Abstract
Study Objectives:
Our ongoing longitudinal study has shown that NREM delta (1-4 Hz) and theta (4-8 Hz) power measured at C3 and C4 decrease by more than 60% between ages 11 and 17 years. Here, we investigate the age trajectories of delta and theta power at frontal, central, and occipital electrodes.
Design:
Baseline sleep EEG was recorded twice yearly for 6 years in 2 cohorts, spanning ages 9-18 years, with overlap at 12-15 years.
Setting:
Sleep EEG was recorded in the subjects’ homes with ambulatory recorders.
Participants:
Sixty-seven subjects in 2 cohorts, one starting at age 9 (n = 30) and one at age 12 years (n = 37).
Measurements and Results:
Sleep EEG recorded from Fz, Cz, C3, C4, and O1 was referred to mastoids. Visual scoring and artifact elimination was followed by FFT power analysis. Delta and theta EEG power declined steeply across this age range. The maturational trajectories of delta power showed a “back to front” pattern, with O1 delta power declining earliest and Fz delta power declining latest. Theta EEG power did not show this topographic difference in the timing of its decline. Delta, and to a lesser extent, theta power became frontally dominant in early adolescence.
Conclusions:
We maintain our interpretation that the adolescent decline in EEG power reflects a widespread brain reorganization driven by synaptic pruning. The late decline in frontally recorded delta power indicates that plasticity is maintained in these circuits until a later age. Although delta and theta have similar homeostatic properties, they have different age and topographic patterns that imply different functional correlates.
Citation:
Feinberg I; de Bie E; Davis NM; Campbell IG. Topographic differences in the adolescent maturation of the slow wave EEG during NREM sleep. SLEEP 2011;34(3):325-333.
Keywords: Adolescence, sleep, pruning, maturation, FFT, longitudinal
INTRODUCTION
We recently reported the longitudinal trajectories of NREM sleep delta (1-4 Hz) and theta (4-8 Hz) EEG power across childhood and adolescence.1 NREM delta has been of special interest for two biologically related reasons: (1) it behaves homeostatically,2,3 and (2) it changes markedly with age.3–6 Delta's homeostatic behavior is demonstrated by its increase in intensity with prior waking duration and subsequent decline across sleep.3,7 This behavior suggests that some substrate or neuronal change produced by waking brain activity is being metabolized or reversed during NREM sleep.3,8 NREM delta is also the most age-dependent sleep EEG frequency. Cross-sectional studies with both visual scoring9,10 and computer measurement11–14 demonstrate that delta EEG intensity declines steeply across adolescence. This decline is one of the most striking manifestations of human postnatal brain maturation. Computer EEG analysis has demonstrated that NREM theta EEG displays homeostatic7 and age-dependent properties13,14 resembling those of delta.
Longitudinal studies can detect age-related patterns that are difficult to discern in cross-sectional data. Thus, our ongoing longitudinal study has revealed previously unknown details about the pattern of delta EEG maturation.1 Delta power recorded from C3 or C4 does not decline between ages 6 and 11 years. Between 11 and 12 years, delta power at these leads begins a precipitous decline, falling 65% from its childhood level by age 16.5 years. Around this age, its rate of decline slows markedly. Theta power manifests a somewhat different pattern. Our cross-sectional data reveal a significant decline between age 6 and 9 years, and our longitudinal data demonstrate a further decline between 9 and 11 years. Theta power then falls massively during adolescence, declining by 60% between 11 and 16.5 years of age. Like delta power, the theta power decline decelerates around age 16.5-17 years.1
The maturational curves for delta and theta we have published thus far are based on data from C3 and C4 referred to the contralateral mastoid. However, power in different EEG frequencies is not uniformly distributed across the scalp.15 There have been several descriptions of NREM EEG topography in baseline sleep16,17 and in recovery from sleep deprivation.18,19 Data from these studies are somewhat conflicting, at least in part because of differing electrode montages. NREM EEG topography has also been studied during early adolescence in one cross-sectional20 and one short-term longitudinal study.21 The cross-sectional study found no anterior-posterior differences in the decline of delta power, but its authors acknowledged that a longitudinal study over a larger age range was needed. The short-term longitudinal study found that delta power declined in both central and occipital recordings and that the declines were largest for the left central and right occipital EEG.
Here we compare longitudinal age trajectories of NREM delta and theta EEG across 9 years of adolescence, recorded referentially from leads at anterior, central, and occipital locations. We recognize that EEG waves are volume conducted15 and that electrical activity recorded at the scalp cannot simply be attributed to the directly underlying cerebral cortex. Nevertheless, these widely spaced electrodes provide an initial test of whether the maturational trajectories of homeostatic frequencies of NREM EEG differ across cortical areas. Specifically, our goals in this study were to compare, between electrode sites, the magnitude, rate, and timing of delta and theta power changes across the crucial years of adolescent brain development.
METHODS
The data presented below are from our ongoing longitudinal study of sleep and EEG changes across childhood and adolescence. Our methods, described in several previous publications,1,22,23 are briefly summarized here.
Subjects
We studied 94 subjects in 3 age cohorts. The C9 cohort (n = 30, 15 boys) was studied for 6 years starting at approximately age 9 years. The C12 cohort (n = 37, 18 boys) was also studied for 6 years, beginning at approximately age 12 years. Thus, these 2 cohorts overlapped between ages 12 and 15 years. Sixty-seven subjects in the C9 and C12 cohorts completed the first 3 years of study; 56 of them completed the full 6 years. This high retention rate and the 6-year study period make this a unique dataset. Data from all 67 subjects are used in the analyses presented below. The C6 cohort (27 subjects, 17 boys) was initiated more recently. They entered the study at approximately age 6 years and, thus far, have been followed for 1.5 years.
Experimental Design
Sleep EEG was recorded in subjects' homes while they slept in their own beds. In all 3 cohorts, EEG was recorded twice per year. For subjects in the C6 cohort, sleep EEG was recorded for 2 consecutive nights with the subjects maintaining their habitual weekday sleep schedules. For subjects in the C9 and C12 cohorts, sleep EEG was recorded on 4 consecutive nights. On the first 2 nights, subjects continued their habitual school day bed and wake times. On the third and fourth nights, subjects in the C9 and C12 cohorts retired at their habitual school day bed time but attempted to sleep as long as possible, for up to 12 hours. Subjects in all 3 cohorts were required to maintain their habitual weekday sleep schedule for 5 days prior to EEG recording. Naps were prohibited. Subjects wore actigraphy watches (A16, Mini Mitter) to confirm sleep schedule compliance. Recordings were canceled and rescheduled if the actigraphy recordings revealed that subjects napped or otherwise deviated from the stipulated schedules.
EEG Recording
EEG electrodes were applied according to the 10-20 electrode system at Fz, Cz, C3, C4, O1, and either Pz or O2 with mastoid electrodes at A1 and A2. EOG electrodes at the right and left outer canthi were referred to the forehead. Ground electrodes were applied to the head and face. For all electrodes, impedance was < 5 kΩ at the beginning of the recording. For C9 and C12 subjects, the first 9 semiannual EEG recordings used Grass H2O EEG recorders. During the tenth semiannual recording, we replaced the H2O recorders with Grass Aura ambulatory EEG recorders. This was necessary because the Grass Corporation discontinued support of the H2O and replaced it with the Aura model. Aura recorders were used for the 10th through 12th semiannual recordings for C9 and C12 and all recordings for C6. The H2O recorder uses an average of the 6 EEG electrodes as a reference. The Aura recorder uses a separate electrode glued to the scalp as its reference. Signals were recorded versus reference, and electrode pairs were obtained by subtraction (e.g., Fz-A1 is Fz-ref minus A1-ref). Digitization rates were 200 Hz for the H2O and 400 Hz for the Aura. Both recorders have single-pole low-frequency filters with a −3 dB point at 0.5 Hz and a 6 dB per octave slope. We tested the frequency responses of the H2O and Aura. Their filter characteristics were virtually identical for signals between 1 and 8 Hz, the EEG frequencies presented here (see Supplemental Figure from Campbell et al.22).
Sleep EEG Analysis
Using a computer display of the digitized data, each 20-sec epoch of EEG was scored as wake, stage 1, NREM, REM, or movement, based on Rechtschaffen and Kales24 criteria, modified by collapsing stages 2, 3, and 4 into one NREM stage. Each record was checked by a second scorer, and differences between scorers were reconciled by a senior lab scientist. Here we analyzed Fz, Cz, C3, C4, and O1 versus mastoid. For Fz and Cz, we used the mastoid that provided the cleaner recording. For C3, C4, and O1, we used only the contralateral mastoid. Epochs with artifact were detected by an automated program based on high power in 0-0.3 Hz (sweat and movement artifact) and high power in 50-100 Hz (EMG and 60 Hz). Nights in which > 10% of the epochs contained artifacts were not used. All artifact-free epochs in the first 5 h of NREM sleep were analyzed using a fast Fourier transform (PassPlus, Delta Software, St. Louis) with the following parameters: 5.12-sec Welch tapered windows with 2.62 sec of overlap yielding 8 windows per 20-sec epoch. Average power (μV2) in the delta (1.07 to 4.00 Hz, rounded to 1-4 Hz) and theta (4.00 to 7.91 Hz, rounded to 4-8 Hz) bands was calculated as total spectral energy (μV2•sec) in each band divided by seconds of artifact-free EEG. All nights of usable baseline data were averaged for each subject at each recording period. “Baseline” data were nights 1 and 2 for cohort C6, and nights 1, 2, and 3 for cohorts C9 and C12. Although night 3 was an extended night, the first 5 h of NREM provided baseline data since they preceded sleep extension.
Statistical Analysis
The age changes in delta power at the 5 derivations could be fit by a Gompertz-type growth equation25,26 shown below, using a negative coefficient, A, to describe a declining function rather than growth. Essentially, Gompertz growth equations fit patterns characterized by an initial plateau followed by a period of rapid change until a second plateau is reached. The equation we used has the form Power =  with the parameters of the equation as follows:
with the parameters of the equation as follows:
D = level of the upper asymptote (μV2)
A = difference between the upper and lower asymptotes (μV2)
M = age at which power is declining most rapidly (years)
C = relative decline rate at age M (1/years), i.e., C divided by the exponential number e equals the rate of decline divided by A
Another informative descriptor of growth or decay functions is lag or age at which the precipitous change begins. For the Gompertz equation, lag equals M-1/C.26,27
Gompertz equations were fit to the longitudinal delta and theta power data from the C9 and C12 cohorts using the SAS nonlinear mixed effect analysis module NLMIXED. Mixed effect analysis is analogous to regression analysis but is more suitable for longitudinal studies because it takes into account the inherent correlation of repeated measurements on the same subject.28,29 A second advantage of mixed effects analysis is that it effectively accommodates missing data, which are virtually inevitable in longitudinal studies.
The output of the NLMIXED procedure provides estimates of the parameters along with their standard errors and confidence intervals. The confidence intervals make it possible to evaluate the statistical significance of differences in the age-related declines in power of EEG recorded from the 5 sites. Differences in parameter M reveal differences in the timing of the power decline. Differences in parameter C indicate differences in the relative rates of decline.
We further compared differences in the maturational trends in frontal and central EEG power by determining whether the ratio of power at the midline sites Fz and Cz changed across the age range spanned by the combined cohorts (9-18 years). We used linear mixed effect analysis (SAS proc MIXED) to test whether the ratio Fz:Cz changed across that age span for delta and theta EEG power.
RESULTS
NREM Delta EEG Maturation
Delta power at Fz, Cz, C3, C4, and O1 remained stable at high levels from age 6 to age 10 years. Between 10 and 12 years, depending on the electrode site, delta power began to decrease. It then dropped steeply until age 16.5-17 years, when its decline slowed markedly. This overarching pattern was found at all 5 sites. The general shapes of the age curves at each site were quite similar and were closely fit by the Gompertz equation. The curves for Fz, Cz, and O1 are shown in Figure 1. While the Gompertz equation closely fit the longitudinal data at all 5 sites, the age of steepest delta decline (M) differed regionally (Table 1). The age of steepest delta decline at Fz occurred 1 year later than at Cz and 1.5 years later than at O1. These age differences in M were statistically significant since the 95% confidence intervals for M did not overlap. M did not differ between the 3 central sites, Cz, C3, and C4. The composite term “lag” (designating onset of decline) also was earliest at O1 and latest at Fz. Thus, the timing of the delta power decline as indexed both by “lag” and M followed a “back to front” pattern.
Figure 1.
Average (± SE) delta power at each semiannual recording is plotted against age for Fz (A), Cz (B), and O1 (C). A Gompertz function calculated with SAS nonlinear mixed effect analysis is fit to the data from the C9 (triangles) and C12 (circles) cohorts. Data from the C6 (squares) cohort are shown but were not used to generate the function. In all three derivations, delta power declined steeply across adolescence but the magnitude and timing differed between sites (see text). The decline began earliest at the O1 electrode and latest at the Fz electrode. The curves for the C9 and C12 cohorts showed excellent agreement in the ages of overlap (12-15 years).
Table 1.
Parameter estimates and confidence intervals from Gompertz functions fit to age-related declines in power of delta EEG recorded at 5 electrode sites
| Estimate |
|||||
|---|---|---|---|---|---|
| Parameter | Fz | Cz | C4 | C3 | O1 |
| D (μV2) | 1688 | 1964 | 1277 | 1250 | 813.0 |
| A (μV2) | 1304 | 1683 | 1030 | 975 | 665.7 |
| C | 0.4792 | 0.4471 | 0.4972 | 0.4966 | 0.5131 |
| M (years) | 14.07 | 13.06 | 13.38 | 13.37 | 12.49 |
| Lag (years) | 11.98 | 10.82 | 11.37 | 11.35 | 10.54 |
|
95% Confidence Interval |
|||||
| Parameter | Fz | Cz | C4 | C3 | O1 |
| D (μV2) | 1576 – 1800 | 1851 – 2078 | 1197 – 1358 | 1167 – 1333 | 747.5 – 878.5 |
| A (μV2) | 1152 – 1455 | 1532 – 1834 | 920 – 1140 | 866 – 1085 | 566.0 – 765.3 |
| C | 0.4104 – 0.5480 | 0.3925 – 0.5016 | 0.4184 – 0.5760 | 0.4182 – 0.5750 | 0.3842 – 0.6419 |
| M (years) | 13.83 – 14.32 | 12.84 – 13.28 | 13.16 – 13.60 | 13.11 – 13.63 | 12.15 – 12.84 |
Power =  D, upper asymptote; A, drop to lower asymptote; C, relative decline rate; M, age at which decline rate is maximal; Lag, M-1/C.
D, upper asymptote; A, drop to lower asymptote; C, relative decline rate; M, age at which decline rate is maximal; Lag, M-1/C.
The Gompertz equation parameters D and A for delta power also differed significantly between sites Fz, Cz, and O1 (Table 1). The upper asymptote for Cz was significantly higher than that for Fz, which was significantly higher than that for O1. Similarly, the magnitude of the drop (A) to the lower asymptote was greater for Cz than for Fz, which, in turn, exceeded that for O1. Equation parameters D and A were also lower for O1 than for C3 and C4. These parameters did not differ between C3 and C4, but the parameters for both central lateral sites were lower than for the midline central site (Cz).
The derivatives of the Gompertz equations indicate rates of power decline. The plots of absolute and relative decline rates in Figures 2A and B show that Cz had the most rapid absolute rate of decline in delta power and that O1 had the highest relative decline rate. However, the relative rate of decline in delta power did not differ significantly among sites, i.e., the 95% confidence intervals (Table 1) for the C parameter of the Gompertz equation, overlapped for all 5 electrode sites. The C parameter defines relative rate of decline only at age M. To compare the average change in delta power across the 9 years covered by the C9 and C12 cohorts, we calculated the percent decline in power from the first recording in the C9 cohort (age 9.3 years) to the twelfth recording in the C12 cohort (age 17.9 years). The greatest percent decline (Table 2) occurred at the occipital electrode (even though it had the lowest initial level of delta power), and the smallest percent decline was at the frontal electrode.
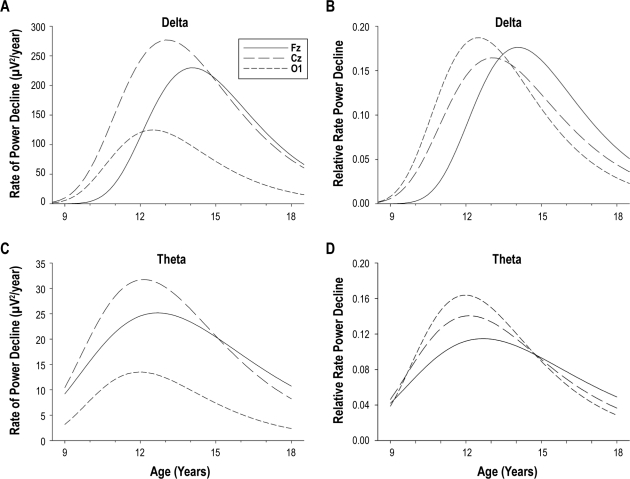
Figure 2.
Absolute (A) and relative (B) rate of decline of delta power and theta power (C, D) plotted against age. Fz, Cz, and O1 are plotted in the same panels for comparison. Absolute rates of decline are the first derivatives (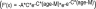 ) of the Gompertz equations plotted in Figure 1 (or Figure 4 for theta). The peak rate of delta power decline (2A) was greatest at electrode Cz. Relative rates of decline (2B, 2D) are the absolute rate of decline divided by the Gompertz parameter A. The relative rates of delta power decline (2B) were similar for the 3 sites. The earlier peak rate of delta power decline in O1 is evident in both Figures 2A and 2B. For theta power, the peak rate of decline was also greatest at Cz and smallest at O1, but the timing of the decline did not differ significantly between sites.
) of the Gompertz equations plotted in Figure 1 (or Figure 4 for theta). The peak rate of delta power decline (2A) was greatest at electrode Cz. Relative rates of decline (2B, 2D) are the absolute rate of decline divided by the Gompertz parameter A. The relative rates of delta power decline (2B) were similar for the 3 sites. The earlier peak rate of delta power decline in O1 is evident in both Figures 2A and 2B. For theta power, the peak rate of decline was also greatest at Cz and smallest at O1, but the timing of the decline did not differ significantly between sites.
Table 2.
Comparison of power declines at 5 different electrode sites
| Delta Power |
Theta Power |
|||||
|---|---|---|---|---|---|---|
| Site | C9 (9.3 yrs) | C12 (17.9 yrs) | Percent Decline | C9 (9.3 yrs) | C12 (17.9 yrs) | Percent Decline |
| Fz | 1599 μV2 | 641 μV2 | 59.9% | 226 μV2 | 81 μV2 | 64.3% |
| Cz | 1921 μV2 | 500 μV2 | 74.0% | 249 μV2 | 77 μV2 | 69.0% |
| C4 | 1324 μV2 | 357 μV2 | 73.0% | 163 μV2 | 53 μV2 | 67.5% |
| C3 | 1264 μV2 | 366 μV2 | 71.0% | 167 μV2 | 56 μV2 | 66.5% |
| O1 | 828 μV2 | 196 μV2 | 76.4% | 114 μV2 | 47 μV2 | 58.8% |
Average delta and theta power are listed for the first recording (mean age = 9.3 years) from the C9 cohort and for the twelfth recording (mean age = 17.9 years) from the C12 cohort.
To further test the back to front pattern of the brain maturation reflected in the delta EEG power decline, we examined age effects on the ratio of frontal to central power, Fz:Cz (Figure 3A). The ratio of frontal to central EEG delta power increased significantly (F1,603 = 197, P < 0.0001) from ∼ 0.85 at age 9.3 years to ∼ 1.3 at age 17.9 years. Delta power was greater at Cz than Fz until about age 12 years, after which power at Fz exceeded that at Cz, producing frontal delta predominance.
Figure 3.
Age-related change in the ratio of frontal (Fz) to central (Cz) power for (A) delta and (B) theta EEG using the same format as Figure 1. For delta power, the Fz:Cz ratio was well below 1.0 at age 9 years and increased steeply across adolescence, surpassing 1.0 at about age 12 years. For theta power the Fz:Cz ratio also began below 1.0 and increased significantly but more slowly than the ratio for delta power.
NREM Theta EEG Maturation
Contrary to the delta power trajectory, theta power at all 5 sites declined from the first measurements at age 6 years (not statistically analyzed) and continued to decline into adolescence. During adolescence, the theta decline, like that of delta, was massive (Figure 4). As was true for delta, the decline in theta power slowed markedly around age 16.5-17 years. Despite its relatively linear decline with age, a Gompertz equation for theta provided a close fit for all 5 EEG leads. The Gompertz equation accommodated the rapid rate of decline in mid-adolescence followed by a relative leveling off. In contrast to delta power, the timing of the theta power decline did not differ significantly among EEG leads. The 95% confidence intervals for M (Table 3) overlapped at all 5 sites, and the “lags” were very similar. As with delta, the shape of the theta curve was similar for the 5 sites. Comparing the confidence intervals for parameter M for theta and delta (Tables 1 and 3) shows that the age of peak rate of decline occurred significantly earlier for theta than for delta at Fz, Cz, C3, and C4, but not at O1.
Figure 4.
Average (± SE) theta power at each semiannual recording is plotted against age for Fz (A), Cz (B), and O1 (C) using the same format as Figure 1. In all 3 derivations, theta power declined steeply across adolescence. Comparing Figure 4 to Figure 1 demonstrates that the theta decline began much earlier than the delta decline at all electrode sites.
Table 3.
Parameter estimates and confidence intervals from Gompertz functions fit to age-related declines in power of theta EEG recorded at 5 electrode sites
| Estimate |
|||||
|---|---|---|---|---|---|
| Parameter | Fz | Cz | C4 | C3 | O1 |
| D (μV2) | 250.0 | 270.3 | 164.3 | 176.3 | 118.7 |
| A (μV2) | 219.2 | 225.7 | 126.4 | 131.0 | 82.5 |
| C | 0.3121 | 0.3820 | 0.4189 | 0.4331 | 0.4452 |
| M (years) | 12.68 | 12.13 | 12.49 | 12.04 | 11.98 |
| Lag (years) | 9.48 | 9.51 | 10.10 | 9.73 | 9.73 |
|
95% Confidence Interval |
|||||
| Parameter | Fz | Cz | C4 | C3 | O1 |
| D (μV2) | 222.1 – 277.9 | 243.9 – 296.7 | 149.1 – 179.5 | 157.6 – 195.0 | 104.7 – 132.8 |
| A (μV2) | 174.0 – 264.4 | 194.1 – 257.3 | 108.0 – 144.8 | 109.5 – 152.5 | 65.1 – 99.9 |
| C | 0.2333 – 0.3909 | 0.3226 – 0.4415 | 0.3413 – 0.4966 | 0.3519 – 0.5143 | 0.3195 – 0.5710 |
| M (years) | 12.18 – 13.18 | 11.77 – 12.49 | 12.14 – 12.85 | 11.65 – 12.43 | 11.41 – 12.55 |
Power =  D, upper asymptote; A, drop to lower asymptote; C, relative decline rate; M, age at which decline rate is maximal; Lag, M-1/C
D, upper asymptote; A, drop to lower asymptote; C, relative decline rate; M, age at which decline rate is maximal; Lag, M-1/C
Although the timing of the theta power decline did not differ between sites, there were site differences in the magnitude of the decline. The upper plateau (D) and the drop (A) to the lower asymptote for Fz and Cz were higher than for C3 and C4, which were higher than for O1. D and A did not differ between Fz and Cz nor between C3 and C4. Figure 2C shows that theta power at Cz had the highest absolute rate of decline. As with delta, the highest relative rate of theta decline was at O1 (Figure 2D), but site differences in relative rates of decline were not significant; the 95% confidence intervals for the C parameter of the Gompertz equations overlapped across the 5 sites. In contrast to delta power, theta power at O1 showed the smallest percent decline (Table 2) from the 1st C9 recording to the 12th C12 recording, i.e., across ages 9.3 to 17.9 years. The ratio of Fz to Cz theta power (Figure 3B) increased significantly (F1,603 = 42, P < 0.0001) across these years. Thus, theta power became more frontally dominant across adolescence. However, the 14% increase in the Fz:Cz theta power ratio was much smaller than the 56% increase in the Fz:Cz delta power ratio.
The maturational changes in the delta and theta Fz:Cz power ratios raise the possibility that all EEG frequencies become more frontal with adolescent brain maturation. To investigate this possibility we computed changes in the Fz:Cz ratio for alpha (8-12 Hz) power since NREM alpha power also declines during adolescence.13,21 Our data show that the Fz:Cz alpha power ratio decreases significantly between age 9 and 18 (F1,603 = 7.12, P = 0.0078), i.e., NREM alpha becomes more central rather than more frontal during adolescence.
DISCUSSION
The maturational pattern of delta EEG power across adolescence differs by electrode location as does, to a lesser extent, the maturational pattern of theta EEG power. The delta and theta patterns also differ from each other. These differences have implications for sleep as well as for brain maturation since delta and theta are the main homeostatic frequencies of NREM sleep. In what follows, we discuss separately the longitudinal patterns of delta and theta topography across ages 9-18 years and then speculate on the theoretical significance of these results. In considering this discussion, the reader should bear in mind a limitation of our topographic data. We report data for only five referential electrode sites; two of these sites (Fz,Cz) are midline, and the other three (C3, C4, O1) are lateral. We realized when we designed this study that a more extensive montage would have yielded additional important information. However, we also knew that a larger montage would increase the burden on our subjects and diminish the probability that subjects would complete the study. Subject retention is the essential requirement of longitudinal research. By minimizing the time requirement and establishing positive relationships with the subjects and their families, we achieved excellent retention, allowing us to meet our main goal: defining the longitudinal trajectories of sleep and sleep EEG across adolescence.
Topographic Patterns of Delta EEG Maturation
The overall trajectories of the declining delta power curves across adolescence are similar at the frontal, central, and occipital electrode sites. At these sites, delta power maintains a high level between ages 9 and 11 years and then declines rapidly until age 16.5-17 years, when the decline slows markedly. Despite similar trajectories, the sites differ in the timing of the decline, relative amounts of delta power, and in their rates of change. The Gompertz parameter (M) and the composite term “lag” confirm that the delta decline begins earliest at O1 and latest at Fz. Figure 1 suggests that we might find even greater topographic differences in the timing of delta maturation when we can add the C6 longitudinal data to the analyses. Fz delta power appears to remain unchanged between 6 and 12 years, whereas delta power in O1 may begin its decline before the “lag” (10.5 years) calculated from the C9 and C12 data.
The topographic pattern of delta power decline found here supports the general back to front maturational patterns of cortical thinning found in cross-sectional and longitudinal MRI studies.30,31 Cortical thinning, generally interpreted as reflecting synaptic pruning, occurs earliest in posterior cortical regions and then moves anteriorly across childhood-adolescence, occurring latest in the frontal cortex.30 Limited cross-sectional anatomic data show that synaptic density decreases earlier in occipital than in frontal cortex32,33 and that post-natal myelination occurs last in the frontal lobe.34
Among the five electrode sites studied, the percent decline in delta power was greatest at the O1 lead and smallest at the Fz lead. Occipital delta power also manifests the most rapid relative rate of decline. Since O1 has the lowest and Cz the highest initial level of delta power, these findings are the opposite of what would occur if adolescent changes in EEG power represented regressions toward the mean. Instead, the findings show that delta becomes frontally dominant during the course of adolescence. Thus, at age 9 years, delta power is lower in Fz than in Cz (with O1 by far the lowest). As cerebral maturation continues, Fz delta power becomes equal to Cz delta power at age 12 years. By age 17 years, Fz power substantially exceeds Cz power. Although Fz and Cz delta power both decline during adolescence, Cz power declines more rapidly, inverting the ratio of power in the two leads. Thus, the pattern of frontal delta dominance, long familiar in studies of adult NREM EEG, is not present in childhood but emerges during early adolescent brain development. Again, we interpret these changes as reflecting regional differences in the rates of synaptic pruning and, probably, neuronal commitment (plasticity).
Higher delta power in the frontal than in the occipital cortex presumably reflects a greater need for homeostatic recuperation in anterior brain regions. According to our 1974 homeostatic model, higher delta power in the frontal cortex would indicate a higher proportion of plastic neurons. Greater plastic neuronal activity during waking would increase the need (substrate) for homeostatic recuperation. The later and smaller percent decline in the frontal areas would indicate the prolonged plasticity of this cortical region. Some investigators have proposed that topographic differences in delta activity reflect differences in “use-dependent” sleep regulation.16,17 We believe that these topographic variations reflect differences in the cell biology of the regions producing the EEG rather than the effects of imposed activity. For a semiquantitative model relating age changes in NREM delta to the proportion of intensely plastic, uncommitted neurons in a neuronal population, see Feinberg et al.35
For the central leads, we found that the Gompertz parameters D and A for the midline lead, Cz, were greater than D and A for the lateral leads, C3 and C4. We cannot conclude whether the higher power recorded at Cz reflects a greater homeostatic need for midline brain areas or is a property of volume conduction within the skull. However, it is important to note that the percent declines in midline and lateral sites are virtually identical.
In a cross-sectional study, Jenni et al.20 compared sleep EEG topography in 20 less mature (Tanner 1-2, mean age 11.4) and 20 more mature (Tanner 4-5, mean age 14.1) subjects using a bipolar electrode montage. Although Jenni et al. reported that the maturational declines in delta power were independent of electrode site, they recommended a longitudinal study over a wider age range. Our longitudinal data across ages 9 to 18 years, indeed, demonstrate clear topographic differences in delta EEG maturation. In a short-term longitudinal study, Tarokh and Carskadon21 compared age changes in EEG power from C3, C4, O1, and O2. They found that delta EEG power declined in all leads but that the decline was greatest in C3 and O2. They interpreted these data as demonstrating hemispheric differences in adolescent EEG maturation. Our data do not suggest hemispheric asymmetry in the decline of delta power at C3-A2 vs. C4-A1: the delta decline across ages 9-18 years was virtually identical in these two leads. A long-term longitudinal study over this age range with a more extensive electrode array is needed to fully describe topographic changes during adolescent EEG maturation.
Topographic Patterns of Theta EEG Maturation
Fz and Cz data confirm our published finding, based on C3 and C4 recordings, that theta power declines earlier than does delta power. Moreover, the trajectories of the theta decline are similar in the five leads, and their timing does not differ significantly by electrode site as it does for delta. Specifically, the theta power decline does not show the back to front progression found for delta power. We have suggested that the earlier theta power decline indicates that the brain circuits generating theta EEG begin synaptic pruning before those that generate delta EEG.1
Although the timing of the theta power decline does not differ significantly by site, theta EEG manifests a shift in the ratio of Fz:Cz power resembling that of delta EEG, but much smaller. Theta power at age 9 years is initially higher at Cz than at Fz, and the ratio of Fz:Cz power increases across adolescence. The Fz:Cz ratio increase for theta power, while statistically robust, is much smaller than the Fz:Cz ratio increase for delta: a 14% increase for theta as compared to a 56% increase for delta. As is the case for delta power, Fz theta power eventually exceeds Cz power because Cz power declines more rapidly. Our finding that frontal theta power greatly exceeds occipital theta power contradicts two previous studies that found higher theta power in posterior than in anterior bipolar recordings.16,17 The difference can be explained by our use of referential (e.g., frontal vs mastoid) recordings rather than bipolar (e.g., parietal vs occipital). Another study using referential recordings also found much higher theta power in frontal than occipital leads.19
We previously proposed that the adolescent decline in theta power reflects maturation of allocortical (3-layer) structures, whereas the decline in delta power reflects five-layer isocortex maturation. This speculation was based on longitudinal MRI studies showing that most allocortical structures mature earlier than neocortical structures and have more linear trajectories.36 Our finding here that theta EEG does not show topographic differences is consistent with this speculation. It is tempting to hypothesize that the cortical theta power we measure during NREM sleep is driven by the hippocampus, a major allocortical structure, and that theta's earlier and topographically uniform decline signifies earlier synaptic elimination in the hippocampus than in the cerebral cortex. However, we recognize that the powerful hippocampal theta recorded in rodents has not been demonstrated in primates nor has any homology been established between the theta recorded in human and animal studies; these issues are thoroughly discussed in the masterly review of Mitchell et al.37
Theoretical Significance
In the absence of concomitant longitudinal measurements of sleep EEG and cognitive functions, the functional significance of the striking brain decline in delta EEG power must remain speculative. When we proposed the homeostatic model of NREM slow waves,3 we hypothesized that delta waves reflect recuperation of plastic neurons from waking information processing. In our model, the amount of recuperation required is proportional to both prior waking duration and the intensity of waking brain activity, with intensity indexed by waking brain metabolic rate. In early papers,3,10 we noted that the decline in NREM delta across adolescence might reflect the diminishing brain plasticity that is manifested functionally by reduced ability to recover from brain injury, to learn complex motor skills, and to learn to speak new languages with native-like inflection. Later, we hypothesized that adolescent brain reorganization driven by synaptic pruning underlies the emergence of adult problem-solving skills and that subtle errors in this process cause mental illness, notably schizophrenia.38 We believe that both hypotheses remain viable. A first step toward their empirical validation requires longitudinal studies that combine anatomic, electrophysiologic, and cognitive measures. The present findings raise the question of what cognitive capacities might be maturing earlier in theta circuits than in delta systems. Recognizing the speculative nature of such theorizing, we tentatively propose that the earlier theta development underlies earlier maturation of memory consolidation systems. The sponge-like absorption of information by children, highlighted by such phenomena as eidetic imagery, diminishes over childhood and might parallel the maturational pattern of theta found here. The maturation of delta-generating circuits, which begins at 10-11 years of age, might reflect the development of complex systems that integrate the executive and planning functions of basic ganglia and cerebral cortex. It is possible that the period of intense delta decline (11-17 years) is the main brain correlate of behavioral adolescence.
We add one final point important for conceptualizing sleep and brain ontogeny. Although we show here that the declines in delta and theta power slow markedly around age 17 years, they clearly do not stop. In a cross-sectional study of the age-related changes in delta EEG using computer measurement, our laboratory demonstrated a robust negative correlation between delta integrated amplitude and age over the range 18-22 years.39 (Delta integrated amplitude is a period-amplitude measure closely correlated with delta spectral power.40) Thus, delta continues a slow but measurable decline well into middle age. This continued decline was firmly demonstrated by early studies of visually scored stage 4 EEG3,41 and, later, by computer measurement.5,13
Sleep researchers have traditionally interpreted the delta decline across adolescence as “developmental” and its decline during adulthood as due to “aging” or degenerative brain changes. We have argued for an alternative possibility: that the delta decline in adults is produced by the same qualitative process that produces the adolescent decline, but occurring at a slower rate.42 The hyperbolic decline of delta intensity (measured as stage 4) across age approaches its asymptote in early middle age.10 In addition, there is little change in computer-measured power between ages 40-60 years.11 Thus, the delta power decline in adulthood is essentially complete before the neuronal or cognitive signs of “aging” become prominent. Our view that the same regressive processes that produce the delta decline of adolescence continue into early adulthood has clinical as well as basic neuroscience implications. Major mental illnesses frequently begin in the second and third decades of life. If the continuation of the delta decline into the third decade is maturational, the onset of mental illness in the third decade, as well as during the second, could be caused by the same sort of errors in late neurodevelopment we originally hypothesized.38
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This research was supported by United States Public Health Service grant R01 MH62521. Rahman Azari, PhD, of the UC Davis Department of Statistics provided valuable statistical assistance with the non-linear mixed effect analysis. Adair McPherson, PhD, provided proofreading and editing help. We thank the subjects and their families for their participation in this study.
REFERENCES
- 1.Campbell IG, Feinberg I. Longitudinal trajectories of non-rapid eye movement delta and theta EEG as indicators of adolescent brain maturation. Proc Natl Acad Sci U S A. 2009;106:5177–80. doi: 10.1073/pnas.0812947106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 3.Feinberg I. Changes in sleep cycle patterns with age. J Psychiatr Res. 1974;10:283–306. doi: 10.1016/0022-3956(74)90011-9. [DOI] [PubMed] [Google Scholar]
- 4.Agnew HW, Jr, Webb WB. The displacement of stages 4 and REM sleep with a full night of sleep. Psychophysiol. 1968;5:142–8. doi: 10.1111/j.1469-8986.1968.tb02811.x. [DOI] [PubMed] [Google Scholar]
- 5.Carrier J, Land S, Buysse DJ, Kupfer DJ, Monk TH. The effects of age and gender on sleep EEG power spectral density in the middle years of life (ages 20-60 years old) Psychophysiol. 2001;38:232–42. [PubMed] [Google Scholar]
- 6.Ehlers CL, Kupfer DJ. Effects of age on delta and REM sleep parameters. Electroencephalogr Clin Neurophysiol. 1989;72:118–25. doi: 10.1016/0013-4694(89)90172-7. [DOI] [PubMed] [Google Scholar]
- 7.Borbély AA, Baumann F, Brandeis D, Strauch I, Lehmann D. Sleep-deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol. 1981;51:483–93. doi: 10.1016/0013-4694(81)90225-x. [DOI] [PubMed] [Google Scholar]
- 8.Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62:143–50. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Coble PA, Kupfer DJ, Taska LS, Kane J. EEG sleep of normal healthy children. Part I: findings using standard measurement methods. Sleep. 1984;7:289–303. doi: 10.1093/sleep/7.4.289. [DOI] [PubMed] [Google Scholar]
- 10.Feinberg I, Carlson VR. Sleep variables as a function of age in man. Arch Gen Psychiatr. 1968;18:239–50. [Google Scholar]
- 11.Coble PA, Reynolds CF, III, Kupfer DJ, Houck P. Electroencephalographic sleep of healthy children. Part II: findings using automated delta and REM sleep measurement methods. Sleep. 1987;10:551–62. [PubMed] [Google Scholar]
- 12.Feinberg I, March JD, Flach K, Maloney T, Chern W-J, Travis F. Maturational changes in amplitude, incidence and cyclic pattern of the 0 to 3 Hz (delta) electroencephalogram of human sleep. Brain Dysfunc. 1990;3:183–92. [Google Scholar]
- 13.Gaudreau H, Carrier J, Montplaisir J. Age-related modifications of NREM sleep EEG: from childhood to middle age. J Sleep Res. 2001;10:165–72. doi: 10.1046/j.1365-2869.2001.00252.x. [DOI] [PubMed] [Google Scholar]
- 14.Jenni OG, Carskadon MA. Spectral analysis of the sleep electroencephalogram during adolescence. Sleep. 2004;27:774–83. [PubMed] [Google Scholar]
- 15.Niedermeyer E. Maturation of the EEG: development of waking and sleep patterns. In: Niedermeyer E, Lopes Da Silva F, editors. Electroencephalography. Basic principles, clinical applications, and related fields. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 209–34. [Google Scholar]
- 16.Werth E, Achermann P, Borbély AA. Brain topography of the human sleep EEG: antero-posterior shifts of spectral power. Neuroreport. 1996;8:123–7. doi: 10.1097/00001756-199612200-00025. [DOI] [PubMed] [Google Scholar]
- 17.Werth E, Achermann P, Borbély AA. Fronto-occipital EEG power gradients in human sleep. J Sleep Res. 1997;6:102–12. doi: 10.1046/j.1365-2869.1997.d01-36.x. [DOI] [PubMed] [Google Scholar]
- 18.Achermann P, Finelli L, Borbely AA. Unihemispheric enhancement of delta power in human frontal sleep EEG by prolonged wakefulness. Brain Res. 2001;913:220–3. doi: 10.1016/s0006-8993(01)02796-2. [DOI] [PubMed] [Google Scholar]
- 19.Marzano C, Ferrara M, Curcio G, Gennaro LD. The effects of sleep deprivation in humans: topographical electroencephalogram changes in non-rapid eye movement (NREM) sleep versus REM sleep. J Sleep Res. 2010;19:260–8. doi: 10.1111/j.1365-2869.2009.00776.x. [DOI] [PubMed] [Google Scholar]
- 20.Jenni OG, van Reen E, Carskadon MA. Regional differences of the sleep electroencephalogram in adolescents. J Sleep Res. 2005;14:141–7. doi: 10.1111/j.1365-2869.2005.00449.x. [DOI] [PubMed] [Google Scholar]
- 21.Tarokh L, Carskadon MA. Developmental changes in the human sleep EEG during early adolescence. Sleep. 2010;33:801–9. doi: 10.1093/sleep/33.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell IG, Darchia N, Higgins LM, et al. Adolescent changes in homeostatic regulation of EEG activity in the delta and theta frequency bands during non-rapid eye movement sleep. Sleep. 2011;34:83–91. doi: 10.1093/sleep/34.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feinberg I, Higgins LM, Khaw WY, Campbell IG. The adolescent decline of NREM delta, an indicator of brain maturation, is linked to age and sex but not to pubertal stage. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1724–9. doi: 10.1152/ajpregu.00293.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring systems for sleep stages of human subjects. Washington, DC: Public Health Services, U.S. Government Printing Office; 1968. [Google Scholar]
- 25.Laird AK. Dynamics of tumor growth. Br J Cancer. 1964;13:490–502. doi: 10.1038/bjc.1964.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zwietering MH, Jongenburger I, Rombouts FM, van't Riet K. Modeling of the bacterial growth curve. Appl Environ Microbiol. 1990;56:1875–81. doi: 10.1128/aem.56.6.1875-1881.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gibson AM, Bratchell N, Roberts TA. The effect of sodium chloride and temperature on the rate and extent of growth of Clostridium botulinum type A in pasteurized pork slurry. J Appl Bacteriol. 1987;62:479–90. doi: 10.1111/j.1365-2672.1987.tb02680.x. [DOI] [PubMed] [Google Scholar]
- 28.Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. J Educ Behav Stat. 1998;23:323–55. [Google Scholar]
- 29.Twisk JWR. Applied longitudinal data analysis for epidemiology. Cambridge: Cambridge University Press; 2003. [Google Scholar]
- 30.Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–87. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- 32.Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 33.Huttenlocher PR, de Courten C. The development of synapses in striate cortex of man. Hum Neurobiol. 1987;6:1–9. [PubMed] [Google Scholar]
- 34.Yakovlev PI, Lecours AR. Myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional development of the brain in early life. Philadelphia: Davis Co.; 1967. pp. 3–70. [Google Scholar]
- 35.Feinberg I, Thode HC, Jr, Chugani HT, March JD. Gamma distribution model describes maturational curves for delta wave amplitude, cortical metabolic rate and synaptic density. J Theor Biol. 1990;142:149–61. doi: 10.1016/s0022-5193(05)80218-8. [DOI] [PubMed] [Google Scholar]
- 36.Shaw P, Kabani NJ, Lerch JP, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–94. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchell DJ, McNaughton N, Flanagan D, Kirk IJ. Frontal-midline theta from the perspective of hippocampal “theta”. Prog Neurobiol. 2008;86:156–85. doi: 10.1016/j.pneurobio.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982/1983;17:319–34. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 39.Feinberg I, March JD, Fein G, Floyd TC, Walker JM, Price L. Period and amplitude analysis of 0.5-3 c/sec activity in NREM sleep of young adults. Electroencephalogr Clin Neurophysiol. 1978;44:202–13. doi: 10.1016/0013-4694(78)90266-3. [DOI] [PubMed] [Google Scholar]
- 40.Uchida S, Feinberg I, March JD, Atsumi Y, Maloney T. A comparison of period amplitude analysis and FFT power spectral analysis of all-night human sleep EEG. Physiol Behav. 1999;67:121–31. doi: 10.1016/s0031-9384(99)00049-9. [DOI] [PubMed] [Google Scholar]
- 41.Agnew HW, Jr, Webb WW, Williams RL. Sleep patterns in late middle age males: an EEG study. Electroencephalogr Clin Neurophysiol. 1967;23:168–71. doi: 10.1016/0013-4694(67)90107-1. [DOI] [PubMed] [Google Scholar]
- 42.Feinberg I. Slow wave sleep and release of growth hormone. JAMA: 2000;284:2717–8. [PubMed] [Google Scholar]