Abstract
Study Objectives:
Prior studies, all using SPECT techniques, failed to find any differences for dopamine transporter (DAT) in restless legs syndrome (RLS) subjects. The distinct pharmacokinetic properties associated with SPECT-determined DAT along with rapid biodynamic changes in DAT may, however, have missed membrane-bound DAT differences. The current studies assessed real-time DAT binding potentials (BP) in striatum of RLS patients using 11C-methylphenidate and PET techniques.
Design:
RLS medications were stopped at least 11 days prior to the PET study. Clinical severity of RLS was also assessed. PET scans were performed at 2 different times of day (starting at 08:30 and 19:30) in separate groups of subjects. The primary outcome measure was total striatal DAT BP.
Participants:
Thirty-six patients with primary RLS and 34 age- and gender-matched controls.
Results:
RLS subjects had significantly lower DAT binding in the striatum compared to controls on both the Day and the Night scans. DAT was decreased in putamen and caudate but not the ventral striatum of RLS subjects. There were no diurnal differences in DAT for the total group or for control and RLS separately. DAT BP did not correlate with any clinical measures of RLS.
Conclusion:
The current study found a significant decrease in DAT BP in two independent studies. These results when viewed along with prior RLS SPECT and autopsy studies of DAT, and cell culture studies with iron deficiency and DAT, suggest that membrane-bound striatal DAT, but not total cellular DAT, may be decreased in RLS.
Citation:
Earley CJ; Kuwabara H; Wong DF; Gamaldo C; Salas R; Brasic J; Ravert HT; Dannals RF; Allen RP. The dopamine transporter is decreased in the striatum of subjects with restless legs syndrome. SLEEP 2011;34(3):341-347.
Keywords: Restless leg syndrome, dopamine transporter, striatum, positron emission tomography
INTRODUCTION
Restless legs syndrome (RLS) is a sensory-motor disorder that is reported to affect between 5% and 10% of the population.1 In large part because of the substantial dramatic symptomatic response to L-DOPA and dopaminergic (DAergic) agonist, a role for the DAergic system in the pathophysiology of RLS has been suggested.2 Aside from these therapeutic implications, there is some limited, though conflicting, scientific evidence for DAergic pathology in RLS.3 These studies include positron emission tomography (PET) and single positron emission, computed tomography (SPECT) studies of dopamine-2/3 receptor (D2R) binding potential (BP), which have shown decreases, increases, and no change.4 They also include autopsy studies that have found decreased D2R in the putamen for RLS cases, which correlated negatively with RLS symptom severity.5
Pertinent to the current study are 3 imaging studies of DAT in RLS, all using SPECT techniques,6–8 and all reporting no differences from controls. The SPECT DAT BP techniques used in these 3 studies involve a 24-h period between isotope infusion and SPECT BP determination. Studies in cultured cells have demonstrated that membrane-bound DAT rapidly turnovers with potential internalization of the bound ligand.9,10 If that is the case in vivo, then what is happening to the SPECT ligand for that 24-h period prior to BP determinations? The ligand has almost certainly been internalized, and thus the findings represent essentially a gross determination of the total DAT pool. The SPECT findings using measures obtained after a 24-h delay do not reflect the short-term state of membrane-bound DAT. PET techniques, on the other hand, identify membrane-bound DAT in real time. Studies have suggested that there is little difference between SPECT and PET scans for DAT determination in Parkinson disease.11 Given the substantial neuronal loss associated with even early Parkinson disease, it should not be surprising that both PET and SPECT provide similar values for such severe underlying pathology. As there is no indication of DAergic cell loss in RLS,2,12,13 we cannot assume that SPECT-determined DAT BP in RLS will provide identical information as PET-determined DAT BP, given the distinctly different pharmacokinetic assumptions of the 2 techniques.
The current studies were undertaken to evaluate DAT in striatum of RLS subjects using PET techniques. These studies were performed starting at 2 different times of day in separate groups of subjects to also evaluate for potential diurnal changes in DAT. The rational for assessing diurnal differences is that a primary and essential feature of RLS is the distinct circadian-dependent nature of the symptoms, being worse at night and quiescent in the morning.14 The theoretical construct for this diurnal affect is that DAergic activity decreases at night to a much greater extent for RLS than non-RLS subjects, contributing to the development of evening and night symptoms.2 The question is whether DAT might play a role in the diurnal change in DAergic activity and whether that differs for the RLS group.
MATERIALS AND METHODS
Two separate and independent studies were completed, one performed in the morning and one in the evening. The studies were started 2 years apart, using separate sets of subjects, but the overall methods including PET camera and analyses were the same. Any differences between the 2 studies are outlined below.
All subjects were screened using a general medical history questionnaire, an RLS diagnostic questionnaire, leg meters, blood work, apnea monitoring where indicated, and the validated Hopkins telephone diagnostic interview (TDI)15 performed by an RLS specialist (RPA). RLS subjects had to have all 4 defining features of RLS16 and not have other symptoms or conditions that might mimic RLS in order to have a “definite” RLS diagnosis. Controls subjects had to have no positive responses to any of the 4 defining RLS features in order to have a definite NOT-RLS diagnosis. Subjects were excluded if apnea-hypopnea index was > 25/h (on polysomnography) or if medical conditions (e.g., chronic inflammation, or renal or hepatic failure) or medications (e.g., antidepressant, neuroleptic) were considered likely to compromise our ability to make the diagnosis of RLS or to perform or interpret the PET results. Control subjects were excluded if a family history of RLS was reported or if periodic leg movements (PLMs) by leg meter were > 10/h on any one night over a 4-5 night evaluation at home. RLS subjects were included only if untreated symptoms were daily or if symptoms were 2-6 times per week and PLMs were ≥ 10/h on average over 4-5 nights. RLS subjects were excluded for any suspected secondary cause of the RLS other than iron deficiency. PLMs were determined for these screening evaluations using a small watch-size device that was worn on each ankle that records and stores specific leg movements (PLMS/h)17 when the subject is lying down. Subjects were withdrawn from all CNS active medications including RLS medications ≥ 11 days prior to the study or ≥ 6 half-lives, whichever was longer. Control subjects were age-, and gender-matched to the RLS cohort.
All subjects were admitted to the Johns Hopkins Bayview General Clinical Research Center (GCRC) 3 days prior to the PET study. At that time, the medical and RLS history were reviewed and the Hopkins TDI15 (a face-to-face assessment) was performed to insure that the subject was either definite RLS or definite NOT-RLS. While in the GCRC subjects had a suggested immobilization test (SIT) followed by a full nighttime polysomnogram on 2 sequential nights. These were performed and scored as previously described.18 RLS subjects also filled out RLS-related rating scales and sleep diaries as previously described.18
PET scans were performed on the GE Advance whole body PET scanner (GE Medical Systems, Waukesha, WI, USA) at the Johns Hopkins PET Center. The morning scan was started at 08:30, and the evening scan was started at 19:30. All subjects had venous and arterial lines placed ≥ 2 h prior to the scan. All subjects were fitted for a thermoplastic mask individually molded to his/her face to reduce head movement during the scan. After the transmission scan using a rotating germanium-68 source, an emission scan was started in a 3D mode with a bolus intravenous injection of [11C]-d-threo-methylphenidate (MP)19–21 (dose range: 15.07-19.72 mCi; injected specific activity range: 3113-23270.4 mCi/micromole). This scan lasted 90 min. The frame schedule consisted of four 15-sec, four 30-sec, three 1-min, two 2-min, five 4-min, and twelve 5-min frames, for a total of 30 frames. While lying on the table during the scan, all subjects had a wedge shaped pillow flexing their legs at the knees. We found this helped reduce frequency and intensity of leg symptoms in the RLS subjects. PET images were reconstructed using the back projection algorithm with a ramp filter using the software provided by the manufacturer correcting for attenuation, scatter, and dead-time. The radioactivity was corrected for physical decay to the injection time. Each PET frame consisted of 128 (left-to-right) by 128 (nasion-to-inion) by 35 (neck-to-cranium) voxels. Volumes of interest (VOIs) were defined on individual subject's MRI for putamen (PU), caudate nucleus (CN) (left and right separately), and cerebellum (whole only) using an interactive-threshold method of MRI intensity. Striatal VOIs were separated into 5 functional subdivisions using anterior-commissural plane and published anatomical guidance based on postmortem cytological classification.22,23 The subdivisions were: anterior dorsal caudate nucleus (aCN), posterior dorsal caudate nucleus (pCN), anterior dorsal putamen (aPU) (associative striatum), posterior dorsal putamen (pPU) (motor striatum), and ventral striatum (VS) (limbic striatum/nucleus accumbens).24 VOIs were transferred from MRI to PET space using MRI-PET coregistration parameters given by the SPM5 coregistration routine,25 and applied on individual PET frames to obtain time-(radio)activity curves (TACs) of regions. Binding potential (BP; precisely, BPND as defined by Innis et al.26) of regions were estimated by multi-linear reference tissue method with 2 parameters27 using cerebellum as a receptor-free reference region.
DAT BP Analysis
Our primary hypothesis was DAT would be decreased in the striatum of RLS subjects. This is based on the iron-deficiency (ID)-rodent model, where brain ID leads to a decrease in DAT in the striatum (caudate-putamen). Our secondary hypothesis was DAT would be decreased in the putamen and in the caudate. No predictions were made for the ventral striatum. Our other primary hypothesis was the DAT would differ between morning and evening studies. We believe the direction of circadian change for DAT would be the same in controls and in RLS subjects but the amount of circadian change would differ. Multi-variant ANOVA for time of day and diagnosis was used for the primary and secondary analyses. Chi-square was used to assess gender balance.
RLS Clinical Measures and Analysis
Our primary clinical measures of RLS were: (1) PLMS and (2) sleep efficiency on the second night PSG; (3) the number of hours of RLS symptoms reported on an RLS-sleep diary during the stay in the GCRC; and (4) the score on the IRLS severity scale obtained the day before the scans. Secondary measures include age of symptom onset, duration of RLS symptoms, Johns Hopkins RLS severity scale, PLM on an evening SIT, and serum measures of iron status (early morning fasting ferritin, iron, TIBC, and % iron saturation). Pearson correlation coefficient was used to look at the relation between DAT BP in the striatum and primary and secondary measures of disease state.
RESULTS
Subject Age and Gender Characteristics
The age and gender for the RLS and Control groups in the Day and Night studies are presented in Table 1. Thirty-six RLS subjects (20 Day; 16 Night) and 34 Control subjects (20 Day; 14 Night) completed the PET studies. A multi-variant ANOVA with age as the dependent variable and disease (RLS-Control: F = 0.03, P = 0.85), study time (Day-Night: F = 2.16, P = 0.15), and interaction (disease × study time: F = 0.16, P = 0.69) effects as independent variables, found no significant differences. There were no significant gender differences found between RLS and control cohorts when Day-study subjects (χ2 = 2.56, P = 0.11), Night-study subjects (χ2 = 0.07, P = 0.80), and all subjects (χ2 = 2.0, P = 0.16) were assessed. The polysomnogram (PSG) measures for the control and RLS groups are given in Table 2. As expected, the RLS group had significantly higher PLMS rates and lower total sleep time than controls. In the RLS-Day group, PLMS rates were higher than the 10/h cutoff for controls because of 3 subjects who PLMS rates of > 50, skewed the data (median value for the PLMS was 3.2). Control subjects older than 75 years of age were needed to match RLS subjects of similar advanced age. Most of the control subjects who were over 75 years of age had > 20-30 PLM on home monitoring. These 3 subjects had no RLS symptoms, and on 5 nights using leg activity meters, they met the set criteria (< 10 PLM on any given night); therefore they were included in the study, despite the PSG findings. In the worst case scenario, if these 3 subjects represented subclinical RLS cases, then the study bias would be against us finding a difference.
Table 1.
The number of subjects (in parentheses) and the mean age plus standard deviation for study time by disease by gender subgroups
| Day |
Night |
|||
|---|---|---|---|---|
| Control | RLS | Control | RLS | |
| Males | (11) 60 ± 9.2 | (6) 59 ± 7.1 | (5) 56 ± 6.4 | (5) 55 ± 10.1 |
| Females | (9) 60 ± 7.1 | (14) 62 ± 9.2 | (9) 59 ± 6.0 | (11) 58 ± 10.3 |
| All | (20) 60 ± 8.1 | (20) 61 ± 8.6 | (14) 58 ± 6.1 | (16) 57 ± 10.1 |
Table 2.
Polysomnogram measures (mean ± standard deviations) for Control and RLS groups
| PSG sleep measure | Control | RLS | t, P values |
|---|---|---|---|
| Total sleep time (min) | 334.6 ± 55.3 | 285.4 ± 74.6 | 3.0, 0.035 |
| PLMS/h | 11.8 ± 19.8 | 61.2 ± 53.9 | 4.8, 0.001 |
| Sleep onset (min) | 10.6 ± 11.9 | 7.6 ± 9.6 | 1.1, ns |
| sleep efficiency % | 90.1 ± 7.2 | 84.7 ± 13.3 | 2.0, 0.048 |
| WASO (min) | 36.8 ± 27.1 | 44.9 ± 37.1 | 1.0, ns |
| N1% | 7.7 ± 4.0 | 11.9 ± 13.9 | 1.6, 0.111 |
| N2% | 53.2 ± 11.1 | 48.1 ± 11.3 | 1.8, 0.068 |
| N3% | 15.5 ± 10.5 | 14.9 ± 12.2 | 0.2, ns |
| REM% | 23.6 ± 8.4 | 25.1 ± 9.7 | 0.7, ns |
WASO, wake after sleep onset; N1%, percent of stage 1 NREM sleep; N2%, percent of stage 2 NREM sleep; N3%, percent of stage 3/4 NREM sleep). The corresponding t-test score, (t) and probability, (P) value for control versus RLS group comparison for the individual PSG values are in the far right column. ns = P > 0.2.
DAT Binding Potentials in the Total Striatum
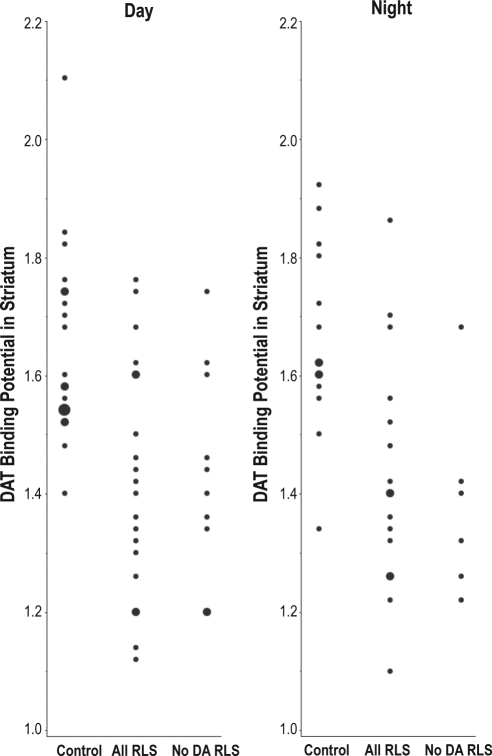
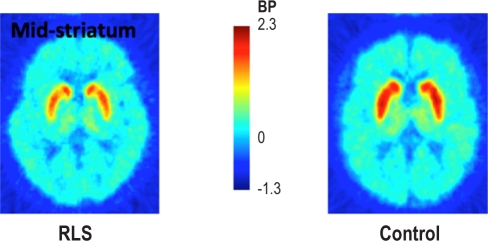
Figure 1 presents the individual striatal DAT BP values for both Day and Night studies. Figure 2 shows a representative PET image of MP binding in the mid-striatum from an RLS subject and a Control subject. The actual RLS-control group means and standard deviation values for striatal DAT BP can be found in Table 3. A multi-variant ANOVA for disease, study time, and interaction effects found the RLS group had significantly decreased DAT BP compared to the Control group (F = 27.4, P < 0.0001; effect size = 1.3). There was, however, no effect of time of study (F = 0.035, P = 0.85), nor was there any interaction effect (F = 0.013, P = 0.91). The DAT BP in the striatum was significantly decreased in the RLS group compared with control for the Day-study group (F = 11.9, P = 0.0015; effect size = 1.3) and also the Night-study group (F = 12.4, P = 0.0015; effect size = 1.3), evaluated separately.
Figure 1.
DAT binding potential in the striatum from the Day and the Night studies for all control, all RLS and dopaminergic-drug naive (No DA) RLS subjects. Individual data points are presented as smallest dots, two overlapping data are presented as medium dots and three overlapping data are presented as the largest dot.
Figure 2.
Representative example of [11C]methylphenidate binding potential in the striatum of a RLS and Control subject.
Table 3.
The mean ± standard deviation of DAT binding potentials in total striatum and in subregions of the striatum from RLS and Control subjects during day and night assessments
| ST | aPU | pPU | aCN | pCN | VS | |
|---|---|---|---|---|---|---|
| Control–Day (N = 20) | 1.65 ± 0.16 | 1.83 ± 0.21 | 1.84 ± 0.16 | 1.65 ± 0.18 | 1.06 ± 0.21 | 1.13 ± 0.28 |
| RLS-Day (N = 20) | 1.43 ± 0.19 | 1.65 ± 0.22 | 1.61 ± 0.22 | 1.44 ± 0.27 | 0.95 ± 0.27 | 1.07 ± 0.24 |
| ANOVA | F = 11.9 | F = 6.0 | F = 10.5 | F = 6.7 | F = 0.68 | F = 0.36 |
| P = 0.0015 | P = 0.020 | P = 0.0026 | P = 0.014 | P = 0.42 | P = 0.55 | |
| Control-Night (N = 14) | 1.66 ± 0.16 | 1.80 ± 0.17 | 1.87 ± 0.15 | 1.66 ± 0.21 | 1.16 ± 0.17 | 1.12 ± 0.25 |
| RLS-Night (N = 16) | 1.43 ± 0.19 | 1.58 ± 0.19 | 1.56 ± 0.21 | 1.41 ± 0.24 | 0.93 ± 0.25 | 1.03 ± 0.27 |
| ANOVA | F = 12.4 | F = 10.5 | F = 20.6 | F = 9.71 | F = 7.98 | F = 0.96 |
| P = 0.0015 | P = 0.0031 | P = 0.0001 | P = 0.0042 | P = 0.0086 | P = 0.34 | |
| All Control vs All RLS | F = 27.4 | F = 16.7 | F = 34.5 | F = 17.8 | F = 8.9 | F = 1.6 |
| P < 0.0001 | P = 0.0001 | P < 0.0001 | P < 0.0001 | P = 0.004 | P = 0.2 | |
| Day vs Night | F = 0.035 | F = 1.08 | F = 0.05 | F = 0.03 | F = 0.48 | F = 0.12 |
| P = 0.85 | P = 0.3 | P = 0.8 | P = 0.9 | P = 0.5 | P = 0.7 | |
| Interaction | F = 0.013 | F = 0.05 | F = 0.39 | F = 0.21 | F = 1.07 | F = 0.05 |
| P = 0.91 | P = 0.8 | P = 0.8 | P = 0.7 | P = 0.3 | P = 0.8 | |
ST, total striatum; aPU, anterior putamen; pPu, posterior putamen; aCN, anterior caudate nucleus; pCN, posterior caudate nucleus; VS, ventral striatum.
Because of the possibility that prior DAergic-drug experience may have had a sustained biasing effect on DAT BP, we performed a post hoc analysis using 16 RLS subjects who had never previously use DAergic agents and compared them to the 34 subjects in the control group. Analysis by ANOVA found no RLS-Control (F = 0.24, P = 0.62), Day-Night (F = 1.84, P = 0.18), or interaction (F = 0.42, P = 0.52) effects for age for these 2 groups. Chi-square analysis for gender found no difference between these RLS and control cohorts when Day-study subjects (χ2 = 1.67, P = 0.20), Night-study subjects (χ2 = 0.01, P = 0.92), and all subjects (χ2 = 1.12, P = 0.29) were assessed. The 2 groups appeared well balanced for age and gender. The DAT BP in the striatum in these DAergic-drug-naïve RLS subjects (Figure 1) was still significantly lower (F = 23.1, P < 0.0001) than control subjects (Table 4). The decrease was significant for Day (F = 10.8, P = 0.0027) and also Night (F = 12.8 16.7, P = 0.0022) studies, evaluated separately. Therefore prior use of DAergic drugs cannot account for the effect seen with the total RLS group.
Table 4.
The mean ± standard deviation of DAT binding potentials in total striatum and in subregions of the striatum from dopaminergic-naïve RLS and Control subjects during day and night assessments
| ST | aPU | pPU | aCN | pCN | VS | |
|---|---|---|---|---|---|---|
| Control–Day (N = 20) | 1.83 ± 0.21 | 1.83 ± 0.21 | 1.84 ± 0.16 | 1.65 ± 0.18 | 1.06 ± 0.21 | 1.13 ± 0.28 |
| RLS-Day (N = 10) | 1.66 ± 0.25 | 1.66 ± 0.25 | 1.66 ± 0.23 | 1.47 ± 0.25 | 1.00 ± 0.30 | 1.00 ± 0.29 |
| Control-Night (N = 14) | 1.80 ± 0.17 | 1.80 ± 0.17 | 1.87 ± 0.15 | 1.66 ± 0.21 | 1.16 ± 0.17 | 1.12 ± 0.25 |
| RLS-Night (N = 6) | 1.55 ± 0.16 | 1.55 ± 0.16 | 1.53 ± 0.20 | 1.36 ± 0.19 | 0.85 ± 0.22 | 0.97 ± 0.22 |
| All Control vs All RLS | F = 23.1 | F = 10.8 | F = 23.3 | F = 14.1 | F = 6.73 | F = 3.0 |
| P < 0.0001 | P = 0.0019 | P < 0.0001 | P = 0.0005 | P = 0.013 | P = 0.09 | |
| Day vs Night | F = 1.6 | F = 1.2 | F = 0.82 | F = 0.61 | F = 0.18 | F = 0.07 |
| P = 0.69 | P = 0.3 | P = 0.4 | P = 0.4 | P = 0.7 | P = 0.8 | |
| Interaction | F = 0.43 | F = 0.4 | F = 2.1 | F = 1.07 | F = 3.3 | F = 0.03 |
| P = 0.52 | P = 0.6 | P = 0.2 | P = 0.3 | P = 0.08 | P = 0.9 | |
ST, total striatum; aPU, anterior putamen; pPu, posterior putamen; aCN, anterior caudate nucleus; pCN, posterior caudate nucleus; VS, ventral striatum.
There were 9 RLS subjects (7 Day; 2 Night) who were completely drug-naïve, having never experienced any treatment for their RLS symptoms. A comparison of these 9 RLS subjects to the 34 control subjects (Table 5) still showed the significant decrease in striatal DAT BP (F = 16.7, P = 0.0002). This further supports the concept that prior drug-exposure is not the primary factor in creating the striatal DAT decrease seen in RLS cohort.
Table 5.
The mean ± standard deviation of DAT binding potentials in total striatum and in subregions of the striatum from drug-naïve RLS and Control subjects
| ST | aPU | pPU | aCN | pCN | VS | |
|---|---|---|---|---|---|---|
| Control (N = 34) | 1.65 ± 0.16 | 1.83 ± 0.21 | 1.84 ± 0.16 | 1.65 ± 0.18 | 1.06 ± 0.21 | 1.13 ± 0.28 |
| RLS (N = 9) | 1.40 ± 0.20 | 1.62 ± 0.27 | 1.61 ± 0.28 | 1.46 ± 0.26 | 0.98 ± 0.31 | 0.95 ± 0.29 |
| ANOVA: Control-RLS | F = 16.7 | F = 6.18 | F = 12.5 | F = 6.06 | F = 2.24 | F = 3.25 |
| P = 0.0002 | P = 0.017 | P = 0.001 | P = 0.018 | P = 0.14 | P = 0.08 | |
ST, total striatum; aPU, anterior putamen; pPu, posterior putamen; aCN, anterior caudate nucleus; pCN, posterior caudate nucleus; VS, ventral striatum.
DAT Binding Potentials in the Subregions of the Striatum
Secondary analysis of the DAT BP includes subregions of the striatum: the anterior (aPU) and posterior (pPU) putamen, the anterior (aCN) and posterior (pCN) caudate, and the ventral striatum (VS). The mean DAT BP for RLS and Control groups from the Day study and from the Night study are presented for each striatal subregion in Table 3. Analysis by multi-variant ANOVA showed a significant decrease for RLS compared to Controls in DAT BP for the anterior (F = 16.7, P = 0.0001) and posterior (F = 34.5, P < 0.0001) putamen, and for the anterior (F = 17.8, P < 0.0001) and posterior (F = 8.9, P = 0.004) caudate nucleus.
No diagnosis differences were found for the ventral striatum. Neither study time nor interaction of diagnosis and study-time effects showed any significance difference for any of the subregions assessed. Therefore, no diurnal effect on DAT BP was found within the striatal subregions, even for controls. When study-time groups were analyzed separately, DAT BPs for RLS were still decreased in the aPU (Day: F = 6.0, P = 0.02; Night: F = 10.5, P = 0.0031), pPU (Day: F = 10.5, P = 0.0026; Night: F = 20.6, P = 0.0001), and the aCN (Day: F = 607, P = 0.014; Night: F = 9.71, P = 0.0042) in both the Day and the Night study groups. There were no differences found between RLS-Control in the VS DAT BP (Day: F = 0.36, P = 0.55; Night: F = 0.96, P = 0.34) for either the Day or Night groups. Mixed results were found with pCN: no difference in DAT BP for the Day-study cohort (F = 0.68, P = 0.42) but a significant decrease at Night (F = 7.98, P = 0.0086) for RLS compared to Control subjects.
Post hoc analysis of the data comparing DAergic-drug-naïve RLS group to the control group was also performed (Table 4). A multi-variant ANOVA for disease, study time, and interaction effects found that the decreases in DAT BP found in the full RLS cohort were still present in the DAergic-drug-naïve subgroup. DAT BP was decreased in the aPU (F = 10.8, P = 0.0019), pPU (F = 23.3, P < 0.0001), aCN (F = 14.1, P = 0.0005), and pCN (F = 6.73, P = 0.013), but not the VS (F = 3.0, P = 0.09). A comparison of the 9 RLS subjects (Table 5) who were completely drug-naïve to the 34 control subjects, still showed the significant decrease in aPU (F = 6.18, P = 0.017), pPU (F = 12.5, P = 0.001), and aCN (F = 6.06, P = 0.018), but not the pCN (F = 2.24, P = 0.14) or VS (F = 3.25, P = 0.08).
DAT Binding Potential in Relation to Clinical Aspects of RLS
No correlations were found for DAT BP in total striatum and the primary clinical measures or for the secondary measures as defined above in the methods. No correlations were found between DAT BP in any of the substriatal areas and any of the primary and secondary clinical measures.
DISCUSSION
Despite the substantial and dramatic symptomatic response to L-DOPA seen in patients with this disease, there is surprising little DAergic pathology that has been found to be consistent or reproducible across and within methodological approaches.3 Several studies have evaluated the tubero-infundibular DAergic system by direct evaluation of prolactin secretion. However, these studies have consistently shown marginally nonsignificant differences between RLS and control subjects in serum prolactin.28–30 Some studies have found an increase in CSF 3-O-methyl dopamine (3OMD) and tetrahydrobiopterin in idiopathic RLS subjects, the interpretation being that an increase in DA synthesis may underlie some aspect of RLS pathology.31,32 Similar large increases in CSF 3OMD and BH4 compared to controls were reported by Stiasny-Kolster et al.,33 but their results were not statistically significant. PET/SPECT studies of D2R binding potential have shown decreases, increases, and no change.4 Two Fluoro-DOPA PET studies found a decrease F-DOPA accumulation curve in RLS compared to controls.34,35 Studies in postmortem brain sections found increased total and phosphorylated tyrosine hydroxylase (TH) in the putamen and substantia nigra5 and decreased D2R in the putamen for the RLS group. The putamen D2R concentrations also showed a strong negative correlation (r = −0.8) to premortem RLS severity on the International RLS severity scale.5 A reasonable concern in interpreting these autopsy findings is the potential effects of DAergic medications that had been in used in 5 of the 8 cases prior to death.
Thus we have, at best, patchy and limited support for an underlying pathological problem in the DAergic system in RLS. An important limitation of all the PET/SPECT studies, including the current study, is regional selectivity and the sensitivity and specificity of the methods used. The PET/SPECT studies of the DAergic system have in large part been confined to striatum with one notable exception.36 Other regions of interest for which the DAergic system could be involved in RLS include the hypothalamus, thalamus, frontal cortex, and spinal pathways. Negative or positive findings for striatum do not exclude the possibility of a more pertinent role of alternative DAergic pathways in RLS. PET/SPECT binding studies do not adequately assess receptor affinity, turnover, or competitive binding from endogenous DA; thus the presence or absences of an effect often leaves one unsure as to the nature of the finding: no difference in binding does not exclude differences in turnover; differences in binding leave us with unclear casual links. Despite these limitations, some effort at interpreting the current findings against the background of relevant and pertinent research is warranted.
In contrast to our current findings, three prior studies of DAT in RLS subjects have found no differences.6–8 Two of these three studies used drug-naïve RLS subjects.6,7 Two studies were done in the day, but one of the studies was done in the evening.7 The significant difference between the prior studies and the current study, however, is that all three prior studies used SPECT techniques In these SPECT studies, an [123I]iodinated-tropane ligand was infused intravenously, and only after a 24-hour period was the DAT BP determined. Thus these SPECT studies did not measure real-time BP, but instead measured the long-term effects of a ligand reaching some equilibrium in the system. With the current PET studies, [11C]-d-threo-methylphenidate was infused and the subject immediately scanned over the next 90 minutes to create DAT BP curve, thus capturing real-time BP. We know from cell and animal studies that DAT rapidly turns over and is internalized, with some DAT recycled back to the membrane while some is degraded.9,10 For example, cocaine when binding to DAT is internalized and, based on timing of the studies, will show different responses if assessed for membrane-bound cocaine versus total cocaine.10 Therefore, given these factors and the pharmacokinetic differences between PET and SPECT methodologies (ligands, camera, modeling, etc.) used in all of the prior RLS-DAT studies, the current and prior DAT studies could be interpreted as showing decreased membrane bound DAT (PET studies) but a nonsignificant change in total cellular DAT (SPECT studies) in RLS subjects. The later statement is in part supported by an autopsy study which determined total putamen DAT concentrations and found no difference between RLS and controls.5
An alternative interpretation of the decreased DAT-BP is that it reflects increased competition from endogenous synaptic DA. Prior data from CSF (increased tetrahydrobiopterin and 3OMD)31,32 and autopsy (increased phosphorylated tyrosine hydroxylase)5 studies in RLS have suggested that there may be an increase production of DA.37 Brain ID, like that found in RLS, will produce in animals, increased extracellular DA.38 In nonhuman primates, PET studies with cocaine as the DAT ligand found a small but significant increase in BP when the animals were challenged with 3 different drugs known to reduce synaptic dopamine,39 suggesting cocaine binding may be sensitive to synaptic DA concentrations. Sheffel et al.40 using in vivo experiments in mice to study the effects of L-DOPA (plus benserazide) on BP of [3H]-WIN35,428, a cocaine analog, found that 50 mg/kg dose of L-DOPA or higher significantly decreased [3H]-WIN35,428 BP, while a 25 mg/kg dose had no effect. Gatley et al.41 performed similar in vivo studies in mice but found no effect of 50 mg/kg L-DOPA (plus benserazide) on either WIN35,428 or d-threo-methylphenidate DAT BP. When clorgyline, a MAO A inhibitor, was added to L-DOPA plus benserazide treatment, there was, however, an approximate 13% reduction in DAT BP for both ligands.41 MAO inhibition, of course, is associated with a 20-fold increase in synaptic DA.42 More relevant to the current PET study results is a baboon study, showing that 50 mg/kg L-DOPA plus benserazide had no effect on BP of [11C]-d-threo-methylphenidate in the striatum,41 as determined by PET scanning techniques. The studies, therefore, suggest that large, nonphysiological concentrations of synaptic dopamine may have a small effect on methylphenidate DAT BP. As we currently have no reason to believe that concentrations of synaptic DA achieve such levels in RLS, we have no reason to believe that the decrease in DAT BP seen in RLS is attributable to increased synaptic DA.
A potential confound in our results, which could account for the findings, is that some of the RLS subjects had been on DAergic agonists 11 days prior to the study. Thus one interpretation of the decreased DAT is that it reflects DA sensitivity following drug withdrawal: a decrease in DAT might be expected if the DA system experienced a relative loss of DA activity (withdrawal of DA agonist). The counter to this argument is that analysis of those who were not on DA agonists showed the same significant decrease (and same effect size) as those who had received DA agonists. One DAT study using SPECT techniques also found no difference between drug-naïve and prior-drug-treated RLS groups.6
Another relevant issue is the potential effects of sleep loss on the DAergic system. Most of the RLS subjects in the current two studies had reduced total sleep time or reported sleep disruption, especially those individuals who had to come off of medications. Therefore, the RLS and control groups differed on the basis of sleep quality and quantity. Prior studies have shown that although D2R BP may decrease with acute sleep loss that DAT BP did not change.43,44 Furthermore, we found no correlation between DAT BP and any measure of sleep quality. However, one cannot fully exclude sleep loss as a possible confounding effect.
Finally, radionuclide brain imaging of patients with Parkinson disease has been associated with a decrease in DAT, which is considered to reflect DAergic neuronal loss.11 In idiopathic RLS, there have been several autopsy studies none of which imply the presence of cell loss in the basal ganglia. General histological evaluation of tissue section with H&E staining as well as immunostaining for tyrosine hydroxylase failed to identify significant neuronal loss in any brain region, but specifically none in the substantia nigra, putamen, or caudate.5,12 Although diminished iron stores were the predominant findings in these studies, cell size and morphology were normal, and there was no indication of gliosis. One recent study of the A11 DAergic cell group in the hypothalamus actually determines cell volume and numbers and found no difference for RLS or controls.13 This same area in Parkinson disease shows gross DAergic neuronal cell loss.45
Our interpretation of these results, in light of prior SPECT and autopsy results, is that membrane-bound striatal DAT, but not total cellular DAT, is decreased in RLS. Therefore, diminished DAT bound to the cell surface membrane in the striatum may be an important component of the DAergic pathology in RLS. Independent of the interpretations of the results, the current findings of decrease in striatal DAT provide further support for the iron deficiency rodent model as a valid model for predicting the biological underpinning of RLS.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Earley is member of DSMB for a Merck-sponsored study in insomnia. Dr. Kuwabara has received research support from Roche, Sanofi-Aventis, Otsuka, and Eli Lilly. Dr. Wong has received research support from Avid, GE, Intracellular, Lilly, Lundbeck, Merck, Orexigen, Otsuka, Roche, and Sanofi-Aventis. Dr. Allen has received research support from GlaxoSmithKline, and Pharmacosmos, and has consultant for GlaxoSmithKline, Xenoport, Boehringer-Ingelheim, Jazz, UCB, Orion, Novartis, Pfizer, EMD-Serono, Neurogen, Luitpold, and Pharmacosmos. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This research was supported by NIH/NCRR grant M01-RR00052 and M01-RR02719 received by Johns Hopkins GCRC; and NIH grant R01-NS42857 and PO1-AG21190 received by Dr. Earley and K24 DA000412 received by Dr. Wong. We wish to thank David J. Clough and Daniel P. Holt for technical support and effort in PET scanning and radiochemical synthesis.
REFERENCES
- 1.Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005;165:1286–92. doi: 10.1001/archinte.165.11.1286. [DOI] [PubMed] [Google Scholar]
- 2.Earley CJ, Allen RP, Beard JL, Connor JR. Insight into the pathophysiology of restless legs syndrome. J Neurosci Res. 2000;62:623–8. doi: 10.1002/1097-4547(20001201)62:5<623::AID-JNR1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 3.Allen RP. Controversies and challenges in defining the etiology and pathophysiology of restless legs syndrome. Am J Med. 2007;120:S13–21. doi: 10.1016/j.amjmed.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Trenkwalder C, Earley CJ. Neuroimaging in restless legs syndrome. In: Hening WA, Allen RP, Chokroverty S, Earley CJ, editors. Restless legs syndrome. Philadelphia: Saunders Elsevier; 2009. pp. 78–82. [Google Scholar]
- 5.Connor JR, Wang XS, Allen RP, et al. Altered dopaminergic profile in the putamen and substantia nigra in restless leg syndrome. Brain. 2009;132:2403–12. doi: 10.1093/brain/awp125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisensehr I, Wetter TC, Linke R, et al. Normal IPT and IBZM SPECT in drug-naive and levodopa-treated idiopathic restless legs syndrome. Neurology. 2001;57:1307–9. doi: 10.1212/wnl.57.7.1307. [DOI] [PubMed] [Google Scholar]
- 7.Michaud M, Soucy JP, Chabli A, Lavigne G, Montplaisir J. SPECT imaging of striatal pre- and postsynaptic dopaminergic status in restless legs syndrome with periodic leg movements in sleep. J Neurol. 2002;249:164–70. doi: 10.1007/pl00007859. [DOI] [PubMed] [Google Scholar]
- 8.Mrowka M, Jobges M, Berding G, Schimke N, Shing M, Odin P. Computerized movement analysis and beta-CIT-SPECT in patients with restless legs syndrome. J Neural Transm. 2005;112:693–701. doi: 10.1007/s00702-004-0217-9. [DOI] [PubMed] [Google Scholar]
- 9.Zahniser NR, Sorkin A. Rapid regulation of the dopamine transporter: role in stimulant addiction? Neuropharmacology. 2004;47(Suppl 1):80–91. doi: 10.1016/j.neuropharm.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Eriksen J, Rasmussen SGF, Rasmussen TN, et al. Visualization of dopamine transporter trafficking in live neurons by use of fluorescent cocaine analogs. J Neurosci. 2009;29:6794–808. doi: 10.1523/JNEUROSCI.4177-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felicio AC, Shih MC, Godeiro-Junior C, Andrade LA, Bressan RA, Ferraz HB. Molecular imaging studies in Parkinson disease: reducing diagnostic uncertainty. Neurologist. 2009;15:6–16. doi: 10.1097/NRL.0b013e318183fdd8. [DOI] [PubMed] [Google Scholar]
- 12.Connor JR, Boyer PJ, Menzies SL, Dellinger B, Allen RP, Earley CJ. Neuropathological examination suggests impaired brain iron acquisition in restless legs syndrome. Neurology. 2003;61:304–9. doi: 10.1212/01.wnl.0000078887.16593.12. [DOI] [PubMed] [Google Scholar]
- 13.Earley CJ, Allen RP, Connor JR, Ferrucci L, Troncoso J. The dopaminergic neurons of the A11 system in RLS autopsy brains appear normal. Sleep Med. 2009;10:1155–7. doi: 10.1016/j.sleep.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen RP, Earley CJ. Restless legs syndrome: a review of clinical and pathophysiologic features. J Clin Neurophysiol. 2001;18:128–47. doi: 10.1097/00004691-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Hening WA, Allen RP, Washburn M, Lesage S, Earley CJ. Validation of the Hopkins telephone diagnostic interview for restless legs syndrome. Sleep Med. 2008;9:283–9. doi: 10.1016/j.sleep.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 16.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 17.Allen RP, Hening WA. Restless legs syndrome. Philadelphia: Saunders Elsevier; 2009. Actigraph assessment of periodic leg movements and restless legs syndrome. In: Hening WA, Allen RP, Chokroverty S, Earley CJ, eds; pp. 142–9. [Google Scholar]
- 18.Earley CJ, Horska A, Mohamed MA, Barker PB, Beard JL, Allen RP. A randomized, double-blind, placebo-controlled trial of intravenous iron sucrose in restless legs syndrome. Sleep Med. 2009;10:206–11. doi: 10.1016/j.sleep.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding YS, Fowler JS, Volkow ND, et al. Pharmacokinetics and in vivo specificity of [11C]dl-threo-methylphenidate for the presynaptic dopaminergic neuron. Synapse. 1994;18:152–60. doi: 10.1002/syn.890180207. [DOI] [PubMed] [Google Scholar]
- 20.Wang GJ, Volkow ND, Fowler JS, et al. Comparison of two pet radioligands for imaging extrastriatal dopamine transporters in human brain. Life Sci. 1995;57:PL187–91. doi: 10.1016/0024-3205(95)02099-5. [DOI] [PubMed] [Google Scholar]
- 21.Volkow ND, Ding Y-S, Fowler JS, et al. A new PET ligand for the dopamine transporter: studies in the human brain. J Nucl Med. 1995;36:2162–8. [PubMed] [Google Scholar]
- 22.Baumann B, Danos P, Krell D, et al. Reduced volume of limbic system-affiliated basal ganglia in mood disorders: preliminary data from a postmortem study. J Neuropsychiatry Clin Neurosci. 1999;11:71–8. doi: 10.1176/jnp.11.1.71. [DOI] [PubMed] [Google Scholar]
- 23.Oswald LM, Wong DF, McCaul M, et al. Relationships among ventral striatal dopamine release, cortisol secretion, and subjective responses to amphetamine. Neuropsychopharmacology. 2005;30:821–32. doi: 10.1038/sj.npp.1300667. [DOI] [PubMed] [Google Scholar]
- 24.Martinez D, Slifstein M, Broft A, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- 25.Ashburner JT, Friston KJ. Rigid body registration. In: Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE, editors. Statistical parametric mapping: the analysis of functional brain images: Academic Press; 2007. pp. 49–62. [Google Scholar]
- 26.Innis RB, Cunningham VJ, Delforge J, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–9. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 27.Ichise M, Toyama H, Innis RB, Carson RE. Strategies to improve neuroreceptor parameter estimation by linear regression analysis. J Cereb Blood Flow Metab. 2002;22:1271–81. doi: 10.1097/01.WCB.0000038000.34930.4E. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Borreguero D, Larrosa O, Granizo JJ, de la Llave Y, Hening WA. Circadian variation in neuroendocrine response to L-dopa in patients with restless legs syndrome. Sleep. 2004;27:669–73. [PubMed] [Google Scholar]
- 29.Garcia-Borreguero D, Serrano C, Larrosa O, Jose Granizo J. Circadian effects of dopaminergic treatment in restless legs syndrome. Sleep Med. 2004;5:413–20. doi: 10.1016/j.sleep.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Wetter TC, Collado-Seidel V, Oertel H, Uhr M, Yassouridis A, Trenkwalder C. Endocrine rhythms in patients with restless legs syndrome. J Neurol. 2002;249:146–51. doi: 10.1007/pl00007857. [DOI] [PubMed] [Google Scholar]
- 31.Earley CJ, Hyland K, Allen RP. CSF dopamine, serotonin, and biopterin metabolites in patients with restless legs syndrome. Mov Disord. 2001;16:144–9. doi: 10.1002/1531-8257(200101)16:1<144::aid-mds1009>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 32.Allen RP, Connor JR, Hyland K, Earley CJ. Abnormally increased CSF 3-Ortho-methyldopa (3-OMD) in untreated restless legs syndrome (RLS) patients indicates more severe disease and possibly abnormally increased dopamine synthesis. Sleep Med. 2009;10:123–8. doi: 10.1016/j.sleep.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stiasny-Kolster K, Moller JC, Zschocke J, et al. Normal dopaminergic and serotonergic metabolites in cerebrospinal fluid and blood of restless legs syndrome patients. Mov Disord. 2004;19:192–6. doi: 10.1002/mds.10631. [DOI] [PubMed] [Google Scholar]
- 34.Turjanski N, Lees AJ, Brooks DJ. Striatal dopaminergic function in restless legs syndrome: 18F-dopa and 11C-raclopride PET studies. Neurology. 1999;52:932–7. doi: 10.1212/wnl.52.5.932. [DOI] [PubMed] [Google Scholar]
- 35.Ruottinen HM, Partinen M, Hublin C, et al. An FDOPA PET study in patients with periodic limb movement disorder and restless legs syndrome. Neurology. 2000;54:502–4. doi: 10.1212/wnl.54.2.502. [DOI] [PubMed] [Google Scholar]
- 36.Cervenka S, Palhagen SE, Comley RA, et al. Support for dopaminergic hypoactivity in restless legs syndrome: a PET study on D2-receptor binding. Brain. 2006;129:2017–28. doi: 10.1093/brain/awl163. [DOI] [PubMed] [Google Scholar]
- 37.Allen RP, Earley CJ. Dopamine and iron in restless legs syndrome. In: Chockroverty S, Hening W, Walters AS, editors. Sleep and movement disorders. Philadelphia: Butterworth Heinemann; 2003. pp. 333–40. [Google Scholar]
- 38.Bianco LE, Wiesinger J, Earley CJ, Jones BC, Beard JL. Iron deficiency alters dopamine uptake and response to L-DOPA injection in Sprague-Dawley rats. J Neurochem. 2008;106:205–15. doi: 10.1111/j.1471-4159.2008.05358.x. [DOI] [PubMed] [Google Scholar]
- 39.Gatley SJ, Volkow ND, Fowler JS, Dewey SL, Logan J. Sensitivity of striatal [11C]cocaine binding to decreases in synaptic dopamine. Synapse. 1995;20:137–44. doi: 10.1002/syn.890200207. [DOI] [PubMed] [Google Scholar]
- 40.Scheffel U, Steinert C, Kim SE, Ehlers MD, Boja JW, Kuhar MJ. Effect of dopaminergic drugs on the in vivo binding of [3H]WIN 35,428 to central dopamine transporters. Synapse. 1996;23:61–9. doi: 10.1002/(SICI)1098-2396(199606)23:2<61::AID-SYN1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 41.Gatley SJ, Ding YS, Volkow ND, Chen R, Sugano Y, Fowler JS. Binding of d-threo-[11C]methylphenidate to the dopamine transporter in vivo: insensitivity to synaptic dopamine. Eur J Pharmacol. 1995;281:141–9. doi: 10.1016/0014-2999(95)00233-b. [DOI] [PubMed] [Google Scholar]
- 42.Wachtel SR, Abercrombie ED. L-3,4-dihydroxyphenylalanine-induced dopamine release in the striatum of intact and 6-hydroxydopamine-treated rats: differential effects of monoamine oxidase A and B inhibitors. J Neurochem. 1994;63:108–17. doi: 10.1046/j.1471-4159.1994.63010108.x. [DOI] [PubMed] [Google Scholar]
- 43.Volkow ND, Tomasi D, Wang G-J, et al. Hyperstimulation of striatal D2 receptors with sleep deprivation: implications for cognitive impairment. NeuroImage. 2009;45:1232–40. doi: 10.1016/j.neuroimage.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volkow ND, Wang G-J, Telang F, et al. Sleep deprivation decreases binding of [11C]raclopride to dopamine D2/D3 receptors in the human brain. J Neurosci. 2008;28:8454–61. doi: 10.1523/JNEUROSCI.1443-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Langston WJ, Forno LS. The hypothalamus in Parkinson disease. Ann Neurol. 1978;3:129–33. doi: 10.1002/ana.410030207. [DOI] [PubMed] [Google Scholar]