Abstract
Introduction:
Functional magnetic resonance imaging (fMRI) studies enable the investigation of neural correlates underlying behavioral performance. We investigate the working memory (WM) function of patients with untreated obstructive sleep apnea (OSA) from the view point of task positive and default mode networks (TPN and DMN, respectively) and compare the results to those of healthy controls (HC).
Methods:
A parametric fMRI experiment with 4 levels of visuospatial N-back task was used to investigate the pattern of cortical activation in 17 men with untreated moderate or severe OSA and 7 age-matched HC. Categorical and parametrical analysis of the data was performed. Multiple regression analysis of fMRI data of OSA patients was performed with AHI, nocturnal desaturation time, and BMI as covariates.
Results:
OSA patients demonstrate compensatory spatial recruitment of the TPN (maximal at 3-back) and of the DMN (maximal at 2-back). HC had a different patten of spatial recruitment and deactivation of the DMN at the maximal load of task (3-back). Nocturnal desaturation had significant positive correlation with BOLD signal in bilateral frontal, temporal, and occipital regions, and negative correlations in bilateral frontal and left parietal regions; whereas BMI showed only negative correlations with BOLD signal, predominantly in the PFC. AHI was positively correlated with BOLD signal in bilateral frontal regions.
Conclusion:
Both TPN and DMN are affected in OSA patients, with nocturnal desaturation affecting both networks; whereas BMI appears to be the major negative factor influencing the TPN and has a significant negative correlation with behavioral performance.
Citation:
Prilipko O; Huynh N; Schwartz S; Tantrakul V; Kim JH; Peralta AR; Kushida C; Paiva T; Guilleminault C. Task positive and default mode networks during a parametric working memory task in obstructive sleep apnea patients and healthy controls. SLEEP 2011;34(3):293-301.
Keywords: Obstructive sleep apnea, cognitive function, fMRI, working memory, default network
INTRODUCTION
Obstructive sleep apnea syndrome (OSAS) results in recurrent hypoxic episodes during sleep, fragmented sleep, cardiovascular comorbidities, neurocognitive impairment during the day, and excessive daytime sleepiness.1–6 It is a frequent but insufficiently recognized disorder that involves at least 2% to 4% of subjects between 30 to 60 years old and up to 15% of the elderly population, with a 2:1 men/women ratio in Caucasians.7 Despite discrepancies between studies due to variation in tasks and patient samples, several aspects of cognitive function are impaired, but the susceptibility of specific cognitive functions to impairment due to OSAS seems variable. Whereas verbal memory seems to be relatively preserved, the executive function is reported as the most consistently and severely impaired aspect of cognition in patients with OSAS.8–10 Several studies have demonstrated deficits on a variety of cognitive tasks of executive function in OSAS patients (n-back, PASAT, Trail B), which were not always reversible after CPAP treatment.11
Working memory (WM) is commonly the most studied aspect of executive function in neuroimaging and behavioral literature, often using the n-back task that requires subjects to compare the current stimulus with the one presented n-stimuli back. N-back tasks assume involvement of WM functions by maintaining stimulus information until the response can be executed and allow a parametric modulation of WM load by increasing n.12
To date, 3 neuroimaging studies have examined aspects of executive function in OSA patients, with varying results. Patterns of reduced or increased brain activation have been reported in association with decreased or comparable behavioral performance relative to that of healthy controls.13–16 However, most of the interest has been directed at the network of brain regions known to be consistently activated during sustained attention and performance of cognitive tasks and referred to as the task positive network (TPN), which comprise a set of frontal and parietal cortical regions.17,18
In particular, the dorsolateral prefrontal cortex (DLPFC) is known to be a key region for performance of executive function tasks but is also the region primarily affected in aging subjects, schizophrenic patients, and in healthy subjects after total sleep deprivation, all of whom manifest a certain degree of executive function impairment.19–26 The high sensitivity of this brain region to the effects of sleep deprivation was well demonstrated by several studies done on healthy volunteers,24,27 making the DLPFC a region of special interest in cognition-related research in OSA patients.
However, there is increasing evidence that examination of activation in TPN alone does not yield a comprehensive view of cerebral response to cognitive demand. Contrary to TPN, it has been demonstrated that a set of brain regions referred to as the default mode network (DMN), exhibits tonic activation at rest and responds with progressive deactivation as the brain engages into a goal-directed activity.28,29 There is growing evidence that DMN plays a key role in enabling optimal cognitive functioning and that DMN dysfunction, as well as DMN versus TPN anti-correlation imbalance, are associated with cognitive performance impairment.30 Several studies demonstrate that both networks and their reciprocal relationship contribute to the behavioral deficits observed in several pathological conditions as well as normal aging.
In OSAS patients, repetitive hypoxia and repetitive disruption of sleep associated with sleep disordered breathing have been considered as potential factors in induction of cognitive deficit; however, given their simultaneous occurrence, teasing apart the degree to which each of those factors influences cognition as well as what aspects of cognition are preferentially influenced by which OSAS factor is a challenge. Obesity is known to be associated with OSA, and the complex syndrome involving both abdominal obesity and OSA may lead to the metabolic syndrome (MetS), which comprises insulin resistance, central obesity, HTN, glucose intolerance, and dyslipidemia.31,32 Several components of MetS are also associated with cognitive dysfunction. In particular, there is increasing evidence that obesity as expressed by the body mass index (BMI) is associated with cognitive impairment and is a risk factor for developing dementia.33–35 Since sleep fragmentation, intermittent hypoxia, and obesity are all factors commonly associated with OSA, it is likely that each of those factors has an impact on cognitive function of OSA patients and that their association may have a synergistic effect.
Therefore in an attempt to provide a more comprehensive view of neuronal activation changes in OSA patients in our study we are investigating changes in both WM-activation related (TPN) and deactivation related (DMN) networks and examine the independent contributions of sleep fragmentation (SF), intermittent hypoxia (IH), and BMI on brain activation of untreated patients with OSAS during the performance of a parametric working memory (WM) task in respect to both TPN and DMN.
The aims of this study were: (a) to examine the behavioral performance and the underlying brain activation and deactivation patterns in TPN- and DMN-related regions of a sample of untreated OSA patients on a parametric WM n-back task including a limit-of-capacity 3-back level with functional magnetic resonance imaging (fMRI); (b) to compare these patterns to findings in a matched group of healthy controls (HC); and (c) to examine the possible correlations between SF, IH, and BMI and brain activation in TPN and DMN.
Based on results of previous literature, we hypothesized that:
OSA patients will have larger areas of activation in the TPN than HC (spatial recruitment) in order to compensate for a possibly impaired function of the TPN, which would be manifested by higher BOLD signal in HC than in OSA patients in the TPN regions.
OSA patients will have larger areas of deactivation in the DMN (compensatory spatial recruitment) as compared to HC in order to compensate for possibly ineffective deactivation of DMN, which would be manifested by higher BOLD signal in OSA patients than in HC in the DMN regions.
METHODS
Subjects
Seventeen men diagnosed with moderate to severe OSAS were recruited from the Stanford Sleep Clinic and surrounding area via advertisement. All participants were right-handed nonsmokers and were screened for current or previous neurological and psychiatric disorder as determined by history, clinical evaluation, and Hamilton Depression Scale score. All participants reported regular sleep schedules with ≥ 6 h of sleep per night as determined by sleep habits questionnaires. Seven age-matched subjects without history of sleep disorders were recruited from the community as HC; and absence of sleep pathology, including sleep disordered breathing (SDB), was confirmed by an overnight polysomnography (PSG: AHI < 5) (Table 1).
Table 1.
Sample characteristics
| Age | 43.2 (± 8.4) |
| BMI | 27.8 (± 4) |
| AHI | 39.7 (± 22.8) |
| Ethnicity | 4 Asian, 12 Caucasian, 1 Hispanic |
| TST | 376.8 (± 71.1) |
| Sleep efficiency | 83.1 (± 13.1) |
| REM | 17.9 (± 4.9) |
| Stage 1 | 12.0 (± 7.3) |
| Stage 3+4 | 7.9 (± 8.9) |
| Min SpO2 | 87.8 (± 8.9) |
BMI, body mass index; AHI, apnea hypopnea index; TST, total sleep time.
The study was approved by the Stanford Institutional Review Board, and all subjects signed informed consent.
Polysomnography (PSG)
Overnight PSGs were performed in all subjects. The following variables were systematically monitored: EEG, electro-oculogram, electrocardiogram, chin and leg myogram, nasal air flow with nasal cannula, abdominal and thoracic respiratory movements with piezo-electrical belts, and pulse oximetry. Studies were scored by independent technicians and reviewed by a qualified sleep medicine physician according to the AASM scoring criteria.
Experimental Procedure and n-Back Task
All subjects were instructed to abstain from ingestion of any caffeinated beverages ≥ 9 h prior to scanning.
During the fMRI session, participants performed 2 sessions of visuospatial n-back task that was generated with E-Prime 1.0 software and visually projected on a mirror in front of the subject's eyes. They performed two 9 min 40 sec sessions of a block-designed parametric n-back task with 4 levels of difficulty (0-back, 1-back, 2-back, and 3-back). Each session comprised 2 blocks of each WM condition (1-, 2-, and 3-back) of 63 sec duration, separated by 5 blocks of the baseline condition (0-back) of 25.2 sec duration each (0-1-0-2-0-3-0-1-0-2-0-3). During the task, a white dot flashed for 200 ms in 6 pseudo-random locations on a black screen. Fifty percent of stimuli were matches.
Subjects were instructed to respond with their right hand (index finger for matches and middle finger for mismatch) whether the dot appeared on the left or on the right side of the screen in the 0-back condition, or whether each given dot appeared in the same or different location as the dot n-dots before for the 1-, 2-, and 3-back conditions, respectively. Subjects' responses during the n-back sessions and their response times (RT) in the scan were recorded via a custom-made response box in E-Prime Data Aid software.
fMRI Data Acquisition
Functional MRI data was acquired on a 3.0T GE (Milwaukee, WI) whole-body scanner with a custom quadrature bird-cage head coil. Head movement was minimized with foam padding. Thirty oblique axial slices were taken parallel to the anterior/posterior commissure plane (AC-PC) with 4-mm slice thickness, 1-mm interslice gap. High resolution T2 weighted fast spin echo structural images were acquired for anatomical reference. A T2*-sensitive gradient echo spiral in/out pulse sequence36 was used for functional imaging (TR = 2000 ms, TE = 30 msec, flip angle = 70, FOV = 24 cm, matrix = 64 × 64). An automated high-order shimming procedure based on spiral acquisitions was used to reduce B0 heterogeneity.37 A high resolution T1 volume scan (124 slices, 1.2-mm thickness) was collected for every subject using an IR-prep FSPGR sequence for T1 contrast.
fMRI Data Analysis
Functional MRI data were preprocessed and analyzed using Statistical Parametric Mapping software (Welcome Department of Cognitive Neurology, London) and custom MATLAB routines (MathWorks Natick, MA). The preprocessing steps consisted of realignment of all images to the first image, normalization to MNI template, and spatial smoothing with a Gaussian filter of 6 mm full-width-half-maximum.
To test for the effect of each task load, we used a standard general linear approach with 4 regressors for the 3 task load and baseline conditions, modeled as a boxcar function convolved with the canonical HRF. The 6 motion parameters from the realignment were added as 6 regressors of no interest. Statistical analysis at the single-subject level treated each voxel according to a general linear model.38 Individual contrast images were created by computing each WM task load versus the 0-back load baseline (1 > 0, 2 > 0, 3 > 0). OSA and HC groups were then compared for each WM task load by entering individual contrast images in a 2-sample t-test. For each of the 3 main contrasts, we also used an ANOVA and performed an exclusive masking procedure to reveal any voxel significantly activated in one group but not in the other group (Figure 1).
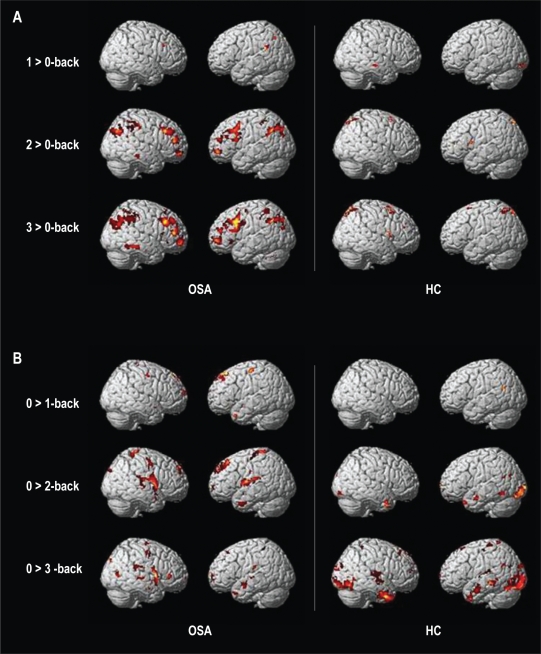
Figure 1.
Spatial recruitment of (A) TPN in OSA patients after exclusive masking by brain activation of the HC group (left) and spatial recruitment of TPN in HC after exclusive masking by brain activation of OSA group (right); (B) DMN in OSA patients after exclusive masking by brain deactivation of the HC group (left) and spatial recruitment of DMN in HC after exclusive masking by brain deactivation of OSA group (right).
In order to minimize the type I error, a cluster threshold method was used,39 requiring that any given voxel be significantly activated at P level of 0.001 and be a part of a cluster of ≥ 10 contiguous significantly activated voxels.
To test for correlation of a general task load effect with clinical measures, we performed a second analysis on the patient dataset using a multiple regression approach. We first designed a parametric model where all task blocks were modelled as one single regressor, with 2 additional regressors modeling a linear modulation of the task-related activity by load level (1-, 2-, and 3-back), a quadratic modulation of the task-related activity by load level, and 7 regressors of no interest (behavioral performance per task block and 6 motion correction parameters). Contrast images generated by the linear load regressor from each subject entered the multiple regression group analysis with AHI, time spent under 90% SpO2, and BMI as covariates. These analyses were performed on the data from the OSA patients only.
RESULTS
Clinical Measure and Behavioral Performance
For 17 OSA patients, the mean AHI was 39.7 (± 22.8), the mean Epworth score was 7.3 (± 4), and the mean BMI was 27.8 (± 4) kg/m2. Mean minimal nocturnal oxygen saturation was 87.8% (± 8.9%). Details of sleep quality and sleep stages are reported in Table 1.
During scanning subjects' accuracy decreased and response time increased with increasing n-back load. There was no difference in RT between OSA patients and HC on any level of the task load. OSA patients performed significantly worse than HC on the 3-back (79% versus 89% correct, P = 0.005), but there was no significant difference in performance for the other levels of task load (Table 2). Significant negative correlation between BMI and behavioral performance was found at the 3-back level (r = −0.7).
Table 2.
Behavioral performance in OSA patients and HC
| OSA patients | HC | P value | |
|---|---|---|---|
| Accuracy 0-back | 99.0 (± 2.5) | 99.5 (± 0.8) | 0.5 |
| RT 0-back | 466.9 (± 125.6) | 446.2 (± 117.5) | 0.7 |
| Accuracy 1-back | 96.7 (± 4.7) | 96.3 (± 4.3) | 0.9 |
| RT 1-back | 762.7 (± 167) | 671.3 (± 182.8) | 0.3 |
| Accuracy 2-back | 91.4 (± 6.8) | 93.6 (± 5.3) | 0.4 |
| RT 2-back | 984.6 (± 318.2) | 806.8 (± 274.2) | 0.19 |
| Accuracy 3-back | 78.9 (± 10.6) | 89.0 (± 5.2) | 0.005 |
| RT 3-back | 1197.1 (± 322) | 992.1 (± 239.6) | 0.1 |
HC, healthy controls; RT, reaction time.
In order to exclude an influence of sleepiness on brain activation and performance, we examined a possible relationship between RT during the 0-back epochs throughout both sessions of the n-back task with task duration. There was no correlation between RT and task duration for either session.
fMRI
Working memory load
Comparing activity during each WM load versus baseline revealed 2 distinct networks. Significant activations were found in several brain regions previously described to be involved in WM processing in other fMRI studies using n-back tasks. Those included bilateral SMA, bilateral DLPFC, anterior cingulate cortex (ACC), and parietal cortex bilaterally.
On the other hand, significant deactivations with increasing load were found in the medial PFC, posterior cingulate cortex, bilateral insular and hippocampal cortex (DMN).
Both activation and deactivation networks showed a progressively marked pattern with increasing task load (see Supplementary Material, Figure S1).
Comparison between OSA Patients and Healthy Controls
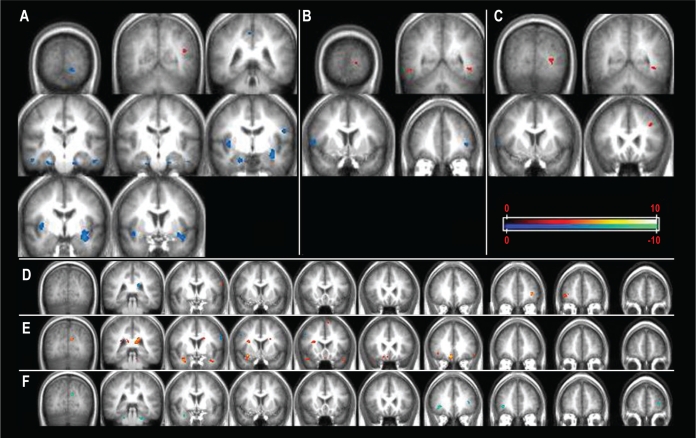
For the 1-back versus baseline comparison, significantly higher activation was found in HC than OSA patients in bilateral parahippocampal regions, right insula, bilateral claustrum, left precentral gyrus (BA 4), and right precuneus (BA 7) (Figure 2A, Table 3). Significantly higher activation was found in the left temporo-occipital area (BA19) in OSA patients than in HC.
Figure 2.
Brain regions significantly higher activated in HC compared to OSA patients in cold scheme; brain regions significantly higher activated in OSA patients as compared to HC in warm color scheme. (A) 1- versus 0-back comparison, (B) 2- versus 0-back comparison, (C) 3- versus 0-back comparison. Correlations between cerebral activation during WM task in OSA patients and (D) AHI, (E) nocturnal desaturation time (min), (F) BMI. Images presented in radiologic convention.
Table 3.
Talairach coordinates of brain regions showing significant differences in cerebral activation between OSA patients and healthy controls (t-test analysis)
| X | Y | Z | Region |
|---|---|---|---|
| OSA > HC 1- vs 0-back | |||
| −42 | −59 | 18 | L BA 19 |
|
HC > OSA 1- vs 0-back | |||
| −30 | −6 | −11 | L BA 34,38 |
| −40 | 2 | −11 | |
| −32 | 1 | −9 | |
| 49 | −19 | −18 | R BA 20 |
| 20 | −9 | −18 | R BA 34 |
| 27 | −20 | −22 | R BA 36 |
| 18 | −14 | −24 | R BA 28 |
| 36 | −8 | 8 | R claustrum |
| 45 | −7 | 1 | R insula |
| −45 | −18 | −22 | L BA 20 |
| −17 | −16 | −23 | L BA 28 |
| −51 | −11 | 26 | L BA 4 |
| −12 | −88 | −13 | L BA 18 |
| 38 | 4 | −4 | R claustrum |
| 2 | −44 | 47 | R BA 7 |
|
OSA > HC 2- vs 0-back | |||
| 6 | −33 | 17 | R pulvinar |
| 51 | −50 | −9 | R BA 37 |
| −44 | −49 | −8 | L BA 37 |
| −12 | −95 | −3 | L BA 17 |
|
HC > OSA 2- vs 0-back | |||
| −38 | 39 | 9 | L BA 10 |
| 51 | 3 | 13 | R BA 44 |
|
OSA > HC 3- vs 0-back | |||
| −27 | −76 | −5 | L BA 19 |
| −35 | 18 | 38 | L BA 8 |
|
HC > OSA 3- vs 0-back | |||
| 56 | 7 | 11 | R BA 44 |
P = 0.001, voxel extent threshold = 10.
For the 2-back versus baseline comparison, HC had higher activation than OSA patients in right precentral (BA 44) and left middle frontal gyrus (BA 10), whereas OSA patients had higher activation in bilateral temporal (BA 37) and left occipital (BA 17) regions (Figure 2B, Table 2).
In the 3-back versus baseline comparison, HC had higher activation than OSA patients in right precentral gyrus (BA 44), whereas OSA patients had higher activations in left middle frontal (BA 8) regions (Figure 2C, Table 2).
Using exclusive masking of WM activated regions in OSA patients by activation in HC, we found a higher number of activated regions in OSA group at both 2- and 3- versus 0-back loads, with maximal effect at the highest difficulty level (3- vs 0-back) in TPN-related regions (Figure 3A). DMN pattern showed a different pattern with higher number of deactivated regions in OSA patients as compared to HC in the 2- vs 0-back comparison and a recruitment of a different set of deactivated regions in HC at the 3- vs 0-back comparison, consisting of lateral temporal regions (BA 38) and bilateral posterior fusiform and lingual gyri (Figure 3B). At the 3-back level, OSA patients still showed more widespread DMN recruitment in the bilateral insulas, anterior midline and posterior parahippocampal regions as compared to HC.
Multiple Regression Analysis
Multiple regression analysis yielded significant positive correlation between AHI and brain activation across WM task loads in the left and right frontal regions (L BA 6, 10, R BA 10) (Figure 2A, Table 4).
Table 4.
Talairach coordinates of brain regions showing significant correlation with AHI, desaturation time, BMI, and cerebral activation during the WM task
| X | Y | Z | Region |
|---|---|---|---|
| Positive correlations with AHI | |||
| −32 | 37 | 9 | L BA 10 |
| −57 | −2 | 27 | L BA 6 |
| 38 | 43 | 7 | R BA 10 |
|
Negative correlations with AHI | |||
| −16 | −30 | −19 | culmen |
|
Positive correlations with desaturation time | |||
| −49 | 29 | −3 | L BA 47 |
| 24 | 34 | 29 | R BA 47 |
| −7 | 9 | 64 | L BA 6 |
| 5 | 28 | −15 | R BA 11 |
| −1 | 17 | −12 | L BA 25 |
| 5 | 31 | −5 | R BA 24 |
| −9 | −13 | 24 | L BA 23 |
| 6 | −69 | 21 | R BA 31 |
| −16 | −48 | 14 | L BA 29 |
| −43 | 17 | −16 | L BA 38 |
| 38 | 19 | −13 | R BA 38 |
| −9 | −73 | 21 | L BA 18 |
| 29 | 2 | −17 | R BA 34 |
| −14 | −31 | −21 | Culmen |
|
Negative correlations with desaturation time | |||
| 45 | 11 | 42 | R BA 8 |
| −57 | −2 | 30 | L BA 6 |
| −29 | −50 | 39 | L BA 7 |
| −48 | −16 | 48 | L BA 2 |
|
Negative correlations with BMI | |||
| −29 | 50 | 12 | L BA 10 |
| −11 | −73 | 21 | L BA 18 |
| −23 | −50 | −3 | L BA 19 |
| 19 | −52 | 0 | R BA 19 |
| −34 | −12 | −5 | L claustrum |
| 29 | 2 | −18 | R BA 34 |
| −23 | −34 | −28 | L cerebellum |
| −19 | −39 | −23 | L cerebellum |
| 20 | −33 | −26 | R cerebellum |
| 23 | −38 | −30 | R cerebellum |
P = 0.001, voxel extent threshold = 10.
We found a positive correlation between brain activation across WM task loads and sleep time spent under 90% SpO2 in bilateral inferior frontal lobe regions (BA 47), right medial frontal gyrus (BA 11), right anterior and bilateral posterior cingulate (R BA 24, L BA 23, 29), left subcallosal gyrus (BA 25), left precentral region (BA 6), bilateral temporal poles (BA 38), right parahippocampal gyrus (BA 34), bilateral occipital regions (L BA 18, R BA 31), and left cerebellar regions (culmen). Negative correlation was found in the bilateral frontal (L BA 6, R BA 8) and left parietal lobes (BA 2,7) (Figure 2B, Table 4).
Significant negative correlations have been found for BMI in left frontal cortex (BA 10), right temporal (BA 42), left claustrum, bilateral occipital (BA 18, 19), and cerebellar regions. There was no positive correlation between BMI and brain activation across the WM task loads.
DISCUSSION
Recent research on task positive and default mode networks in the human brain indicates that dysfunction of both those networks, as well as the imbalance of the antagonistic relationship between them, can lead to behavioral impairment and are in fact observed in several conditions associated with cognitive deficit, such as normal aging, Alzheimer disease, schizophrenia, ADHD, and depression.40 In particular, it has been shown that the extent of task-related deactivations in the DMN could differentiate between subjects with mild cognitive impairment, Alzheimer disease, and healthy controls, with lesser deactivation in MCI than healthy controls and in Alzheimer patients than in MCI.41 Furthermore, Lawrence et al. have demonstrated that good behavioral performance on a sustained attention/WM task can arise from stronger activation in the TPN, stronger deactivation in the DMN, or a mixture of strong levels of activation and deactivation.42
In our study, both groups demonstrated a progressive increase of spatial recruitment of regions within and adjacent to the TPN from 1- to 3-back level of task, probably reflecting a continuous compensatory effort, with a larger recruitment in OSA subjects as compared to HC. However, the groups differed in the task-related deactivation of the DMN with more widespread deactivation (DMN spatial recruitment) in OSA than in HC at the 2-back level and a different pattern of compensatory recruitment in the two groups at the 3-back level, in spite of a continuous spatial recruitment of DMN regions in both groups with increasing load (Figure S1).
Therefore, significantly worse behavioral performance of OSA patients at the 3-back level appears to be related more to a different pattern of recruitment and deactivation of DMN-related regions than a defective TPN.
Our finding of positive correlation between cerebral activation during the n-back task and the measure of nocturnal desaturation in a number of regions of and adjacent to the DMN (particularly in bilateral temporal poles and mesial temporal regions) suggests intermittent hypoxia to be a major factor for the DMN dysfunction in OSA patients. IH was also found to have a negative impact on TPN regions, with a negative correlation between IH and cerebral activation in bilateral frontal and left parietal regions.
Defective deactivation of a network of brain regions commonly more active during rest than during a cognitive task (DMN) has been demonstrated to be related to worse behavioral performance in a number of conditions associated with cognitive impairment and sleep disturbances (normal aging, Parkinson disease, schizophrenia, ADHD), suggesting that inability to reallocate neuronal resources and suppress activity in neighboring regions plays a role in cognitive impairment.43–46 Disruption of task-related deactivation within the midline regions of DMN has been reported after acute sleep deprivation,47 and slower reaction times on a psychomotor vigilance task have also been associated with increased activation in midline DMN regions after total sleep deprivation,48 suggesting that sleep deprivation has a direct impact on DMN. Similarly, reduced deactivation in the posterior regions of DMN has been shown to be related to attentional lapses in healthy subjects.49 In regard to those findings, the observed recruitment in middle regions of DMN in OSA patients in our study together with the absence of changes in reaction times suggests that sleep deprivation is not a mechanism that dysregulates DMN in OSA patients.
We found significantly increased brain activation in HC as compared to OSA patients in several regions of the limbic system at the 1- versus 0-back comparison. The analysis of parameter estimates trend revealed a peak of BOLD signal at 1-back in HC that is absent in OSA patients. Several of those brain regions are known to be implicated in emotional and autonomic nervous system control.50–53 It is well established that OSAS is associated with autonomic nervous system dysfunction.54,55 Therefore, we believe that the observed difference in activation reflects a blunted autonomic response in OSA patients. Indeed, the design of our task means that each session began with the 1-back task as the first load block (see Methods), clearly enhancing the risk of task-related stress due to the new situation during this test. This proposition is further supported by a re-analysis of data that considered only the second part of each session, in which there was no difference between patients and HC in those regions. Further studies including autonomic nervous system measures are needed in order to evaluate more in depth this specific aspect of the testing situation.
Finally, more and more attention is being directed to the effect of obesity per se on cognitive function, as it has been shown that excess body weight is associated with brain structural and functional alterations56 and a higher risk of developing dementia in later life.33 Higher BMI was found to be associated with hypometabolism as well as reduced NAA/Cho ratio of the prefrontal and cingulated regions.57,58 Behaviorally, several studies find a significant relationship between high BMI and cognitive dysfunction in otherwise healthy adults or after controlling for BP, age, and diabetes.33,34,59 Since obesity is strongly associated with OSA due to patient recruitment, it is important to examine the independent effect of BMI on cognitive function of OSA patients and to systematically account for its effect in cognition studies in these subjects. Our results are in line with previous literature, as we found only negative correlations between BMI and brain activation during the WM task in a number of brain regions of the TPN, in particular in bilateral DLPFC and occipital and cerebellar regions. Not surprisingly, BMI and desaturation time were significantly correlated in our sample, hence using a multiple regression model allowed us to tease apart their independent influence on cerebral activation during the WM task. However, despite the significant correlation between BMI and desaturation time, only BMI was significantly negatively correlated with behavioral performance at the limit-of-capacity level of task (3-back), suggesting that BMI has a more deleterious role on WM function in OSA than sleep fragmentation and nocturnal desaturation. This finding and the fact that only negative correlations were found between BMI and cerebral activation suggests that BMI has a structural impact on the gray matter of the TPN. Thus, further studies are needed to investigate the independent impact of BMI and the effect of obesity treatment on cognitive function in OSA.
Several neuroimaging studies have previously investigated executive function aspects in OSA patients. Thomas et al. reported a decreased brain activation in anterior cingulate, bilateral DLPFC, and posterior parietal regions in OSA patients during the performance of a 2-back verbal task, which paralleled a significantly worse behavioral performance as compared to healthy controls.13 Ayalon et al., using a Go-no-Go task, reported a decreased cerebral activation in several regions typically involved in attentional tasks in patients compared to controls, whereas there was no significant difference in behavioral performance and response time between the two groups for the Go versus rest (although there was a trend towards worse performance in patients for the No-go versus rest comparison).14,15 Castronovo et al. have reported an increased activation in OSA patients versus HC in left frontal regions, precuneus, putamen, hippocampus, and cerebellum, as well as decreased activation in brainstem, left occipital, and right orbital regions at similar behavioral performance at a 2-back task.16
Our study differs from previous studies on several points: (a) it considers a visuospatial (and not a verbal) n-back task, (b) it involves 4 levels of difficulty, and (c) it examines BOLD signal changes in not only the TPN but also the DMN regions. It is therefore more difficult to draw direct comparisons between our results and results of previous studies. However, similar to previous studies we found an increase in spatial recruitment of TPN, in particular of the left hemisphere, in OSA patients as compared to HC, as well as a decrease in activation in frontal regions involved in WM function, particularly at more difficult task levels. Moreover, Castronovo et al. have reported an increased activation in the precuneus in their OSA group, which partly corroborates our results of decreased deactivation in posterior regions of DMN.
Our study has limitations: we have examined only male subjects, as controversy exists about gender effects on brain activation during cognitive performance; therefore our results cannot be extended to female OSA patients. Our patient and HC groups also significantly differed in the average of years of schooling, introducing the question of contribution of intelligence level (when based on years of schooling) to the observed behavioral and neuronal differences. We believe however that this bias is not as important as it seems: although statistically significant, the schooling difference between the groups was small, and both groups had a high educational level (mean of 16.5 and 18.9 years). Having a more reliable measure of fluid intelligence than the education level would be certainly more relevant.
Whereas the design of our task demanded an alternation between four task levels—the 1-, 2-, and 3-back blocks having a duration of 1 min for a total of 4 min on each task level per patient not allowing observation of potential effects due to a long time-on-task—it enabled us to include a limit-of-capacity 3-back level in our parametric design, requesting more effort from patients and HC. As our primary interest was the difference in WM function, shorter block duration gave us the advantage of not having the confounding effect of fatigue, typically seen in sleep deprived subject in designs with long time-on-task blocks. But the fixed order of increasing load difficulty in our task design, while likely enabling us to uncover a different trend of response to stress between the two groups, may have masked more WM-related effects at the low-load level. We believe that differences in task designs between various studies, although making the result comparison more difficult, yield complementary information on brain function in OSAS.
In conclusion, our study shows impairment of both TPN and DMN in our OSA patients, although to a different extent and by different factors: DMN is more severely affected with a different regional pattern of recruitment at the limit-of-capacity- 3-back level, and this impairment is mostly related to the level of nocturnal desaturation. Nocturnal desaturation has a negative impact on both TPN and DMN by decreasing cerebral activation in the former and increasing it in the latter. BMI per se has a negative effect, predominantly on prefrontal regions, decreasing cerebral activation in the TPN and showing a significant negative correlation with behavioral performance at the maximal level of WM load. Cerebral activation in the TPN network is negatively affected by both nocturnal desaturation and the BMI, even though it appears to be relatively better preserved as is demonstrated by increasing compensatory recruitment as compared to the DMN.
DISCLOSURE STATEMENT
This study was supported by FSBMB and Swiss National Foundation for Scientific Research (FNS), Covidien, Respironics and Resmed. Dr. Kushida Has received research support through grants to Stanford University from Ventus Medical, Merck, Respironics, Jazz, Cephalon, GlaxoSmithKline, Pacific Medico, and Xenoport. The other authors have indicated no other financial conflicts of interest.
ACKNOWLEDGMENTS
Authors are also particularly grateful to Professor G. Glover, PhD; Dr. M. Thomason, PhD; Dr. F. Hoeft, MD, PhD; P. Mazaika, PhD; E. Leary; and D. Nichols for their help and support.
Cerebral activation (warm color scheme) and deactivation (cold color scheme) in (A) OSA patients and (B) healthy controls for 1-, 2-, and 3 versus 0-back contrasts.
REFERENCES
- 1.Caples SM, Gami AS, Somers VK. Obstructive sleep apnea. Ann Intern Med. 2005;142:187–97. doi: 10.7326/0003-4819-142-3-200502010-00010. [DOI] [PubMed] [Google Scholar]
- 2.Wolk R, Gami AS, Garcia-Touchard A, Somers VK. Sleep and cardiovascular disease. Curr Probl Cardiol. 2005;30:625–62. doi: 10.1016/j.cpcardiol.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Yaggi H, Mohsenin V. Obstructive sleep apnoea and stroke. Lancet Neurol. 2004;3:333–42. doi: 10.1016/S1474-4422(04)00766-5. [DOI] [PubMed] [Google Scholar]
- 4.Parish JM, Somers VK. Obstructive sleep apnea and cardiovascular disease. Mayo Clin Proc. 2004;79:1036–46. doi: 10.4065/79.8.1036. [DOI] [PubMed] [Google Scholar]
- 5.Coccagna G, Pollini A, Provini F. Cardiovascular disorders and obstructive sleep apnea syndrome. Clin Exp Hypertens. 2006;28:217–24. doi: 10.1080/10641960600549090. [DOI] [PubMed] [Google Scholar]
- 6.Bedard MA, Montplaisir J, Richer F, Rouleau I, Malo J. Obstructive sleep apnea syndrome: pathogenesis of neuropsychological deficits. J Clin Exp Neuropsychol. 1991;13:950–64. doi: 10.1080/01688639108405110. [DOI] [PubMed] [Google Scholar]
- 7.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 8.Kim HC, Young T, Matthews CG, Weber SM, Woodward AR, Palta M. Sleep-disordered breathing and neuropsychological deficits. A population-based study. Am J Respir Crit Care Med. 1997;156:1813–9. doi: 10.1164/ajrccm.156.6.9610026. [DOI] [PubMed] [Google Scholar]
- 9.Ferini-Strambi L, Baietto C, Di Gioia MR, et al. Cognitive dysfunction in patients with obstructive sleep apnea (OSA): partial reversibility after continuous positive airway pressure (CPAP) Brain Res Bull. 2003;61:87–92. doi: 10.1016/s0361-9230(03)00068-6. [DOI] [PubMed] [Google Scholar]
- 10.Beebe DW, Groesz L, Wells C, Nichols A, McGee K. The neuropsychological effects of obstructive sleep apnea: a meta-analysis of norm-referenced and case-controlled data. Sleep. 2003;26:298–307. doi: 10.1093/sleep/26.3.298. [DOI] [PubMed] [Google Scholar]
- 11.Felver-Gant JC, Bruce AS, Zimmerman M, Sweet LH, Millman RP, Aloia MS. Working memory in obstructive sleep apnea: construct validity and treatment effects. J Clin Sleep Med. 2007;3:589–94. [PMC free article] [PubMed] [Google Scholar]
- 12.Lis S, Krieger S, Hennig D, et al. Executive functions and cognitive subprocesses in patients with obstructive sleep apnoea. J Sleep Res. 2008 doi: 10.1111/j.1365-2869.2008.00660.x. [DOI] [PubMed] [Google Scholar]
- 13.Thomas RJ, Rosen BR, Stern CE, Weiss JW, Kwong KK. Functional imaging of working memory in obstructive sleep-disordered breathing. J Appl Physiol. 2005;98:2226–34. doi: 10.1152/japplphysiol.01225.2004. [DOI] [PubMed] [Google Scholar]
- 14.Ayalon L, Ancoli-Israel S, Drummond SP. Altered brain activation during response inhibition in obstructive sleep apnea. J Sleep Res. 2009;18:204–8. doi: 10.1111/j.1365-2869.2008.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ayalon L, Ancoli-Israel S, Aka AA, McKenna BS, Drummond SP. Relationship between obstructive sleep apnea severity and brain activation during a sustained attention task. Sleep. 2009;32:373–81. doi: 10.1093/sleep/32.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castronovo V, Canessa N, Strambi LF, et al. Brain activation changes before and after PAP treatment in obstructive sleep apnea. Sleep. 2009;32:1161–72. doi: 10.1093/sleep/32.9.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 18.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 19.Smith EE, Geva A, Jonides J, Miller A, Reuter-Lorenz P, Koeppe RA. The neural basis of task-switching in working memory: effects of performance and aging. Proc Natl Acad Sci U S A. 2001;98:2095–100. doi: 10.1073/pnas.98.4.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reuter-Lorenz PA, Jonides J, Smith EE, et al. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neurosci. 2000;12:174–87. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- 21.Morcom AM, Good CD, Frackowiak RS, Rugg MD. Age effects on the neural correlates of successful memory encoding. Brain. 2003;126:213–29. doi: 10.1093/brain/awg020. [DOI] [PubMed] [Google Scholar]
- 22.Chee MW, Choo WC. Functional imaging of working memory after 24 hr of total sleep deprivation. J Neurosci. 2004;24:4560–7. doi: 10.1523/JNEUROSCI.0007-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jansma JM, Ramsey NF, van der Wee NJ, Kahn RS. Working memory capacity in schizophrenia: a parametric fMRI study. Schizophr Res. 2004;68:159–71. doi: 10.1016/S0920-9964(03)00127-0. [DOI] [PubMed] [Google Scholar]
- 24.Drummond SP, Brown GG, Stricker JL, Buxton RB, Wong EC, Gillin JC. Sleep deprivation-induced reduction in cortical functional response to serial subtraction. Neuroreport. 1999;10:3745–8. doi: 10.1097/00001756-199912160-00004. [DOI] [PubMed] [Google Scholar]
- 25.Drummond SP, Brown GG, Gillin JC, Stricker JL, Wong EC, Buxton RB. Altered brain response to verbal learning following sleep deprivation. Nature. 2000;403:655–7. doi: 10.1038/35001068. [DOI] [PubMed] [Google Scholar]
- 26.Drummond SP, Brown GG. The effects of total sleep deprivation on cerebral responses to cognitive performance. Neuropsychopharmacology. 2001;25:S68–73. doi: 10.1016/S0893-133X(01)00325-6. [DOI] [PubMed] [Google Scholar]
- 27.Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–52. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 28.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–94. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 30.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jun J, Polotsky VY. Metabolic consequences of sleep-disordered breathing. ILAR J. 2009;50:289–306. doi: 10.1093/ilar.50.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levy P, Bonsignore MR, Eckel J. Sleep, sleep-disordered breathing and metabolic consequences. Eur Respir J. 2009;34:243–60. doi: 10.1183/09031936.00166808. [DOI] [PubMed] [Google Scholar]
- 33.Wolf PA, Beiser A, Elias MF, Au R, Vasan RS, Seshadri S. Relation of obesity to cognitive function: importance of central obesity and synergistic influence of concomitant hypertension. The Framingham Heart Study. Curr Alzheimer Res. 2007;4:111–6. doi: 10.2174/156720507780362263. [DOI] [PubMed] [Google Scholar]
- 34.Cournot M, Marquie JC, Ansiau D, et al. Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology. 2006;67:1208–14. doi: 10.1212/01.wnl.0000238082.13860.50. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Dai Q, Jackson JC, Zhang J. Overweight is associated with decreased cognitive functioning among school-age children and adolescents. Obesity (Silver Spring) 2008;16:1809–15. doi: 10.1038/oby.2008.296. [DOI] [PubMed] [Google Scholar]
- 36.Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med. 2001;46:515–22. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- 37.Kim DH, Adalsteinsson E, Glover GH, Spielman DM. Regularized higher-order in vivo shimming. Magn Reson Med. 2002;48:715–22. doi: 10.1002/mrm.10267. [DOI] [PubMed] [Google Scholar]
- 38.Worsley KJ, Liao CH, Aston J, et al. A general statistical analysis for fMRI data. Neuroimage. 2002;15:1–15. doi: 10.1006/nimg.2001.0933. [DOI] [PubMed] [Google Scholar]
- 39.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 40.Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33:279–96. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Rombouts SA, Barkhof F, Goekoop R, Stam CJ, Scheltens P. Altered resting state networks in mild cognitive impairment and mild Alzheimer's disease: an fMRI study. Hum Brain Mapp. 2005;26:231–9. doi: 10.1002/hbm.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lawrence NS, Ross TJ, Hoffmann R, Garavan H, Stein EA. Multiple neuronal networks mediate sustained attention. J Cogn Neurosci. 2003;15:1028–38. doi: 10.1162/089892903770007416. [DOI] [PubMed] [Google Scholar]
- 43.Grady CL, Protzner AB, Kovacevic N, et al. A multivariate analysis of age-related differences in default mode and task-positive networks across multiple cognitive domains. Cereb Cortex. 2010;20:1432–47. doi: 10.1093/cercor/bhp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Eimeren T, Monchi O, Ballanger B, Strafella AP. Dysfunction of the default mode network in Parkinson disease: a functional magnetic resonance imaging study. Arch Neurol. 2009;66:877–83. doi: 10.1001/archneurol.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant default mode functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–7. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- 46.Castellanos FX, Margulies DS, Kelly C, et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–7. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gujar N, Yoo SS, Hu P, Walker MP. The unrested resting brain: sleep deprivation alters activity within the default-mode network. J Cogn Neurosci. 2010;22:1637–48. doi: 10.1162/jocn.2009.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drummond SP, Bischoff-Grethe A, Dinges DF, Ayalon L, Mednick SC, Meloy MJ. The neural basis of the psychomotor vigilance task. Sleep. 2005;28:1059–68. [PubMed] [Google Scholar]
- 49.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–8. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 50.Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ. Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigation in humans. J Physiol. 2000;523(Pt 1):259–70. doi: 10.1111/j.1469-7793.2000.t01-1-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Critchley HD, Melmed RN, Featherstone E, Mathias CJ, Dolan RJ. Volitional control of autonomic arousal: a functional magnetic resonance study. Neuroimage. 2002;16:909–19. doi: 10.1006/nimg.2002.1147. [DOI] [PubMed] [Google Scholar]
- 52.Napadow V, Dhond R, Conti G, Makris N, Brown EN, Barbieri R. Brain correlates of autonomic modulation: combining heart rate variability with fMRI. Neuroimage. 2008;42:169–77. doi: 10.1016/j.neuroimage.2008.04.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheshire WP, Jr, Saper CB. The insular cortex and cardiac response to stroke. Neurology. 2006;66:1296–7. doi: 10.1212/01.wnl.0000219563.87204.7d. [DOI] [PubMed] [Google Scholar]
- 54.Narkiewicz K, Somers VK. Cardiovascular variability characteristics in obstructive sleep apnea. Auton Neurosci. 2001;90:89–94. doi: 10.1016/S1566-0702(01)00272-7. [DOI] [PubMed] [Google Scholar]
- 55.Leung RS. Sleep-disordered breathing: autonomic mechanisms and arrhythmias. Prog Cardiovasc Dis. 2009;51:324–38. doi: 10.1016/j.pcad.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 56.Gazdzinski S, Millin R, Kaiser LG, et al. BMI and neuronal integrity in healthy, cognitively normal elderly: a proton magnetic resonance spectroscopy study. Obesity (Silver Spring) 2010;18:743–8. doi: 10.1038/oby.2009.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Volkow ND, Wang GJ, Telang F, et al. Inverse association between BMI and prefrontal metabolic activity in healthy adults. Obesity (Silver Spring) 2009;17:60–5. doi: 10.1038/oby.2008.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gazdzinski S, Kornak J, Weiner MW, Meyerhoff DJ. Body mass index and magnetic resonance markers of brain integrity in adults. Ann Neurol. 2008;63:652–7. doi: 10.1002/ana.21377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Gordon E. Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Compr Psychiatry. 2007;48:57–61. doi: 10.1016/j.comppsych.2006.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cerebral activation (warm color scheme) and deactivation (cold color scheme) in (A) OSA patients and (B) healthy controls for 1-, 2-, and 3 versus 0-back contrasts.