Abstract
Study Objectives:
Sleep loss has pro-inflammatory effects, but the roles of specific cell populations in mediating these effects have not been delineated. We assessed the modulation of the electroencephalographic and molecular responses to sleep deprivation (S-DEP) by minocycline, a compound that attenuates microglial activation occurring in association with neuroinflammatory events.
Design:
Laboratory rodents were subjected to assessment of sleep and wake in baseline and sleep deprived conditions.
Participants:
Adult male CD-1 mice (30-35 g) subjected to telemetric electroencephalography.
Interventions:
Minocycline was administered daily. Mice were subjected to baseline data collection on the first day of minocycline administration and, on subsequent days, 2 S-DEP sessions, 1 and 3 h in duration, followed by recovery sleep. Following EEG studies, mice were euthanized either at the end of a 3 h S-DEP or as time-of day controls for sampling of brain messenger RNAs. Gene expression was measured by real-time polymerase chain reaction.
Measurements and Results:
Minocycline-treated mice exhibited a reduction in time spent asleep, relative to saline-treated mice, in the 3-h interval immediately after administration. S-DEP resulted in an increase in EEG slow wave activity relative to baseline in saline-treated mice. This response to S-DEP was abolished in animals subjected to chronic minocycline administration. S-DEP suppressed the expression of the microglial-specific transcript cd11b and the neuroinflammation marker peripheral benzodiazepine receptor, in the brain at the mRNA level. Minocycline attenuated the elevation of c-fos expression by S-DEP. Brain levels of pro-inflammatory cytokine mRNAs interleukin-1β (il-1β), interleukin-6 (il-6), and tumor necrosis factor-α (tnfα) were unaffected by S-DEP, but were elevated in minocycline-treated mice relative to saline-treated mice.
Conclusions:
The anti-neuroinflammatory agent minocycline prevents either the buildup or expression of sleep need in rodents. The molecular mechanism underlying this effect is not known, but it is not mediated by suppression of il-1β, il-6, and tnfα at the transcript level.
Citation:
Wisor JP; Schmidt MA; Clegern WC. Evidence for neuroinflammatory and microglial changes in the cerebral response to sleep loss. SLEEP 2011;34(3):261-272.
Keywords: Microglia, neuroinflammation, CD11b, cytokines, real-time polymerase chain reaction
INTRODUCTION
Insufficient sleep and poor sleep quality are risk factors for inflammation-related conditions such as cardiovascular disease,1 immune dysfunction,2,3 and metabolic disorders.1,4 These relationships are likely to be causal rather than simply correlational, as S-DEP has a well-documented pro-inflammatory effect.5–10 The pro-inflammatory effect of S-DEP extends to brain tissues, as pro-inflammatory cytokines are expressed in the rodent brain in association with sleep need and promote sleep.2,11,12 There is thus a need for further studies on inflammatory mechanisms as a possible mediator of the negative health consequences of S-DEP.
The roles of neuronal populations in mediating effects of sleep loss on brain function have been studied exhaustively,13 but with rare exceptions,14,15 the regulation of sleep by non-neuronal cell populations in the brain is an unexplored possibility. Microglia, a cell population that drives neuroinflammatory events in the brain, make up 5% to 20% of glia16 and roughly 3.5% to 7% of total cells in the CNS.17 Converging lines of evidence provide a compelling rationale for studies designed to ascertain the role of cerebral glia, and microglia in particular, as a sleep-regulatory population. First, microglia are a significant source of pro-inflammatory cytokines.18,19 Second, transcripts that are markers for microglial activation are upregulated in the brain in response to intracerebral administration of substances that promote sleep.20 Third, administration of minocycline, a compound known to attenuate microglial function in the brain,21 inhibits slow wave activity in humans subjected to polysomnographic measurement of sleep.21,22 Fourth, S-DEP elevates some chemical signals (including adenosine, cytokines, interferons, and heat shock proteins), which are known to trigger changes in microglial function.23–25 Fifth, long-term S-DEP (5 days using the disk-over-water method) has been reported to induce morphological changes in cerebral microglia that are indicative of increased microglial activation.26 In view of these observations, we hypothesized that modulation of microglial function within the brain is a necessary precondition for the EEG changes that occur as a consequence of sleep loss. A manipulation that attenuates microglial activation should be expected to attenuate the electroencephalographic (EEG) and biochemical responses to S-DEP. Microglial activation can be assessed by changes in microglial morphology and microglial-enriched transcripts.27–29 Systemic administration of the tetracycline derivative, minocycline, attenuates these morphological and biochemical changes.21,30,31 We hypothesized that minocycline would attenuate both EEG measures of sleep need and pro-inflammatory cytokine expression in microglia. Here, we assessed the effect of systemic minocycline administration on the EEG at baseline and after S-DEP and measured the effect of minocycline on the expression of microglial-enriched transcripts. The data are compatible with the concept that S-DEP induces changes in microglial function.
METHODS
Experimental Subjects and Setting
Male mice of the outbred CD-1 strain were obtained from an in-house breeding colony. The individuals used in these experiments were second generation offspring of founder mice obtained from Charles River (Wilmington, MA; strain code 022) and weighed 30-35 g at the time of surgery, roughly 4-6 weeks of age. CD-1 mice were subjected to experimentation because their rapid growth (see http://www.criver.com/SiteCollectionDocuments/rm_rm_c_CD_1_mice.pdf) facilitates surgical implantation of telemetered transmitters and because, as an outbred strain, they are a useful genetic resource.32 Mice were housed in an LD12:12 cycle with lights-on at 06:00 and lights-off at 18:00 throughout experimentation. Ambient temperature was 22.6 ± 1 degrees Celsius. Relative humidity was maintained at 52% throughout experimentation.
Surgical Procedures
All procedures were approved by the Institutional Animal Care and Use Committee at Washington State University and adhered to the National Research Council Guide for the Care and Use of Laboratory Animals.33 Animals were surgically implanted with a telemetry device (FT-20EET, Data Sciences Inc, St Paul, MN) capable of collecting electroencephalographic (EEG) and electromyographic (EMG) potentials through stainless steel wires that enter the body of the device. Body temperature (Tb) and locomotor activity (LMA) data were collected by sensors embedded in the device. FT-20EET transmitters were implanted into the peritoneal cavity under isoflurane anesthetic (5% induction, 1% to 2.5% maintenance). Body temperature was maintained throughout surgery using a circulating water blanket. After induction of anesthesia, the fur was shaved from the top of the head and from the mid-abdominal region. After iodine wash and 70% alcohol rinse of the shaved areas, midline incisions were made through the skin on both shaved areas and through the abdominal musculature. The transmitter was then inserted into the peritoneal cavity and sutured into the abdominal musculature with Vicryl absorbable suture (Ethicon, NJ). The wire leads for the EEG and EMG exited the peritoneal cavity via the suture site. The abdominal skin was closed using Ethilon non-absorbable suture (Ethicon, NJ). The wires were strung subcutaneously dorsal to the forelimb and between the ears up to the incision on the top of the head. The insulating sheath was removed from the end of each EEG and EMG lead, leaving approximately 0.3 cm of exposed wire at their ends. The EEG lead wires were bent into a hairpin loop for insertion into the skull. Holes were drilled bilaterally through the skull (2.0 mm lateral and 1.0 mm anterior from lambda) with a 0.7 mm diameter stainless steel burr. The EEG leads were inserted into the holes and affixed to the skull with Scotchbond Dual-Cure (3M, St Paul, MN) and composite dental restorative material (Patterson Dental Supply, Inc, St. Paul, MN). The EMG leads were inserted through the neck musculature at locations roughly 0.5 cm apart and held in place by a suture knot on the end of the lead where it exited the muscle. The incision on the head and neck was closed with Ethilon non-absorbable suture (Ethicon, NJ). Buprenorphine was administered as a long-lasting analgesia (0.05 mg/kg SC) for 3 consecutive days post-surgery. Animals were monitored closely following surgery until they were ambulatory.
EEG Data Collection and Analysis
EEG (digitization rate, 500 Hz) and EMG (digitization rate, 500 Hz) were collected with Dataquest ART software and processed with Neuroscore 2.01 software (Data Sciences Inc, St Paul, MN). Digitized EEG (bandpass, 1-30 Hz) and integrated EMG (high pass, 10 Hz), were processed in 10-s epochs, each of which was classified as wake, rapid eye movement sleep (REMS), or non-REMS (NREMS) by individuals expert in rodent sleep-state classification. EEG data were fast Fourier transformed in 10-s epochs by an algorithm embedded in the Neuroscore software. NREMS delta power (EEG power in the 0.75-4 Hz range) was calculated for each NREMS epochs and averaged over all NREMS epochs within each time interval subjected to analysis. Delta power was computed only for those hours during which the animal spent ≥ 10% of epochs in NREMS, as this measure is unreliable when < 10% of time is spent in NREMS. This threshold resulted in missing values for some animals when data were assessed in 1-h bins. NREMS delta power values were processed in 2 ways prior to statistical analysis, the purpose of which was to eliminate missing data points from the statistical analysis. First, NREMS delta power values from the baseline recording session and the entire 6-h post-S-DEP recording session were averaged separately into 3-h bins for analysis. In the event that one of 3 hourly delta power values was missing because of < 10% of epochs being scored as NREMS, the average of the values from the 2 remaining hours was used as the value for that 3-h interval. Second, to assess acute S-DEP-induced changes in NREMS delta power, average NREMS delta power was calculated from the first 360 epochs of sleep subsequent to termination of S-DEP and the analogous time of day on the baseline day.
Experiment 1- Effects of minocycline on baseline sleep and the EEG response to S-DEP
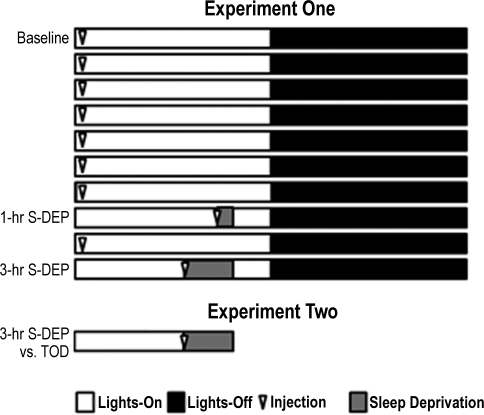
Eleven mice were subjected to minocycline treatment, (45 mg/kg; Sigma-Aldrich, St Louis, MO) administered intraperitoneally in saline in a protocol known to attenuate the neuroinflammatory effects of experimental stroke.31 The protocol consisted of once-daily administration, at the start of S-DEP on S-DEP days and at lights-on on other days, over 10 days of experimentation (Figure 1). The control group (n = 11) received saline injections at the same time minocycline was administered to the experimental group. The 24-h baseline sleep recordings were performed on the first day of minocycline administration. On days 8 and 10 of minocycline administration, mice were subjected to S-DEP sessions of 1- and 3-h duration, respectively. S-DEP of 3-h duration produces robust, reliable changes in EEG delta power and state consolidation, whereas 1-h S-DEP is marginally effective at increasing EEG delta power above baseline levels.34 Collectively, these durations provided a dose-response curve for homeostatic sleep regulation across strains. On S-DEP days, injections were administered at the onset of S-DEP rather than the onset of light. The injections were delayed on S-DEP days so as to avoid producing the transient decrease in sleep that was observed on the baseline day in minocycline-treated mice. If minocycline had been administered at the onset of light on S-DEP days, minocycline-treated animals would have undergone an additional sleep loss during the first 3 h after administration, in excess of the sleep loss incurred as a consequence of S-DEP. The resulting difference between the minocycline group and the saline group in net sleep loss would confound between-group comparisons of recovery measures. During S-DEP, wakefulness was maintained by visual inspection of the animals, sensory stimulation, and introduction of novel objects into the cages. S-DEP sessions ended 8 h into the light phase of the LD cycle (ZT8). Recovery sleep was monitored for the remainder of the light portion of the LD cycle (4 h) and the first 2 h of the dark phase. We did not counterbalance the order of the 2 sleep deprivations across animals. While it is possible that the EEG effect of S-DEP may be modified with repeated exposure to S-DEP, it is common to subject animals to a series of S-DEP sessions without randomizing the order of the S-DEP sessions within experimental groups.34–37
Figure 1.
Experimental procedures. In experiment 1, experimental data were collected over a 10-day interval. Baseline data were collected during day 1, beginning immediately after intraperitoneal administration of saline (control group) or minocycline, 45 mg/kg (experimental group) at lights-on. Administration continued on a daily basis throughout experimentation. S-DEP sessions of 1-h and 3-h duration occurred on days 8 and 10 of experimentation, respectively. In experiment 2, half of the mice within each of the 2 pharmacological groups in experiment 1 (saline vs. minocycline) were randomly assigned to an S-DEP group (3-h S-DEP) and the other half to a time of day control group (TOD). The 3-h S-DEP animals were euthanized for gene expression assays at the end of the 3-h S-DEP, and TOD animals were euthanized at the same time after sleeping spontaneously.
Experiment 2- Effects of S-DEP and minocycline on gene expression
Subsequent to daily administration of minocycline or saline over 10 days, mice were subjected to a terminal S-DEP experiment (3 h, ending at ZT8), after which brain tissues were collected for biochemical assays. The experimental facility used in these experiments included only one room instrumented for sleep recordings. Because it was essential for time of day control mice within each pharmacological group to remain undisturbed in their home cages until the time of euthanasia, it was necessary to remove the S-DEP mice from that room. Consequently, biopotential data were not collected on the day of the terminal S-DEP experiment. At the end of S-DEP, S-DEP (n = 8 per pharmacological group) and time of day control animals (n = 8 per pharmacological group) were euthanized by CO2 asphyxiation and cervical dislocation. Brains were flash frozen on dry ice and stored at −80°C.
Brain tissue was homogenized with an automated tissue grinder. Total RNA was isolated using the Trizol (Invitrogen, Carlsbad, CA) method according to manufacturer's specifications. First strand cDNA was prepared from RNA samples using the SuperScript First Strand Synthesis System (Invitrogen, Carlsbad, CA) according to manufacturer's instructions. The resulting cDNA was diluted 5-fold with nuclease free water and stored at −20°C for further use. For each real-time polymerase chain reaction (PCR), the target cDNA of interest along with reference cDNA (18S) were simultaneously amplified in 8-well strip tubes (Bio-Rad, Hercules, CA) using an MJ Research PTC200 Peltier Real-Time Thermal Cycler coupled to a Bio-Rad Chromo4 continuous fluorescence detector. Taqman primer/probe sets purchased from Applied Biosystems were as follows: c-fos (Mm00487425_m1), cd11b (Mm00434455_m1), interleukin-1β (il-1β; Mm00434228_m1), interleukin-6 (il-6; Mm00446190_m1), peripheral benzodiazepine receptor (pbr; Mm00437828_m1), tumor necrosis factor-α (tnfα; Mm00443258_m1), and 18S rRNA (4319413E). Levels of the internal control transcript, 18S rRNA (VIC fluorophore), were measured simultaneously with each target transcript (FAM fluorophore). Real-time reaction products were subjected to normalization by the comparative CT method, also referred to as the 2-ΔΔCT method.38 A threshold fluorescence value (CT) was chosen by visual inspection of the FAM and VIC fluorescence saturation curves for all reactions in a given assay run. This threshold was placed at a value that optimized the dynamic range of values across all samples. The fold-change of target transcript relative to internal control was measured for each sample by the following formula: fold-change (i.e., 2-ΔΔCT) = (CT target gene from experimental sample –CT internal control from experimental sample) / (mean of all CT target gene values – mean of all CT internal control values). Finally, to account for greatly reduced levels of target transcripts relative to 18S (a housekeeping gene), fold-change values were normalized to the mean of the saline-treated time of day control group within each target gene assay. The fold-change value of one pbr amplification reaction exceeded the group mean by > 6 standard deviations; this sample was removed from the data set prior to statistical analysis.
Experiment 3- Gene expression profiling in affinity-purified CD11b-positive cells
CD11b is a cell surface protein that is highly enriched on the surface of cells of the hematopoietic monocyte lineage, including microglia, the only population of the monocyte lineage resident in the brain parenchyma.27 The presence of CD11b on the cell membrane of microglia can be used to isolate them from CD11b-negative neuronal and astroglial populations in dissociated brain samples. The tissues used for these assays were from surgically-naive adult male C57BL/6 mice euthanized after spontaneous sleep. Subsequent to CO2 euthanasia, brains were subjected to a neuronal dissociation protocol (Miltenyi, Inc, Auburn, CA). Anti-CD11b immunoglobulins conjugated to magnetic beads (Miltenyi, Inc., Auburn, CA) were used to select CD11b-positive cells out of dissociated brain tissues per manufacturer instructions. mRNAs were purified from CD11b-positive and CD11b-negative pools of cells and subjected to real-time PCR using the above described techniques.
Statistics
Baseline values for spontaneous sleep variables measured in 3-h bins were subjected to 2-way analysis of variance (ANOVA) with pharmacological treatment (minocycline vs. saline) as a grouping factor and time of day as a repeating factor. Post-S-DEP values for dependent variables measured on an hourly basis were subjected to 3-way ANOVA with pharmacological treatment (minocycline vs. saline) and S-DEP duration (0 h vs. 1 h vs. 3 h) as grouping factors and time interval relative to the end of S-DEP (abbreviated as “time” hereafter) as a repeating factor. NREMS delta power during the first hour of sleep accumulated after S-DEP sessions was subjected to repeated-measures ANOVA with pharmacological treatment as a grouping factor and S-DEP duration as a repeating factor. Real-time PCR data were subjected to ANOVA with pharmacological treatment (minocycline vs. saline) and S-DEP duration (0 h vs. 3 h) as grouping factors. When ANOVA yielded statistically significant F values, t-tests with Bonferroni correction were performed to determine which experimental groups exhibited significant treatment effects on dependent variables. Error bars in all figures represent standard error of the mean. Statistics were performed with Statistica software (version 9.0).
RESULTS
Experiment 1- Effects of minocycline on baseline sleep and the EEG response to S-DEP
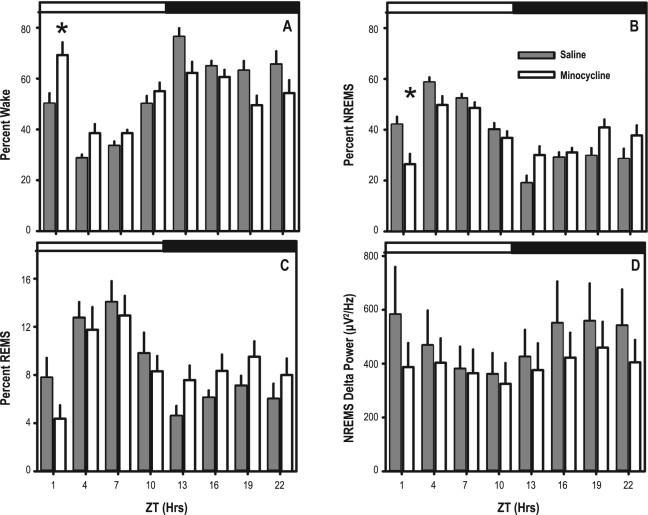
Administration of minocycline to previously pharmacologically-naive animals resulted in an acute transient increase in wakefulness (Figure 2). ANOVA yielded significant time of day × pharmacological treatment interaction for wake (F7,140 = 6.20, P < 0.001), NREMS (F7,140 = 6.02, P < 0.001) and REMS as a percent of time (F7,140 = 2.63, P < 0.014). Post hoc contrasts indicated a significant difference between the 2 pharmacological treatment groups in NREMS as a percentage of time and wake as a percentage of time only in the 3-h interval immediately after minocycline administration at light onset (Figure 2A, B). When data were assessed across the two 12-h phases of the light/dark cycle (light vs. dark; Table 1), minocycline resulted in a redistribution of the timing of wake (F1,20 = 39.36, P < 0.001), NREMS (F1,20 = 45.040, P < 0.001), and REMS (F1,20 = 7.13, P < 0.015). Time spent in NREMS was significantly less in minocycline-treated animals than saline-treated animals during the light phase and greater in minocycline-treated animals than saline-treated animals during the dark phase; inverse changes occurred in the timing of wake in response to minocycline (Figure 2A, B, and Table 1). The total time spent in each of the 3 sleep states over the entire 24-h recording session was not altered by minocycline (Table 1). Thus, the transient loss of sleep after minocycline administration at light onset was recovered over the day. Although average NREMS delta power, averaged across the entire 24-h baseline, was 23% lower in minocycline-treated animals relative to wild type animals (Figure 2D), the main effect for pharmacological treatment on NREMS delta power was not statistically significant.
Figure 2.
Baseline sleep parameters in 3-h bins: (A-C) Wake (A), NREMS (B) and REMS (C) as a percentage of each 3-h bin. (D) NREMS EEG delta power averaged across each 3-h bin. Animals were subjected to i.p. saline (gray bars) or minocycline (white bars) at light onset. The timing of the light/dark cycle is indicated by the white and dark bars at the top of the each graph.
Table 1.
Sleep parameters during the 12-h light and dark phases and across the entire 24-h baseline recording session
| Light Phase |
Dark Phase |
24-h Avg. |
||||
|---|---|---|---|---|---|---|
| Saline | Minocycline | Saline | Minocycline | Saline | Minocycline | |
| Wake (%) | 41 ± 2 | 50 ± 2* | 68 ± 2 | 57 ± 2* | 54 ± 2 | 54 ± 2 |
| NREMS (%) | 48 ± 1 | 40 ± 2* | 27 ± 2 | 35 ± 1* | 37 ± 1 | 38 ± 1 |
| REMS (%) | 11 ± 1 | 9 ± 1 | 6 ± 1 | 8 ± 1 | 9 ± 1 | 9 ± 1 |
| Delta Power (μV2/Hz) | 440 ± 111 | 372 ± 86 | 518 ± 133 | 416 ± 90 | 478 ± 121 | 393 ± 87 |
ANOVAs for repeated measures yielded significant treatment × time of day interaction for wake and NREMS (P < 0.05).
P < 0.05, saline vs. minocycline with Bonferroni correction.
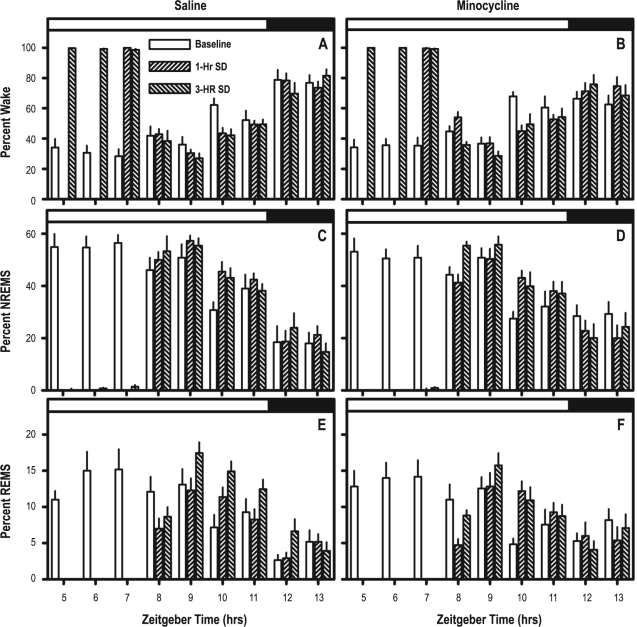
Time spent awake during S-DEP was not significantly modulated by pharmacological treatment: animals of both treatment groups spent more than 98% of time awake during all hours of both 1-h and 3-h S-DEP (Figure 3A, B). Sleep timing in the 6-h interval subsequent to S-DEP was modulated as a consequence of recovery from S-DEP. ANOVAs with pharmacological treatment as a grouping factor and S-DEP duration (0, 1, and 3 h, with 0 being data from the baseline day) and time lapsed after S-DEP (“time”) as repeating factors yielded significant S-DEP duration × time interaction for percent of time spent in wake (F10,200 = 3.25, P < 0.001), NREMS (F10,200 = 2.52, P = 0.007), and REMS (F10,200 = 6.05, P < 0.001). The absence of significant interaction of pharmacological treatment × time × S-DEP duration for all 3 states indicates that the effect of S-DEP on timing of subsequent sleep was not modulated by minocycline (Figure 3).
Figure 3.
Comparison of sleep parameters during baseline, 1-h S-DEP and 3-h S-DEP. A, C, E contain data from saline-treated mice; B, D, F contain data from minocycline-treated mice. Repeated measures ANOVA with pharmacological treatment as a grouping factor and S-DEP duration (0, 1, 3) and time of day as repeating factors yielded significant main effect of S-DEP on all parameters, but no S-DEP × pharmacological treatment interactions. The timing of the light/dark cycle is indicated by the white and dark bars at the top of the each graph.
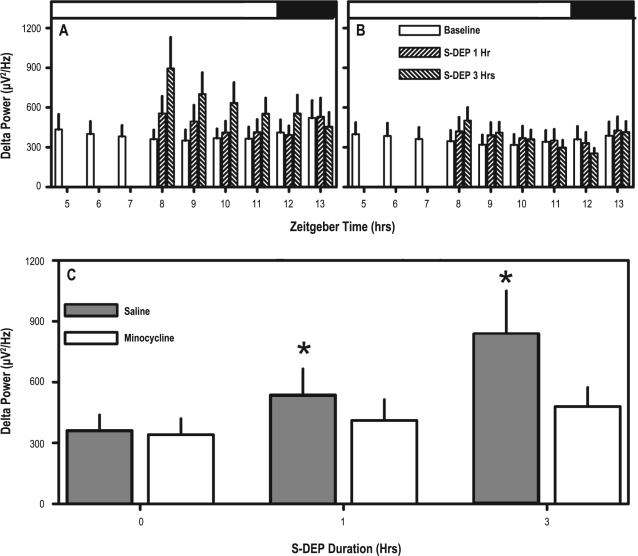
Minocycline did, however, strongly attenuate the increase in NREMS delta power after S-DEP relative to baseline. ANOVA with pharmacological treatment as a grouping factor and S-DEP duration and time as repeating factors yielded a significant interaction of pharmacological treatment × time × S-DEP duration affecting NREMS delta power (F2,40 = 3.71, P = 0.033; Figure 4A, B). Additionally, when peak NREMS delta power (i.e., delta power during the first 360 accumulated epochs of sleep) was subjected to analysis, ANOVA yielded a significant pharmacological treatment × S-DEP duration interaction (F2,40 = 3.50, P = 0.040). Both durations of S-DEP resulted in increased NREMS delta power in saline-treated animals but failed to cause an increase in NREMS delta power in minocycline-treated animals (Figure 4C).
Figure 4.
Effect of S-DEP on NREMS delta power. (A, B) NREMS delta power within each h of clock time from S-DEP onset through 6 h post-S-DEP. Data are from baseline, 1-h S-DEP and 3-h S-DEP. (A) Data from saline-treated mice. (B) Data from minocycline-treated mice. (C) Average NREMS delta power measured across the first 360 accumulated epochs of sleep subsequent to 1- or 3-h S-DEP, and during the analogous time of day on the baseline day in saline-treated mice (gray) and minocycline-treated mice (white). *P < 0.05 vs. baseline, same group, with Bonferroni correction. The timing of the light/dark cycle is indicated by the white and dark bars at the top of A and B.
Experiment 2- Effects of S-DEP and minocycline on gene expression
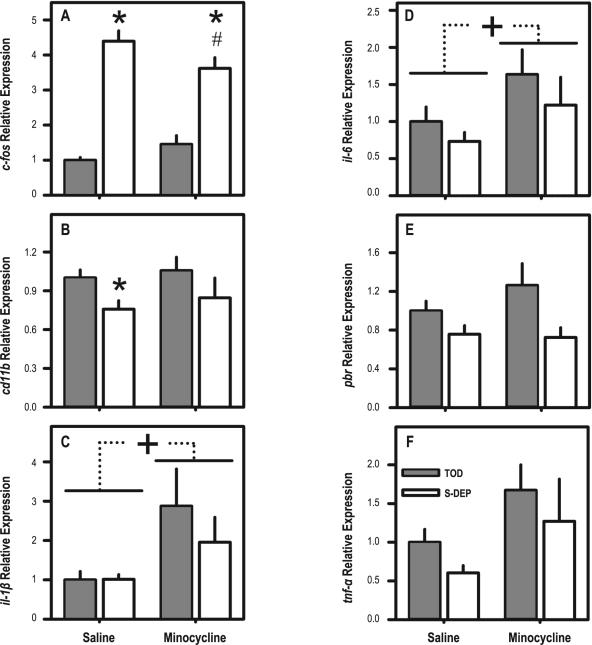
Real-time PCR was used to assess changes in one positive control transcript known to be elevated the brain in during S-DEP (c-fos) and 5 transcripts that are related to neuroinflammatory events (cd11b, il-1β, il-6, pbr, and tnfα). Expression of il-1β, il-6, and tnfα was unaffected by S-DEP. Two-way ANOVAs yielded a significant effect of S-DEP on the expression of c-fos (F1,28 = 123.03, P < 0.001), pbr (F1,27 = 7.37, P = 0.011), and cd11b (F1,28 = 4.88, P = 0.0035), in whole brain tissue samples (Figure 5; Table 2). The effect of S-DEP on c-fos expression was modulated by minocycline treatment (interaction F1,28 = 5.98, P = 0.021), and was significantly blunted in the minocycline-treated S-DEP group relative to the saline-treated S-DEP group. Whereas c-fos expression was elevated > 4-fold in sleep deprived saline-treated mice, it was elevated < 2.5-fold in sleep-deprived minocycline-treated mice relative to spontaneously sleeping controls (Figure 5A).
Figure 5.
Effects of S-DEP and minocycline on gene expression in brain tissues from mice euthanized after 3-h S-DEP and spontaneous sleep controls. See Table 2 for statistical analyses. *P < 0.05 vs. time of day control, Bonferroni corrected. #P < 0.05 vs. S-DEP-saline group. +P < 0.05 for main effect of minocycline.
Table 2.
Effects of S-DEP and minocycline on gene expression in brain tissues
| Target Gene | Main Effect, S-DEP (df = 1,28) | Main Effect, Minocycline (df = 1,28) | Interaction (df = 1,28) |
|---|---|---|---|
| c-fos | F = 123.0, P < 0.001 | NS | F = 6.0, P = 0.021 |
| cd11b | F = 4.9, P = 0.035 | NS | NS |
| il-1β | NS | F = 5.9, P = 0.022 | NS |
| il-6 | NS | F = 4.8, P = 0.037 | NS |
| pbr | F = 7.4, P = 0.011* | NS | NS |
| tnfα | NS | F = 4.1, P = 0.053 | NS |
df = 1,27 for pbr
Expression of cd11b was reduced in S-DEP animals relative to time of day controls (F1,28 = 4.88, P = 0.035), by 24% in saline-treated mice and 20% in minocycline-treated mice (Figure 5B). This reduction was significant only in saline-treated mice after Bonferroni correction. pbr expression was also reduced in S-DEP animals relative to time of day controls (F1,27 = 7.37, P = 0.011), by 24% in saline-treated mice and 28% in minocycline-treated mice (Figure 5E).
There were significant effects of minocycline on expression of il-1β (F1,28 = 5.89, P = 0.022), and il-6 (F1,28 = 4.81, P = 0.037). Both of these transcripts were elevated in minocycline-treated animals relative to controls (Figure 5C, D). The greater than 80% elevation of tnfα expression in minocycline-treated mice relative to saline-treated mice (Figure 5F) was nearly statistically significant (F1,28 = 4.06, P = 0.053).
Experiment 3- Gene expression profiling in affinity-purified CD11b-positive cells
Measurement of gene expression in CD11b-positive and CD11b-negative cell pools from minocycline-naive mice demonstrated that S-DEP- and minocycline-regulated genes were enriched ≥ 10-fold in the microglial (CD11b-positive) pool of brain cells relative to the CD11b-negative pool (Table 3). The 2622-fold enrichment for the mRNA encoding CD11b demonstrates that the sorting of cells for CD11b immunopositivity was successful. Also enriched in the CD11b-positive cell pool relative to the CD11b-negative pool were the S-DEP-induced transcript c-fos (11-fold) and the genes up-regulated in minocycline-treated mice relative to saline-treated mice: il-1β (633-fold) and il-6 (53-fold) and tnfα (696-fold).
Table 3.
Enrichment of neuroinflammation-related transcripts in CD11b-positive vs. CD11b-negative brain tissue pools
| Target Gene CT |
Target Gene | |||
|---|---|---|---|---|
| Mean ± SEM |
Fold Difference | |||
| Target Gene | CD11b+ | CD11b− | ANOVA (df = 1,42) | (Positive/Negative) |
| c-fos | 20.6 ± 0.3 | 23.8 ± 0.5 | F = 32.1, P < 0.001 | 11.18 |
| cd11b | 21.1 ± 0.3 | 32.2 ± 0.4 | F = 327.1, P < 0.001 | 2622.41 |
| il-1β | 23.5 ± 0.3 | 30.8 ± 1.5 | F = 22.2, P < 0.001 | 633.00 |
| il-6 | 27.9 ± 0.3 | 33.9 ± 0.5 | F = 92.4, P < 0.001 | 52.56 |
| pbr | 30.1 ± 0.2 | 29 ± 0.3 | F = 8.17, P = 0.007 | 0.56 |
| tnfα | 23.3 ± 0.2 | 32.5 ± 0.6 | F = 183.4, P < 0.001 | 696.22 |
Fold difference values > 1 indicate enrichment in the CD11b-positive cell pool; fold difference values < 1 indicate enrichment in the CD11b-negative cell pool.
DISCUSSION
Sleep is known to be influenced by the immune system,2,39 but whether microglia, the innate immune cells of the brain, regulate sleep is an unanswered question. Here, we have demonstrated an effect of sleep deprivation on the expression of cd11b, a molecular marker for microglia. Additionally, we have shown that minocycline suppresses sleep in baseline conditions and sleep slow waves after S-DEP. Minocycline is known to act directly on microglial cells, to suppress production of cytokines, nitric oxide and prostaglandins, and to attenuate cerebral neurochemical changes mediated by these microglial products.21,40–42 The current study documents effects of minocycline on mRNAs encoding cytokines that promote sleep (il-1β, il-6, and tnfα).2,12 We measured mRNAs for these cytokines with two hypotheses: (1) that cytokine mRNAs would increase in concentration as a consequence of sleep loss (as in previous reports43,44) and (2) that the increase resulting from sleep loss would be attenuated by administration of minocycline, in parallel with the attenuated delta power response to S-DEP. Since the first hypothesis was not supported by the data, it was not possible to test the second hypothesis. IL-1β44 and TNFα43 are elevated in the brain subsequent to 6-h S-DEP relative to time of day controls. In the current study 1- and 3-h S-DEP failed to elevate these mRNAs. It is likely that the duration of S-DEP used, while sufficient to detect an effect of minocycline on the EEG, was not sufficient to drive changes in these mRNAs. Therefore, the effect of minocycline on the EEG is not likely to be mediated by suppression of cytokine-encoding mRNAs.
In fact, only the positive control mRNA (c-fos), a molecule that is reliably upregulated by sleep loss,45–47 was elevated in response to S-DEP. This effect of S-DEP was attenuated by minocycline. The simultaneous attenuation of c-fos expression and EEG delta power show that minocycline suppresses the cerebral response to S-DEP at both molecular and electrophysiological levels. Minocycline inhibits the activity of poly-ADP ribose polymerase (PARP).48 PARP activates the p38 kinase, a critical mediator of Ca2+-induced intracellular signaling.49 PARP inhibition by minocycline consequently attenuates p38 kinase activity.49,50 Direct inhibition of p38 activity with the compound SB203580 suppresses Ca2+ mediated transcriptional changes, including upregulation of c-fos51, so it is a reasonable assumption that PARP inhibition by minocycline should do the same. Given that wake-related transmitters elevate intracellular Ca2+ in microglia (see below) and neurons alike, the suppression of c-fos expression by minocycline is likely an indication of a general suppression of transcriptional activation normally associated with wakefulness. Since c-fos is an indicator of a broader cellular transcriptional response to wake-related excitation,52,53 the attenuation of this transcriptional response by minocycline may be one mechanism by which minocycline attenuates the EEG response to S-DEP.
We used a chronic minocycline protocol because this protocol was previously shown to suppress neuroinflammation-dependent processes in vivo.31,54 The neuroinflammatory event studied previously, stroke-induced neuronal degeneration, emerges over a period of several days, and thus was studied in the context of chronic minocycline treatment. By contrast, all the effects of minocycline on sleep timing and the sleep EEG were acute, occurring within 4 h of administration. We are unaware of any data on the half-life of minocycline in the CD-1 mouse, but it seems likely that the effects on sleep timing and sleep EEG are dependent on a pharmacologically active concentration of minocycline being present, rather than a long-term change in cellular physiology as a consequence of transient minocycline exposure.
Long-term effects of chronic minocycline exposure have been documented in the context of neurotoxicity54 or neurodegeneration,31 conditions which, in and of themselves, cause long-term changes in neuronal function. Thus, a transient neuroprotective effect of minocycline in neurotoxicity or neurodegeneration has long-term manifestations in the viability of neurons. Since sleep is a homeostatic process subject to relatively short term (i.e., ultradian) regulation, the effects of minocycline on sleep state timing and EEG might only be expected to occur in the short-term. In vitro data also demonstrate acute effects of minocycline on inflammatory markers.40 Therefore, the chronic exposure to minocycline was probably not necessary for the experimental effects that were measured after S-DEP in the current study. Whether chronic and acute exposures to minocycline have distinct effects on the EEG or neurochemical markers of sleep loss remains to be determined.
The complexity of the response to minocycline makes it difficult to attribute the neuroprotective effects of minocycline to specific mechanisms,55 the effects on the EEG and c-fos expression are not necessarily mediated by direct actions of minocycline within microglia. For instance, minocycline is toxic to gram negative bacteria.56 S-DEP (albeit of durations far greater than those used in the current study) is known to facilitate the translocation of bacteria from the intestinal lumen into the peritoneal cavity57 and to increase tissue leukocyte numbers,58 a possible sign of increased bacterial load. These effects may contribute to the increased severity of sepsis caused by S-DEP in rodents subjected to cecal puncture and ligation.59 Minocycline may, by virtue of its toxicity to bacteria, reduce the systemic bacterial load, and thereby reduce systemic exposure to the somnogenic bacterial products. Whether the attenuation of S-DEP effects on EEG by minocycline is due to reduced systemic concentrations of bacterial factors is a possibility that cannot be ruled out. Further studies will be necessary to ascertain whether changes in microglial function alone are sufficient to modulate the EEG response to sleep loss.
A wealth of data demonstrate that IL-1β, IL-6, and TNFα promote sleep.2,12 Levels of these substances in the brain increase during infectious states characterized by excessive sleepiness and in response to sleep loss. Experimentally induced increases in the levels of these substances in the brain promote sleep, while genetic inactivation or antibody-induced blockade of their receptors suppresses sleep. The transcripts encoding IL-1β, IL-6, and TNFα were enriched in the microglial CD11b-positive population of cells by 50- to 600-fold relative to the CD11b-negative pool (Table 3). This observation alone demonstrates that microglia are potentially a significant source of sleep regulatory factors in the brain. We hypothesized that these mRNAs would be suppressed in minocycline-treated mice in parallel with the suppression of sleep and S-DEP-induced slow waves. In contrast to expectations, these mRNAs were elevated by minocycline. We conclude that either these cytokines do not mediate the effect of minocycline on sleep, or the effect is mediated by changes at the protein level distinct from those at the mRNA level. Regarding the latter possibility, cytokine mRNAs and protein levels do not necessarily change in lock-step. Attenuation of cytokine release is a well-documented effect of minocycline.41,60 The expression of pro-inflammatory cytokines at the mRNA level is sensitive to negative feedback from cell signaling pathways activated by cell surface receptors.61 The increase in il-1β and il-6 mRNAs (and near significant 80% increase in tnfα) in minocycline-treated mice relative to saline-treated mice is therefore not necessarily an indicator of increased cytokine protein levels, but, in fact, may be a consequence of disinhibition of transcription due to minocycline-induced reductions in extracellular levels of these inflammatory mediators. From a technical standpoint, we only measured mRNA levels. We did not directly measure synthesis or release of the proteins they encode. Additionally, mice were euthanized after 3 h of S-DEP because this duration matched that of the longest S-DEP duration in the EEG study. It would be useful to determine whether a longer duration of S-DEP would result in increased expression of genes related to neuroinflammation and microglial activation, and whether minocycline attenuates this response. However, short of such information, the biochemical data provided here do not support the hypothesis that minocycline acts through downregulation of cytokine mRNAs to suppress EEG slow wave activity. Other actions of minocycline that were not measured, including the effects on cerebral cytokine synthesis at the protein level,60 cytokine release,41,62,63 and production of nitric oxide42,63 and prostaglandins,42 might mediate its effects on sleep.
The suppression of cd11b mRNA by S-DEP demonstrates for the first time that microglia detect and react to short-term sleep loss. But what can explain the decrease in cd11b and pbr expression in association with sleep deprivation? This change may be an effect of wake-related neurotransmission on microglial physiology. Acetylcholine levels in the cerebral cortex are elevated in wakefulness relative to slow wave sleep.64 Acetylcholine acts through microglial muscarinic receptors to increase intracellular Ca2+ concentration.65 Increased intracellular Ca2+ activates protein kinase C.66 Protein kinase C triggers an increase in the binding activity of the transcriptional repressor ZBP-89 to regulatory sequences on the cd11b promoter, resulting in transcriptional repression of the cd11b gene,67 an effect mediated by inhibition of the transcriptional activator SP1.68 Microglia also express receptors for noradrenaline and glutamate.69,70 Noradrenaline and glutamate levels are elevated in the brain during wakefulness relative to SWS and can affect intracellular Ca2+ signaling pathways in ways analogous to acetylcholine.71,72
The transcriptional regulatory element conferring protein kinase C sensitivity to cd11b (an SP1 promoter element) also exists in the pbr promoter.73 Therefore, downregulation of pbr is regulated by the same signaling cascade. Given that pbr expression is, in fact, enriched in non-microglial cells (most likely astrocytes74) despite expectations to the contrary,75 the relevance of S-DEP-induced suppression of pbr expression to microglial function is questionable.
Adenosine and its metabolic precursor, adenosine triphosphate, may also play a role in the context of cd11b downregulation after S-DEP. Adenosine is elevated in the brain during S-DEP.76 Microglia are enriched for adenosine receptors and for purinergic receptors activated by ATP. Microglia react to adenosine receptor activation by reducing their scavenging activity in the brain parenchyma.77 Reduced cd11b expression in microglial cells during S-DEP may be a manifestation of the microglial response to increased adenosine.
Cd11b downregulation may, in turn, underlie changes in sleep propensity that occur as a consequence of S-DEP. CD11b blocks the intracellular signaling pathways that promote the synthesis and release of pro-inflammatory cytokines.78 S-DEP-induced downregulation of cd11b is thus expected to disinhibit pro-inflammatory cytokine production in microglia, thereby elevating the propensity for sleep. Additionally, CD11b protein, together with CD18, forms the integrin needed for the adhesion of microglia to other cells in their environment.79 Such interactions at the microglial cell surface are necessary for synaptic elimination,80 a posited function of sleep.81 Reduced phagocytotic activity, as a consequence of reduced integrin signaling, may contribute to the putative neuroprotective effect of S-DEP in cerebral ischemia.82
In addition to their role in immune surveillance within the brain, microglia also produce growth factors and neurotrophins, and perform glutamate uptake.83–85 They can thus be thought of as critical support cells for neurotransmission. Microglia are derived from the myeloid hematopoietic lineage and consequently are very similar, morphologically and biochemically, to macrophages and related inflammatory cell types.16 Given the increasing certainty with which sleep insufficiency has been linked to disorders of inflammation in both the brain86 and elsewhere,1,58 further studies are needed on the roles of microglia in mediating physiological and pathological responses to sleep loss.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
These experiments were supported by a Washington State University, Spokane Faculty Seed grant.
REFERENCES
- 1.Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009;10:199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lange T, Dimitrov S, Fehm HL, Westermann J, Born J. Shift of monocyte function toward cellular immunity during sleep. Arch Intern Med. 2006;166:1695–700. doi: 10.1001/archinte.166.16.1695. [DOI] [PubMed] [Google Scholar]
- 4.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–78. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irwin MR, Wang M, Ribeiro D, et al. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008;64:538–40. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haensel A, Bardwell WA, Mills PJ, et al. Relationship between inflammation and cognitive function in obstructive sleep apnea. Sleep Breath. 2009;13:35–41. doi: 10.1007/s11325-008-0198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30:1145–52. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frey DJ, Fleshner M, Wright KP., Jr The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun. 2007;21:1050–7. doi: 10.1016/j.bbi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–62. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 10.van Leeuwen WM, Lehto M, Karisola P, et al. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PLoS One. 2009;4:e4589. doi: 10.1371/journal.pone.0004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marshall L, Born J. Brain-immune interactions in sleep. Int Rev Neurobiol. 2002;52:93–131. doi: 10.1016/s0074-7742(02)52007-9. [DOI] [PubMed] [Google Scholar]
- 12.Krueger JM. The role of cytokines in sleep regulation. Curr Pharm Des. 2008;14:3408–16. doi: 10.2174/138161208786549281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones BE. Basic mechanisms of sleep-wake states. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 3rd ed. Philadelphia: WB Saunders; 2000. pp. 134–54. [Google Scholar]
- 14.Fellin T, Halassa MM, Terunuma M, et al. Endogenous nonneuronal modulators of synaptic transmission control cortical slow oscillations in vivo. Proc Natl Acad Sci U S A. 2009;106:15037–42. doi: 10.1073/pnas.0906419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halassa MM, Florian C, Fellin T, et al. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–9. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tambuyzer BR, Ponsaerts P, Nouwen EJ. Microglia: gatekeepers of central nervous system immunology. J Leukoc Biol. 2008:352–70. doi: 10.1189/jlb.0608385. 352–70. [DOI] [PubMed] [Google Scholar]
- 17.Watkins LR, Milligan ED, Maier SF. Spinal cord glia: new players in pain. Pain. 2001;93:201–5. doi: 10.1016/S0304-3959(01)00359-1. [DOI] [PubMed] [Google Scholar]
- 18.Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J Leukoc Biol. 2008;84:932–9. doi: 10.1189/jlb.0208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watkins LR, Maier SF. Immune regulation of central nervous system functions: from sickness responses to pathological pain. J Intern Med. 2005;257:139–55. doi: 10.1111/j.1365-2796.2004.01443.x. [DOI] [PubMed] [Google Scholar]
- 20.Terao A, Huang ZL, Wisor JP, et al. Gene expression in the rat brain during prostaglandin D2 and adenosinergically-induced sleep. J Neurochem. 2008;105:1480–98. doi: 10.1111/j.1471-4159.2008.05257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim HS, Suh YH. Minocycline and neurodegenerative diseases. Behav Brain Res. 2009;196:168–79. doi: 10.1016/j.bbr.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 22.Nonaka K, Nakazawa Y, Kotorii T. Effects of antibiotics, minocycline and ampicillin, on human sleep. Brain Res. 1983;288:253–9. doi: 10.1016/0006-8993(83)90101-4. [DOI] [PubMed] [Google Scholar]
- 23.Toth LA. Strain differences in the somnogenic effects of interferon inducers in mice. J Interferon Cytokine Res. 1996;16:1065–72. doi: 10.1089/jir.1996.16.1065. [DOI] [PubMed] [Google Scholar]
- 24.Terao A, Steininger TL, Hyder K, et al. Differential increase in the expression of heat shock protein family members during sleep deprivation and during sleep. Neuroscience. 2003;116:187–200. doi: 10.1016/s0306-4522(02)00695-4. [DOI] [PubMed] [Google Scholar]
- 25.Cirelli C, Tononi G. Gene expression in the brain across the sleep-waking cycle. Brain Res. 2000;885:303–21. doi: 10.1016/s0006-8993(00)03008-0. [DOI] [PubMed] [Google Scholar]
- 26.Hsu JC, Lee YS, Chang CN, Chuang HL, Ling EA, Lan CT. Sleep deprivation inhibits expression of NADPH-d and NOS while activating microglia and astroglia in the rat hippocampus. Cells Tissues Organs. 2003;173:242–54. doi: 10.1159/000070380. [DOI] [PubMed] [Google Scholar]
- 27.Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–45. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 28.Lynch MA. The multifaceted profile of activated microglia. Mol Neurobiol. 2009;40:139–56. doi: 10.1007/s12035-009-8077-9. [DOI] [PubMed] [Google Scholar]
- 29.Sugama S, Takenouchi T, Fujita M, Conti B, Hashimoto M. Differential microglial activation between acute stress and lipopolysaccharide treatment. J Neuroimmunol. 2008;207:24–31. doi: 10.1016/j.jneuroim.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 30.He Y, Appel S, Le W. Minocycline inhibits microglial activation and protects nigral cells after 6-hydroxydopamine injection into mouse striatum. Brain Res. 2001;909:187–93. doi: 10.1016/s0006-8993(01)02681-6. [DOI] [PubMed] [Google Scholar]
- 31.Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci U S A. 1998;95:15769–74. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aldinger KA, Sokoloff G, Rosenberg DM, Palmer AA, Millen KJ. Genetic variation and population substructure in outbred CD-1 mice: implications for genome-wide association studies. PLoS ONE. 2009;4:e4729. doi: 10.1371/journal.pone.0004729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Institute of Laboratory Animal Resources CoLS, National Research Council. Guide for Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- 34.Wisor JP, Pasumarthi RK, Gerashchenko D, et al. Sleep deprivation effects on circadian clock gene expression in the cerebral cortex parallel electroencephalographic differences among mouse strains. J Neurosci. 2008;28:7193–201. doi: 10.1523/JNEUROSCI.1150-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wisor JP, O'Hara BF, Terao A, et al. A role for cryptochromes in sleep regulation. BMC Neurosci. 2002;3:20. doi: 10.1186/1471-2202-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franken P, Chollet D, Tafti M. The homeostatic regulation of sleep need is under genetic control. J Neurosci. 2001;21:2610–21. doi: 10.1523/JNEUROSCI.21-08-02610.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huber R, Deboer T, Tobler I. Effects of sleep deprivation on sleep and sleep EEG in three mouse strains: empirical data and simulations. Brain Res. 2000;857:8–19. doi: 10.1016/s0006-8993(99)02248-9. [DOI] [PubMed] [Google Scholar]
- 38.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 39.Bentivoglio M, Kristensson K. Neural-immune interactions in disorders of sleep-wakefulness organization. Trends Neurosci. 2007;30:645–52. doi: 10.1016/j.tins.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Nutile-McMenemy N, Elfenbein A, Deleo JA. Minocycline decreases in vitro microglial motility, beta1-integrin, and Kv1.3 channel expression. J Neurochem. 2007;103:2035–46. doi: 10.1111/j.1471-4159.2007.04889.x. [DOI] [PubMed] [Google Scholar]
- 41.Saito O, Svensson CI, Buczynski MW, et al. Spinal glial TLR4-mediated nociception and production of prostaglandin E and TNF. Br J Pharmacol. 2010;160:1754–64. doi: 10.1111/j.1476-5381.2010.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsui T, Svensson CI, Hirata Y, Mizobata K, Hua XY, Yaksh TL. Release of prostaglandin E2 and nitric oxide from spinal microglia is dependent on activation of p38 mitogen-activated protein kinase. Anesth Analg. 2010;111:554–60. doi: 10.1213/ANE.0b013e3181e3a2a2. [DOI] [PubMed] [Google Scholar]
- 43.Veasey SC, Mackiewicz M, Fenik P, Ro M, Ogilvie MD, Pack AI. IL1β knockout mice lack the TNFα response to sleep deprivation but have normal sleep and sleep recovery. Soc Neur Abs. 1997;23:792. [Google Scholar]
- 44.Taishi P, Chen Z, Obal F, Jr, et al. Sleep-associated changes in interleukin-1beta mRNA in the brain. J Interferon Cytokine Res. 1998;18:793–8. doi: 10.1089/jir.1998.18.793. [DOI] [PubMed] [Google Scholar]
- 45.O'Hara BF, Young KA, Watson FL, Heller HC, Kilduff TS. Immediate early gene expression in brain during sleep deprivation: preliminary observations. Sleep. 1993;16:1–7. doi: 10.1093/sleep/16.1.1. [DOI] [PubMed] [Google Scholar]
- 46.Pompeiano M, Cirelli C, Tononi G. Immediate-early genes in spontaneous wakefulness and sleep: expression of c-fos and NGFI-A mRNA and protein. J Sleep Res. 1994;3:80–96. doi: 10.1111/j.1365-2869.1994.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 47.Cirelli C, Pompeiano M, Tononi G. Neuronal gene expression in the waking state: a role for the locus coeruleus. Science. 1996;274:1211–5. doi: 10.1126/science.274.5290.1211. [DOI] [PubMed] [Google Scholar]
- 48.Alano CC, Kauppinen TM, Valls AV, Swanson RA. Minocycline inhibits poly(ADP-ribose) polymerase-1 at nanomolar concentrations. Proc Natl Acad Sci U S A. 2006;103:9685–90. doi: 10.1073/pnas.0600554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mester L, Szabo A, Atlasz T, et al. Protection against chronic hypoperfusion-induced retinal neurodegeneration by PARP inhibition via activation of PI-3-kinase Akt pathway and suppression of JNK and p38 MAP kinases. Neurotox Res. 2009;16:68–76. doi: 10.1007/s12640-009-9049-6. [DOI] [PubMed] [Google Scholar]
- 50.Corsaro A, Thellung S, Chiovitti K, et al. Dual modulation of ERK1/2 and p38 MAP kinase activities induced by minocycline reverses the neurotoxic effects of the prion protein fragment 90-231. Neurotox Res. 2009;15:138–54. doi: 10.1007/s12640-009-9015-3. [DOI] [PubMed] [Google Scholar]
- 51.Lee SA, Park JK, Kang EK, Bae HR, Bae KW, Park HT. Calmodulin-dependent activation of p38 and p42/44 mitogen-activated protein kinases contributes to c-fos expression by calcium in PC12 cells: modulation by nitric oxide. Brain Res Mol Brain Res. 2000;75:16–24. doi: 10.1016/s0169-328x(99)00280-6. [DOI] [PubMed] [Google Scholar]
- 52.Cirelli C. Cellular consequences of sleep deprivation in the brain. Sleep Med Rev. 2006;10:307–21. doi: 10.1016/j.smrv.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 53.Cirelli C, Tononi G. Locus ceruleus control of state-dependent gene expression. J Neurosci. 2004;24:5410–9. doi: 10.1523/JNEUROSCI.0949-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Padi SS, Kulkarni SK. Minocycline prevents the development of neuropathic pain, but not acute pain: possible anti-inflammatory and antioxidant mechanisms. Eur J Pharmacol. 2008;601:79–87. doi: 10.1016/j.ejphar.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 55.Domercq M, Matute C. Neuroprotection by tetracyclines. Trends Pharmacol Sci. 2004;25:609–12. doi: 10.1016/j.tips.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 56.Norskov-Lauritsen N, Marchandin H, Dowzicky MJ. Antimicrobial susceptibility of tigecycline and comparators against bacterial isolates collected as part of the TEST study in Europe (2004-2007) Int J Antimicrob Agents. 2009;34:121–30. doi: 10.1016/j.ijantimicag.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 57.Everson CA, Toth LA. Systemic bacterial invasion induced by sleep deprivation. Am J Physiol Regul Integr Comp Physiol. 2000;278:R905–16. doi: 10.1152/ajpregu.2000.278.4.R905. [DOI] [PubMed] [Google Scholar]
- 58.Everson CA, Thalacker CD, Hogg N. Phagocyte migration and cellular stress induced in liver, lung, and intestine during sleep loss and sleep recovery. Am J Physiol Regul Integr Comp Physiol. 2008;295:R2067–R74. doi: 10.1152/ajpregu.90623.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Friese RS, Bruns B, Sinton CM. Sleep deprivation after septic insult increases mortality independent of age. J Trauma. 2009;66:50–4. doi: 10.1097/TA.0b013e318190c3a1. [DOI] [PubMed] [Google Scholar]
- 60.Cai ZY, Yan Y, Chen R. Minocycline reduces astrocytic reactivation and neuroinflammation in the hippocampus of a vascular cognitive impairment rat model. Neurosci Bull. 2010;26:28–36. doi: 10.1007/s12264-010-0818-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tan JC, Rabkin R. Suppressors of cytokine signaling in health and disease. Pediatr Nephrol. 2005;20:567–75. doi: 10.1007/s00467-004-1766-8. [DOI] [PubMed] [Google Scholar]
- 62.Bernardino AL, Kaushal D, Philipp MT. The antibiotics doxycycline and minocycline inhibit the inflammatory responses to the Lyme disease spirochete Borrelia burgdorferi. J Infect Dis. 2009;199:1379–88. doi: 10.1086/597807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci. 2001;21:2580–8. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marrosu F, Portas C, Mascia MS, et al. Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep-wake cycle in freely moving cats. Brain Res. 1995;671:329–32. doi: 10.1016/0006-8993(94)01399-3. [DOI] [PubMed] [Google Scholar]
- 65.Zhang L, McLarnon JG, Goghari V, Lee YB, Kim SU, Krieger C. Cholinergic agonists increase intracellular Ca2+ in cultured human microglia. Neurosci Lett. 1998;255:33–6. doi: 10.1016/s0304-3940(98)00706-x. [DOI] [PubMed] [Google Scholar]
- 66.Purves D, Augustine GJ, Fitzpatrick D, et al. Neurotransmitters and their receptors. Neuroscience. Sunderland, MA: Sinauer Associates; 2008. [Google Scholar]
- 67.Park H, Shelley CS, Arnaout MA. The zinc finger transcription factor ZBP-89 is a repressor of the human beta 2-integrin CD11b gene. Blood. 2003;101:894–902. doi: 10.1182/blood-2002-03-0680. [DOI] [PubMed] [Google Scholar]
- 68.Zhang X, Diab IH, Zehner ZE. ZBP-89 represses vimentin gene transcription by interacting with the transcriptional activator, Sp1. Nucleic Acids Res. 2003;31:2900–14. doi: 10.1093/nar/gkg380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mori K, Ozaki E, Zhang B, et al. Effects of norepinephrine on rat cultured microglial cells that express alpha1, alpha2, beta1 and beta2 adrenergic receptors. Neuropharmacology. 2002;43:1026–34. doi: 10.1016/s0028-3908(02)00211-3. [DOI] [PubMed] [Google Scholar]
- 70.Noda M, Nakanishi H, Nabekura J, Akaike N. AMPA-kainate subtypes of glutamate receptor in rat cerebral microglia. J Neurosci. 2000;20:251–8. doi: 10.1523/JNEUROSCI.20-01-00251.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park SP. In vivo microdialysis measures of extracellular norepinephrine in the rat amygdala during sleep-wakefulness. J Korean Med Sci. 2002;17:395–9. doi: 10.3346/jkms.2002.17.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dash MB, Douglas CL, Vyazovskiy VV, Cirelli C, Tononi G. Long-term homeostasis of extracellular glutamate in the rat cerebral cortex across sleep and waking states. J Neurosci. 2009;29:620–9. doi: 10.1523/JNEUROSCI.5486-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Giatzakis C, Papadopoulos V. Differential utilization of the promoter of peripheral-type benzodiazepine receptor by steroidogenic versus nonsteroidogenic cell lines and the role of Sp1 and Sp3 in the regulation of basal activity. Endocrinology. 2004;145:1113–23. doi: 10.1210/en.2003-1330. [DOI] [PubMed] [Google Scholar]
- 74.Chen MK, Baidoo K, Verina T, Guilarte TR. Peripheral benzodiazepine receptor imaging in CNS demyelination: functional implications of anatomical and cellular localization. Brain. 2004;127:1379–92. doi: 10.1093/brain/awh161. [DOI] [PubMed] [Google Scholar]
- 75.Sekine Y, Ouchi Y, Sugihara G, et al. Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci. 2008;28:5756–61. doi: 10.1523/JNEUROSCI.1179-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–8. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gyoneva S, Orr AG, Traynelis SF. Differential regulation of microglial motility by ATP/ADP and adenosine. Parkinsonism Relat Disord. 2009;15(Suppl 3):S195–9. doi: 10.1016/S1353-8020(09)70813-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Han C, Jin J, Xu S, Liu H, Li N, Cao X. Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat Immunol. 2010;11:734–42. doi: 10.1038/ni.1908. [DOI] [PubMed] [Google Scholar]
- 79.Rotshenker S. The role of galectin-3/MAC-2 in the activation of the innate-immune function of phagocytosis in microglia in injury and disease. J Mol Neurosci. 2009;39:99–103. doi: 10.1007/s12031-009-9186-7. [DOI] [PubMed] [Google Scholar]
- 80.Stevens B, Allen NJ, Vazquez LE, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–78. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 81.Tononi G. Slow wave homeostasis and synaptic plasticity. J Clin Sleep Med. 2009;5:S16–S9. [PMC free article] [PubMed] [Google Scholar]
- 82.Hsu JC, Lee YS, Chang CN, Ling EA, Lan CT. Sleep deprivation prior to transient global cerebral ischemia attenuates glial reaction in the rat hippocampal formation. Brain Res. 2003;984:170–81. doi: 10.1016/s0006-8993(03)03128-7. [DOI] [PubMed] [Google Scholar]
- 83.Lambertsen KL, Clausen BH, Babcock AA, et al. Microglia protect neurons against ischemia by synthesis of tumor necrosis factor. J Neurosci. 2009;29:1319–30. doi: 10.1523/JNEUROSCI.5505-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Streit WJ, Xue QS. Life and death of microglia. J Neuroimmune Pharmacol. 2009;4:371–9. doi: 10.1007/s11481-009-9163-5. [DOI] [PubMed] [Google Scholar]
- 85.Ronnback L, Hansson E. On the potential role of glutamate transport in mental fatigue. J Neuroinflammation. 2004;1:22. doi: 10.1186/1742-2094-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kang JE, Lim MM, Bateman RJ, et al. Amyloid-β dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–7. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]