Abstract
Background:
Severe sleep disruption is a well-documented problem in mechanically ventilated, critically ill patients during their time in the intensive care unit (ICU), but little attention has been paid to the period when these patients become clinically stable and are transferred to a step-down unit (SDU). We monitored the 24-h sleep pattern in 2 groups of patients, one on mechanical ventilation and the other breathing spontaneously, admitted to our SDU to assess the presence of sleep abnormalities and their association with mechanical ventilation.
Methods:
Twenty-two patients admitted to an SDU underwent 24-h polysomnography with monitoring of noise and light.
Results:
One patient did not complete the study. At night, 10 patients showed reduced sleep efficiency, 6 had reduced percentage of REM sleep, and 3 had reduced percentage of slow wave sleep (SWS). Sleep amount and quality did not differ between patients breathing spontaneously and those on mechanical ventilation. Clinical severity (SAPSII score) was significantly correlated with daytime total sleep time and efficiency (r = 0.51 and 0.5, P < 0.05, respectively); higher pH was correlated with reduced sleep quantity and quality; and higher PaO2 was correlated with increased SWS (r = 0.49; P = 0.02).
Conclusions:
Patients admitted to an SDU after discharge from an ICU still have a wide range of sleep abnormalities. These abnormalities are mainly associated with a high severity score and alkalosis. Mechanical ventilation does not appear to be a primary cause of sleep impairment.
Citation:
Fanfulla F; Ceriana P; Lupo ND; Trentin R; Frigerio F; Nava S. Sleep disturbances in patients admitted to a step-down unit after ICU discharge: the role of mechanical ventilation. SLEEP 2011;34(3):355-362.
Keywords: Chronic obstructive pulmonary disease, mechanical ventilation, polysomnography, sleep alterations, sleep disordered breathing, step-down unit
INTRODUCTION
Severe sleep disruption is a well-documented problem among critically ill patients staying in an intensive care unit (ICU) and may impair psychological and physiological well-being, with effects such as increased protein catabolism, decreased immune function, and altered respiratory mechanics, which could affect weaning from mechanical ventilation.1
Some studies have identified poor sleep as one of the most frequent complaints among patients who have survived a critical illness, often persisting for a long period after discharge.2–4 Previous polysomnographic studies performed in both ventilated and spontaneously breathing ICU patients have revealed that such patients have decreased total sleep time and abnormal sleep architecture.5–7 Several hypotheses have been forwarded to explain the sleep disturbances in these patients: severity of the illness, environmental noise, light intensity, types and modes of mechanical ventilation, and drugs.5–11
Some patients admitted to an ICU because of respiratory failure are not discharged directly home, but transferred to a step-down unit (SDU) once they are clinically stable, in order to speed the weaning process or, if breathing spontaneously, to continue pharmacological treatment and/or rehabilitation.12 The transfer from ICU to SDU is mainly based upon clinical parameters such as arterial blood gases, level of consciousness, hemodynamic stability, and the need for invasive monitoring, while little or no attention has been given to the problem of sleep that has been shown to be associated with poor outcome.13 Sleep data on patients admitted to SDUs or similar environments are very scarce; the single study on this aspect was limited to polysomnographic recording during only night hours.14
Our hypothesis was that once transferred to an SDU, most of these patients continue to experience sleep abnormalities, and that it would be possible to detect factors influencing sleep quality. Mechanical ventilation has been recognized as a possible cause of sleep disruption in critically ill patients8,10,13,15 as well as in patients with acute or chronic respiratory failure.16–18
The aim of this prospective study was therefore to monitor the 24-h sleep pattern in 2 groups of patients admitted to our SDU, one group still receiving continuous invasive mechanical ventilation and the other breathing spontaneously, in order to assess the presence of sleep abnormalities, their association with mechanical ventilation, and the potential influence of risk factors known to negatively affect sleep quality. Sleep data were also correlated with others factors known to negatively influence sleep quality in the ICU: clinical severity, environment, and the patient's care.
PATIENTS AND METHODS
Patients
We studied 22 patients admitted to our SDU for intensive monitoring (n = 11) or weaning from mechanical ventilation delivered through a tracheotomy (n = 11). One patient in the mechanically ventilated group did not complete the sleep study because 3 hours after the start of the study, he developed acute lung edema that required intensive pharmacological treatment and a change in ventilation settings.
Exclusion criteria were a comatose state, severe neurological disorders potentially affecting the sleep architecture, and the presence of delirium or psychomotor agitation requiring pharmacological treatment. The patients' clinical characteristics are shown in Table 1. The severity of the patients' clinical status at admission was evaluated using the SAPSII index.19 Blood gas analysis was performed on the second morning of recordings at 08:00.
Table 1.
Clinical and physiological data of the patients enrolled into the study
| MV | SB | Entire Group | P | |
|---|---|---|---|---|
| Age (years) | 70.8 ± 8.5 | 71.7 ± 8.4 | 71.8 ± 8 | n.s |
| SAPS index | 31.8 ± 7.3 | 28.2 ± 8.2 | 29.9 ± 7.8 | n.s |
| BZP therapy (n) | 6 | 8 | 14 | n.s. |
| LOS ICU | 41.2 ± 20.5 | 46.5 ± 26.6 | 43.9 ± 23.3 | n.s. |
| Cause of acute respiratory failure (n) | n.s. | |||
| COPD | 3 | 1 | 4 | |
| Sepsis with MOF | 1 | 1 | 2 | |
| Post-surgery | 3 | 5 | 8 | |
| Restrictive thoracic diseases | 3 | 1 | 4 | |
| Pneumonia | 1 | 1 | 2 | |
| Heart failure | 0 | 1 | 1 | |
| PaO2(mm Hg) | 80.5 ± 23.4 | 78.5 ± 13.6 | 79.7 ± 18.5 | n.s. |
| PaO2/FiO2 | 272.6 ± 96.5 | 304 ± 72.9 | 289 ± 84.3 | n.s. |
| PaCO2(mm Hg) | 42.2 ± 6.2 | 38.3 ± 9.7 | 40.2 ± 8.2 | n.s. |
| SaO2(%) | 95.8 ± 2 | 96.1 ± 1.7 | 96 ± 1.8 | n.s. |
| pH | 7.44 ± 0.05 | 7.46 ± 0.04 | 7.45 ± 0.05 | n.s. |
| Base excess (mmol/L) | 5.39 ± 3.7 | 3.44 ± 5.8 | 4.37 ± 4.9 | n.s. |
| HCO3-(mmol/L) | 29.5 ± 3.6 | 27.6 ± 5.2 | 28.5 ± 4.5 | n.s. |
MV, mechanical ventilation; SB, spontaneous breathing; LOS ICU, length of stay in intensive care unit; BZP (benzodiazepine) therapy, number of patients who received benzodiazepine therapy; COPD, chronic obstructive pulmonary disease; MOF, multi-organ failure.
The patients were studied 10.2 ± 8.7 days after admission, after they had become acclimatized to the new environment and had started a rehabilitation program. The study was approved by the local ethics committee, and patients gave written informed consent to their participation.
Sleep Study
All patients underwent standard 24-h polysomnography (Embla N-S 7000, Denver, CO, USA) with simultaneous monitoring of noise and light. Sleep studies were scored manually according to standard criteria20 and reviewed by the same operator (FF). Data on sleep architecture were compared with those reported in normal subjects21 of the same age group. Patient-ventilator asynchronies were identified as previously reported.15 Nurses were asked to monitor all care activities for each patient: i.e., toilette, blood tests, chest radiographs, endobronchial suction, changes to ventilator devices, assessment of vital signs, and administration of medication.
Environmental Measures
The noise level, expressed in decibels (dB) and weighted in frequency with an “A” curve staged by the American National Standard Institute (ANSI), was assessed with a sound level meter with a real-time spectrum analyzer (LD 2900 B, Larson & Davis Inc. Depew, NY, USA) with a precision of ± 0.5 dB (A) and analyzed with Noise Works for Windows (Lake View Software, Como, Italy). The noise level is expressed by the mean level, the mean value of maximum and minimum levels, and the L95 and L5, represent the sound level exceeded for 95% and 5%, respectively, of the duration of measurement.
Illumination was measured by a digital lux meter (Climalux, LSI, Settala, Italy) located at the patient's bedside. All the rooms in our SDU have very large windows and face southeast and south. Patients were not directly exposed to light, since the beds are oriented away from the windows and curtains are used when light levels are high. The intensity of light exposure was, therefore, similar for all patients.
The noise and light data were continuously integrated with the polysomnographic traces to assess the impact of noise and light impulses on sleep. Arousals or awakenings caused by noise or light were defined as those occurring within 3 to 5 seconds of the termination of a peak of noise or light. Noise data were available for only 16 patients.
Statistical Analysis
Results are presented as means and standard deviations (SD). Differences in physiological indices between groups were analysed using one-way analysis of variance (ANOVA). Single correlations between polysomnographic indices and factors known to influence the amount and the quality of sleep in an ICU—blood gases, SAPSII score, and length of time spent in the ICU— were evaluated by Pearson product-moment correlation coefficient. The χ2 test was performed to evaluate differences in benzodiazepine assumption between patients on mechanical ventilation and those breathing spontaneously.
All the analyses were performed using the STATISTICA/W statistical package (Tulsa, OK, USA); P values < 0.05 were considered statistically significant.
RESULTS
The 10 mechanically ventilated patients required continuous pressure support ventilation except for one patient who received mechanical ventilation only at night. The mean inspiratory support was 14.4 ± 2.9 cm H2O with a PEEPe of 3.9 ± 0.3 cm H2O and a back-up rate of 8-10 bpm. The ventilatory parameters were chosen by experienced physicians and were based on arterial blood gases, expired tidal volume, and patient comfort. Four patients were finally weaned from ventilation at discharge. No differences in age, blood gases, or clinical severity were found between the mechanically ventilated patients and those breathing spontaneously (Table 1).
Sleep Architecture and Sleep Disruption
All patients had scorable EEG data for the entire duration of the recordings; these data are summarized in Table 2. Most patients showed abnormal sleep architecture. Indeed, although the mean total sleep time (10.8 ± 5.4 h) was in the normal range, there was marked variability in sleep duration and in sleep pattern: 12 patients had 24-h sleep efficiency < 40%, and 2 patients did not show REM phase sleep. Considering only the data obtained during the night, 10 patients showed reduced sleep efficiency (< 70%), 6 patients had a reduced REM percentage or no REM sleep, and 3 had reduced or absent slow wave sleep (SWS). The mean value of the overall arousal index was 24.7 ± 17 with a very large scatter of data, ranging from 4.9 to 63.2 events/hour.
Table 2.
24-hour sleep and wake data
| MV | SB | Total | ANOVA (P) | |
|---|---|---|---|---|
| Total time of recording (min) | 1458 ± 32 | 1449 ± 32 | 1454 ± 31.7 | n.s. |
| WASO (min) | 562 ± 389 | 730 ± 223 | 650 ± 316.5 | n.s. |
| TST (min) | 613 ± 249 | 645 ± 326 | 644 ± 326.4 | n.s. |
| NREM1 (% of TST) | 14.4 ± 10.5 | 13.1 ± 12.8 | 13.7 ± 11.4 | n.s. |
| NREM2 (% of TST) | 50.9 ± 12.3 | 40.9 ± 13.5 | 45.7 ± 13.7 | n.s. |
| SWS (% of TST) | 23.8 ± 13.8 | 31.9 ± 15 | 28.1 ± 14.7 | n.s. |
| REM (% of TST) | 10.8 ± 7.4 | 14.2 ± 8.7 | 12.5 ± 8.1 | n.s. |
| Cycles (n) | 6.3 ± 7.5 | 6 ± 4.5 | 6.1 ± 5.9 | n.s. |
| Spontaneous arousals index (ev/h) | 20.9 ± 16.6 | 22 ± 16.2 | 21.5 ± 16 | n.s. |
| Asynchrony arousals (ev/h) | 3.4 ± 4.9 | |||
| Noise arousals (ev/h) | 3.6 ± 5.9 | 2.9 ± 3.8 | 3.2 ± 4.8 | n.s. |
| Ineffective efforts index (ev/h) | 45.3 ± 66 | |||
| SaO2 (%) | 95.9 ± 2.7 | 96.2 ± 2.1 | 96.1 ± 2.4 | n.s. |
| Awake SaO2 (%) | 95.6 ± 2.3 | 95.6 ± 2.2 | 95.6 ± 2.2 | n.s. |
| NREM SaO2 (%) | 96 ± 2.5 | 96.2 ± 2 | 96.1 ± 2.2 | n.s. |
| REM SaO2 | 96 ± 3.7 | 96.1 ± 2.5 | 96 ± 3 | n.s. |
| TST90 (% of TST) | 5.7 ± 11.8 | 3.3 ± 7.5 | 4.4 ± 9.6 | n.s. |
| ODI (ev/h) | 4.6 ± 5 | 21.8 ± 31.5 | 13.6 ± 24.2 | n.s. |
| SaO2 nadir (%) | 86 ± 7.5 | 83.1 ± 9 | 84.5 ± 8.2 | n.s. |
MV, mechanical ventilation; SB, spontaneous breathing; WASO, wake after sleep onset; TST, total sleep time; SWS, slow wave sleep; TST90, total sleep time with SaO2 < 90%; ODI, oxygen desaturation index.
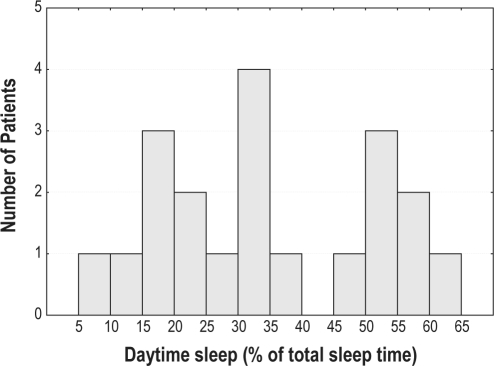
We observed patients with 2 different sleep patterns: “long sleepers” with sleep prevalently during the daytime and patients with a preserved wake/sleep rhythm but a highly fragmented sleep pattern (Figure 1).
Figure 1.
Distribution of daytime sleep time (expressed as % of total sleep time) in all patients.
Differences in sleep data collected during the night (21:00–07:00, according to light intensity, Figure 2) and those collected during the day are summarized in Table 3. There was a predominance of stage 1 sleep in comparison to SWS and REM sleep during the day, as well as a lower number of sleep cycles. Sleep was more disrupted during the day, as demonstrated by the higher arousal index. The amount of sleep time (expressed as a percentage of total sleep time) was inversely correlated with PaO2 (r = −0.44; P = 0.049).
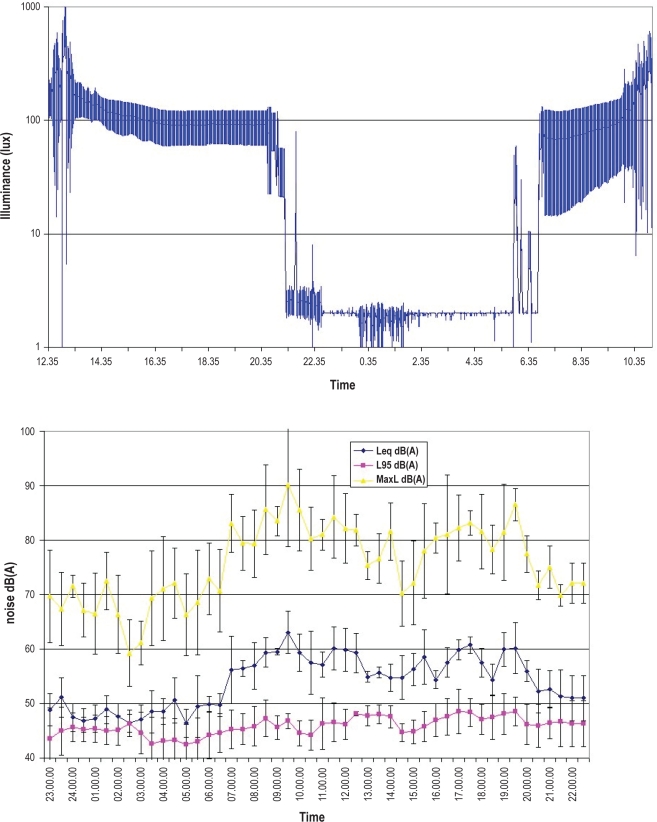
Figure 2.
Mean light intensity (top) and noise levels (bottom) along the 24-h recordings.
Table 3.
Distribution of sleep indices in the daytime and nighttime
| Daytime | Nighttime | P | |
|---|---|---|---|
| Time of recording (min) | 836 ± 174 | 580 ± 32.2 | < 0.001 |
| Total sleep time (min) | 248 ± 239 | 398 ± 125 | 0.002 |
| Sleep efficiency (%) | 28.1 ± 26.4 | 68.6 ± 21.7 | < 0.001 |
| Sleep latency (min) | 57.5 ± 65.8 | 65.6 ± 81.1 | n.s. |
| NREM1 (% of TST) | 22 ± 16.6 | 10.4 ± 10.8 | < 0.001 |
| NREM2 (% of TST) | 46.6 ± 18.1 | 44.6 ± 15 | n.s. |
| SWS (% of TST) | 21.3 ± 17.6 | 29.8 ± 15 | 0.05 |
| REM (% of TST) | 6 ± 10.5 | 15.1 ± 8 | < 0.001 |
| Cycles (n) | 1.7 ± 2.7 | 4.5 ± 3.7 | < 0.001 |
| REM latency (min) | 125.8 ± 145 | 91.2 ± 59 | n.s. |
| Arousals index (ev/h) | 30.6 ± 25.7 | 22.5 ± 16.8 | n.s |
| Noise arousals (ev/h) | 6.6 ± 9.6 | 2.4 ± 3 | n.s. |
| TST90 (% of TST) | 67.7 ± 21.7 | 5.28 ± 11.4 | n.s. |
| ODI (ev/h) | 9.6 ± 17.8 | 9.1 ± 14.6 | n.s. |
SWS, slow wave sleep; TST, total sleep time; TST90, total sleep time with SaO2 < 90%; ODI, oxygen desaturation index.
Therapy with benzodiazepines (14 patients received lorazepam 1 mg in the evening, and 4 patients received alprazolam 0.5 mg in the evening) did not influence the sleep data: patients receiving benzodiazepine therapy showed a higher sleep efficiency (72.8% ± 18.6%) than those who did not (60.3% ± 26.3%), but this difference was not statistically significant.
Mechanical Ventilation
Patients requiring mechanical ventilation had sleep indices similar to those breathing spontaneously, suggesting that mechanical ventilation was not a factor that influenced sleep quality in these patients. Furthermore, ventilatory parameters did not influence sleep quality in patients requiring mechanical ventilation. Many patients showed ineffective efforts that produced only a small number of arousals from sleep (3 ± 4.9 events/h). Benzodiazepine therapy was equally distributed between patients on mechanical ventilation and those breathing spontaneously (6/10 vs. 8/11, respectively; χ2 0.38, P = n.s.).
Clinical Severity
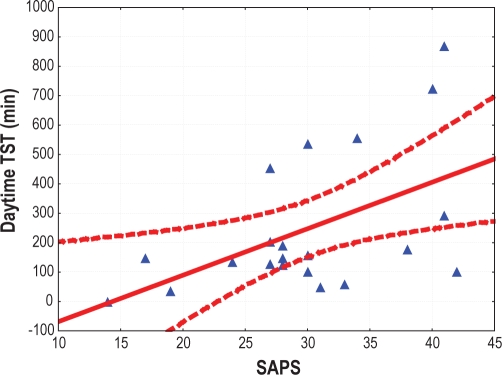
The SAPSII score was significantly correlated with daytime total sleep time (r = 0.51, P = 0.01) (Figure 3) and sleep efficiency (r = 0.5, P = 0.02). Higher values of PaO2 were associated with an increased percentage of SWS along the entire recording (r = 0.49, P = 0.02) and during the daytime (r = 0.5; P = 0.03). Patients sleeping mostly during the day (i.e., > 50% of the total sleep time occurring during the daytime) had higher SAPSII scores than those sleeping mostly during the night (35 ± 5.9 vs. 27.5 ± 7.65, respectively; ANOVA F 4.72, P = 0.04).
Figure 3.
Correlation between daytime total sleep time (TST) and SAPSII score. Dashed lines represent the 95% confidence interval.
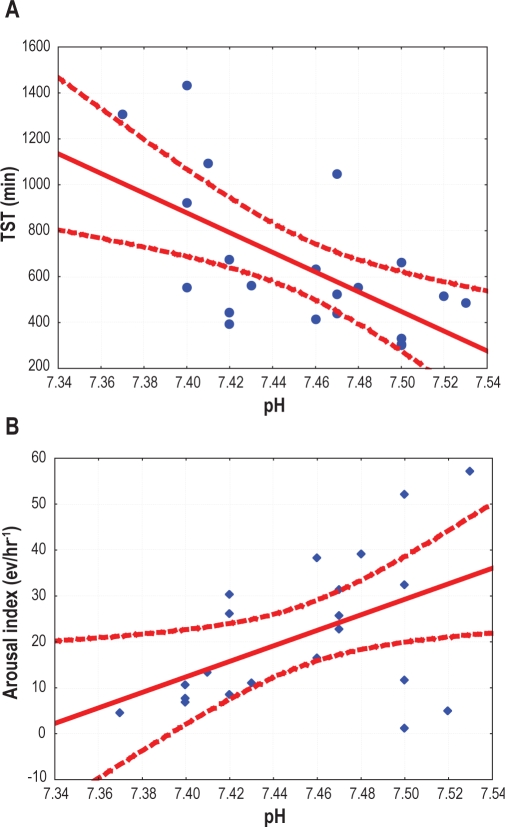
The acid-base balance, expressed by the pH value, was strictly correlated with indices of sleep quality for the whole 24-h recording and separately for the night and day, as shown in Table 4 and Figure 4. A higher pH was associated with reduced sleep quantity and quality.
Table 4.
Correlations matrix (r and P values) between pH and several sleep indices.
| Entire Recording | Day | Night | |
|---|---|---|---|
| TST | −0.60(0.004) | −0.55(0.01) | −0.53(0.01) |
| SE | −0.57(0.007) | −0.56(0.08) | −0.49(0.02) |
| NREM1 | 0.55(0.01) | 0.28(n.s.) | 0.56(0.008) |
| SWS | −0.47(0.03) | −0.58(0.006) | −0.4(n.s.) |
| Arousal index | 0.48(0.026) | 0.52(0.019) | 0.5 (0.02) |
TST, total sleep time; SE, sleep efficiency; SWS, slow wave sleep.
Figure 4.
Correlation between pH and total sleep time (A) or arousal index (B) calculated for the entire recording. Dashed lines represent the 95% confidence interval.
The number of major nursing care activities was 3.1 ± 2 for the entire recording and 0.4 ± 0.9 during the night: no statistically significant differences were found between ventilated and non-ventilated patients (3.3 ± 1.4 vs. 2.8 ± 2.6, respectively, for the entire recording; 0.6 ± 0.7 vs. 0.6 ± 1, respectively, during the night)
Noise and Light Data
Table 5 shows the environmental noise and illumination data separately for the day and night. Noise decreased during the night but still remained above recommended levels.18,19 No sound peaks > 80 dB(A) were recorded during the night. When setting a threshold at 50 dB(A) to define “peaks” of noise, some sleep events appeared to be temporally related to the peaks: 20% and 10% of the arousals that occurred during the day and night, respectively, were related to noise peaks. The 24-h trend in light exposure was characterized by a marked drop at 21:00 (lights off) and very negligible light peaks during the night (Figure 2). We found a great variability in daytime light exposure due to the variability and intensity of sunlight. There were virtually no arousals from sleep due to light peaks.
Table 5.
Environmental noise and light data during the daytime and nighttime
| Night | Day | |
|---|---|---|
| Noise | ||
| EqL (dB) | 49.5 ± 1.08 | 57.9 ± 0.95 |
| Lmin(dB) | 42.3 ± 4.5 | 42.9 ± 5 |
| L95 (dB) | 44.3 ± 3.75 | 46.3 ± 4.7 |
| L5 (dB) | 49 ± 3.5 | 60.8 ± 8.41 |
| Lmax (dB) | 68.7 ± 1.77 | 78.8 ± 0.28 |
| N. peaks > 50dB (ev/h) | 21.8 ± 19.8 | |
| N. peaks > 80dB (ev/h) | < 1 | 17.8 ± 14.2 |
| Light | ||
| Mean (lux) | 2.5 ± 0.5 | 107.1 ± 45 |
| Mean minimum (lux) | 1.3 ± 0.9 | 2.29 ± 0.6 |
| Mean maximum (lux) | 47.3 ± 34.0 | 524.1 ± 751.3 |
EqL, equivalent level; L95, sound threshold exceeded for more than 95% of the duration of the measurement; L5, sound threshold exceed for 5% of the duration of the measurement; Lmin and Lmax, minimum and maximum level of noise.
No statistically significant differences were found for light and noise exposure in ventilated and non-ventilated patients: the mean light exposure during the day was 100 ± 30.9 lux in ventilated vs. 116 ± 58 lux in non-ventilated patients, while mean light exposure during the night was 2.8 ± 0.44 lux in ventilated patients vs. 2.4 ± 0.54 in non-ventilated patients; the mean noise exposure (EqL) was 58.2 ± 0.39 dB in ventilated vs. 58.1 ± 1.02 dB in non-ventilated patients during the day; during the night, these levels were 50.2 ± 0.2 dB vs. 48.9 ± 0.3 dB.
DISCUSSION
In this study, we found that in patients transferred to an SDU in the phase of recovery from an episode of acute respiratory failure, mechanical ventilation was not a main determinant of sleep abnormalities. Patients in an SDU still have sleep abnormalities, but these are mainly associated with clinical severity or alkalosis. Noise, illumination, and patient care contributed little to the abnormalities.
Mechanical ventilation has been recognized to disrupt sleep in patients with acute or chronic respiratory failure though different mechanisms: level of inspiratory support, development of central apneas and/or Cheyne-Stokes breathing, patient-ventilatory asynchronies (particularly ineffective inspiratory efforts), and the presence of an endotracheal tube.5,7,8,14,15 Parthasarathy et al. found that critically ill patients experience greater fragmentation of sleep during pressure support ventilation than during assist-control ventilation because of the development of central apneas.15 They concluded that the selection of the ventilator mode may have a great influence on the quality of sleep of patients. Similarly, Bosma et al., in a study performed to investigate the role of patient-ventilator asynchrony in the etiology of sleep disruption and to determine whether optimizing patient-ventilator interactions by using proportional assist ventilation could improve sleep7 found that patient-ventilator discordance caused sleep disruption and that proportional assist ventilation seemed more efficacious than pressure support ventilation in matching ventilatory requirements with ventilator assistance, resulting in fewer patient-ventilator asynchronies and better quality of sleep.
In contrast, Cabello et al., in a study designed to compare the influence of three common ventilation modes on sleep quality, observed that the ventilatory mode did not influence sleep pattern.12 Nonetheless, sleep quality was impaired, with a low percentage of REM sleep and with high arousal and awakening indices. We observed similar data in our patients. Indeed, the sleep pattern was similar in patients requiring mechanical ventilation and in those breathing spontaneously. The role of patient-ventilator asynchronies, in particular ineffective efforts, was negligible (˜10% of total arousals). Possible explanations for the differences between our data and those previously reported may be related to differences in clinical severity (increased respiratory drive during acute respiratory failure and during acute inflammatory states), etiology of acute respiratory failure (in particular acute cardiac failure or sepsis), and mechanical respiratory properties.6,16,22,23
Knowledge about the sleep/wake cycle in patients admitted to an ICU is limited, since the three published studies enrolled a total of only 49 patients who underwent 24-h polysomnography2–4 and concluded that ICU patients suffer from qualitative, but not quantitative, sleep deprivation, and that environmental factors are responsible for only a part of the sleep disruption.
Very few data on sleep-wake cycles are available in patients admitted to a SDU, where theoretically the patient's stable clinical condition and the environmental situation may help patients to recover more physiological sleep architecture. A small study (6 patients), in whom polysomnography was performed only during the night, showed that environmental noise may be an important cause of sleep disruption in an SDU.18
The average total sleep time we observed was considerably longer than that reported in other studies: 11.5 h/24 h in our study vs. 6 to 8.8 h/24 h in ICU patients.2–4 Similarly, the average nocturnal sleep was 6.7 h/night, longer than that previously reported in a respiratory ICU (5.4 h/night).18 We confirmed the presence of a large variability of sleep-wake data in our patients, many of whom experienced selective deprivation of SWS or REM sleep and/or moderate to severe sleep fragmentation.
We found two different sleep patterns in our patients: one group of patients were “long sleepers” who slept for more time during the day than during the night; the other group had a preserved wake/sleep rhythm but highly fragmented sleep pattern.
Long Sleepers
The influence of an illness itself on sleep deprivation is not entirely clear and, indeed, is difficult to establish, given the others factors mentioned above.22 A significant increase in sleep disruption has been found in patients who have higher disease severity score,16 which is usually associated with a high awakening index, shorter sleep time, and decreased SWS.4 However, our data contrast with these findings, since we observed a statistically significant correlation between the indices of sleep quantity and the severity of the patients' clinical condition. In particular, patients with a higher SAPSII score slept for longer during the day. We can assume that patients with more severe disease reaching clinical stability and remaining in a more physiological environment may experience sleep recovery. Recovery from sleep deprivation is associated with an increased propensity to daytime sleep, shortened sleep onset latency, improved sleep efficiency, increased total sleep time, and an increase in SWS.24,25 Jay et al. demonstrated that recovery from sleep deprivation may be obtained only when opportunities to fall asleep are not restricted.24 Similarly, Åkerstedt et al. demonstrated that individuals who were subjected to repeated partial sleep deprivation had a reduced latency for SWS during the recovery phase, suggesting an increased pressure for sleep, as well as a gradual increase and subsequent recovery to normality of the total amount of SWS.26 Similar findings were made by Cajochen et al. in older subjects.27 Furthermore, we found that the patients with the greatest improvement of clinical status, as determined by the level of arterial oxygenation, were those who had the most SWS. Sustained hypoxia has been demonstrated to alter normal circadian oscillations and may contribute to the development of sleep fragmentation or daytime fatigue.28 In a mouse model, Polotsky et al. demonstrated that exposure to hypoxia determines overall deficits in the EEG delta power of NREM sleep and in the amount of REM sleep.29 Acute changes in CO2 and pH have been demonstrated to induce selective homeostatic activation of hypothalamic orexin neurons: acidification increases intrinsic excitability.30 One can hypothesize that during the recovery phase from an acute respiratory decompensation the activity of orexin neurons may decrease, leaving patients to recover from sleep deprivation. Indeed, in a mouse model, Terada et al. found that prolonged exposure to hypoxia induced a significant reduction in SWS that progressively recovered during normoxic respiration.31 Finally, in less severely ill patients, the presence of a more physiological environment in the SDU (i.e., more physiological light and noise) may favor better sleep quality during the night, thus reducing the need for diurnal naps.32 Our results expand the observation that the levels of both noise and illumination are lower and more physiological in a SDU than in an ICU, with absence of noise peaks above 80 dB during the night.3–4,7,13 Light exposure in our SDU reflects the circadian rhythm: patients received natural illumination (range 100–3000 lux), which is usually higher than the artificial level (300 lux), and with a sudden change from day to night. This pattern of exposure may help to restore circadian secretion of melatonin, which had been demonstrated to be abolished in ICU patients.33,34
Poor Sleepers
Our data demonstrate a clear relationship between sleep quality and acid-base imbalance, which had not been previously described in the literature. A higher pH was associated with a reduced quantity and poorer quality of sleep, in terms of less SWS and more arousals.35,36 The presence of alkalosis, including respiratory alkalosis with hypocapnia, increases neuronal excitability, seizure activity, and spontaneous and/or asynchronous firing of cortical neurones.37 Intracellular pH seems to play a key role in mediating the effect of hypocapnia or alkalosis on neuronal excitability. In particular, low levels of PaCO2 with alkalosis decrease intracortical inhibition by modulating intrinsic neuronal circuits.38
Further studies are needed to explain the mechanism of alkalosis in patients recovering from acute life-threatening respiratory failure, and the roles of the severity of the acute decompensation, alterations in homeostatic systems, subclinical kidney impairment, pharmacological therapy, and prolonged stressful condition in determining sleep instability. It has been demonstrated that stressful conditions activate orexin neurons that usually show state-dependent activity: orexin levels increase just before awakening, remain high during wakefulness, increase considerably during exercise and/or in heightened alertness, decrease during SWS, and increase again during REM sleep.39 Orexinergic connections are involved in sleep-wake regulation and emotional stress-induced behavioral changes that modulate cardiorespiratory homeostasis: several effects have been measured, such as changes in alertness or arousability level, blood pressure, heart rate, respiratory frequency, and tidal volume, as well as the development of long-term facilitation after prolonged hypoxia stimulus and baroreceptor reflex suppression.39
Limitations of the Study
Some confounding factors must be discussed. Most of the patients included in our study, were over 60 years old, so that age-related physiological changes of sleep should be considered.27,40 Important changes in sleep structure occur with age, including a reduction in the total amount of time spent asleep, as well as a reduction in the total amount of SWS. However, in our analysis of sleep architecture we compared the data from our patients with those reported for subjects of the same age in the general population.21 Furthermore, insomnia is quite frequent in older people, as a primary sleep disturbance or as a consequence of underlying diseases or drug use.41 Finally, underlying disease may be responsible for poor sleep quality. Sleep complaints and alterations have been described in patients with chronic obstruction pulmonary disease as well as in patients with other chronic diseases with respiratory impairment, such as chronic heart failure, kyphoscoliosis, and neuromuscular disorders.42
In conclusion, our data show that patients admitted to an SDU and transferred from an ICU still have sleep abnormalities. Dependence on a ventilator does not appear to be relevant. The presence of alkalosis seems to be an important cause of poor quality sleep, whereas the improvement of clinical conditions and the more physiological SDU environment is associated with sleep recovery.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Weinhouse GL, Schawab RJ. Sleep in the critically ill patient. Sleep. 2006;29:707–16. doi: 10.1093/sleep/29.5.707. [DOI] [PubMed] [Google Scholar]
- 2.Freedman NS, Kotzer N, Schwab RJ. Patient perception of sleep quality and etiology of sleep disruption in the Intensive Care Unit. Am J Respir Crit Care Med. 1999;159:1155–62. doi: 10.1164/ajrccm.159.4.9806141. [DOI] [PubMed] [Google Scholar]
- 3.Rotondi AJ, Chelluri L, Sirio C, et al. Patients' recollections of stressful experiences while receiving prolonged mechanical ventilation in an intensive care unit. Crit Care Med. 2002;30:746–52. doi: 10.1097/00003246-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Nelson JE, Meier DE, Oei EJ, et al. Self-reported symptom experience of critically ill cancer patients receiving intensive care. Crit Care Med. 2001;29:277–82. doi: 10.1097/00003246-200102000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Cooper AB, Thornley KS, Young GB, Slutsky AS, Stewart TE, Hanly PJ. Sleep in critically ill patients requiring mechanical ventilation. Chest. 2000;117:809–18. doi: 10.1378/chest.117.3.809. [DOI] [PubMed] [Google Scholar]
- 6.Freedman NS, Gazendam J, Levan L, Pack AI, Schwab RJ. Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 2001;163:51–7. doi: 10.1164/ajrccm.163.2.9912128. [DOI] [PubMed] [Google Scholar]
- 7.Gabor JY, Cooper AB, Crombach SA, et al. Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. Am J Respir Crit Care Med. 2003;167:708–15. doi: 10.1164/rccm.2201090. [DOI] [PubMed] [Google Scholar]
- 8.Parthasarathy S, Tobin MJ. Effect of ventilator mode on sleep quality in critically ill patients. Am J Respir Crit Care Med. 2002;166:1423–9. doi: 10.1164/rccm.200209-999OC. [DOI] [PubMed] [Google Scholar]
- 9.Thille AW, Rodriguez P, Cabello B, Lellouche F, Brochard L. Patient-ventilator asynchrony during assisted mechanical ventilation. Intensive Care Med. 2006;32:1515–22. doi: 10.1007/s00134-006-0301-8. [DOI] [PubMed] [Google Scholar]
- 10.Bosma K, Ferreyra G, Ambrogio C, et al. Patient-ventilator interaction and sleep in mechanically ventilated patients: pressure support versus proportional assist ventilation. Crit Care Med. 2007;35:1048–54. doi: 10.1097/01.CCM.0000260055.64235.7C. [DOI] [PubMed] [Google Scholar]
- 11.Toublanc B, Rose D, Glerant JC, et al. Assist-control ventilation vs. low levels of pressure support ventilation on sleep quality in intubated ICU patients. Intensive Care Med. 2007;33:1148–54. doi: 10.1007/s00134-007-0659-2. [DOI] [PubMed] [Google Scholar]
- 12.Nava S. Rehabilitation of patients admitted to a respiratory intensive care unit. Arch Phys Med Rehabil. 1998;79:849–54. doi: 10.1016/s0003-9993(98)90369-0. [DOI] [PubMed] [Google Scholar]
- 13.Roche Campo F, Drouot X, Thille AW, et al. Poor sleep quality is associated with late non-invasive ventilation failure inpatients with acute hypercapnic respiratory failure. Crit Care Med. 2010;38:477–85. doi: 10.1097/CCM.0b013e3181bc8243. [DOI] [PubMed] [Google Scholar]
- 14.Aaron JN, Carlisle CC, Carskadon MA, Meyer TJ, Hill NS, Millman RP. Environmental noise as a cause of sleep disruption in an intermediate respiratory care unit. Sleep. 1996;19:707–10. doi: 10.1093/sleep/19.9.707. [DOI] [PubMed] [Google Scholar]
- 15.Cabello B, Thille AW, Druot X, et al. Sleep quality in mechanically ventilated patients: comparison of three ventilatory mode. Crit Care Med. 2008;36:1749–55. doi: 10.1097/CCM.0b013e3181743f41. [DOI] [PubMed] [Google Scholar]
- 16.Ambrogio C, Lowman X, Kuo M, Malo J, Prasad AR, Parthasarathy S. Sleep and non-invasive ventilation in patients with chronic respiratory failure insufficiency. Intensive Care Med. 2009;35:306–13. doi: 10.1007/s00134-008-1276-4. [DOI] [PubMed] [Google Scholar]
- 17.Fanfulla F, Delmastro M, Berardinelli A, D'Artavilla Lupo N, Nava S. Effects of different ventilator settings on sleep and inspiratory effort in patients with neuromuscular disease. Am J Respir Crit Care Med. 2005;172:619–24. doi: 10.1164/rccm.200406-694OC. [DOI] [PubMed] [Google Scholar]
- 18.Parthasarathy S. Sleep during mechanical ventilation. Curr Opinion Pulm Med. 2004;10:488–94. doi: 10.1097/01.mcp.0000143691.94442.fa. [DOI] [PubMed] [Google Scholar]
- 19.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–63. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 20.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 21.Walsleben JA, Kapur VK, Newman AB, et al. Sleep and reported daytime sleepiness in normal subjects: the Sleep Heart Health Study. Sleep. 2004;27:293–8. doi: 10.1093/sleep/27.2.293. [DOI] [PubMed] [Google Scholar]
- 22.Friese RS. Sleep and recovery from critical illness and injury: a review of theory, current practice, and future directions. Crit Care Med. 2008;36:697–705. doi: 10.1097/CCM.0B013E3181643F29. [DOI] [PubMed] [Google Scholar]
- 23.Ackland GL, Kasymov V, Gourine AV. Physiological and pathophysiological roles of extracellular ATP in chemosensory control of breathing. Biochem Soc Trans. 2007;35:1264–8. doi: 10.1042/BST0351264. [DOI] [PubMed] [Google Scholar]
- 24.Jay SM, Lamond N, Ferguson SA, Dorrian J, Jones CB, Dawson D. The characteristics of recovery sleep when recovery opportunity is restricted. Sleep. 2007;30:353–60. doi: 10.1093/sleep/30.3.353. [DOI] [PubMed] [Google Scholar]
- 25.Dijk DJ, Groeger JA, Stanley N, Deacon S. Age-related reduction in daytime propensity and nocturnal slow wave sleep. Sleep. 2010;33:211–23. doi: 10.1093/sleep/33.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Åkerstedt T, Kecklund G, Ingre M, Lekander M, Axelsson J. Sleep homeostasis during repeated sleep restriction and recovery: support form EEG dynamics. Sleep. 2009;32:217–22. doi: 10.1093/sleep/32.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cajochen C, Münch M, Knoblauch V, Blatter K, Wirz-Justice A. Age-related changes in the circadian and homeostatic regulation of human sleep. Chronobiol Int. 2006;23:461–74. doi: 10.1080/07420520500545813. [DOI] [PubMed] [Google Scholar]
- 28.Mortola JP. Hypoxia and circadian patterns. Respir Physiol Neurobiol. 2007;158:274–79. doi: 10.1016/j.resp.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Polotsky VY, Rubin AE, Balbir A, et al. Intermittent hypoxia causes REM sleep deficits and decreases EEG delta power in NREM sleep in the C57BL/6J mouse. Sleep Med. 2006;7:7–16. doi: 10.1016/j.sleep.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. PNAS. 2007;104:10685–9. doi: 10.1073/pnas.0702676104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terada J, Nakamura A, Zhang W, Yanagisawa M, et al. Ventilator long-term facilitation in mice can be observed during both sleep and wake periods and depends on orexin. J Appl Phyisiol. 2008;104:499–507. doi: 10.1152/japplphysiol.00919.2007. [DOI] [PubMed] [Google Scholar]
- 32.Boivin DB, Duffy JF, Kronauer RE, Czeiler CA. Dose-response relationships for resetting of human circadian clock by light. Nature. 1996;379:540–2. doi: 10.1038/379540a0. [DOI] [PubMed] [Google Scholar]
- 33.Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev. 2005;9:11–24. doi: 10.1016/j.smrv.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Olofsson K, Alling C, Lundberg D, Malmros C. Abolished circadian rhythm of melatonin secretion in sedated and artificially ventilated intensive care patients. Acta Anaesthesiol Scand. 2004;48:679–84. doi: 10.1111/j.0001-5172.2004.00401.x. [DOI] [PubMed] [Google Scholar]
- 35.Laffey JG, Kavanagh BP. Hypocapnia. N Engl J Med. 2002;347:43–53. doi: 10.1056/NEJMra012457. [DOI] [PubMed] [Google Scholar]
- 36.Lovering AT, Frainge JJ, Dunin-Barkowski WL, Vidruk EH, Orem JM. Hypocapnia decreases the amount of rapid eye movement sleep in cats. Sleep. 2003;26:961–7. doi: 10.1093/sleep/26.8.961. [DOI] [PubMed] [Google Scholar]
- 37.Lee J, Taira T, Pihlaja P, Ransom BR, Kaila K. Effects of CO2 on excitatory transmission apparently caused by changes in intracellular pH in the rat hippocampal slice. Brain Res. 1996;706:210–6. doi: 10.1016/0006-8993(95)01214-1. [DOI] [PubMed] [Google Scholar]
- 38.Kuwaki T, Zhang W. Orexin neurons as arousal-associated modulators of central cardiorespiratory regulation. Respir Physiol Neurobiol. 2010 doi: 10.1016/j.resp.2010.04.018. (in press). Doi: 10.1016/j.respir.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 39.Sparing R, Dafotakis M, Buelte D, Meister IG, Noth J. Excitability of human motor and visual cortex before, during, and after hyperventilation. J Appl Physiol. 2007;102:406–11. doi: 10.1152/japplphysiol.00770.2006. [DOI] [PubMed] [Google Scholar]
- 40.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 41.Wolkove N, Elkholy O, Baltzan M, Palayew M. Sleep and aging: sleep disorders commonly found in older people. CMAJ. 2007;176:1299–304. doi: 10.1503/cmaj.060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fanfulla F. Other respiratory conditions and disorders. In: Kushida C, editor. Obstructive sleep apnea – diagnosis and treatment. New York: Informa Healthcare; 2007. pp. 333–46. [Google Scholar]