Abstract
Study Objectives:
The objective of this study was to assess the cumulative impact of 1 hour of nightly sleep restriction over the course of 6 nights on the neurobehavioral functioning (NBF) of children with attention deficit hyperactivity disorder (ADHD) and healthy controls.
Design:
Following 6 nights of actigraphic monitoring of sleep to determine baseline sleep duration, children were asked to restrict sleep duration by 1 hour for 6 consecutive nights. NBF was assessed at baseline (Day 6) and following sleep manipulation (Day 12).
Setting:
A quiet location within their home environments.
Participants:
Forty-three children (11 ADHD, 32 Controls, mean age = 8.7 years, SD = 1.3) between the ages of 7 and 11 years.
Interventions:
NA
Measurements:
Sleep was monitored using actigraphy. In addition, parents were asked to complete nightly sleep logs. Sleepiness was evaluated using a questionnaire. The Conners' Continuous Performance Test (CPT) was used to assess NBF.
Results:
Restricted sleep led to poorer CPT scores on two-thirds of CPT outcome measures in both healthy controls and children with ADHD. The performance of children with ADHD following sleep restriction deteriorated from subclinical levels to the clinical range of inattention on two-thirds of CPT outcome measures.
Conclusions:
Moderate sleep restriction leads to a detectable negative impact on the NBF of children with ADHD and healthy controls, leading to a clinical level of impairment in children with ADHD.
Citation:
Gruber R; Wiebe S; Montecalvo L; Brunetti B; Amsel R; Carrier J. Impact of sleep restriction on neurobehavioral functioning of children with attention deficit hyperactivity disorder. SLEEP 2011;34(3):315-323.
Keywords: ADHD, Continuous Performance Test, sleep restriction, children
INTRODUCTION
Attention deficit hyperactivity disorder (ADHD) is a developmental disorder characterized by an unusually high and chronic level of inattention, impulsivity, and hyperactivity. It is one of the most prevalent conditions in child psychiatry, and has been estimated to occur in 3% to 7.5% of school-age children.1 ADHD routinely continues into adolescence and adulthood2,3 and, if untreated, may result in impairments in crucial areas of life.4
An estimated 25% to 50% of children and adolescents with ADHD experience sleep problems, particularly difficulties in initiating and maintaining sleep.5 Approximately a third of medication-free children with ADHD experience chronic sleep-onset insomnia (SOI).6–8 Other common sleep problems in children with ADHD include bedtime resistance, night awakening, restless sleep, difficulties in awakening in the morning, shorter sleep duration,9,10 unstable sleep patterns or inconsistency in sleep and wake times,11,12 and higher levels of nocturnal activity.13 These sleep problems can lead to chronic sleep deprivation, which can in turn lead to “sleep” debt and significant neurobehavioral impairments. It has been shown that partial sleep restriction for one week results in performance deficits equivalent to those seen following one to two nights of total sleep deprivation.13
Abundant pediatric, clinical, and research data suggest that inadequate sleep results in tiredness and daytime difficulties with focused attention, learning, and impulse modulation.7,14–16 In 1991, Dahl and colleagues noted that the symptoms of sleep deprivation were similar to the core symptoms of ADHD.17 In particular, both groups of patients have impaired executive functions and arousal.18 Hence, sleep difficulties or insufficient sleep in children with ADHD may exacerbate daytime ADHD symptoms. This presents a considerable challenge for clinicians who are attempting to treat children with ADHD and improve their neurobehavioral functioning (NBF).5
The association between sleep and NBF in children with ADHD and sleep disordered breathing (ADHD-SDB) and in children with ADHD and restless leg syndrome/periodic leg movement disorder (RLS/PLMD) has been studied by several researchers.4,14,19–21 These studies showed that in these populations, sleep disruption was associated with hyperactivity and inattention.21 Although these studies provide convincing evidence that sleep and NBF interact in children with ADHD and primary sleep disorders (i.e., SDB, RLS/PLMD), it is unknown whether this is true for the larger cohort of children who have ADHD but do not suffer from sleep apnea, SDB, or RLS/PLMD. Few studies have examined the association between sleep and NBF in children with ADHD and no breathing problems (ADHD-NBP), and no studies have used experimental manipulation of sleep to study this population. Therefore, in the present study, we examined the impact of sleep restriction on the NBF of children with ADHD-NBP.
Recent studies suggest that tracking the neurobehavioral consequences of changes in sleep duration may be dependent on the tested domain.22,23 Hence, in the present study we aimed to examine the impact of mild changes in sleep duration by using a task that captures neurobehavioral deficits that characterize children with ADHD. The Continuous Performance Test (CPT) is an objective measure that has been widely used to assess attention in children with ADHD and shows significant differences between individuals with ADHD and controls.24 Therefore, the CPT was used as the NBF outcome measure of interest.
Causal associations between sleep duration and NBF were examined by comparing performance on the Conners CPT before and after sleep restriction. The participants were children with ADHD who had no comorbid conditions that might affect their sleep, and healthy controls. Our hypothesis was that sleep restriction would impair NBF in both groups.
METHODS
Participants
A sample of 43 children (11 ADHD, 32 Controls), aged 7-11 years old (mean age = 8.7, SD = 1.3), participated in the study. The diagnosis of ADHD was determined by criteria from DSM-IV, using the Diagnostic Interview Schedule for Children (DISC-IV),25 which was administered to parents. The diagnosis generated by the DISC-IV was then confirmed after reviewing behavior rating scales obtained from parents, including the Child Behavior Checklist26 and the Conners' Parent Rating Scales.27
Seven (63.6%) of the children with ADHD were males, and 20 (62.5%) controls were males. Nine (81.8%) of the children with ADHD had Combined subtype ADHD, 1 (9.1%) was Inattentive subtype, and 1 (9.1%) was Undefined. As well, 36.4% of the children with ADHD had used medication for ADHD symptoms (Ritalin, Concerta, or Strattera).
Participants were excluded from the study if they had: (1) A history of mental retardation with an IQ ≤ 80, as measured by the Wechsler Intelligence Scale for Children-IV (WISC-IV)28; (2) a history of autism, Tourette syndrome, pervasive developmental disorder, or psychosis; (3) any medical or psychiatric condition that interferes with the ability to sleep; (4) a major medical condition or impairment that would interfere with the ability to complete testing; and (5) RLS/PLMD or SDB, as determined by polysomnography criteria. Screening for sleep problems was performed in 2 stages:
Stage 1: During our initial contact with the children, when participant eligibility was determined prior to enrolment in the study (Figure 1), screening for sleep disordered breathing (SDB) and periodic leg movement disorder (PLMD) was conducted using 2 validated questionnaires. The first was the Pediatric Sleep Questionnaire29 exploring SDB, snoring, and sleepiness. The instrument contains a validated, reliable, 22-item SDB scale. The second questionnaire was that of Chervin and Hedger, which explores leg restlessness, experience of growing pains in bed, and the presence of insomnia and morning headache.30 The response data were used as an initial screen for PLMS/PLMD. Children scoring ≥ 0.33 (i.e., 33% of the 22 question-items answered positively) on one or both of the scales were excluded from the study.
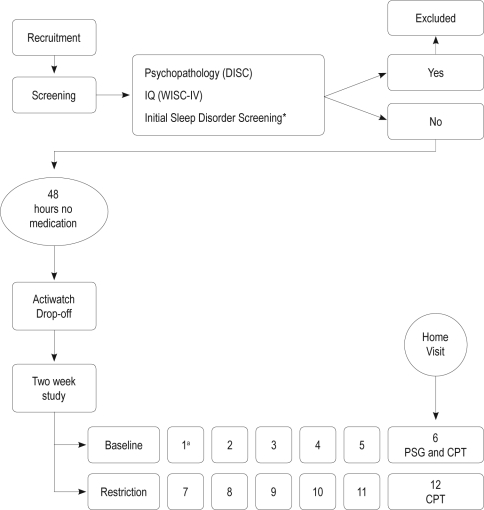
Figure 1.
Diagram of the study flow, showing the timing of polysomnography (PSG) screening and Continuous Performance Test (CPT) assessments. *Pediatric Sleep Questionnaire (PSQ) pertaining to sleep disordered breathing (Chervin, Hedger, & Dillon, 2000) and periodic leg movements subscale (Chervin & Hedger, 2001).
aNumber refers to the day of protocol.
Stage 2: On the sixth night of the study, children meeting inclusion criteria underwent 1 night of polysomnography (PSG) conducted at home and commencing at the habitual bedtime (Figure 1). Signals recorded using a digital ambulatory sleep recorder (Vitaport-3 System; TEMEC Instruments, Kerkrade, The Netherlands) were used to assess sleep quality. A nasal/oral thermistor, a piezo sensor-containing respiratory belt (TEMEC), and EMG leg electrodes were employed to screen for disorders of breathing and leg movements during sleep. The respiratory disturbance index was defined as the sum of obstructive apnea and hypopnea events per hour of sleep. Each event ≥ 1 sec was counted. A diminution ≥ 50% in respiratory signal was considered to reflect hypopnea, whereas a complete halt in signal was defined as apnea. Participants with a respiratory disturbance index > 1/h were considered to have SDB, whereas RLS/PLMD was diagnosed if > 5 leg movements were evident per hour of sleep.
Children were recruited through local schools, psychologists, and centers aimed at helping children with ADHD. The study was approved by the Research Ethics Board of the Douglas Hospital. Parents signed informed consent forms, and all of the children assented to participation in the study.
Thirty-one (72.1%) of the children were of Caucasian descent, 2 (4.7%) were of African descent, 5 (11.6%) were of a multi-ethnic background, and 5 (11.6%) had unknown backgrounds. The majority of children (65.1%) came from families where the parents were married, 6 (13.9%) came from families where the parents were separated or divorced, 2 (4.7%) came from families where parents had remarried, and 5 (11.6%) came from families with a single mother. Twenty-six children came from homes were the mother was currently in or had completed a university education (60.4%), 14 (32.6%) had mothers who were either in or had completed college, and 1 (2.3%) came from a family where the mother had completed high school.
Study Design
Study Design is presented in Figure 1. Before participation, children were screened for eligibility to participate in the study. Eligible participants were asked to discontinue medication 48 h before the first day of the study. In addition, all subjects were told to avoid products containing caffeine (e.g., chocolate or cola). Each child completed a period of baseline protocol and a period of moderate nightly sleep restriction, in which sleep was evaluated objectively in the participants' natural home environment. During the experimental period, children were asked to eliminate 1 h of sleep (relative to their average baseline sleep duration on weekdays) by going to sleep 1 h later than usual. At the beginning of each period, participants received a package that included an Actiwatch and daily report forms used by parents to assess clinical characteristics, sleep and sleepiness. NBF was measured on the sixth day (baseline) and on the twelfth day using the CPT (see Figure 1). To increase compliance, a research assistant called the parents during the experimental week to remind them of the experimental sleep schedule.
MEASURES
Behavioral and Physical Measures
Behavioral
Overall behavioral functioning was examined using the Child Behavioral Checklist (CBCL),26 a 113-item parental questionnaire assessing behavioral and emotional problems. Using this measure, externalizing and internalizing symptoms were differentiated at the level of global scores. The CBCL yields good metric characteristics and excellent representative norms, and its reliability and validity have been repeatedly established. The Revised Conners' Parent Rating Scale (CPRS-R),27 which contains a subscale for ADHD behavior, was also administered to determine behavioral functioning.
Puberty
A modified version of Petersen's puberty development scale31 was used to assess pubertal development. The PDS has a male and female version and asks participants to report about physical changes associated with puberty. Development on each characteristic is rated on a 4-point scale ranging from 1 (no development) to 4 (development is completed), with the exception of menarche, which is scored dichotomously (1 = has not occurred or 4 = has occurred).
Socioeconomic status (SES)
Information regarding education, marital status, income, and profession was collected through a background questionnaire, and an SES score was calculated based on the 4-factor index developed by Hollingshead,32 which assigns certain scores to various professions and education levels and incorporates income and marital status to obtain a family SES.
Sleep Assessment
Actigraphy
Actigraphs (AW64 series) were used to assess participants' sleep patterns in their natural home environments. These computerized wristwatch-like devices collect data generated by movements. They are minimally invasive and allow sleep to be recorded reliably without interfering with the family's routine. Actigraphy has been widely used to assess sleep and has been validated against polysomnography with agreement rates for minute-by-minute sleep-wake identification > 90%.33,34 One-minute epochs were used to analyze actigraphic sleep data. The reported bedtime and wake time (provided by the sleep logs) were used as the start and end times for the analyses. For each 1-min epoch, the total sum of activity counts was computed. If they exceeded a threshold (threshold sensitivity value = mean score in active period/45), then the epoch was considered waking. If it fell below that threshold, then it was considered sleep.
The actigraphic sleep measures used in this study included parameters pertaining to estimated and actual time spent asleep during the night, along with sleep efficiency, sleep latency, and sleep fragmentation. Actigraphic data were analyzed using sleep software (AW64 series, Mini-Mitter), and included the following parameters; (a) Assumed Sleep Time—sleep period; (b) Actual Sleep Time—the amount of time, between Sleep Start and Sleep End, scored as sleep according to the Actiware-Sleep algorithm; (c) Sleep Efficiency—sleep percent excluding all periods of wakefulness; (d) Sleep Latency—the amount of time taken from bedtime until the subject falls asleep; (e) Fragmentation Index—the extent to which sleep is fragmented based on an Actiware-Sleep algorithm.
Sleepiness
A modified version of the Epworth Sleepiness Scale (ESS),21 an 8-item questionnaire assessing an individual's likeliness to fall asleep during common situations, was used to measure children's general level of daytime sleepiness.
Daily sleep logs
Daily sleep logs were completed by parents and included information regarding children's bedtimes and waking times.
Neurobehavioral Functioning
Continuous Performance Test
The Continuous Performance Test (CPT)35 is a standardized computer-administered test in which single letters are presented on a computer screen at 3 different rates: once per second, once every 2 s, or once every 4 s. Over the course of the test (∼ 15 min), the participant is asked to press a button in response to every signal except the target signal (an X). The utilized CPT measures included the total number of omissions (missed targets), total number of commissions (false hits), reaction time (RT), RT variability, RT change by inter-stimulus interval (ISI), and signal detectability (d').
The Conners CPT indices are presented in both raw score and T-score formats. The T-Score approach allows comparison of a performance level to existing validated norms, and was, therefore, used as the outcome of interest. The mean T-score for a comparison group is 50 (SD = 10). According to the CPT manual,36 a T-score ≥ 1 SD above the mean (i.e., ≥ 60) is considered to be high and to indicate a problem.
Analyses
Demographic, intellectual, and psychiatric characteristics were considered to be dependent variables and were compared across the groups using either one-way analyses of variance (ANOVAs) or χ2 analysis, depending on the nature of the data. Changes in sleep patterns or in reported sleepiness of children with ADHD and Controls following the experimental sleep restriction were examined using mixed design multivariate analyses of variance (MANOVAs), with Period (Baseline, Restriction) as the within-subjects factor and Group (ADHD, Control) as the between-subjects factor.
When examining the impact of sleep restriction on CPT performance, 2 separate questions were addressed. First, and as in previous studies exploring the neurocognitive consequences of sleep deprivation on performance, we explored whether a statistically significant change in neurocognitive performance was evident following experimental manipulation. The second question addressed the clinical meaning of the observed changes.
To measure statistical differences in the performance of children following sleep restriction, mixed design MANOVAs were conducted on CPT T-Score data, using Group (ADHD or Control) as a between-subject factor and Period (Baseline or Restriction) as a within-subject factor. Post hoc power analyses were also conducted.
The clinical meaning of changes in performance following experimental sleep restriction was determined using the recommended clinical cutoff score (T-Score ≥ 60).36 Change was considered to be clinically meaningful if T-Scores were ≥ 60. Such analyses can yield 3 potential outcomes. First, a change might be both statistically (the CPT score at baseline differed from the score obtained during the experimental week) and clinically meaningful (T-Score ≥ 60). Second, an observed change might be statistically significant (the CPT score at baseline differed from the score obtained during the experimental week), but not clinically meaningful (T-Score < 60). Third, an observed change might not be statistically significant (the CPT score at baseline did not differ from the score obtained during the experimental week) and also lack clinical meaning (T-Score < 60).
SPSS Version 15.0 for Windows (SPSS Inc., Chicago, IL) was used for all statistical analyses and P-values < 0.05 were considered to indicate statistical significance.
RESULTS
Demographic and Clinical Characteristics
Means and standard deviations (SD) of demographic and clinical characteristics of children with ADHD and Controls are presented in Table 1. No significant differences were found between groups for age, pubertal development, SES or IQ and gender and race distributions were also shown to be similar across groups.
Table 1.
Demographic and clinical characteristi cs of children with ADHD-no breathing problems and Controls
| Variable | ADHD (n = 11) | Control (n = 32) | Test Statistic | P |
|---|---|---|---|---|
| Gender (M/F) | 7/4 | 20/12 | χ2 (1) = 0.005 | 0.95 |
| Age | 8.7 (1.3) | 8.8 (1.3) | F1,41 = 0.002 | 0.96 |
| Ethnic Background | χ2 (3) = 3.89 | 0.27 | ||
| Caucasian | 7 | 24 | ||
| African | 0 | 2 | ||
| Multi-ethnic | 3 | 2 | ||
| Other | 1 | 2 | ||
| SES | 52.2 (13.4) | 49.2 (10.4) | F1,20 = 0.36 | 0.56 |
| IQ | 103.9 (17.3) | 103.8 (14.6) | F1,38 = 0.001 | 0.98 |
| Pubertal Development | 6.6 (1.0) | 7.3 (2.3) | F1,31 = 0.74 | 0.40 |
| DISC-IV Inattention items | 6.45 (2.51) | 0.59 (1.48) | F1,41 = 88.38 | <0.001 |
| DISC-IV Hyperactivity items | 4.27 (1.95) | 0.28 (0.58) | F1,41 = 109.91 | <0.001 |
| DISC-IV total ADHD items | 10.91 (2.47) | 0.72 (1.57) | F1,41 = 253.70 | <0.001 |
| Comorbid ODD | 4 | 2 | χ2 (1) = 6.18 | 0.01 |
| Comorbid CD | 1 | 0 | χ2 (1) = 2.98 | 0.08 |
| Conners' ADHD score | 69.82 (12.79) | 48.71 (5.86) | F1,40 = 54.32 | <0.001 |
| CBCL | ||||
| Internalizing | 58.45 (9.25) | 49.28 (8.56) | F1,41 = 9.01 | 0.005 |
| Externalizing | 57.82 (10.00) | 48.75 (9.37) | F1,41 = 7.41 | 0.009 |
| Total | 63.00 (8.81) | 48.22 (7.59) | F1,41 = 28.65 | <0.001 |
ADHD, attention deficit hyperactivity disorder; SES, socioeconomic status; DISC-IV, Diagnostic Interview Schedule for Children, Fourth Edition; ODD, oppositional defiant disorder; CD, conduct disorder; CBCL, Child Behavior Checklist. All means are T-score transformed.
The Effects of Sleep Restriction on Sleep and Sleepiness
In Table 2, we present the means and SDs of the sleep and sleepiness measures of children with ADHD and Controls at baseline and following sleep restriction. The mixed design MANOVAs that were conducted to determine differences on sleep measures between baseline and the period of sleep restriction revealed a significant Period main effect on measures of actual sleep time, sleep quality, and sleepiness (F5, 34 = 31.30, P < 0.001), whereby all children had shorter sleep duration (F1, 38 = 40.89, P < 0.001, β > 0.99), shorter sleep latency (F1, 38 = 46.87, P < 0.001, β > 0.99), higher sleep efficiency (F1, 38 = 86.18, P < 0.001, β > 0.99), decreased Fragmentation Index (F1, 38 = 12.22, P = 0.001, β = 0.93), and increased sleepiness scores (F1, 38 = 7.37, P = 0.01, β = 0.75) during the sleep restriction period compared to baseline. These effects resulted from significant changes in sleep onset time (mean = 45 min later) and in sleep ending time (mean = 29 min earlier) during the restriction period.
Table 2.
Means and (standard deviations) for sleep measures
| Measure | ADHD |
Control |
||
|---|---|---|---|---|
| Baseline | Restriction | Baseline | Restriction | |
| Sleep Log | ||||
| Bedtime | 21:11 (0:36) | 22:14 (1:06) | 21:01 (0:44) | 21:59 (0:44) |
| Wake time | 7:42 (0:41) | 6:57 (0:38) | 7:04 (0:28) | 6:37 (0:26) |
| Sleep duration (min) | 630.1 (33.3) | 522.6 (44.1) | 603.1 (35.0) | 517.6 (38.3) |
| Sleepiness | 2.82 (2.64) | 4.91 (3.81) | 3.55 (3.33) | 5.52 (4.70) |
| Actigraphy | ||||
| Duration (min) | 487.75 (27.21) | 433.07 (45.97) | 478.81 (29.55) | 444.67 (39.42) |
| Latency (min) | 43.01 (23.32) | 10.80 (7.70) | 30.26 (20.40) | 9.38 (6.27) |
| Efficiency | 76.497 (4.00) | 83.88 (2.87) | 79.22 (4.45) | 86.05 (4.02) |
| Fragmentation | 32.69 (7.32) | 28.36 (9.12) | 36.52 (7.96) | 29.86 (10.89) |
Sleepiness Scores are based on a modified Epworth Sleepiness Scale. All values are mean values, and standard deviations are indicated in parentheses.
Impact of Sleep Restriction on Performance on the CPT
Means and SDs of the CPT measures of children with ADHD and Controls at baseline and following sleep restriction are presented in Table 3.
Table 3.
Means and (standard deviations) for the Continuous Performance Test
| ADHD |
Control |
|||
|---|---|---|---|---|
| Measure | Baseline | Restriction | Baseline | Restriction |
| Omission | 60.51 (26.06) | 75.50 (34.05) | 51.57 (12.66) | 56.41 (22.40) |
| Commission | 54.33 (9.64) | 48.18 (13.67) | 51.75 (9.27) | 48.17 (12.08) |
| RT (ms) | 54.33 (20.02) | 63.97 (16.68) | 50.03 (11.83) | 54.33 (13.54) |
| Variability | 55.75 (10.99) | 62.34 (9.93) | 51.88 (11.33) | 54.52 (9.94) |
| Detectability | 55.42 (8.93) | 47.15 (15.76) | 52.89 (9.82) | 51.54 (14.94) |
| RT by ISI (ms) | 56.92 (20.61) | 63.45 (19.76) | 54.06 (11.42) | 59.04 (10.53) |
All scores have been transformed to T-Scores for comparison purposes.
Examining Statistical Significance of Changes
Mixed design MANOVAs were conducted to determine differences in sleep measures taken at baseline and during the period of sleep restriction, and revealed a significant Period main effect with differences in performance following sleep restriction, compared to performance at baseline (F6,36 = 4.06, P = 0.003). Children in both groups committed a greater number of Omission Errors (F1,41 = 8.68, P = 0.005, β = 0.82), a lower number of Commission Errors (F1,41 = 11.37, P = 0.002, β = 0.91), had slower reaction times (RTs) (F1,41 = 16.58, P < 0.001, β = 0.98), showed a change in RT on the various ISIs tested (F1,41 = 7.02, P = 0.01) and in Detectability (d') (F1,41 = 4.15, P < 0.05, β = 0.51), and had a higher Variability in ISIs (F1,41 = 7.38, P = 0.01, β = 0.76) during the week of sleep restriction. A main effect of diagnostic group was also found (F6,36 = 3.11, P = 0.02), with children with ADHD generally committing more Omission Errors than controls (F1,41 = 4.21, P < 0.05, β = 0.52).
Examining the Clinical Significance of Changes
Comparison between CPT outcome scores and clinical cutoff scores of children with ADHD and controls, after the period of sleep restriction, are presented in Table 4. Although the scores of children in the control group, deteriorated by an average of almost 5 points during the restriction week, all scores nonetheless remained below 60. However, the deterioration of ∼6-15 points in scores for Omission Errors, RT, change in RT, and Variability during the week of sleep restriction in children with ADHD resulted in reduction in performance from subclinical levels of inattention to scores higher than or equal to a T-Score of 60 on two-thirds of CPT outcome measures.
Table 4.
Comparison of CPT scores obtained after partial sleep restriction to the clinical cutoff score (T = 60)
| ADHD |
Control |
|||
|---|---|---|---|---|
| Measure | Restriction | Comparison to Clinical Cutoff Score (T = 60) | Restriction | Comparison to Clinical Cutoff Score |
| Omission | 75.50 (34.05)* | > | 56.41 (22.40) | < |
| Commission | 48.18 (13.67) | < | 48.17 (12.08) | < |
| RT (ms) | 63.97 (16.68)* | > | 54.33 (13.54) | < |
| Variability | 62.34 (9.93)* | > | 54.52 (9.94) | < |
| Detectability | 47.15 (15.76) | < | 51.54 (14.94) | < |
| RT by ISI (ms) | 63.45 (19.76)* | > | 59.04 (10.53) | < |
All scores have been transformed to T-Scores for comparison purposes.
P ≤ 0.05.
**P ≤ 0.001.
DISCUSSION
The main goal of this study was to determine the effects of modest reduction in sleep duration on the NBF of children with ADHD and control children without ADHD. The main strengths of this study are that we: (i) implemented experimental restriction of sleep duration; (ii) assessed sleep in participants' natural home environments over consecutive nights, thereby allowing accurate and objective determination of sleep duration in an ecologically valid setting; (iii) implemented moderate reductions in sleep duration to mimic changes that are common in everyday life; (iv) used an objective methodology to assess sleep duration and NBF; and (iv) controlled for multiple potential confounding variables by studying a control group of healthy children with no primary sleep disorders and no physical or psychological conditions that might affect sleep.
Our main finding was that cumulative reduction in sleep duration of 40.7 minutes was associated with detectable deterioration in most of the CPT variables. The CPT measures vigilance and sustained attention, key processes that are essential for optimal cognitive and academic success in the classroom. More specifically, there was deterioration in the performance of healthy controls and of children with ADHD with regard to measures of sustained attention and vigilance. In children with ADHD, this deterioration of performance led to a diagnostic change, from the subclinical range to the clinical range, based on CPT norms. Given that two-thirds of their CPT measure scores were surpassing the T-score of 60, changes in the scores of ADHD children after sleep restriction were both statistically robust and clinically meaningful.
This finding is important because sleep problems are common in children with ADHD. Despite the relatively high prevalence of ADHD and sleep abnormalities in children, it was not clear whether these problems would affect their NBF. The present study indicates that sleep deprivation does have a significant negative impact on NBF in children with ADHD even in the absence of breathing problems such as sleep apnea or SDB.
Performance on the commission score improved on the second week of the study. This might be related to the general decrease in response level and reduced task engagement that were observed following sleep restriction. Children became more inattentive, and were less responsive to stimuli, as reflected in a higher level of omission errors and slow and variable reaction times. Hence, it is reasonable to conclude that vigilance decreased and that children became generally less responsive. They developed a general tendency to respond less, which was reflected both when a response was necessary (as indicated by the presence of more omission errors) and when a response was not necessary (resulting in fewer commission errors). These findings are consistent with data from another reported experimental study in which the impact of partial sleep deprivation on neurobehavioral functioning was examined in healthy school-age children,22 where no change in the level of commission errors was noted when baseline and test values after sleep restriction were compared. The results from both the cited and present study suggest that the response of children to fatigue is not similar to that of adults, and that the impact of experimental change in sleep duration could be dependent on the tested domain.22,23 Future work should examine developmental changes in vulnerability and resilience to the impact of sleep deprivation on performance. Furthermore, examining the role of underlying brain mechanisms that are not fully developed in school-age children, such as those of the prefrontal cortex, may help to better understand the cognitive responses to sleep deprivation.
Previous studies have suggested that the frontal-parietal regions of the brain are involved in CPT task performance in children with ADHD and in normal control individuals.37 Other studies have documented significant deactivation in the prefrontal38 and posterior parietal39 cortices of sleep deprived individuals. Hence, sleep deprivation seems to affect brain circuits that are implicated in the attentional networks that are measured by the CPT (sustained attention and vigilance). Previous studies have demonstrated that sleep has a restorative effect on brain functions,40,41 and that increased stimulation during wakefulness is followed by an increased intensity of sleep in corresponding cortices.42,43 Brain areas that form the attentional network require more sleep for recovery, so it is possible that these regions are extremely vulnerable to sleep deprivation in school-age children. These findings are consistent with the evidence that frontal, dorsolateral prefrontal, ventrolateral prefrontal, and lateral temporal and parietal regions44–46 are implicated in the pathophysiology of ADHD.
Our experimental alterations of sleep time resulted in an average reduction of 40.7 minutes of habitual sleep duration. This indicates that children in both groups were able to make small changes to sleep time. Sleep restriction resulted in shorter sleep latency and higher sleep efficiency. These effects are consistent with the results of previous studies that used experimental changes in sleep duration,22,47 and explained the results in terms of the two-process model of sleep regulation.48 According to this model, a homeostatic component regulates sleep by increasing sleep pressure when there is more awake time, as indicated by shorter sleep latency, increased time spent asleep as a percentage of the total time spent in bed (based on actigraphy), and lower sleep fragmentation index. Our findings indicate that the decrease in sleep duration overrode improvements in sleep quality, so that our study participants had poorer performance following sleep restriction, despite the improved quality of sleep.
Our finding that partial cumulative sleep restriction had a significant impact on NBF in both groups is in agreement with the results of previous studies in healthy adults, which showed that total and partial sleep restriction was associated with significant deficits in NBF.49–51
It is important to note that the reduction in sleep duration in our study was modest and similar to the sleep deprivation that might occur in daily life. Thus, even small changes in dinner time, computer time, or staying up to do homework could result in poorer NBF the following day and affect sustained attention and vigilance, which are essential for optimal academic performance.
Clinical Implications and Future Directions
Reduction in sleeping hours has become a characteristic of modern society and decreases in sleep time and increasingly delayed bedtimes have led to sleep deprivation in preadolescents.52,53 The findings of the present study indicate that sleep deprivation affects the NBF of children with ADHD and healthy children. Hence, an important implication of the present study is that investments in programs that aim to decrease sleep deprivation may lead to improvements in NBF and academic performance. Therefore, we suggest that knowledge transfer tools that address the problem of inadequate sleep in students should be prioritized, and that initial efforts should be directed toward the educational system. For example, it has been recommended that a program designed by sleep researchers and implemented by classroom teachers, students, and parents could reduce sleep deprivation in adolescents. We are currently implementing such a program—“Sleep for Success”—in elementary schools in Quebec. It has also been shown that delaying school start time by one hour was associated with an average increase in sleep time of 50 minutes,54 improved daily attendance, decreased drop-out rates, improved behavior, and lowered incidences of depression.55 Finally, it has previously been shown that sleep extension in normal school-age children led to improved CPT performance.22
ADHD is the most commonly diagnosed neurobehavioral disorder in children and adolescents, but there is limited consensus concerning the tests needed for accurate diagnosis. In recent years, evaluators have increasingly included the CPT in the basic neurobehavioral battery administered for evaluation of ADHD. Our finding that sleep duration can affect CPT performance suggests that it may be useful to include objective sleep assessments in the clinical assessment of ADHD.
The finding that sleep restriction is associated with NBF in children indicates that researchers should stringently control for sleep duration when examining NBF, both in general and in populations that are prone to present with deficits in NBF and sleep problems, such as individuals with bipolar disorder or schizophrenia.
Sleep-onset insomnia is a common childhood problem, as well as the most prevalent sleep condition in children with ADHD. Empirically supported psychological and behavioral approaches to treatment include several cognitive-behavioral components, among which is sleep restriction.56 Generally, treatment of insomnia focuses on changing maladaptive sleep behavior that contributes to the problem. Although a great deal of empirical support indicates that treatment of adult insomnia using behavioral approaches is valuable, no report has yet evaluated the efficacy of this treatment modality in children or adolescents. The findings of the present study highlight two important results of such intervention. On the one hand, it was evident that when children were sleep restricted, sleep efficiency increased and sleep latency decreased by 10 minutes. It, thus, appeared that sleep restriction would be of potential benefit in the treatment of sleep-onset insomnia. On the other hand, sleep reduction significantly compromised the daytime functioning of the children studied. These findings do not preclude the use of sleep restriction therapy as a possible therapeutic modality for either normal or ADHD children, but do indicate that when sleep restriction is considered in efforts to reduce sleep-onset insomnia, it will be important to monitor the effect of this approach on the child's daytime functioning in school. An evaluation of each individual child is required to determine whether decreasing sleep latency by means of sleep restriction is acceptable with respect to the extent of daytime impairment that might be caused or exacerbated. Future studies considering the daytime consequences of sleep intervention methods that have been used in adults, including sleep restriction, are required to determine the optimal treatment for sleep disorders in school-age children.
Limitations and Future Directions
This study has limitations that should be noted and addressed in future work. First, although statistical power analysis indicated that our sample size was sufficient for the detection of significant effects, the size of our sample was relatively small, so we suggest that our results be considered as preliminary. Second, although polysomnography was used as a screening tool, it was only used post-CPT performance during one week of the study and, therefore, was not able to be used as a measure of sleep quality and duration. Actigraphy allows the reliable, continuous recording of a child's sleep in his or her home environment, but does not allow recording of sleep architecture. Future studies would benefit from the use of polysomnography in conjunction with actigraphy to investigate the association of sleep and NBF in school-age children.
It is important to note that rapid withdrawal of medications right before the baseline could have affected the results. The “wash-out” period of 48 hours was chosen because the half-life of the stimulant methylphenidate is, on average, 2.4 hours when either of the two dosage forms (long- or short-acting) is employed,57 and our choice of wash-out time was consistent with that of previous studies examining the association between sleep and cognition in children with ADHD58 and with the pharmacodynamic effects on behavior, appearing within 30 minutes, reaching a peak within 1 to 3 hours, and gone by 4 to 6 hours.59,60 Therefore, this wash-out period was sufficient for the purposes of the study, while allowing minimization of its impact on everyday functioning of the participants.
Finally, although our findings support the combined impact of the prefrontal cortex on attention and arousal systems, future research more precisely identifying the brain circuits involved in these two systems is critical to further understand the processes that underlie the association between sleep duration and NBF.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We are appreciative for the grants received from the Canadian Institutes of Health Research (CIHR, grant number 153139) and the Fonds de la recherche en santé (FRSQ, grant number 10091) funding agencies and are grateful to the children and families who participated in this study, as well as the research assistants who helped with data collection.
REFERENCES
- 1.Barkley A, Fischer M, Smallish L, Fletcher K. The persistence of attention-deficit/hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder. J Abnorm Psychol. 2002;111:279–89. [PubMed] [Google Scholar]
- 2.Picchietti DL, Underwood DJ, Farris WA. Further studies on periodic limb movement disorder and restless legs syndrome in children with attention-deficit hyperactivity disorder. Mov Disord. 1999;14:1000–7. doi: 10.1002/1531-8257(199911)14:6<1000::aid-mds1014>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 3.Picchietti DL, England SJ, Walters AS, Willis K, Verrico T. Periodic limb movement disorder and restless legs syndrome in children with attention-deficit hyperactivity disorder. J Child Neurol. 1998;13:588–94. doi: 10.1177/088307389801301202. [DOI] [PubMed] [Google Scholar]
- 4.Archbold K, Giordani B, Ruzicka D, Chervin R. Cognitive executive dysfunction in children with mild sleep-disordered breathing. Biol Res Nurs. 2004;5:168–76. doi: 10.1177/1099800403260261. [DOI] [PubMed] [Google Scholar]
- 5.Picchietti MA, Picchietti DL. Restless legs syndrome and periodic limb movement disorder in children and adolescents. Semin Pediatr Neurol. 2008;15:91–9. doi: 10.1016/j.spen.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Gruber R, Xi T, Frenette S, Robert M, Vannasinh P, Carrier J. Sleep disturbances in prepubertal children with attention deficit hyperactivity disorder: a home polysomnography study. Sleep. 2009;32:343–50. doi: 10.1093/sleep/32.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stein M. Unravelling sleep problems in treated and untreated children with ADHD. J Child Adolesc Psychopharmacol. 1999;9:157–68. doi: 10.1089/cap.1999.9.157. [DOI] [PubMed] [Google Scholar]
- 8.Van der Heijden KB, Smits MG, Van Someren EJ, Ridderinkhof KR, Gunning WB. Effect of melatonin on sleep, behavior, and cognition in ADHD and chronic sleep-onset insomnia. J Am Acad Child Adolesc Psychiatry. 2007;46:233–41. doi: 10.1097/01.chi.0000246055.76167.0d. [DOI] [PubMed] [Google Scholar]
- 9.Gruber R, Sadeh A, Raviv A. Instability of sleep patterns in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2000;39:495–501. doi: 10.1097/00004583-200004000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Owens J, Sangal RB, Sutton VK, Bakken R, Allen AJ, Kelsey D. Subjective and objective measures of sleep in children with attention-deficit/hyperactivity disorder. Sleep Med. 2009;10:446–56. doi: 10.1016/j.sleep.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Gruber R, Sadeh A. Sleep and neurobehavioral functioning in children with ADHD. Sleep. 2004;27:267–73. doi: 10.1093/sleep/27.2.267. [DOI] [PubMed] [Google Scholar]
- 12.Konofal E, Lecendreux M, Bouvard MP, Mouren-Simeoni MC. High levels of nocturnal activity in children with attention-deficit hyperactivity disorder: a video analysis. Psychiatry Clin Neurosci. 2001;55:97–103. doi: 10.1046/j.1440-1819.2001.00808.x. [DOI] [PubMed] [Google Scholar]
- 13.Owens J. The ADHD and sleep conundrum: a review. J Devel Behav Pediatr. 2005;26:312–22. doi: 10.1097/00004703-200508000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Chervin R, Dillon J, Bassetti C, Ganoczy D, Pituch K. Symptoms of sleep disorders, inattention, and hyperperactivity in children. Sleep. 1997;20:1185–92. doi: 10.1093/sleep/20.12.1185. [DOI] [PubMed] [Google Scholar]
- 15.Corkum P, Moldofsky H, Hogg-Johnson S, Humphries T, Tannock R. Sleep problems in children with attention-deficit hyperactivity disorder: impact of subtype, comorbidity, and stimulant medication. J Am Acad Child Adolesc Psychiatry. 1999;38:1285–93. doi: 10.1097/00004583-199910000-00018. [DOI] [PubMed] [Google Scholar]
- 16.O'Brien L, Mervis C, Holbrook C, et al. Neurobehavioral correlates of sleep-disordered breathing in children. J Sleep Res. 2004;13:165–72. doi: 10.1111/j.1365-2869.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- 17.Dahl R, Pelham W, Wieron M. The role of sleep disturbances in attention deficit disorder symptoms: a case study. J Pediatr Psychol. 1991;16:229–39. doi: 10.1093/jpepsy/16.2.229. [DOI] [PubMed] [Google Scholar]
- 18.Rowland AS, Lesesne CA, Abramowitz AJ. The epidemiology of attention deficit hyperactivity disorder (ADHD): A public health view. Ment Retard Dev Disabil Res Rev. 2002;8:162–70. doi: 10.1002/mrdd.10036. [DOI] [PubMed] [Google Scholar]
- 19.Bass J, Corwin M, Gozal D, et al. The effect of chronic or intermittent hypoxia on cognition in childhood: a review of the evidence. Pediatrics. 2004;114:805–16. doi: 10.1542/peds.2004-0227. [DOI] [PubMed] [Google Scholar]
- 20.Beebe D, Gozal D. Obstructive sleep apnea and the prefrontal cortex: Towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res. 2002;11:1–16. doi: 10.1046/j.1365-2869.2002.00289.x. [DOI] [PubMed] [Google Scholar]
- 21.Melendres M, Lutz J, Rubin E, Marcus C. Daytime sleepiness and hyperactivity in children with suspected sleep-disordered breathing. Pediatrics. 2004;114:768–75. doi: 10.1542/peds.2004-0730. [DOI] [PubMed] [Google Scholar]
- 22.Sadeh A, Gruber R, Raviv A. The effects of sleep restriction and extension on school-age children: What a difference an hour makes. Child Devel. 2003;74:444–55. doi: 10.1111/1467-8624.7402008. [DOI] [PubMed] [Google Scholar]
- 23.Sadeh A. Consequences of sleep loss or sleep disruption in children. Sleep Med Clin. 2007;2:513–20. [Google Scholar]
- 24.Drummond S, Brown G, Gillin J, Stricker J, Wong E, Buxton R. Altered brain response to verbal learning following sleep deprivation. Nature. 2000;403:655–7. doi: 10.1038/35001068. [DOI] [PubMed] [Google Scholar]
- 25.Shaffer D, Fisher P, Lucas C, Dulcan M, Schwab-Stone M. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Achenbach T. Manual for the child behavior checklist/4-18 and 1991 profile. Burlington: Department of Psychiatry, University of Vermont; 1991. [Google Scholar]
- 27.Conners C, Sitarenios G, Parker J, Epstein J. The revised Conners' Parent Rating Scale (CPRS-R): Factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998;26:257–68. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- 28.Wechsler D. WISC-IV technical and interpretive manual. San Antonio: TX Psychological Corporation; 2003. [Google Scholar]
- 29.Chervin R, Hedger K, Dillon J. Pediatric Sleep Questionnaire (PSQ): Validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med Rev. 2000;1:21–32. doi: 10.1016/s1389-9457(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 30.Chervin R, Hedger K. Clinical prediction of periodic leg movements during sleep in children Sleep Med Rev. 2001;2:501–10. doi: 10.1016/s1389-9457(01)00069-7. [DOI] [PubMed] [Google Scholar]
- 31.Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16:258–62. doi: 10.1093/sleep/16.3.258. [DOI] [PubMed] [Google Scholar]
- 32.Hollingshead AB. Four factor index of social status. Yale University, Department of Sociology; 1975. [Google Scholar]
- 33.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak C. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 34.Sadeh A, Acebo C. The role of actigraphy in sleep medicine. Sleep Med Rev. 2002;6:113–24. doi: 10.1053/smrv.2001.0182. [DOI] [PubMed] [Google Scholar]
- 35.Conners K. Conners' Continuous Performance Test (v 3.0) In. Toronto, CA: Multi-Health Systems; 1994. [Google Scholar]
- 36.Conners CK. Conners' Continuous Performance Test (CPT II) Version 5 for Windows: Technical Guide and Software Manual. Toronto: Multi-Health Systems Inc.; 2000. [Google Scholar]
- 37.Epstein JN, Delbello MP, Adler CM, et al. Neuropediatrics. 2009. Differential patterns of brain activation over time in adolescents with and without attention deficit hyperactivity disorder (ADHD) during performance of a sustained attention task; pp. 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairements during sleepiness. I., Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–52. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 39.Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol. 2004;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- 40.Maquet P. Sleep function(s) and cerebral metabolism. Behav Brain Res. 1995;69:75–83. doi: 10.1016/0166-4328(95)00017-n. [DOI] [PubMed] [Google Scholar]
- 41.Stickgold R, Whidbee D, Schirmer B, Patel V, Hobson JA. Visual discrimination task improvement: A multi-step process occurring during sleep. J Cogn Neurosci. 2000;12:246–54. doi: 10.1162/089892900562075. [DOI] [PubMed] [Google Scholar]
- 42.Kattler H, Dijk D, Borbely A. Effect of unilateral somatosensory stimulation prior to sleep on the sleep EEG in humans. J Sleep Res. 1994;3:159–64. doi: 10.1111/j.1365-2869.1994.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 43.Vyazovskiy V, Borbely A, Tobler I. Unilateral vibrissae stimulation during waking induces interhemispheric EEG asymmetry during subsequent sleep in the rat. Sleep Res. 2000;9:367–71. doi: 10.1046/j.1365-2869.2000.00230.x. [DOI] [PubMed] [Google Scholar]
- 44.Bush G, Valera E, Seidman L. Functional neuroimaging of attention deficit/hyperactivity disorder: a review and suggested future directions. Biol Psychiatry. 2005;57:1273–84. doi: 10.1016/j.biopsych.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 45.Seidman L, Valera E, Makris N. Structural brain imaging of attention deficit hyperactivity disorder. Biol Psychiatry. 2005;57:1263–72. doi: 10.1016/j.biopsych.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 46.Swanson J, Posner M, Cantwell D, et al. Attention deficit hyperactivity disorder: symptom domain, cognitive processes and neural networks. In: Parasuraman R, editor. The attentive brain. Cambridge: MIT Press; 1998. pp. 445–60. [Google Scholar]
- 47.Vgontzas AN, Zoumakis E, Bixler EO, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–26. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 48.Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 49.Dinges DF, Kribbs NB. Performing while sleepy: Effects of experimentally-induced sleepiness. In: Monk TH, editor. Sleep, sleepiness and performance. New York: Wiley; 1991. [Google Scholar]
- 50.Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25:117–29. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- 51.Harrison Y, Horne JA. Sleep loss impairs short and novel language tasks having a prefrontal focus. J Sleep Res. 1998;7:95–100. doi: 10.1046/j.1365-2869.1998.00104.x. [DOI] [PubMed] [Google Scholar]
- 52.Carskadon MA, Wolfson AR, Acebo C, Tzischinsky O, Seifer R. Adolescent sleep patterns, circadian timing, and sleepiness at a transition to early school days. Sleep. 1998;21:871–81. doi: 10.1093/sleep/21.8.871. [DOI] [PubMed] [Google Scholar]
- 53.Owens J. Classification and epidemiology of childhood sleep disorders. Sleep Med Clin. 2007;2:353–61. [Google Scholar]
- 54.Danner FW. High school start time and teen auto crashes. Sleep. 2002;25:A86. [Google Scholar]
- 55.Wahlstrom KL. Accommodating the sleep patterns of adolescents within current educational structures: an uncharted path. In: Carskadon MA, editor. Adolescent sleep patterns: biological, sociological and psychological influences. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- 56.Babson KA, Feldner MT, Badour CL. Cognitive behavioral therapy for sleep disorders. Psychiatr Clin North Am. 2010;33:629–40. doi: 10.1016/j.psc.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 57.Faraj BA, Israili ZH, Perel JM, et al. Metabolism and disposition of methylphenidate-14C: studies in man and animals. J Pharmacol Exp Ther. 1974;191:535–47. [PubMed] [Google Scholar]
- 58.Banaschewski T, Ruppert S, Tannock R, et al. Colour perception in ADHD. J Child Psychol Psychiatry. 2006;47:568–72. doi: 10.1111/j.1469-7610.2005.01540.x. [DOI] [PubMed] [Google Scholar]
- 59.Swanson J, Kinsbourne M, Roberts W, Zucker K. Time-response analysis of the effect of stimulant medication on the learning ability of children referred for hyperactivity. Pediatrics. 1978;61:21–9. [PubMed] [Google Scholar]
- 60.Swanson J, Volkow N. Pharmacodynamics and pharmacokinetics of stimulants in AD/HD. In: Solanto M, Castellanos X, editors. The neuropharmacology of psychostimulant drugs: implications for AD/HD. New York: Oxford University Press; 2000. pp. 101–25. [Google Scholar]