the other, we examined the proportion change scores separately for the AN and AB conditions (see footnote a, page 260). We begin by determining whether the proportion change scores differ significantly from 0. For the Control group, neither proportion change score differed from 0 (AN: t16 = 0.67, NS; AB: t16 = 0.96, NS). For the Sleepless group, both proportion change scores differed significantly from 0 (AN: t17 = 4.00, P < 0.005; AB: t17 = 3.34, P < 0.005). Next, we compared the proportion change scores for the AN and AB tasks as a function of Group. The proportion change was significantly larger for the Sleepless group than for the Control group in the AN task (F1,33 = 4.39, P < 0.05, η2 = 0.108) but did not differ across Sleepless and Control groups in the AB task (F1,33 = 0.78, NS, η2 = 0.023). Thus, the proportion change from Day 1 to Day 2 was significantly larger in the Sleepless group relative to the Control group for the AN task but not for the AB task.
To determine whether a speed-accuracy tradeoff difference might underlie the proportion change score effect, we computed the proportion change in mean reaction time from Day 1 to Day 2 separately for the Sleepless and Control groups in the AN and AB conditions. For the AN and AB tasks, the proportion change did not differ across Sleepless and Control groups (both t values < 1.0), indicating that sleep deprivation did not slow performance relative to rested control subjects, and these results rule out a speed-accuracy tradeoff as an explanation for the accuracy differences.
Locus of the AN Total Sleep Deprivation Effect: Computational Modeling
To explore the locus of the Sleepless versus Control group effect on performance in the AN task, we applied a simple 1-prototype model separately to the data from each participant.12,13,15,32 Suppose that the participant is presented with stimulus x, which is composed of a set of (binary-valued) features on each of 10 dimensions. The model assumes that the participant computes the weighted distance between the stimulus x and the prototype of category A, PA. Specifically, the (Euclidean) distance between x and PA, dxPA is:
where wi denotes the weight placed on dimension i in the distance computation, with larger values denoting greater weight to a particular dimension. Without loss of generality, the weights are assumed to sum to 1, yielding 9 free wi parameters. xi denotes the feature value (0 or 1) on dimension i for stimulus x, and PAi denotes the feature value (0 or 1) on dimension i for PA. The predicted probability of responding A to stimulus x, P(A|x) is:
where ηiA equals e-dxPA and B is a free parameter that determines the similarity threshold above which an A response is most likely and below which a not-A response is most likely. We fit the model using maximum-likelihood procedures.33–35
First, we examined the fit of the model to the data by calculating the average absolute deviation between the observed and predicted probability of responding A. The fits of the model were quite good, with an average deviation between the predicted and observed probability of responding A of 0.039 and 0.007 for the Control participants on Day 1 and Day 2, respectively, and 0.028 and 0.050 for the Sleepless participants on Day 1 and Day 2, respectively.
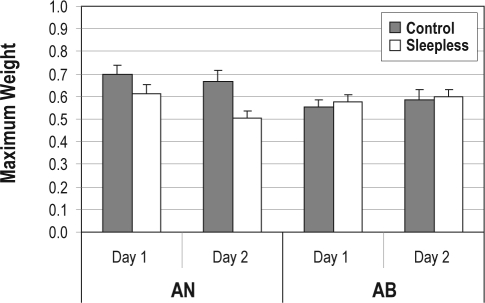
Next, we examined the weight placed on the most highly weighted dimension. This provides a measure of focus on the most highly weighted dimension. The maximum weight values for each participant group on Day 1 and Day 2 are displayed in Figure 4. There was no maximum weight difference between Control and Sleepless participants on Day 1 (t33 = 1.52, NS), and there was no change in maximum weight across days for Control participants (t16 < 1.0). However, the maximum weight dropped from Day 1 to Day 2 for Sleepless participants (t17 = 2.72, P < 0.05), and, on Day 2, the maximum weight for Sleepless participants was significantly smaller than for Control participants (t33 = 2.72, P < 0.01). Thus, 24 hours of total sleep deprivation affected participants' focus, making them less able to focus on specific stimulus dimensions.
Figure 4.
Maximum weight value from the Prototype Model applied to the AN and AB data for Control (gray bars) and Sleepless (white bars) participants on Day 1 and Day 2. Error bars denote SEM.
If one interprets the weight parameter, w, as a measure of attention (as is common in the literature12,13,15,32), then this pattern of results supports the idea that sleep deprivation is associated with changes in attentional focus. Moreover, this would also be consistent with the attentional hypothesis outlined in the Introduction. Changes or “lapses” in attention should be reflected in a reduction of the maximum weight value, and, in the AN condition, these appear to be associated with a large performance drop from Day 1 to Day 2 in the Sleepless, but not the Control, group.
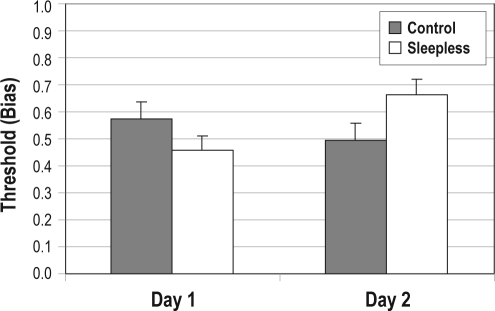
Finally, we examined the threshold parameter. This determines the threshold similarity above which an A response is most likely and below which a non-A response is most likely to occur. In essence, it serves the function of a bias, with larger values leading the participant to be biased toward the non-A category. The threshold/bias values for each participant group on Day 1 and Day 2 are displayed in Figure 5. There was no bias difference between Control and Sleepless participants on Day 1 (t33 = 1.36, NS), and there was no change in the bias across days for Control participants (t16 = 1.09, NS). However, the bias increased from Day 1 to Day 2 for Sleepless participants (t17 = 2.95, P < 0.01), and, on Day 2, the bias for Sleepless participants was (nearly) significantly larger than for Control participants (t33 = 1.99, P = 0.055). Thus, after 24 hours of total sleep deprivation, participants appear to have been less confident during the test phase of the category A information that they had learned about during the training phase, making them less likely to endorse items as members of category A.
Figure 5.
Threshold (Bias) from the Prototype Model applied to the AN data for Control (gray bars) and Sleepless (white bars) participants on Day 1 and Day 2. Error bars denote SEM.
The model-based analyses suggest that the AN performance deficit associated with 24 hours of total sleep deprivation is due to a change in attention consistent with increased lapsing, along with a (response) bias to respond not-A. The response bias is consistent with previous studies that have shown changes in response criteria resulting from sleep deprivation.36 The decrease in the maximum weight that is consistent with an increase in attention lapses is interesting and supports the view that there are significant changes in attention as a result of relatively short-term sleep deprivation.30 Although lapses in attention are not likely to be specific to either the AN or the AB task, as we outline in the Introduction, it is highly likely that the effect of attention lapses on test performance could be more severe in the AN than in the AB condition. This follows because, during learning in the AN task, there is 1 specific set of features that is diagnostic of the category A prototype. Any attention lapses during learning or testing will adversely affect test performance, since only features of the single A prototype are diagnostic. By contrast, in the AB task, there are 2 sets of learned features that can be used to solve the task—features associated with category A and features associated with category B. Thus, the effects of attention lapses during learning or testing will be more broadly distributed across the A and B prototype and should have a smaller effect on test performance, since the participant has both prototypes upon which to rely. Given this difference, lapses in attention associated with sleep deprivation would likely impair AN but not AB prototype learning.
As a test of this hypothesis, we fit the Equation 2, 1-prototype model to the AB data. One version assumed that the single prototype was the A prototype, and the other assumed that the single prototype was the B prototype. We also fit a 2-prototype model to the AB data. This is the more appropriate model, since it assumes that 2 prototypes were learned during training, but we wanted to apply both to determine which provided a better account of the data. In the 2-prototype model, the distance between a stimulus and Prototype A is calculated using Equation 1, and Prototype B replaces Prototype A in Equation 1 when computing the distance between a stimulus and Prototype B. Equation 2 is modified as follows:
where ηiA equals e-dxPA and ηiB equals e-dxPB.
The model-comparison results were clear. For the best-fitting 1-prototype model, the average deviation between the predicted and observed probability of responding A was 0.096 and 0.104 for the Control participants on Day 1 and Day 2, respectively, and 0.078, and 0.094 for the Sleepless participants on Day 1 and Day 2, respectively. On the other hand, for the 2-prototype model, the average deviation between the predicted and observed probability of responding A was 0.061 and 0.047 for the Control participants on Day 1 and Day 2, respectively, and 0.039 and 0.052 for the Sleepless participants on Day 1 and Day 2, respectively. As expected, the 2-prototype model provided a better account of the data (see footnote b, page 260).
Having concluded that the 2-prototype model provides a better fit for the AB task, a test of our attention-lapse hypothesis involved examining the maximum weight values from the 2-prototype model applied to the AB data. These are displayed in Figure 4. As predicted from the attention-lapse hypothesis, there was little difference in the maximum weight values across days or groups, with an average maximum weight on Day 1 of 0.55 and 0.58 for the Control and Sleepless groups, respectively, and an average maximum weight on Day 2 of 0.59 and 0.60 for the Control and Sleepless groups, respectively. None of the relevant comparisons approached significance (all t values < 1.0).
Taken together, the model fits to the AN and AB data suggest that sleep deprivation results in changes to the weighting parameter, previously associated with attention, that have an adverse effect on AN performance but have a smaller effect on AB performance.
DISCUSSION
This article explores the implications of 24 hours of total sleep deprivation on performance on 2 neurally and cognitively dissociable prototype learning tasks. The data suggest that 24 hours of total sleep deprivation adversely affects performance in the AN prototype learning tasks that are thought to be mediated by a perceptual representation memory system7 but does not adversely affect performance in AB prototype learning tasks that are thought to be mediated by a explicit and episodic memory system.7 More importantly, quantitative model-based analyses support the hypothesis that the deficit in AN prototype learning resulted from changes in attention focus, which are consistent with lapses in attention that are associated with sleep deprivation. In addition, there was a corresponding decrease in confidence that was reflected in an increased bias to respond non-A. Although there was no sleep-deprivation deficit in the AB task, modeling was able to demonstrate that this is likely due to the fact that learning on this task is less affected by attention lapses, since there is an increased amount of perceptual information available to solve the task because the 2 training prototypes are relevant.
The effects of sleep deprivation on other learning systems have been examined, and a more complete picture is now beginning to emerge. One learning system that has been examined in sleep deprivation is referred to as rule-based or hypothesis-testing learning. Here one must learn to apply a verbal strategy to separate objects from each category. Rule-based learning is thought to be mediated primarily by frontal brain regions.37–39 Sleep deprivation has been shown to adversely affect rule-based learning. Specifically, Herscovitch, Stuss, and Broughton40 found that sleep-deprived individuals were impaired in the Wisconsin Card Sorting Task, with the ratio of perseverative errors within a category to total perseverative errors increasing after sleep deprivation. Rule-based learning requires focused attention on the specific features that are associated with the critical rules, and, thus, a sleep-deprivation deficit should emerge if sleep deprivation leads to a broadening or “loosening” of attention focus.
A second dissociable learning system is the procedural learning system. Here one learns to apply nonverbal strategies to separate objects. In a recent study from our lab, we examined 24 hours of total sleep deprivation on procedural learning41 and found that individuals who successfully learned the procedural strategy and continued to use it following 24 hours of total sleep deprivation showed no procedural learning with sleep deprivation. Applying nonverbal strategies likely involves a relaxation of attention focus, as evidenced in a previous study in which we showed that including a dual working-memory/attention-demanding task enhanced procedural learning.42 Thus, a sleep-deprivation deficit should not emerge if sleep deprivation leads to a change in attention focus.
The current study suggests 1 common explanation for the various deficits seen with sleep deprivation across multiple learning tasks, namely, a change in attention focus that has been demonstrated to occur with sleep deprivation. Sleep deprivation hinders AN prototype learning and rule-based learning because lapses in attention focus lead to a poor perceptual representation of the single A prototype in AN learning and hinder rule maintenance and a narrowing of attention focus that is required in rule-based learning. On the other hand, sleep deprivation has little effect on AB prototype learning and information-integration learning because lapses in attention focus have less impact when 2 prototypes are relevant, as in AB learning, and because lapses in attention result in a loosening of attention focus, which is advantageous in information-integration learning. These conclusions are in line with a recent meta-analysis that examined the effects of sleep deprivation across multiple cognitive domains.30 The authors surveyed 70 articles that, in total, examined 147 cognitive tests. They found that, although most cognitive domains demonstrated declines in performance with relatively short-term sleep deprivation, it was simple attention and vigilance tasks that demonstrated the largest effects sizes. In the current prototype-learning experiment, if one considers that a difficulty in maintaining focused attention will likely result in changes to the weighting parameter of the most highly attended stimulus feature, then this is what is seen as the effect of sleep deprivation in the AN task. During learning, participants are only exposed to the A category stimuli. Therefore, their attention focus is on 1 set of critical stimulus features, and a lack of attention to these critical features would likely cause participants to miss classifying some trials. Repeated misclassification would result in a shift in the tendency to say “not A”—and it appears that this was reflected in the change in bias scores.
Although a change in attention focus is consistent with the performance decline on the AN task, it was not immediately clear why this would not affect the AB task as well. Computational modeling helped resolve this issue by demonstrating that, when 2 prototypes are relevant, continuous attention focus is not as critical, since information about either prototype can be utilized during task performance. Since participants have learned 2 sets of features, missing 1 at testing would not be detrimental, since 2 sets have been learned. It would be compatible with the results of the current study as well as other studies in the literature to postulate that different performance changes associated with sleep deprivation could be attributed to a common mechanism of changes in simple attention and vigilance, with some tasks more critically affected by these changes than others.
One possible alternative to our proposed attention hypothesis is that, with sleep deprivation, there is increased susceptibility to proactive interference. This is where stimulus features from 1 task get confused with another. Given that 2 stimulus sets were used for the AB and AN task, the possibility exists that 1 task, the AN, is more susceptible to interference. If this were the case, then one might predict a deficit due to sleep deprivation through a mechanism of proactive interference. Although this is a possibility that likely will need to be examined in future research, we do not believe it can account for the current results. First, past research has demonstrated that it is the explicit memory tasks that are more susceptible to proactive interference,43,44 and, if that were the case, then one might expect the deficit to be seen in the AB task, which has been demonstrated to rely on brain structures associated with explicit memory. Second, unpublished research from our own lab has examined whether AN and AB learning can occur on the same stimulus set separated by a single night of sleep. Since there would be 100% overlap of stimulus features, any susceptibility of either AN or AB to proactive interference would become apparent in this design. None was found. Specifically, 5 participants completed the AN task on Day 1 followed by the AB task on Day 2 with stimulus set 1. Five more did the same with stimulus set 2, and 10 others followed the same procedures but with the AB task on Day 1 and the AN task on Day 2. In every condition, there was no significant change in performance from Day 1 to Day 2 (all t values < 1.0).
These findings have implications for training and performance, especially with respect to individuals who perform critical societal tasks under sleep-deprivation conditions. These data suggest that the changes associated with sleep deprivation may favor a prototype-training approach that is dependent on learning multiple categories, such as AB training, and less on single-category training, such as AN training. Those tasks, like the AN task, that require focused attention on a small range of features appear to suffer from relatively short-term sleep deprivation. For example, when training an emergency medical technician to diagnose medical disorders, it might be important to train on subjects with contrasting medical conditions, such as heart attack versus indigestion (as in AB training), where multiple learned features can contribute to the diagnosis, and the technician is not just trained to identify a heart attack (as in AN training). Sleep deprivation is a serious problem faced my many individuals who fulfill critical societal roles (e.g., firefighters, emergency medical personnel, and parents). An understanding of the cognitive processes that are and are not affected by sleep deprivation is critical to improving the effectiveness of these individuals' performances. Studies, like this one, that examine specific experimental effects while holding as many other aspects of the task constant will yield important results and help us to better achieve this mission.
DISCLOSURE STATEMENT
This was not an industry-supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This research was supported by NIH Grant R01 MH077708 to WTM and the Army Grant W911NF-07-2-0023, through The Center for Strategic and Innovative Technologies at The University of Texas at Austin, to WTM and DMS. We thank Andrea Bozoki for providing us with the basis for the stimulus sets and Sean Maddox, Borami Lee, and Cristina Benavides for developing the rest of the stimulus sets.
FOOTNOTES
It is worth mentioning that an omnibus analysis of variance was conducted and the interaction between Group and Condition did not reach significance. Although we believe that separate AN and AB condition analyses are warranted, the lack of an interaction potentially makes the results more tentative.
One might argue that the 2-prototype model should also be applied to the AN data. Although methodologically that is possible, it would assume that AN training led the participant to extract a prototype from unseen stimuli, which does not make theoretical sense.
REFERENCES
- 1.Belenky G, Penetar DM, Thorne D, et al. Food Components to Enhance Performance. Washington, DC: National Academy Press; 1994. The effects of sleep deprivation on preformance during continuous combat operations; pp. 127–35. [Google Scholar]
- 2.Papp KK, Stoller EP, Sage P, et al. The effects of sleep loss and fatigue on resident-physicians: a multi-institutional, mixed-method study. Acad Med. 2004;79:394–406. doi: 10.1097/00001888-200405000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Veasey S, Rosen R, Barzansky B, Rosen I, Owens J. Sleep loss and fatigue in residency training: a reappraisal. JAMA. 2002;288:1116–24. doi: 10.1001/jama.288.9.1116. [DOI] [PubMed] [Google Scholar]
- 4.Takeyama H, Itani T, Tachi N, et al. Effects of shift schedules on fatigue and physiological functions among firefighters during night duty. Ergonomics. 2005;48:1–11. doi: 10.1080/00140130412331303920. [DOI] [PubMed] [Google Scholar]
- 5.Harrison Y, Horne JA. The impact of sleep deprivation on decision making: a review. J Exp Psychol Appl. 2000;6:236–49. doi: 10.1037//1076-898x.6.3.236. [DOI] [PubMed] [Google Scholar]
- 6.Ashby FG, Maddox WT. Human category learning. Ann Rev Psychol. 2005;56:149–78. doi: 10.1146/annurev.psych.56.091103.070217. [DOI] [PubMed] [Google Scholar]
- 7.Zeithamova D, Maddox WT, Schnyer DM. Dissociable prototype learning systems: evidence from brain imaging and behavior. J Neurosci. 2008;28:13194–201. doi: 10.1523/JNEUROSCI.2915-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosch E. On the internal structure of perceptual and semantic categories. New York, NY: Academic Press; 1973. [Google Scholar]
- 9.Rosch E, Mervis CB. Family resemblances: studies in the internal structure of categories. Cogn Psychol. 1975;7:573–605. [Google Scholar]
- 10.Ross GS. Categorization in 1- to 2-year olds. Dev Psychol. 1980;16:391–6. [Google Scholar]
- 11.Strauss MS. Abstraction of prototypical information by adults and 10-month-old infants. J Exp Psychol. 1979;5:618–32. [PubMed] [Google Scholar]
- 12.Ashby FG, Maddox WT. Relations between prototype, exemplar, and decision bound models of categorization. J Math Psychol. 1993;37:372–400. [Google Scholar]
- 13.Posner MI, Keele SW. On the genesis of abstract ideas. J Exp Psychol. 1968;77:304–63. doi: 10.1037/h0025953. [DOI] [PubMed] [Google Scholar]
- 14.Posner MI, Keele SW. Retention of abstract ideas. J Exp Psychol. 1970;83:304–8. doi: 10.1037/h0025953. [DOI] [PubMed] [Google Scholar]
- 15.Smith JD, Minda JP. Prototypes in the mist: The early epochs of category learning. J Exp Psychol. 1998;24:1411–36. [Google Scholar]
- 16.Reber PJ, Squire LR. Intact learning of artificial grammars and intact category learning by patients with Parkinson's disease. Behav Neurosci. 1999;113:235–42. doi: 10.1037//0735-7044.113.2.235. [DOI] [PubMed] [Google Scholar]
- 17.Keri S, Kelemen O, Benedek G, Janka Z. Intact prototype learning in schizophrenia. Schizophr Res. 2001;52:261–4. doi: 10.1016/s0920-9964(00)00092-x. [DOI] [PubMed] [Google Scholar]
- 18.Keri S, Kalman J, Kelemen O, Benedek G, Janka Z. Are Alzheimer's disease patients able to learn visual prototypes? Neuropsychologia. 2001;39:1218–23. doi: 10.1016/s0028-3932(01)00046-x. [DOI] [PubMed] [Google Scholar]
- 19.Knowlton BJ, Squire LR. The learning of categories: Parallel brain systems for item memory and category level knowledge. Science. 1993;262:1747–9. doi: 10.1126/science.8259522. [DOI] [PubMed] [Google Scholar]
- 20.Zaki SR, Nosofsky RM, Jessup NM, Unverzagt FW. Categorization and recognition performance of a memory-impaired group: evidence for single-system models. J Int Neuropsychol Soc. 2003;9:394–406. doi: 10.1017/S1355617703930050. [DOI] [PubMed] [Google Scholar]
- 21.Aizenstein HJ, MacDonald AW, Stenger VA, et al. Complementary category learning systems identified using event-related functional MRI. J Cogn Neurosci. 2000;12:977–87. doi: 10.1162/08989290051137512. [DOI] [PubMed] [Google Scholar]
- 22.Reber PJ, Stark CE, Squire LR. Contrasting cortical activity associated with category memory and recognition memory. Learn Mem. 1998;5:420–8. [PMC free article] [PubMed] [Google Scholar]
- 23.Reber PJ, Stark CE, Squire LR. Cortical areas supporting category learning identified using functional MRI. Proc Natl Acad Sci. 1998;95:747–50. doi: 10.1073/pnas.95.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinha RR. Neuropsychological substrates of category learning. Dissert. Abstr. Int.: Sect. B: Sci. Engineer. 1999 60(5-B), 2381 (UMINo. AEH9932480) [Google Scholar]
- 25.DeGutis J, D'Esposito M. Distinct mechanisms in visual category learning. Cogn Affect Behav Neurosci. 2007;7:251–9. doi: 10.3758/cabn.7.3.251. [DOI] [PubMed] [Google Scholar]
- 26.Seger CA, Poldrack RA, Prabhakaran V, Zhao Z, Glover GH, Gabrieli JDE. Hemispheric asymmetries and individual differences in visual concept learning as measured by functional MRI. Neuropsychologia. 2000;38:1316–24. doi: 10.1016/s0028-3932(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 27.Drummond SP, Brown GG, Gillin JC, Stricker JL, Wong EC, Buxton RB. Altered brain response to verbal learning following sleep deprivation. Nature. 2000;403:655–7. doi: 10.1038/35001068. [DOI] [PubMed] [Google Scholar]
- 28.Drummond SP, Brown GG, Stricker JL, Buxton RB, Wong EC, Gillin JC. Sleep deprivation-induced reduction in cortical functional response to serial subtraction. Neuroreport. 1999;10:3745–8. doi: 10.1097/00001756-199912160-00004. [DOI] [PubMed] [Google Scholar]
- 29.Williamson AM, Feyer A, Mattick RP, Friswell R, Finlay-Brown S. Developing measures of fatigue using an alcohol comparison to validate the effects of fatigue on performance. Accid Anal Prev. 2000;33:313–26. doi: 10.1016/s0001-4575(00)00045-2. [DOI] [PubMed] [Google Scholar]
- 30.Lim J, Dinges DF. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull. 2010;136:375–89. doi: 10.1037/a0018883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bozoki A., Grossman M., Smith E.E. Can patients with Alzheimer's disease learn a category implicitly? Neuropsychologia. 2006;44:816–827. doi: 10.1016/j.neuropsychologia.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Reed SK. Pattern recognition and categorization. Cogn Psychol. 1972;3:382–407. [Google Scholar]
- 33.Ashby FG. Multivariate Probability Distributions. Hillsdale, NJ: Erlbaum; 1992. [Google Scholar]
- 34.Takane Y, Shibayama T. Structure in Stimulus Identification Data. Hillsdale, NJ: Erlbaum; 1992. [Google Scholar]
- 35.Wickens TD. Models for Behavior: Stochastic Processes in Psychology. San Francisco, CA: WH Freeman; 1982. [Google Scholar]
- 36.Roge J, Gabaude C. Deterioration of the useful visual field with age and sleep deprivation: insight from signal detection theory. Percept Mot Skills. 2009;109:270–84. doi: 10.2466/PMS.109.1.270-284. [DOI] [PubMed] [Google Scholar]
- 37.Ashby FG, Alfonso-Reese LA, Turken AU, Waldron EM. A neuropsychological theory of multiple systems in category learning. Psychol Rev. 1998;105:442–81. doi: 10.1037/0033-295x.105.3.442. [DOI] [PubMed] [Google Scholar]
- 38.Anderson SW, Damasio H, Jones RD, Tranel D. Wisconsin Card Sorting Test performance as a measure of frontal lobe damage. J Clin Exp Neuropsychol. 1991;13:909–22. doi: 10.1080/01688639108405107. [DOI] [PubMed] [Google Scholar]
- 39.Monchi O, Petrides M, Petre V, Worsley K, Dagher A. Wisconsin Card Sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. J Neurosci. 2001;21:7733–41. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herscovitch J, Stuss D, Broughton R. Changes in cognitive processing following short-term cumulative partial sleep deprivation and recovery oversleeping. J Clin Neuropsychol. 1980;2:301–19. [Google Scholar]
- 41.Maddox WT, Glass BD, Wolosin SM, et al. The effects of sleep deprivation on information-integration categorization performance. Sleep. 2009;32:1439–48. doi: 10.1093/sleep/32.11.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Filoteo JV, Lauritzen JS, Maddox WT. Removing the frontal lobes: the effects of engaging executive functions on perceptual category learning. Psychol Sci. 2010;21:415–23. doi: 10.1177/0956797610362646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jonides J, Nee DE. Brain mechanisms of proactive interference in working memory. Neuroscience. 2006;139:181–93. doi: 10.1016/j.neuroscience.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 44.Nee DE, Jonides J, Berman MG. Neural mechanisms of proactive interference-resolution. Neuroimage. 2007;38:740–51. doi: 10.1016/j.neuroimage.2007.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]