Abstract
Background
Cassava starch, the economically important agricultural commodity in Thailand, can readily be cast into films. However, the cassava starch film is brittle and weak, leading to inadequate mechanical properties. The properties of starch film can be improved by adding plasticizers and blending with the other biopolymers.
Results
Cassava starch (5%w/v) based films plasticized with glycerol (30 g/100 g starch) were characterized with respect to the effect of carboxymethyl cellulose (CMC) concentrations (0, 10, 20, 30 and 40%w/w total solid) and relative humidity (34 and 54%RH) on the mechanical properties of the films. Additionally, intermolecular interactions were determined by Fourier transform infrared spectroscopy (FT-IR), melting temperature by differential scanning calorimetry (DSC), and morphology by scanning electron microscopy (SEM). Water solubility of the films was also determined. Increasing concentration of CMC increased tensile strength, reduced elongation at break, and decreased water solubility of the blended films. FT-IR spectra indicated intermolecular interactions between cassava starch and CMC in blended films by shifting of carboxyl (C = O) and OH groups. DSC thermograms and SEM micrographs confirmed homogeneity of cassava starch-CMC films.
Conclusion
The addition of CMC to the cassava starch films increased tensile strength and reduced elongation at break of the blended films. This was ascribed to the good interaction between cassava starch and CMC. Cassava starch-CMC composite films have the potential to replace conventional packaging, and the films developed in this work are suggested to be suitable for low moisture food and pharmaceutical products.
Background
The development of biopolymer-based edible films could replace the use of some petrochemicals in the food packaging industry and reduce the negative environmental impact associated with packages from non-renewable and non-recyclable resources. Edible films are generally produced from renewable natural and abundant biodegradable polymeric materials such as polysaccharides, proteins, lipids, or the combination of these components. Some edible films and coatings have been widely used for fresh fruits, vegetables, confectioneries, frozen foods, and meat products [1]. However, many edible films have limitations in mechanical or barrier properties or are prohibitively expensive. Starch films often have good barrier properties to oxygen, carbon dioxide, and lipids and can protect products from lipid oxidation [2]. Films from polysaccharides are also stronger and more extensible than proteinaceous films [3].
Cassava (Manihot esculenta crantz) or tapioca is one of the economically important crops in Thailand and is the cheapest raw material for starch production. Structurally, cassava starch consists of 17% amylose content, and this is responsible for its strong film-forming characteristics [4]. Cassava starch can readily be cast into films. However, the cassava starch film is brittle and weak, leading to inadequate mechanical properties. Overcoming the brittleness of the film can be accomplished by adding plasticizers [5]. Common plasticizers used in the production of starch films are water, glycerol, sorbitol, and other low-molecular weight polyhydroxy compounds [6]. Glycerol and sorbitol are widely used as plasticizers because of their stability and edibility. The addition of plasticizers makes the brittle films more flexible, but also less strong [5] and results in higher moisture permeability [7]. This problem must be addressed to improve the functional properties of cassava starch films. Blending [8] or laminating [9] with other polysaccharide materials could improve cassava starch film mechanical functionality. Carboxymethyl cellulose (CMC), xanthan, guar, and arabic gum, which are water-soluble heteropolysaccharides with high molecular weights, are often used together with starches to provide desirable texture, control moisture and water mobility, and improve overall product quality and/or stability [10]. CMC is an anionic linear polysaccharide derived from cellulose. It is an important industrial polymer with a wide range of applications in flocculation, drug reduction, detergents, textiles, papers, foods, and drugs [11]. CMC is used primarily because it has high viscosity, is non-toxic, and is non-allergenic. The numerous hydroxyl and carboxylic groups in CMC enable water binding and moisture sorption properties. CMC hydrogel has a high water content, good biodegradability, and a wide range of applications due to its low cost [12]. Because of its polymeric structure and high molecular weight, it can be used as a filler in biocomposite films [13]. CMC is able to improve the mechanical and barrier properties of pea starch-based films [14].
Some edible films based on mixtures of multiple polysaccharides such as starch-methylcellulose [15], pullulan-starch [16], chitosan-starch and chitosan-pullulan [17], CMC-rice starch [10], and CMC-pea starch [14] have been investigated. These publications demonstrated that homogeneous structures containing a single-phase of polymeric complexes have been obtained. Depending on the interactions between components, these formulas can improve the mechanical and moisture barrier properties of the edible films in some cases. In a previous study [18], water vapor permeability and moisture sorption isotherms of cassava starch based films blended with CMC were studied. The addition of CMC to cassava starch films had no effect on the WVPs of all films tested (WVP ranged from 0.05 to 0.10 and 0.2 to 0.3 g mm/day m2 mmHg at 33% and 54% RH, respectively), but moisture sorption of cassava starch based films increased with increasing CMC contents. It was suggested that the cassava starch based films with and without CMC had potential for use as biodegradable and edible packaging due to the moderate WVP. However, the mechanical properties of biodegradable films are also important for their applications. There is no data available about the physical properties of cassava starch films blended with CMC, thus the objective of this study was to determine the effect of CMC concentration on mechanical and physical properties of cassava starch based films.
Results and discussion
The influence of CMC concentrations on properties of cassava starch based films was analyzed in terms of FT-IR spectra, mechanical properties of the films, water solubility, DSC thermograms, and film morphology as reported below. All the cassava starch films with and without CMC appeared smooth, clear, and transparent. Film thickness ranged from 120-160 μm.
The FT-IR spectrum of a cassava starch film (without CMC) is shown in Figure 1a. A broad absorption band at 3268 is evident, due to the stretching frequency of the -OH group, and a band at 2920 cm-1 attributable to C-H stretching vibration [11]. The presence of a strong absorption band at 1648 cm-1 confirms the presence of water. The bands around 1413 and 1337 cm-1 are assigned to -CH2 bending in plane and C-OH bending vibration, respectively. The band at 1149 cm-1 is due to C-O-C antisymmetric bridge stretching [19].
Figure 1.
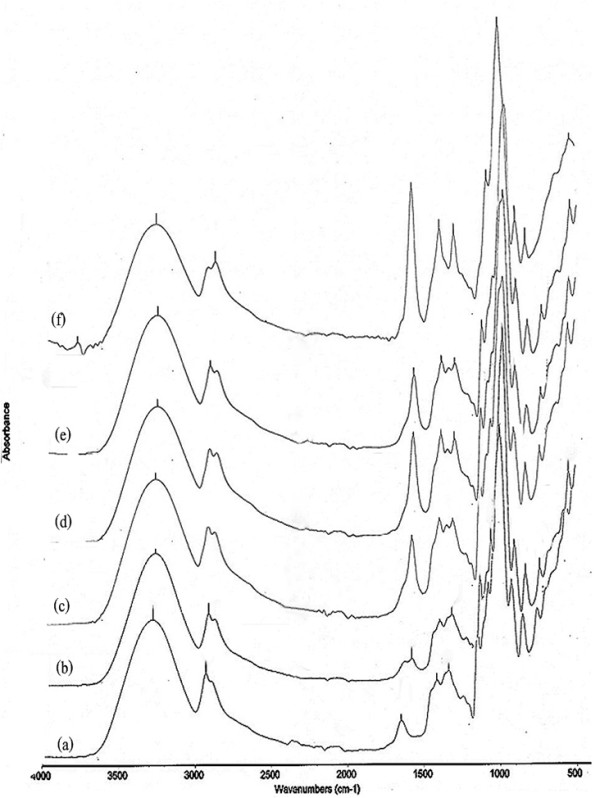
FT-IR spectra of cassava starch film with and without CMC. (a) cassava starch film (control); (b) cassava starch-10% CMC film; (c) cassava starch-20% CMC film; (d) cassava starch-30% CMC film; (e) cassava starch-40% CMC film; and (f) CMC film.
The FT-IR spectrum of a CMC film is shown in Figure 1f. The broad absorption band at 3260 cm-1 is due to the stretching frequency of the -COO group [20] which overlaps with the -OH stretching region at 3480-3440 cm-1. The band at 2876 cm-1 is due to the C-H stretching vibration. The bands around 1412 and 1319 cm-1 are assigned to -CH2 scissoring and -OH bending vibration, respectively. The band at 1060 cm-1 is due to CH-O-CH2 stretching [11]. Spectra of CMC films showed peaks around 1592 cm-1, attributable to antisymmetric vibration of COO- groups [21,22].
Adding a range of CMC concentrations to cassava starch films resulted in similar characteristics in the FT-IR spectra (Figures 1b, c, d and 1e). Blending CMC with cassava starch caused minimal shifting but increased absorption in the COO- band around 1592 cm-1 in cassava starch films containing 10, 20, 30 and 40% CMC, respectively. This indicated that the antisymmetric and symmetric vibrations of C = O and C-O bonds were enhanced, probably due to the disruption of intermolecular H-bonds between carboxylic groups caused by added starch [23]. The symmetric COO- stretching was found at ~1411 cm-1 in all film samples. The water absorption band of cassava starch at 1648 cm-1 disappeared upon addition of CMC. The broad band located around 3270 cm-1 appeared in all films and was caused by O-H stretching and intermolecular/intramolecular hydrogen bonds [23,24]. The O-H band of CMC and cassava starch occurred at 3267 and 3261, respectively. By blending cassava starch with CMC, the O-H band of films shifted to 3265-3272 cm-1. The band of C-OH bending of cassava starch film that appeared at 1337 cm-1 was shifted to 1322-1333 cm-1 with CMC addition. Xu et al. [25] reported that the ester bonds were mostly formed between the hydroxyl groups in amylopectin branches of starch and carboxylic acid groups of CMC, forming a stable cross-linked structure. This is likely what occurred in the composite cassava starch CMC films studied here. These results were correlated with FT-IR spectra of rice flour-CMC blended film [10], chitosan-cassava starch-gelatin films [26], and corn starch-CMC-nanoclay biocomposite films [13].
The effects of CMC concentration and % relative humidity (RH) on tensile strength and elongation at break of cassava starch based films are shown in Figure 2. It was obvious that the tensile strength and elongation at break were strongly influenced by the concentration of CMC in both 34 and 54% RH conditions.
Figure 2.
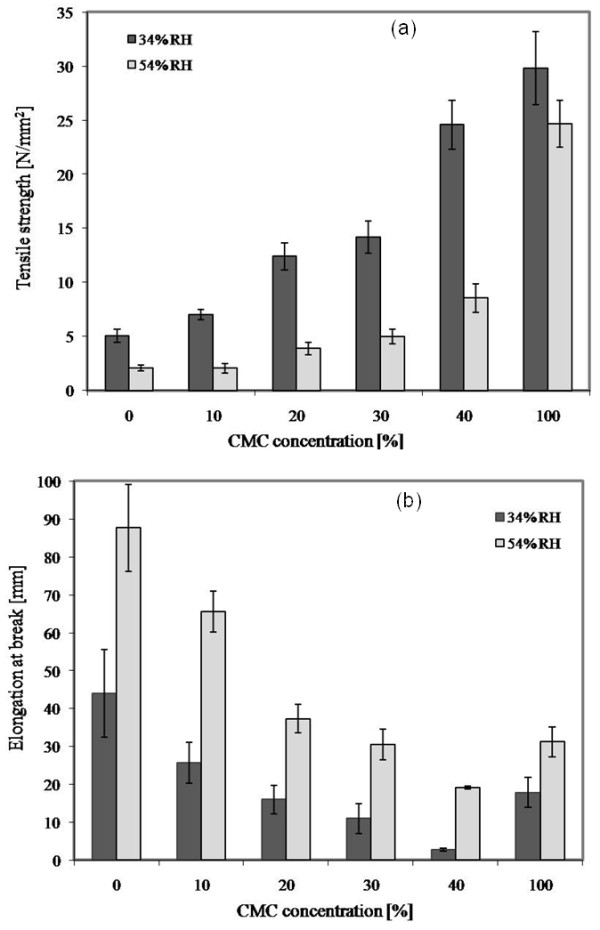
Effect of CMC concentrations on mechanical properties of the films. (a) tensile strength and (b) elongation at break of cassava based films at 34 and 54% RH.
The tensile strength and elongation at break of cassava starch based films were inversely related. At 34% RH, cassava starch based films containing CMC had significantly higher tensile strength but lower elongation at break than control films (cassava starch film without CMC). As the concentration of CMC increased, the tensile strengths of films significantly increased but the elongations at break significantly decreased. These results were consistent with corn starch films [27] and pea starch films [14] which had improved tensile strength as the concentration of added CMC increased. The increasing tensile strength of the cassava starch films with increasing concentrations of CMC is likely attributable to the formation of intermolecular interaction between the hydroxyl group of starch and carboxyl group of CMC [10]. During the processing and drying of the composite films, the original hydrogen bonds formed between starch molecules could be replaced by new hydrogen bonds formed between the hydroxyl groups in starch molecules and the hydroxyl and carboxyl groups in CMC [13]. Intermolecular interaction between starch and CMC resulted in more compact molecule structure of starch-CMC mixture, and tensile strength was therefore increased [10]. This finding corroborates the FT-IR results reported above. These results are similar to reports of the tensile strength of chitosan-starch films [25], rice starch-CMC film [10], and corn starch-CMC-nanoclay films [13]. At 54% RH, the tensile strength of all films decreased but % elongation increased when compared with the films at 34% RH. Gennadios et al. [28] reported that films with higher elongation values usually require a lower load to cause film breakage. These results could be related to structural modification of the starch network by water which causes a greater flexibility in polymer structure. At 54% RH, increasing CMC concentration in the cassava starch films improved the tensile strength but reduced the elongation at break. This result agreed with effect of humidity on tensile strength and % elongation of blended chitosan-methylcellulose films [29].
Solubility in water is an important property of starch-based films. Potential applications may require water insolubility to enhance product integrity, moisture barrier properties, and shelf-life. However, in other cases the water solubility of films before product consumption might be useful, such as for the encapsulation of food ingredients or additives [30].
The water solubility of cassava starch film blends depended on the CMC concentration (Figure 3). The water solubility of the control cassava starch film was about 73%, and film solubility decreased with the increase of CMC concentration. This result was similar to the water solubility of rice starch-CMC films [10], corn starch-CMC films [27] and corn starch-CMC-nanoclay films [13]. The decreased solubility indicated that intermolecular interaction occurred between starch and CMC in the cassava starch-CMC films [10]. The hydroxyl group and carboxyl group of CMC can form strong hydrogen bonds [27,13] and ester bonds [10], respectively, with the hydroxyl groups on starch, thus improving the interactions between molecules, improving the cohesiveness of the biopolymer matrix, and decreasing the water solubility [13]. The intermolecular interaction level depended on the amount of CMC and starch [10]. Besides the intermolecular interaction that occurred between starch and CMC, the decrease in water solubility as a function of CMC concentration could be due to the reduction of the starch concentration itself (as shown in Table 1) in the blended films.
Figure 3.
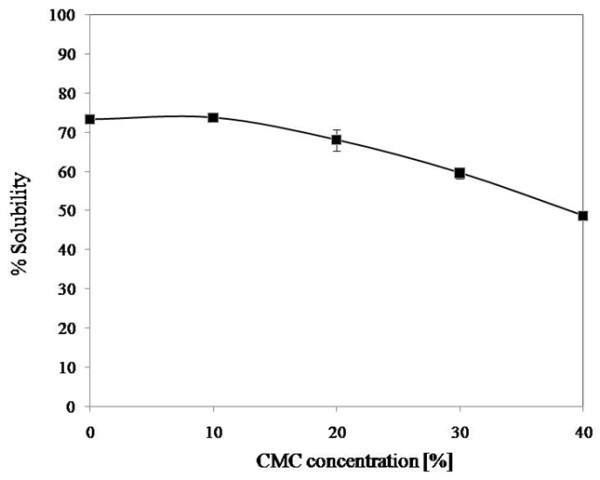
Effect of CMC concentration on cassava starch-CMC blended film solubility at 25°C.
Table 1.
Composition of CMC/cassava starch in 100 ml film solution
| Films | Composition (g/100 ml water) | ||
|---|---|---|---|
| starch | CMC | glycerol | |
| Cassava starch | 5 | 0 | 1.5 |
| 10%CMC | 4.5 | 0.5 | 1.5 |
| 20%CMC | 4 | 1 | 1.5 |
| 30%CMC | 3.5 | 1.5 | 1.5 |
| 40%CMC | 3 | 2 | 1.5 |
| CMC | 0 | 5 | 1.5 |
The melting temperature (Tm) and total heat of fusion (ΔHf) of cassava starch films with and without CMC are presented in Table 2. DSC thermograms of cassava starch film, the blended films, and CMC film are shown in Figure 4. Thermograms of the films blended with CMC (Figure 4b) exhibited a single sharp endothermic peak, which indicated homogeneity of the films. This endothermic peak has been associated with the melting of crystalline starch domains reorganized during retrogradation [13]. This result agreed with DSC thermograms of pea starch-CMC films [14] and corn starch-CMC-nanoclay films [13].
Table 2.
Melting temperature and heat of fusion of cassava starch film without and with CMC
| Film samples | Tm starch-CMC film (°C) | Heat of fusion (ΔHf) of starch-CMC film (J/g) |
|---|---|---|
| Cassava starch: CMC (100:0) | 165.46a | 135.34a |
| Cassava starch: CMC (90:10) | 147.20a, b | 91.98b |
| Cassava starch: CMC (80:20) | 144.53a, b | 92.07b |
| Cassava starch: CMC (70:30) | 128.26b | 160.10a, c |
| Cassava starch: CMC (60:40) | 96.62c | 173.26c |
| Cassava starch: CMC (0:100) | 102.46c | 212.98d |
Different letters in the same column indicate significant differences between the means obtained in Duncan's test (p < 0.05).
Figure 4.
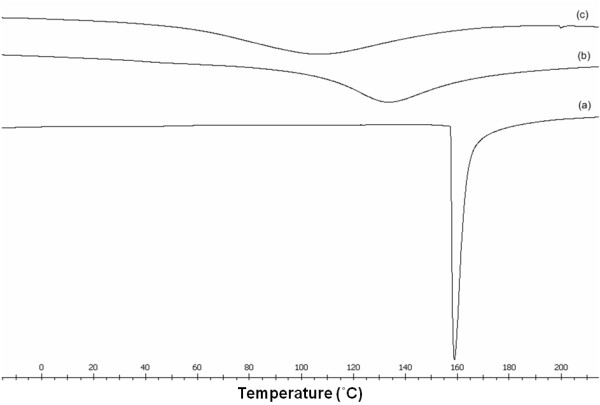
DSC thermograms of cassava starch film, blended film and CMC film. (a) cassava starch film (control); (b) cassava starch-30% CMC film; and (c) CMC film.
The melting temperature (Tm) of cassava starch films blended with CMC were lower than the Tm of cassava starch films but higher than the Tm of CMC films (except for the film with 40% CMC). The Tm of film blends shifted due to the interaction of the two biopolymers [31]. The area under the endothermic peak expressed the total heat of fusion of the films [32,33], which increased with increasing CMC concentrations in cassava starch films. The increased heat of fusion should be the effect of higher crystallization because of the high degree of crystallinity of CMC [34]. The total heat of fusion of blended films was lower than for CMC alone, likely because the interaction between cassava starch and CMC molecules interrupted the rearrangement of polymer chains [33]. Gimeno et al. [34] found that a higher interaction of hydrocolloids and starch retained more water molecules, causing a higher mobility during heating, increasing the kinetic energy, and decreasing the enthalpy value (ΔH). These results were similar to DSC thermograms of HDPE/PP blends [32] and chitosan-MC films with vanillin [33].
Scanning electron micrographs of cassava starch films with and without CMC were observed (Figure 5). All samples presented smooth and compact surface structures. Micrographs of cryogenic fracture surfaces of cassava starch film, cassava starch film with 30% CMC, and CMC film obtained by SEM are shown in Figures 5a, b and 5c, respectively. They showed a dense and smooth cross-section that indicated homogeneous structure. This result was similar to the surface and cross-section morphologies of cassava starch-gum films [35] and pullulan/alginate/CMC film [23].
Figure 5.
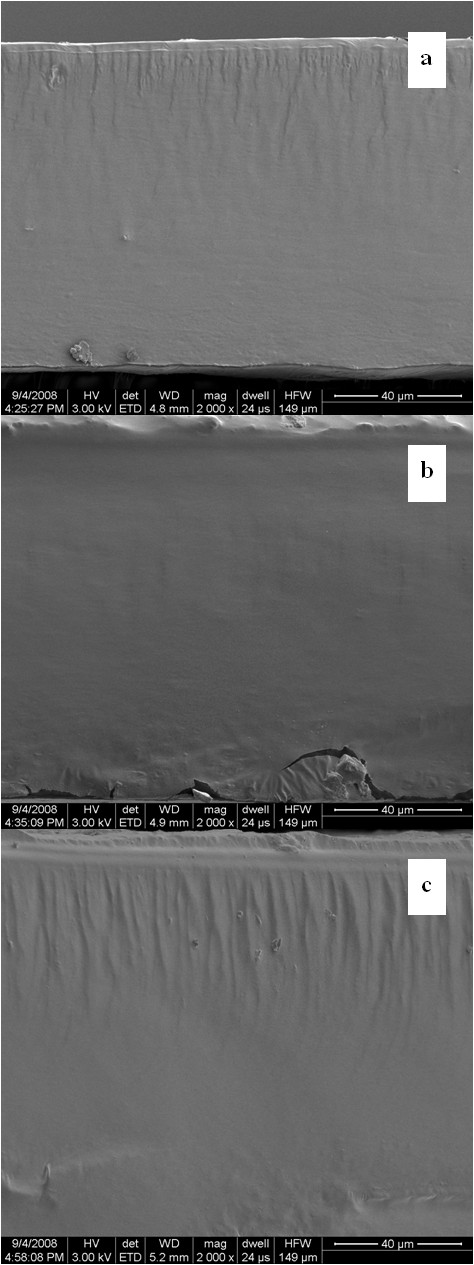
SEM observations of cross-sections of cassava starch film, blended film and CMC film. (a) cassava starch film (control); (b) cassava starch-30% CMC film; and (c) CMC film at 54% RH.
Conclusions
The effects of CMC concentrations and relative humidity on physical properties of cassava starch based films were studied. The addition of CMC to the cassava starch films increased tensile strength and reduced elongation at break of the blended films. This was ascribed to the good interaction between cassava starch and CMC. FT-IR spectra indicated intermolecular interactions between cassava starch-CMC film blends by shifting of carboxyl (C = O) and OH groups. The thermogram of film blends also confirmed chemical interaction of cassava starch-CMC as ΔH decreases. The homogeneity of cassava starch-CMC films was represented as a single melting peak, and SEM micrographs visibly confirmed the homogeneous structure of cassava starch-CMC films. All films stored at 54% RH gave higher elongation but lower tensile strength than films at 34% RH. Increasing CMC concentrations in the cassava starch film decreased water solubility of the blended films. Films containing 30% CMC had better physical properties (high tensile strength, moderate elongation and low water solubility) than the other films. Thus, it seems that the CMC-starch biocomposite films show better physical properties than cassava starch films alone, and cassava starch-CMC composite films have the potential to be used as edible and biodegradable films for low and intermediate moisture products.
Experimental
Materials
Cassava starch (Bangkok Inter Food Co., LTD., Thailand) and glycerol (EM Science, Germany) were employed to produce the films. CMC was donated from Akzo Nobel (Netherlands).
Cassava starch-CMC film preparation
Solutions used to prepare films (5% w solid/v) were prepared by dispersing cassava starch and CMC at different concentrations in distilled water (Table 1). Glycerol (30% w/w solid) was added as the plasticizer. The film solutions were heated to 80°C with constant stirring to obtain starch gelatinization. Then the film-forming solutions (200 ml) were cast on flat 30 × 30 cm Teflon plates. The films were dried at room temperature (25°C) for 24 hours [18].
Mechanical characterization
Films were cut into 25 × 100 mm strips and then equilibrated in desiccators over saturated salt solutions having the desired relative humidity 34% (MgCl2) and 54% RH (Mg(NO3)2) condition at 25°C for 48 hours before testing. The tensile strength and elongation at break of the films were measured using a universal testing machine (Hounsfield, England) according to the ASTM D 882-91 method [36]. The initial gauge length was 50 mm and crosshead speed was 50 mm/min. Ten specimens were tested for each sample.
Water solubility of cassava starch-CMC films
Film solubility in water was measured as percentage of dry matter of the film solubilized in water during a 24 hours period. This method was adapted from method of Phan et al. [37]. The initial dry matter of each film was obtained after drying film specimens at 65°C for 24 hours followed by placement in 0% RH silica gel desiccators for 2 days. Dried films (about 0.3 g) were weighed (initial dry weight) and immersed in beakers containing 50 ml distilled water at 23°C that were then sealed and periodically agitated for 24 hours. The solutions containing film residues were filtered with Whatman filter paper No.1 (previously dried at 105°C for 24 hours and weighed before using), the filters were dried at 80°C for 24 hours, and the dried filters with film residues were weighed to determine the weight of dry matter (final dry weight). Tests were performed in triplicate, and the solubility was calculated using Equation (1):
| (1) |
Fourier transform infrared spectroscopy (FT-IR)
Transmission infrared spectra of the films were recorded at room temperature using a Nicolet 6700 FT-IR spectrometer (Thermo Electron Corporation, USA) in the range of 4000-400 cm-1 with 64 scans, 4 cm-1 resolution, using a DTGS KBr detector and KBr beam splitter. The films were mounted directly in the sample holder.
Differential scanning calorimetry (DSC)
Differential scanning calorimetry was carried out using a Mettler Toledo Schwerzenbach instrument (USA). Samples were previously conditioned at 54% RH and 25°C. Three replicates of film samples (~10 milligrams) contained in aluminum pans were heated in the temperature range -20 to 220°C at a heating rate of 5°C/min in a nitrogen atmosphere (50 ml/min).
Film morphology
Film samples were conditioned at 25°C in 54%RH desiccators for 7 days prior to analysis. The surface and cross-section microstructure of films were observed by Field-Emission Scanning Electron Microscope (FESEM, JOEL JSM-6700F, Japan).
Statistical analysis
ANOVA analysis was performed on all results using a statistical program SPSS v. 10.0 at a confidence interval of 95% to determine the significant difference between group samples.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
WT carried out the study design, film preparation, testing, performed the statistical analysis and drafted the manuscript. LJM has been involved in revising for important intellectual content and structure of manuscript. SW and PS have given final approval of the version to be published. PR has made substantial contributions to conception and design, analysis and interpretation of data, revises for important intellectual content and coordination and has given final approval of the version to be published. All authors read and approved the final manuscript.
Contributor Information
Wirongrong Tongdeesoontorn, Email: wtongdee@hotmail.com.
Lisa J Mauer, Email: mauer@purdue.edu.
Sasitorn Wongruong, Email: sasitorn@chiangmai.ac.th.
Pensiri Sriburi, Email: pensiri@science.cmu.ac.th.
Pornchai Rachtanapun, Email: p.rachta@chiangmai.ac.th.
Acknowledgements
The authors gratefully acknowledge the National Research University Project under Thailand's Office of the Higher Education Commission (CHE), Thailand for financial support. The authors also thank Akzo Nobel (Netherlands) for CMC donation.
References
- Ou S, Wang Y, Tang S, Huang C, Jackson MG. Role of ferulic acid in preparing edible films from soy protein isolate. J Food Eng. 2005;70:205–210. doi: 10.1016/j.jfoodeng.2004.09.025. [DOI] [Google Scholar]
- Banker GS. Film coating theory and practice. J Pharm Sci. 1966;55:81–89. doi: 10.1002/jps.2600550118. [DOI] [PubMed] [Google Scholar]
- Krochta JM, De Mulder CLC. In: Agricultural Materials as Renewable Resources. Fuller G, McKeon TA, Bills DD, editor. Washington, DC.: American Chemical Society; 1995. Biodegradable polymers from agricultural products; pp. 120–140. [Google Scholar]
- Bangyekan C, Aht-Ong D, Srikulkit K. Preparation and properties evaluation of chitosan-coated cassava starch films. Carbohydr Polym. 2006;63:61–71. doi: 10.1016/j.carbpol.2005.07.032. [DOI] [Google Scholar]
- Suppakul P, Chalernsook B, Ratisuthawat B, Prapasitthi S, Munchukangwan K. Plasticizer and Relative Humidity Effects on Mechanical Properties of Cassava Flour Films. The 15th IAPRI World Conference on Packaging; 3 - 5 October 2006 Tokyo, Japan. 2006.
- Rindlav-Westling A, Stading M, Hermansson AM, Gatenholm P. Structure, mechanical and barrier properties of amylose and amylopectin films. Carbohyd Polym. 1998;36:217–224. doi: 10.1016/S0144-8617(98)00025-3. [DOI] [Google Scholar]
- Kester JJ, Fennema O. Edible films and coatings: a review. Food Technol. 1986;40:47–59. [Google Scholar]
- Chandra R, Rustgi R. Biodegradable polymers. Prog Polym Sci. 1998;23:1273–1335. doi: 10.1016/S0079-6700(97)00039-7. [DOI] [Google Scholar]
- Coffin DR, Fishman ML. Viscoelastic properties of pectin/starch blends. J Agr Food Chem. 1993;41:1192–1197. doi: 10.1021/jf00032a005. [DOI] [Google Scholar]
- Li Y, Shoemaker CF, Ma J, Shen X, Zhong F. Paste viscosity of rice starches of different amylose content and carboxymethylcellulose formed by dry heating and the physical properties of their films. Food Chem. 2008;109:616–623. doi: 10.1016/j.foodchem.2008.01.023. [DOI] [Google Scholar]
- Biswal DR, Singh RP. Characterisation of carboxymethyl cellulose and polyacrylamide graft copolymer. Carbohyd Polym. 2004;57:379–387. doi: 10.1016/j.carbpol.2004.04.020. [DOI] [Google Scholar]
- Nie H, Liu M, Zhan F, Guo M. Factors on the preparation of carboxymethycellulose hydrogel and its degradation behaviour in soil. Carbohyd Polym. 2004;58:185–189. doi: 10.1016/j.carbpol.2004.06.035. [DOI] [Google Scholar]
- Almasi H, Ghanbarzadeh B, Entezami AA. Physicochemical properties of starch-CMC-nanoclay biodegradable films. Biol Macromol. 2010;46:1–5. doi: 10.1016/j.ijbiomac.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Ma X, Chang PR, Yu J. Properties of biodegradable thermoplastic pea starch/carboxymethyl cellulose and pea starch/microcrystalline cellulose composites. Carbohyd Polym. 2008;72:369–375. doi: 10.1016/j.carbpol.2007.09.002. [DOI] [Google Scholar]
- Arvanitoyannis I, Biliaderis CG. Physical properties of polyol-plasticized edible blends made of methyl cellulose and soluble starch. Carbohyd Polym. 1999;38:47–58. doi: 10.1016/S0144-8617(98)00087-3. [DOI] [Google Scholar]
- Biliaderis CG, Lazaridou A, Arvanitoyannis I. Glass transition and physical properties of polyol-plasticised pullulan-starch blends at low moisture. Carbohyd Polym. 1999;40:29–47. doi: 10.1016/S0144-8617(99)00026-0. [DOI] [Google Scholar]
- Lazaridou A, Biliaderis CG. Thermophysical properties of chitosan, chitosan-starch and chitosan-pullulan films near the glass transition. Carbohyd Polym. 2002;48:179–190. doi: 10.1016/S0144-8617(01)00261-2. [DOI] [Google Scholar]
- Tongdeesoontorn W, Mauer LJ, Wongruong S, Rachtanapun P. Water vapour permeability and sorption isotherm of cassava starch based films blended with gelatin and carboxymethyl cellulose. Asian J Food Agro-Ind. 2009;2:501–514. [Google Scholar]
- Vicentini NM, Dupuy N, Leitzelman M, Cereda MP, Sobral PJA. Prediction of cassava starch eible film properties by chemometric analysis of infrared spectra. Spectrosc Lett. 2005;38:749–767. doi: 10.1080/00387010500316080. [DOI] [Google Scholar]
- Su JF, Huang Z, Yuan XY, Wang XY, Li M. Structure and properties of carboxymethyl cellulose/soy protein isolate blend edible films crosslinked by Maillard reactions. Carbohyd Polym. 2010;79:145–153. doi: 10.1016/j.carbpol.2009.07.035. [DOI] [Google Scholar]
- Xiao C, Weng L, Zhang L. Improvement of physical properties of crosslinked alginate and carboxymethyl konjac glucomannan blend films. J App Polym Sci. 2002;84:2554–2560. doi: 10.1002/app.10582. [DOI] [Google Scholar]
- Yang F, Li G, He YG, Ren FX, Wang Gx. Synthesis, characterization, and applied properties of carboxymethyl cellulose and polyacrylamide graft copolymer. Carbohyd Polym. 2009;78:95–99. doi: 10.1016/j.carbpol.2009.04.004. [DOI] [Google Scholar]
- Tong Q, Xiao Q, Lim LT. Preparation and properties of pullulan-alginate-carboxymethylcellulose blend films. Food Res Int. 2008;41:1007–1014. doi: 10.1016/j.foodres.2008.08.005. [DOI] [Google Scholar]
- Jiang L, Li Y, Wang X, Zhang L, Wen J, Gong M. Preparation and properties of nano-hydroxyapatite/chitosan/carboxymethyl cellulose composite scaffold. Carbohyd Polym. 2008;74:680–684. doi: 10.1016/j.carbpol.2008.04.035. [DOI] [Google Scholar]
- Xu YX, Kim KM, Hanna MA, Nag D. Chitosan-starch composite film: preparation and characterization. Ind Crop Prod. 2005;21:185–192. doi: 10.1016/j.indcrop.2004.03.002. [DOI] [Google Scholar]
- Zhong QP, Xia WS. Physicochemical properties of edible and preservative films from chitosan/cassava starch/gelatin blend plasticized with glycerol. Food Technol Biotechnol. 2008;46:262–269. [Google Scholar]
- Ghanbarzadeh B, Almasi H, Entezami AA. Physical properties of edible modified starch/carboxymethyl cellulose films. Innov Food Sci Emerg. 2010;11:697–702. doi: 10.1016/j.ifset.2010.06.001. [DOI] [Google Scholar]
- Gennadios A, Weller CL, Testin RF. Property modification of edible wheat gluten films. Trans ASAE. 1993;36:465–470. [Google Scholar]
- Rachtanapun P, Wongchaiya P. Effect of relative humidity on mechanical properties on blended chitosan-methylcellulose film. Mae Fah Luang Symposium; 26-28 Nov Chiang Rai, Thailand. 2008. p. 50.
- Bertuzzi MA, Castro Vidaurre EF, Armada M, Gottifredi JC. Water vapor permeability of edible starch based films. J Food Eng. 2007;80:972–978. doi: 10.1016/j.jfoodeng.2006.07.016. [DOI] [Google Scholar]
- Veiga-Santos P, Oliveira LM, Cereda MP, Scamparini ARP. Sucrose and inverted sugar as plasticizer. Effect on cassava starch-gelatin film mechanical properties, hydrophilicity and water activity. Food Chem. 2007;103:255–262. doi: 10.1016/j.foodchem.2006.07.048. [DOI] [Google Scholar]
- Rachtanapun P, Selke SEM, Matuana LM. Effect of the high-density polyethylene melt index on the microcellular foaming of high-density polyethylene/polypropylene blends. J Appl Polym Sci. 2004;93:364–371. doi: 10.1002/app.20428. [DOI] [Google Scholar]
- Sangsuwan J, Rattanapanone N, Rachtanapun P. Effect of vanillin and plasticizer on properties of chitosan-methyl cellulose based film. J Appl Polym Sci. 2008;109:3540–3545. doi: 10.1002/app.28461. [DOI] [Google Scholar]
- Gimeno E, Moraru CI, Kokini JL. Effect of xanthan gum and CMC on the structure and texture of corn flour pellets expanded by microwave heating. Cereal Chem. 2004;81:100–107. doi: 10.1094/CCHEM.2004.81.1.100. [DOI] [Google Scholar]
- ASTM International. Annual book of ASTM. Philadelphia: American Society for Testing and Materials, D882-91; 1991. Standard test methods for tensile properties of thin plastic sheeting. [Google Scholar]
- Phan TD, Debeaufort F, Luu D, Voilley A. Functional properties of edible agar-based and starch-based films for food quality preservation. J Agr Food Chem. 2005;53:973–981. doi: 10.1021/jf040309s. [DOI] [PubMed] [Google Scholar]
- Veiga-Santos P, Suzuki CK, Cereda MP, Scamparini ARP. Microstructure and color of starch-gum films: Effect of gum deacetylation and additives. Part 2. Food Hydrocolloid. 2005;19:1064–1073. doi: 10.1016/j.foodhyd.2005.02.007. [DOI] [Google Scholar]


