Abstract
Study Objectives:
Epidemiological studies point to a strong association between short sleep duration and the development of diabetes. We examined the hypothesis that short-term sleep loss decreases glucose tolerance and insulin sensitivity and, if so, how these changes relate to hypothalamic-pituitary-adrenal (HPA) secretory activity and markers of subclinical inflammation.
Design:
In a balanced, within-subject design, circulating glucose, insulin, C-peptide, glucagon, ACTH, cortisol, and IL-6 levels were closely monitored during a 15-h daytime period following 2 nights of restricted sleep (02:45–07:00) and 2 nights of regular sleep (bedtime 22:45–07:00), respectively.
Setting:
Time-deprivation suite within a university medical center sleep laboratory.
Participants:
15 healthy, unmedicated normal-weight men.
Intervention:
Sleep restriction.
Measurements and Results:
Pre-breakfast concentrations of blood parameters were unchanged following sleep manipulation (P > 0.30). However, insulin and glucose peak responses to breakfast intake at 08:00 were distinctly increased by sleep restriction in comparison to regular sleep (398.5 ± 57.4 vs. 284.3 ± 51.5 pmol/L and 6.8 ± 0.3 vs. 6.1 ± 0.3 mmol/L, respectively; all P < 0.02), while glucagon responses were blunted by sleep loss (P = 0.03). There were no differences in circulating ACTH, cortisol, and IL-6 concentrations between the 2 conditions (all P > 0.25).
Conclusions:
Data indicate an impairment of glucose tolerance after 2 days of sleep restriction to ˜4 h that appears to be primarily caused by a reduction in insulin sensitivity. Unchanged HPA secretory activity and IL-6 concentrations argue against a mediation of these effects by stress-related or inflammatory mechanisms.
Citation:
Schmid SM; Hallschmid M; Jauch-Chara K; Wilms B; Lehnert H; Born J; Schultes B. Disturbed glucoregulatory response to food intake after moderate sleep restriction. SLEEP 2011;34(3):371-377.
Keywords: Sleep deprivation, insulin resistance, glucose tolerance, insulin sensitivity, glucagon
INTRODUCTION
The prevalence of type 2 diabetes mellitus is on a worldwide rise1,2 while in parallel, average sleep duration has decreased by almost two hours over the past century.3 Cross-sectional and longitudinal studies point to an U-shaped relationship between self-reported sleep duration and the risk of type 2 diabetes mellitus4–7 with the lowest risk between 7 to 8 hours of sleep. However, until now experimental evidence for a causal link between sleep loss and impairments of glucose metabolism has been scarce. Spiegel and coworkers have shown that healthy men display a marked reduction in glucose tolerance as assessed by an intravenous glucose tolerance test (IVGTT) along with a blunted acute insulin response after 6 days of sleep restriction to 4 hours per night as compared with 6 subsequent days of 9-hour recovery sleep.8 In addition to impaired β-cell insulin secretory function, insulin sensitivity was found to be distinctly reduced after 24 h of total sleep deprivation,9 suggesting that insulin resistance critically contributes to the deterioration of glucose metabolism by sleep loss. Furthermore, a most recent study10 indicated a reduction in glucose tolerance and insulin sensitivity after 14 days of bedtime restriction to 5.5 h (vs. 8.5 h) which, however, did not provoke compensation via enhanced insulin secretion in response to an intravenous or oral glucose challenge.
Here we assessed the effects of moderate sleep restriction to 4 h for 2 consecutive days in comparison to regular sleep of 8 h per day on glucose metabolism during a subsequent 15-h daytime period in which subjects were presented with a standardized breakfast buffet at 08:00 followed by a snack buffet and main dishes throughout the day. From the same set of experiments, we have previously reported reduced spontaneous physical activity and unchanged total energy but slightly elevated fat intake in the sleep restriction condition.11 Here we focus on our measurements of relevant parameters of glucose metabolism and of circulating concentrations of ACTH, cortisol, and IL-6. Because alterations in endocrine stress systems12 and proinflammatory markers of the immune system13 are tightly linked to the regulation of glucose homeostasis, it was of particular interest how presumed sleep loss-induced changes in glucose metabolism relate to HPA axis secretory activity and subclinical inflammation.
METHODS
Subjects
The study was carried out in 15 healthy young men aged 20 to 40 years (mean ± SEM: 27.1 ± 1.3 years) with a body mass index between 20.5 and 24.9 kg/m2 (22.9 ± 0.3 kg/m2). All participants had a regular sleep wake cycle without shift-working during the last 4 weeks before the experiments. A standardized interview on sleep habits revealed a habitual sleep duration of 459 ± 7 min (range: 450–540 min) with bedtime starting between 22:00h and 00:00h and wake up time ranging from 0600h to 08:00. Before participating, all subjects spent one night at the laboratory to polysomnographically confirm physiological sleep architecture. Exclusion criteria were chronic or acute illness, current medication of any kind, smoking, alcohol or drug abuse, obesity, and diabetes in first-degree relatives. All investigations were carried out in accordance with the principles of the Declaration of Helsinki as revised in 2000. The study protocol was approved by the ethics committee on research involving humans at the University of Luebeck, and all participants gave written informed consent prior to participation.
Study Design and Procedure
Following an adaptation night in the laboratory that included preparing the subjects for standard polysomnographic recordings, participants were tested in a counter-balanced crossover design on 2 conditions spaced ≥ 6 weeks apart. Assessment of glucose metabolism took place (i) after 2 consecutive nights of 4 h of sleep (“4h-sleep”; bedtime: 02:45 to 07:00) and (ii) after 2 consecutive nights of 8 h of sleep (“8h-sleep”; bedtime: 22:45 to 07:00). During the 15-h daytime assessment period (08:00–23:00), subjects were time-deprived by removing all clocks, radios, and further time cues from the sound-attenuated laboratory room (with adjacent bathroom) without natural light. Subjects were allowed to read and to play video games.
Subjects were instructed to eat a light dinner before arrival at the research unit at 20:00 each laboratory night. Thereafter, they were only allowed to drink water until the next morning. After preparation of polysomnographic recordings, subjects went to bed and lights were turned off at 22:45 in the 8h-sleep condition. In the 4h-sleep condition, subjects remained awake in a sitting position until 02:45. They were allowed to read and to watch non-arousing movies. Brisk physical activities were avoided and subjects were constantly monitored by the experimenters. After each experimental night in both conditions, subjects were woken up at 07:00.
After the first of the 2 consecutive laboratory nights (night 1), subjects in both conditions received a standard breakfast of around 600 kcal and were allowed to leave the laboratory (day 1). They were instructed not to deviate from their usual eating habits and to avoid intense physical activities (e.g., working out) and naps during the day. Compliance with these instructions was confirmed by accelerometric recordings (Actiwatch, CamNtech Ltd., Cambridge, UK). Subjects reported back to the laboratory at 20:00 to be prepared for laboratory night 2 the procedure and setting of which were identical to night 1.
After waking up the subjects at 07:00 at the end of night 2, the experimental day (day 2) started. An intravenous catheter was inserted into a vein of the subject's non-dominant distal forearm to allow the drawing of blood samples at 07:40 and at every full hour between 08:00 and the end of the experiment at 23:00. At 08:00 subjects were presented with a large standardized breakfast buffet (5060 kcal) from which they were allowed to eat ad libitum. At 11:00, the breakfast buffet was replaced by a snack buffet (5010 kcal at first serve) which remained in the experimental room until the end of the day (at 23:00). Buffet components were refilled whenever necessary. In addition to the snack buffet, subjects could select main meals from a menu (1200 kcal at first serve) whenever they chose after 11:00. Food intake was measured outside the experimental room by weighing buffet components before serving and after clearing the table. Nutritional analyses of breakfast food intake were performed using a software program for macronutrient analyses (DGE-PC professional 3.3, Stuttgart, Germany). Results of total food intake during day 2 have been previously reported.11
Sleep Recordings
For standard polysomnography, electrodes were attached to the scalp (C3, C4) for electroencephalographic (EEG) recordings, above, below, and beside the eyes for horizontal and vertical electrooculogram (EOG), and on the chin for electromyogram (EMG). Recordings were performed using a Nihon Kohden amplifier (EEG 4400 series, Nihon Kohden GmbH, Germany). They were scored offline according to standard criteria.14 The following sleep parameters were determined: total sleep time (TST), time spent in sleep stage (S) 1, 2, 3, 4, SWS (i.e., S3 + S4), and REM sleep (all in min and % of TST), time spent awake after sleep onset (WASO), and movement time (MT, in % of TST).
Assays
Blood glucose levels were determined immediately by HemoCue B-Glucose-Analyzer (Ángelholm, Sweden). Concentrations of insulin, C-peptide, glucagon, cortisol, adrenocorticotropin (ACTH), and non-esterified fatty acids (NEFA) were determined from stored (−80°C) serum or plasma samples by enzyme-linked immunoassays as described previously.15,16 Concentrations of interleukin 6 (IL-6; Quantikine human IL-6, R&D Systems Inc., Minneapolis, USA) were also determined by enzyme-linked immunoassays.
Statistical Analyses
Based on power calculations inferred from previous experiments,17 our sample size of 15 provided a power of 0.95 (α = 0.05) to detect meaningful differences in relevant hormonal measures after the respective sleep manipulation. All values are expressed as mean ± SEM. Analyses of sleep data were based on analysis of variance (ANOVA) for repeated measures, including the factors “Condition” (for 4 h vs. 8 h of sleep) and “Night” (for night 1 vs. night 2). Analyses of hormonal data were based on ANOVA for repeated measures, including the factors “Condition” and “Time” (for repeated measurements during the experimental day). Where ANOVA indicated significant main effects, pairwise comparisons of single time point and area under the curve (AUC) values were performed using Student's t-test. A P-value < 0.05 was considered significant.
RESULTS
Sleep
Sleep data recorded during the 2 nights of 4 h of sleep and the 2 nights of 8 h of sleep are summarized in Table 1. In the 4h-sleep condition, subjects slept on average ˜229 min less than in the 8h-sleep condition. The absolute time spent in SWS was identical in both conditions (P = 0.84). The longer sleep duration in the 8h-sleep condition was primarily due to more pronounced shallow sleep, i.e., S1 and S2, as well as REM sleep. Overall, sleep quality improved in the second as compared to the first night, displaying greater amounts of SWS (P = 0.002) and smaller amounts of S2 (P < 0.001). The increase in the relative amount of SWS was more pronounced in the 4h than 8h-sleep condition (P = 0.018).
Table 1.
Sleep parameters during the first and second night of the 4h and 8h-sleep conditions
| 4h-sleep | 8h-sleep | P |
|||
|---|---|---|---|---|---|
| Condition | Night | Night × Condition | |||
| TST (min) | |||||
| Night I | 236 ± 2 | 465 ± 4 | < 0.001 | 0.294 | 0.923 |
| Night II | 238 ± 1 | 467 ± 3 | |||
| WASO (min) | |||||
| Night I | 2 ± 1 | 18 ± 4 | 0.002 | 0.127 | 0.120 |
| Night II | 2 ± 1 | 9 ± 3 | |||
| WASO (%) | |||||
| Night I | 1 ± 0 | 4 ± 1 | 0.012 | 0.149 | 0.143 |
| Night II | 1 ± 0 | 2 ± 1 | |||
| S1 (min) | |||||
| Night I | 18 ± 2 | 52 ± 10 | 0.002 | 0.051 | 0.136 |
| Night II | 16 ± 1 | 37 ± 8* | |||
| S1 (%) | |||||
| Night I | 7 ± 1 | 11 ± 2 | 0.061 | 0.150 | 0.127 |
| Night II | 7 ± 1 | 8 ± 2* | |||
| S2 (min) | |||||
| Night I | 124 ± 7 | 255 ± 9 | < 0.001 | 0.097 | 0.382 |
| Night II | 111 ± 7* | 254 ± 8 | |||
| S2 (%) | |||||
| Night I | 52 ± 3 | 55 ± 2 | 0.054 | < 0.001 | 0.135 |
| Night II | 47 ± 3* | 54 ± 2 | |||
| SWS (min) | |||||
| Night I | 47 ± 7 | 49 ± 6 | 0.838 | 0.001 | 0.067 |
| Night II | 69 ± 7*** | 61 ± 6** | |||
| SWS (%) | |||||
| Night I | 21 ± 3 | 10 ± 1 | < 0.001 | 0.002 | 0.018 |
| Night II | 29 ± 3** | 13 ± 1** | |||
| REM (min) | |||||
| Night I | 43 ± 6 | 87 ± 11 | < 0.001 | 0.146 | 0.029 |
| Night II | 38 ± 5 | 101 ± 8 | |||
| REM (%) | |||||
| Night I | 18 ± 2 | 19 ± 2 | 0.186 | 0.626 | 0.045 |
| Night II | 16 ± 2 | 22 ± 2 | |||
| MT (min) | |||||
| Night I | 2 ± 0 | 4 ± 1 | < 0.001 | 0.130 | 0.223 |
| Night II | 3 ± 0 | 5 ± 1 | |||
| MT (%) | |||||
| Night I | 1 ± 0 | 1 ± 0 | 0.244 | 0.237 | 0.567 |
| Night II | 2 ± 1 | 1 ± 0 | |||
Data are mean ± SEM. TST, total time spent asleep; WASO, wake time after sleep onset; S1, sleep stage 1; S2, sleep stage 2; SWS, slow wave sleep; MT, movement time during TST. P-values are derived from ANOVA including the factors “Condition” for 4h-hour vs. 8h-sleep condition and Night for comparison of night 1 vs. night 2.
P < 0.05;
P < 0.01;
P < 0.001 for Student t-test comparing night 1 vs. night 2 within conditions.
Food Intake and Glucose Metabolism
Nutritional analyses revealed that food intake from the breakfast buffet was identical in the respective 4h and 8h-sleep condition with regard to total energy (6159 ± 507 vs. 6272 ± 532 kJ; P = 0.83), carbohydrate (42.8% ± 2.4% vs. 43.0% ± 2.7%; P = 0.90), fat (42.5% ± 2.0% vs. 42.0% ± 3.1%; P = 0.68), and proteins (14.7% ± 0.6% vs. 15.0% ± 0.6%; P = 0.58). Also, monosaccharides (14.9 ± 3.4 vs. 11.0 ± 2.9 g; P = 0.35), disaccharides (67.5 ± 6.2 vs. 70.3 ± 6.7 g; P = 0.55), and polysaccharides (66.9 ± 5.7 vs. 72.0 ± 7.1 g; P = 0.59) as well as glucose, fructose, starch, and saccharose intake did not differ between conditions (all P > 0.36). Likewise, total energy intake during the whole day was identical between conditions (16618 ± 1080 vs. 17041 ± 1193 kJ; P = 0.70).11
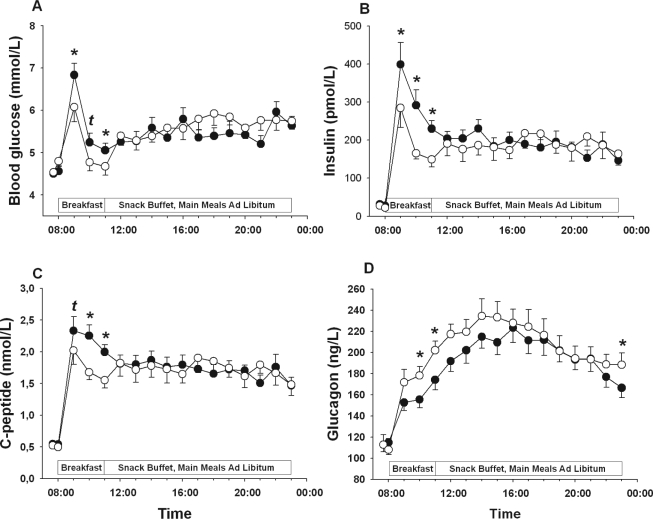
While there was no effect of sleep deprivation on pre-breakfast concentrations of markers of glucose metabolism (P > 0.30 for all comparisons), breakfast-induced increases in blood glucose (P = 0.022 for the respective Condition × Time interaction) as well as serum insulin (P = 0.007) and C-peptide levels (P = 0.022) were distinctly higher in the 4h than the 8h-sleep condition (Table 2 and Figure 1A-C), indicating a pronounced effect of sleep loss on glucose metabolism in response to identical caloric loads. Plasma glucagon levels rapidly increased in response to breakfast and peaked mid-afternoon in both conditions, but sleep loss significantly attenuated this increase (P = 0.032 for Condition), with this effect again being most pronounced during the breakfast period (Table 2 and Figure 1D).
Table 2.
Parameters of glucose metabolism before and in response to breakfast
| 4h-sleep | 8h-sleep | P | |
|---|---|---|---|
| Glucose | |||
| Pre-breakfast (mmol/L) | 4.5 ± 0.1 | 4.5 ± 0.1 | 0.67 |
| Peak (mmol/L) | 6.8 ± 0.3 | 6.1 ± 0.3 | < 0.01 |
| AUC (mmol*min/L) | 552 ± 16 | 514 ± 18 | 0.01 |
| Insulin | |||
| Pre-breakfast (pmol/L) | 31.2 ± 5.0 | 27.9 ± 3.7 | 0.30 |
| Peak (pmol/L) | 398.5 ± 57.4 | 284.3 ± 51.5 | 0.02 |
| AUC (pmol*min/L) | 24855 ± 2131 | 16304 ± 1868 | < 0.001 |
| C-peptide | |||
| Pre-breakfast (nmol/L) | 0.55 ± 0.04 | 0.52 ± 0.03 | 0.50 |
| Peak (nmol/L) | 2.3 ± 0.2 | 2.0 ± 0.2 | 0.10 |
| AUC (nmol*min/L) | 181 ± 11 | 147 ± 11 | < 0.001 |
| Glucagon | |||
| Pre-breakfast (ng/L) | 112.9 ± 6.9 | 112.9 ± 9.6 | 0.99 |
| AUC (ng*min/L) | 14588 ± 713 | 16259 ± 787 | 0.03 |
| ACTH | |||
| Pre-breakfast (pmol/L) | 2.1 ± 0.2 | 1.8 ± 0.2 | 0.42 |
| AUC (pmol*min/L) | 443.0 ± 39.0 | 421.5 ± 30.9 | 0.44 |
| Cortisol | |||
| Pre-breakfast (nmol/L) | 129.1 ± 24.0 | 127.2 ± 20.8 | 0.91 |
| AUC (nmol*min/L) | 33049 ± 1370 | 33207 ± 1499 | 0.88 |
| IL-6 | |||
| Pre-breakfast (pg/mL) | 2.0 ± 0.0 | 2.2 ± 0.2 | 0.28 |
| AUC (pg*min/mL) | 215.0 ± 11.4 | 203.7 ± 3.7 | 0.38 |
Data are mean ± SEM. P-values are derived from Student t-tests. Pre-breakfast values refer to baseline assessments at 07:40 before starting breakfast. Peak values refer to maximum response to breakfast intake; AUC denotes the areas under the curve from 08:00 to 11:00.
Figure 1.
Mean (± SEM) concentrations plasma glucose (A), serum insulin (B), serum C-peptide (C), and plasma glucagon (D) during an experimental day following 2 nights of 8 h of sleep each (white bars/circles) and 2 nights each containing 4 h of sleep (black bars/circles), respectively. N = 15; tP < 0.10; *P < 0.05.
NEFA, HPA Secretory Activity, and IL-6 Levels
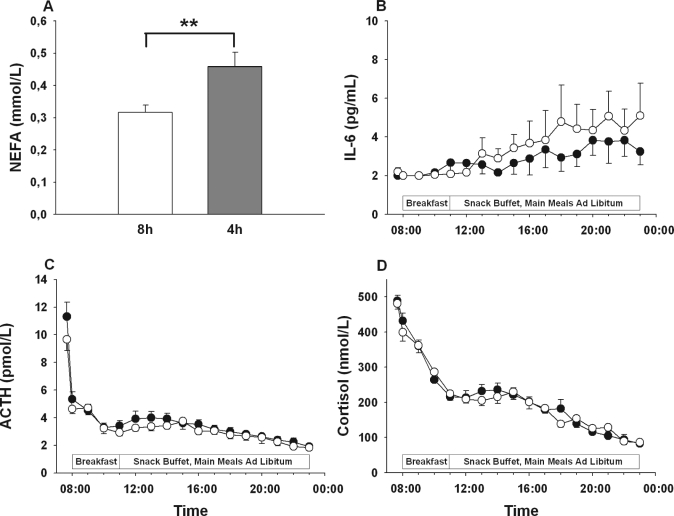
Fasting concentrations of NEFA (Figure 2A) were distinctly elevated in the morning after 2 nights of sleep restriction (0.46 ± 0.04 vs. 0.32 ± 0.02 mmol/L; P = 0.002). Concentrations of ACTH and cortisol showed the characteristic circadian decline across the day (Figure 2B, C) without any differences between conditions (P > 0.25 and P > 0.51 for the respective Condition × Time interactions, P > 0.10 for all single time point comparisons). Serum IL-6 levels slightly increased during the experimental day (Figure 2D) but likewise showed no difference between conditions (P = 0.40 for Condition × Time, P > 0.13 for all single time point comparisons).
Figure 2.
Mean (± SEM) concentrations of serum NEFA (A), ACTH (B), cortisol (C), and IL-6 (D) during an experimental day following 2 nights of 8 h of sleep each (white bars/circles) and 2 nights each containing 4 h of sleep (gray bar/black circles), respectively. N = 15; **P < 0.01
DISCUSSION
Our data indicate a distinct reduction in glucose tolerance in conjunction with elevated insulin and C-peptide concentrations in response to breakfast food intake after two nights of mild sleep restriction to 4 hours in comparison to 8 hours of regular sleep in healthy men. This pattern of effects points to reduced insulin sensitivity (i.e., increased insulin resistance, as an important mechanism that links sleep loss to disturbed glucose metabolism). Furthermore, serum NEFA concentrations were found be elevated in the morning after two nights of mild sleep restriction suggesting a contribution of altered lipid metabolism in post-sleep deprivation insulin resistance. Unchanged concentrations of HPA axis hormones and IL-6 after sleep loss moreover argue against a major contribution of these neuroendocrine and immunological pathways to the reduction in insulin sensitivity after sleep loss.
Our results are at some variance with previous findings of sleep loss-associated reductions in insulin secretion in response to an intravenous glucose challenge.8 Of note, in the same study8 blood glucose concentrations showed a stronger increase in response to a standardized meal test after five days of sleep restriction, whereas circulating insulin levels as compared to responses after five days of extended recovery sleep remained unchanged. Also, a recent study10 found a reduction of glucose tolerance after 14 days of bedtime restriction, but an unaltered insulin response to intravenous and oral glucose loads. However, Bosy-Westphal and coworkers could not detect any change in glucose tolerance during an oral glucose tolerance test after four consecutive nights of stepwise decreases in sleep duration from 8 h during the first night to 4 h during the fourth night,18 whereas in other studies total sleep deprivation for a period of 60 h resulted in elevated morning insulin concentrations and reduced glucose tolerance.19 It may thus be that alterations in insulin action and β-cell function after sleep loss depend on the duration and severity of sleep restriction, which clearly varied between our study and previous studies.8,10,18–20 However, the enhanced insulin response to breakfast intake found in our study after two days of moderate sleep restriction—that was mirrored by enhanced C-peptide concentrations—were so clear-cut that a reduction in insulin sensitivity rather than impaired β-cell function can be safely assumed to be the main cause of the impairment of glucose tolerance observed here. This view is further supported by a very recent study showing reduced insulin sensitivity as measured by hyperinsulinemic euglycemic clamp experiments after one night of sleep restriction.20
Interestingly, the effects of sleep restriction on glucose metabolism were most pronounced in the morning, while in the afternoon and evening there were no detectable differences in parameters of glucose metabolism between the two sleep conditions. Insulin sensitivity and glucose tolerance are well known to show a circadian rhythm21 that is driven by the suprachiasmatic nucleus.22,23 At a first glance, our finding of reduced insulin sensitivity in the morning but not later in the day suggests an interaction of the effects of sleep restriction on glucose homeostasis with circadian pacemakers. However, it needs to be emphasized that subjects were allowed to eat ad libitum after the breakfast period in our study, precluding solid conclusions on glucose metabolism during the second half of the day.
The mechanisms underlying the reduction of insulin sensitivity due to sleep restriction cannot be conclusively derived from our data. Circulating NEFA levels were increased in the morning after two nights of sleep restriction which could result from enhanced lipolysis or reduced NEFA clearance. Considering that increased NEFA levels distinctly reduce insulin sensitivity,24,25 our data point to an important contribution of altered lipid metabolism to the increase in insulin resistance due to sleep restriction.
Reduced physical activity is a well-known pathophysiological mechanism in the development of insulin resistance and type 2 diabetes mellitus.26 As reported elsewhere,11 physical activity during day 1, i.e. after the first night spent in the laboratory, was reduced in the sleep restriction condition as compared to regular sleep condition. Although statistical analyses did not reveal significant interactions between prior-day physical activity and the effects of sleep loss on glucose metabolism, future studies clearly should evaluate to which extent the detrimental influence of sleep restriction on glucose homeostasis may be co-mediated by effects on physical activity.
Previous studies8,27 exploring the effects of more severe sleep restriction reported elevations of evening serum cortisol levels, suggesting that increased HPA axis activity could be a potential mediator of disturbances in glucose metabolism. However, in the present study we were unable to detect any changes in circulating ACTH and cortisol levels after short-term sleep loss, which does not support the assumption that after mild sleep loss as more frequently experienced in everyday life, changes in HPA axis activity contribute to the impairment of insulin sensitivity. In order to synchronize the time point of awakening, thereby avoiding prolonged fasting in the wake state before examinations, subjects were allowed to fall asleep 4 hours later in the sleep restriction than control condition. This sleep restriction schedule did not induce a time shift in HPA-axis secretory activity, which may represent an important difference to foregoing studies on this subject.17,28 However, it cannot be excluded that time shifts in endocrine rhythms apart from HPA-axis activity may have contributed to the reduction in glucose tolerance after sleep restriction. Likewise, unchanged serum IL-6 levels indicate that the effects of sleep restriction on glucose metabolism are not likely to result from the induction of a pro-inflammatory state.13 Several8,10,29 but not all28,30 previous studies have also suggested that sleep loss-associated reductions in insulin sensitivity and glucose tolerance31 may be due to increased activity of the sympathetic nervous system (SNS) that was not assessed in our study.
Most interestingly, a sophisticated study has recently shown that the selective experimental suppression of slow wave sleep (SWS) during three consecutive nights is sufficient to induce a distinct reduction of insulin sensitivity in healthy young men.32 This finding suggests that SWS is the most critical sleep stage for the maintenance of normal glucose homeostasis. Nevertheless, in accordance with previous studies using quantitative sleep shortage,8,33 we found an impairment of glucose metabolism by sleep restriction to 4 hours with a total amount of SWS that was almost identical to that obtained in the control condition. This pattern of results provides evidence that in addition to SWS, an appropriate amount of lighter sleep stages, i.e., S1 and S2, and probably also of REM sleep—all of which were curtailed in our setting—is required to maintain proper insulin sensitivity. This view is further supported by recent findings showing that sympathetic nervous system activity as a possible mechanism modulating insulin sensitivity is down-regulated even by lighter sleep stages and in particular REM sleep.34
A remarkable finding of the present study is the distinct reduction in plasma glucagon concentrations after sleep restriction. Accordingly, a suppressing influence of sleep loss on circulating glucagon levels has also been shown under basal conditions and during insulin-induced hypoglycemia.17,28 At a first glance, reduced glucagon levels may speak against a pathophysiological role of sleep loss in the development of type 2 diabetes since elevated, but not reduced, glucagon levels represent a hallmark of the disease. However, it should be pointed out that the reduction in glucagon levels was observed after two days of moderate sleep restriction and that more chronic forms of sleep loss may yield different effects, since a very recent study in rats showed elevated glucagon concentrations after more severe sleep restriction and sleep deprivation.35 This limitation also applies to our findings and most previous experimental findings17,27,28,36 regarding the impact of sleep loss on glucose homeostasis per se. Still, the consistently found reduction of glucagon levels due to sleep deprivation in humans clearly calls for further investigation on the relationship between sleep and pancreatic α-cell function.
In conclusion, our results provide further experimental evidence for an impairing influence of sleep loss on glucose metabolism and hint at reduced insulin sensitivity as an important underlying mechanism. Although present findings cannot be directly extrapolated to chronic conditions, they point to a crucial role of sleep loss in the development of diabetes that is likewise suggested by a growing number of epidemiological reports.4–7
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Schultes has participated in speaking engagement for Johnson & Johnson, Novo Nordisk, and Abbott. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We are grateful to Mareike Kuck, Elisa Gustke, Claudia Frenzel, Jutta Schwanbohm, and Kathleen Kurwahn for their expert and invaluable laboratory assistance.
Experiments were performed at the University Clinic Schleswig-Holstein & Campus Lubeck, Germany. None of the authors reported financial or other disclosures. Experiments were supported by the Deutsche Forschungsgemeinschaft (SFB 654/B1). Funding of the Deutsche Forschungsgemeinschaft had no influence on design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
REFERENCES
- 1.Lipscombe LL, Hux JE. Trends in diabetes prevalence, incidence, and mortality in Ontario, Canada 1995-2005: a population-based study. Lancet. 2007;369:750–6. doi: 10.1016/S0140-6736(07)60361-4. [DOI] [PubMed] [Google Scholar]
- 2.Burke JP, Williams K, Gaskill SP, Hazuda HP, Haffner SM, Stern MP. Rapid rise in the incidence of type 2 diabetes from 1987 to 1996: results from the San Antonio Heart Study. Arch Intern Med. 1999;159:1450–6. doi: 10.1001/archinte.159.13.1450. [DOI] [PubMed] [Google Scholar]
- 3.Knutson KL, Spiegel K, Penev P, van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–78. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep. 2007;30:1667–73. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mallon L, Broman JE, Hetta J. High incidence of diabetes in men with sleep complaints or short sleep duration: a 12-year follow-up study of a middle-aged population. Diabetes Care. 2005;28:2762–7. doi: 10.2337/diacare.28.11.2762. [DOI] [PubMed] [Google Scholar]
- 6.Ayas NT, White DP, Al Delaimy WK, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–4. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 7.Nilsson PM, Roost M, Engstrom G, Hedblad B, Berglund G. Incidence of diabetes in middle-aged men is related to sleep disturbances. Diabetes Care. 2004;27:2464–9. doi: 10.2337/diacare.27.10.2464. [DOI] [PubMed] [Google Scholar]
- 8.Spiegel K, Leproult R, van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Ortiz M, Martinez-Abundis E, Balcazar-Munoz BR, Pascoe-Gonzalez S. Effect of sleep deprivation on insulin sensitivity and cortisol concentration in healthy subjects. Diabetes Nutr Metab. 2000;13:80–3. [PubMed] [Google Scholar]
- 10.Nedeltcheva AV, Kessler L, Imperial J, Penev PD. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab. 2009;94:3242–50. doi: 10.1210/jc.2009-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmid SM, Hallschmid M, Jauch-Chara K, et al. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am J Clin Nutr. 2009;90:1476–82. doi: 10.3945/ajcn.2009.27984. [DOI] [PubMed] [Google Scholar]
- 12.Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP. Clinical review: The pathogenetic role of cortisol in the metabolic syndrome: a hypothesis. J Clin Endocrinol Metab. 2009;94:2692–701. doi: 10.1210/jc.2009-0370. [DOI] [PubMed] [Google Scholar]
- 13.de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett. 2008;582:97–105. doi: 10.1016/j.febslet.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep of human subjects. Washington DC: US Government Printing Office; 1968. p. 204. NIH publication. [Google Scholar]
- 15.Bremer JP, Baron M, Peters H, et al. Hormonal, subjective, and neurocognitive responses to brief hypoglycemia in postmenopausal women and age-matched men with type 2 diabetes mellitus. Metabolism. 2006;55:331–8. doi: 10.1016/j.metabol.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu S, Tani Y, Yamada H, Tabata M, Murachi T. Enzymatic determination of serum-free fatty acids: a colorimetric method. Anal Biochem. 1980;107:193–8. doi: 10.1016/0003-2697(80)90511-4. [DOI] [PubMed] [Google Scholar]
- 17.Schmid SM, Jauch-Chara K, Hallschmid M, Schultes B. Mild sleep restriction acutely reduces plasma glucagon levels in healthy men. J Clin Endocrinol Metab. 2009;94:5169–73. doi: 10.1210/jc.2009-0969. [DOI] [PubMed] [Google Scholar]
- 18.Bosy-Westphal A, Hinrichs S, Jauch-Chara K, et al. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women. Obes Facts. 2008;1:266–73. doi: 10.1159/000158874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.VanHelder T, Symons JD, Radomski MW. Effects of sleep deprivation and exercise on glucose tolerance. Aviat Space Environ Med. 1993;64:487–92. [PubMed] [Google Scholar]
- 20.Donga E, van DM, van Dijk JG, et al. A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects. J Clin Endocrinol Metab. 2010;95:2963–8. doi: 10.1210/jc.2009-2430. [DOI] [PubMed] [Google Scholar]
- 21.van Cauter E, Polonsky KS, Scheen AJ. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr Rev. 1997;18:716–38. doi: 10.1210/edrv.18.5.0317. [DOI] [PubMed] [Google Scholar]
- 22.la Fleur SE, Kalsbeek A, Wortel J, Fekkes ML, Buijs RM. A daily rhythm in glucose tolerance: a role for the suprachiasmatic nucleus. Diabetes. 2001;50:1237–43. doi: 10.2337/diabetes.50.6.1237. [DOI] [PubMed] [Google Scholar]
- 23.la Fleur SE. Daily rhythms in glucose metabolism: suprachiasmatic nucleus output to peripheral tissue. J Neuroendocrinol. 2003;15:315–22. doi: 10.1046/j.1365-2826.2003.01019.x. [DOI] [PubMed] [Google Scholar]
- 24.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–6. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 25.Delarue J, Magnan C. Free fatty acids and insulin resistance. Curr Opin Clin Nutr Metab Care. 2007;10:142–8. doi: 10.1097/MCO.0b013e328042ba90. [DOI] [PubMed] [Google Scholar]
- 26.Dela F, Mikines KJ, Von LM, Secher NH, Galbo H. Effect of training on insulin-mediated glucose uptake in human muscle. Am J Physiol. 1992;263:E1134–E1143. doi: 10.1152/ajpendo.2006.263.6.E1134. [DOI] [PubMed] [Google Scholar]
- 27.Spiegel K, Leproult R, L'Hermite-Baleriaux M, Copinschi G, Penev PD, van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89:5762–71. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 28.Schmid SM, Hallschmid M, Jauch-Chara K, Bandorf N, Born J, Schultes B. Sleep loss alters basal metabolic hormone secretion and modulates the dynamic counterregulatory response to hypoglycemia. J Clin Endocrinol Metab. 2007;92:3044–51. doi: 10.1210/jc.2006-2788. [DOI] [PubMed] [Google Scholar]
- 29.Irwin M, Thompson J, Miller C, Gillin JC, Ziegler M. Effects of sleep and sleep deprivation on catecholamine and interleukin-2 levels in humans: clinical implications. J Clin Endocrinol Metab. 1999;84:1979–85. doi: 10.1210/jcem.84.6.5788. [DOI] [PubMed] [Google Scholar]
- 30.Kato M, Phillips BG, Sigurdsson G, Narkiewicz K, Pesek CA, Somers VK. Effects of sleep deprivation on neural circulatory control. Hypertension. 2000;35:1173–5. doi: 10.1161/01.hyp.35.5.1173. [DOI] [PubMed] [Google Scholar]
- 31.Reaven GM, Lithell H, Landsberg L. Hypertension and associated metabolic abnormalities--the role of insulin resistance and the sympathoadrenal system. N Engl J Med. 1996;334:374–81. doi: 10.1056/NEJM199602083340607. [DOI] [PubMed] [Google Scholar]
- 32.Tasali E, Leproult R, Ehrmann DA, van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105:1044–9. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spiegel K, Knutson K, Leproult R, Tasali E, Van CE. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99:2008–19. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 34.Rasch B, Dodt C, Molle M, Born J. Sleep-stage-specific regulation of plasma catecholamine concentration. Psychoneuroendocrinology. 2007;32:884–91. doi: 10.1016/j.psyneuen.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 35.Martins PJ, Marques MS, Tufik S, D'Almeida V. Orexin activation precedes increased NPY expression, hyperphagia, and metabolic changes in response to sleep deprivation. Am J Physiol Endocrinol Metab. 2010;298:E726–E734. doi: 10.1152/ajpendo.00660.2009. [DOI] [PubMed] [Google Scholar]
- 36.Spiegel K, Tasali E, Penev P, van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]