Abstract
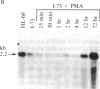
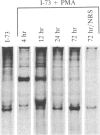
Chronic lymphocytic leukemia cells, representing a clonal population of resting B lymphocytes, were induced to differentiate into immunoglobulin-secreting lymphoblasts and plasmablasts by phorbol 12-myristate 13-acetate. The induction resulted in a rapid increase in the molar ratio of secreted/membrane-bound mu-chain mRNA. Immunoglobulin secretion was preceded by a transition of the cells from the G0 to G1 phase of the cell cycle, as indicated by an increase in RNA and protein synthesis, and an overall increase in cellular RNA. The cells, however, became blocked in G1 and did not enter S phase. The expression of MYC and FOS was rapidly induced by the phorbol 12-myristate 13-acetate treatment. The induction of FOS preceded the shift in secreted/membrane-bound mu-chain mRNA molar ratio, while that of MYC occurred concomitantly with the shift, but prior to induction of total RNA synthesis and immunoglobulin secretion. MYC expression remained at a relatively high level during the whole differentiation process. It is thus concluded that a decline of MYC expression is not a prerequisite for differentiation of the chronic lymphocytic leukemia cells. This suggests that MYC expression may play a different role during differentiation of nonproliferating B cells than in the myelomonocytic cell lines HL-60 and U-937, where MYC expression has been reported to decrease during induced differentiation. The results also show that the expression of the MYC and FOS genes does not result in the transition of these cells into the S phase of the cell cycle.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armelin H. A., Armelin M. C., Kelly K., Stewart T., Leder P., Cochran B. H., Stiles C. D. Functional role for c-myc in mitogenic response to platelet-derived growth factor. Nature. 1984 Aug 23;310(5979):655–660. doi: 10.1038/310655a0. [DOI] [PubMed] [Google Scholar]
- Benjamin D., Magrath I. T., Triche T. J., Schroff R. W., Jensen J. P., Korsmeyer S. J. Induction of plasmacytoid differentiation by phorbol ester in B-cell lymphoma cell lines bearing 8;14 translocations. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3547–3551. doi: 10.1073/pnas.81.11.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Collins S., Groudine M. Amplification of endogenous myc-related DNA sequences in a human myeloid leukaemia cell line. Nature. 1982 Aug 12;298(5875):679–681. doi: 10.1038/298679a0. [DOI] [PubMed] [Google Scholar]
- Cossman J., Neckers L. M., Braziel R. M., Trepel J. B., Korsmeyer S. J., Bakhshi A. In vitro enhancement of immunoglobulin gene expression in chronic lymphocytic leukemia. J Clin Invest. 1984 Feb;73(2):587–592. doi: 10.1172/JCI111247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla-Favera R., Gelmann E. P., Martinotti S., Franchini G., Papas T. S., Gallo R. C., Wong-Staal F. Cloning and characterization of different human sequences related to the onc gene (v-myc) of avian myelocytomatosis virus (MC29). Proc Natl Acad Sci U S A. 1982 Nov;79(21):6497–6501. doi: 10.1073/pnas.79.21.6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla-Favera R., Wong-Staal F., Gallo R. C. Onc gene amplification in promyelocytic leukaemia cell line HL-60 and primary leukaemic cells of the same patient. Nature. 1982 Sep 2;299(5878):61–63. doi: 10.1038/299061a0. [DOI] [PubMed] [Google Scholar]
- Danersund A., Tötterman T. H., Nilsson K., Egle-Jansson I., Kabelitz D., Sjöberg O. Phorbol ester-induced differentiation of chronic B lymphocytic leukaemia cells--regulatory impact of autologous and allogeneic accessory cells. Clin Exp Immunol. 1985 Mar;59(3):644–652. [PMC free article] [PubMed] [Google Scholar]
- Early P., Rogers J., Davis M., Calame K., Bond M., Wall R., Hood L. Two mRNAs can be produced from a single immunoglobulin mu gene by alternative RNA processing pathways. Cell. 1980 Jun;20(2):313–319. doi: 10.1016/0092-8674(80)90617-0. [DOI] [PubMed] [Google Scholar]
- Einat M., Resnitzky D., Kimchi A. Close link between reduction of c-myc expression by interferon and, G0/G1 arrest. Nature. 1985 Feb 14;313(6003):597–600. doi: 10.1038/313597a0. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L., Hyland J. K., Watt R., Rosenberg M., Baserga R. Microinjected c-myc as a competence factor. Science. 1985 Jun 14;228(4705):1313–1315. doi: 10.1126/science.4001943. [DOI] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Klein G., Klein E. Conditioned tumorigenicity of activated oncogenes. Cancer Res. 1986 Jul;46(7):3211–3224. [PubMed] [Google Scholar]
- Kruijer W., Cooper J. A., Hunter T., Verma I. M. Platelet-derived growth factor induces rapid but transient expression of the c-fos gene and protein. Nature. 1984 Dec 20;312(5996):711–716. doi: 10.1038/312711a0. [DOI] [PubMed] [Google Scholar]
- Lacy J., Sarkar S. N., Summers W. C. Induction of c-myc expression in human B lymphocytes by B-cell growth factor and anti-immunoglobulin. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1458–1462. doi: 10.1073/pnas.83.5.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minty A. J., Caravatti M., Robert B., Cohen A., Daubas P., Weydert A., Gros F., Buckingham M. E. Mouse actin messenger RNAs. Construction and characterization of a recombinant plasmid molecule containing a complementary DNA transcript of mouse alpha-actin mRNA. J Biol Chem. 1981 Jan 25;256(2):1008–1014. [PubMed] [Google Scholar]
- Mitchell R. L., Zokas L., Schreiber R. D., Verma I. M. Rapid induction of the expression of proto-oncogene fos during human monocytic differentiation. Cell. 1985 Jan;40(1):209–217. doi: 10.1016/0092-8674(85)90324-1. [DOI] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Müller R., Müller D., Guilbert L. Differential expression of c-fos in hematopoietic cells: correlation with differentiation of monomyelocytic cells in vitro. EMBO J. 1984 Aug;3(8):1887–1890. doi: 10.1002/j.1460-2075.1984.tb02063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson K., Larsson L. G., Carlsson M., Danersund A., Forsbeck K., Hellman L., Tötterman T., Pettersson U. Expression of c-myc and c-fos during phorbol ester induced differentiation of B-type chronic lymphocytic leukemia cells. Curr Top Microbiol Immunol. 1986;132:280–289. doi: 10.1007/978-3-642-71562-4_42. [DOI] [PubMed] [Google Scholar]
- Noma Y., Sideras P., Naito T., Bergstedt-Lindquist S., Azuma C., Severinson E., Tanabe T., Kinashi T., Matsuda F., Yaoita Y. Cloning of cDNA encoding the murine IgG1 induction factor by a novel strategy using SP6 promoter. Nature. 1986 Feb 20;319(6055):640–646. doi: 10.1038/319640a0. [DOI] [PubMed] [Google Scholar]
- Persson H., Gray H. E., Godeau F., Braunhut S., Bellvé A. R. Multiple growth-associated nuclear proteins immunoprecipitated by antisera raised against human c-myc peptide antigens. Mol Cell Biol. 1986 Mar;6(3):942–949. doi: 10.1128/mcb.6.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson H., Hennighausen L., Taub R., DeGrado W., Leder P. Antibodies to human c-myc oncogene product: evidence of an evolutionarily conserved protein induced during cell proliferation. Science. 1984 Aug 17;225(4663):687–693. doi: 10.1126/science.6431612. [DOI] [PubMed] [Google Scholar]
- Rapp U. R., Cleveland J. L., Brightman K., Scott A., Ihle J. N. Abrogation of IL-3 and IL-2 dependence by recombinant murine retroviruses expressing v-myc oncogenes. Nature. 1985 Oct 3;317(6036):434–438. doi: 10.1038/317434a0. [DOI] [PubMed] [Google Scholar]
- Ravetch J. V., Siebenlist U., Korsmeyer S., Waldmann T., Leder P. Structure of the human immunoglobulin mu locus: characterization of embryonic and rearranged J and D genes. Cell. 1981 Dec;27(3 Pt 2):583–591. doi: 10.1016/0092-8674(81)90400-1. [DOI] [PubMed] [Google Scholar]
- Reed J. C., Nowell P. C., Hoover R. G. Regulation of c-myc mRNA levels in normal human lymphocytes by modulators of cell proliferation. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4221–4224. doi: 10.1073/pnas.82.12.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitsma P. H., Rothberg P. G., Astrin S. M., Trial J., Bar-Shavit Z., Hall A., Teitelbaum S. L., Kahn A. J. Regulation of myc gene expression in HL-60 leukaemia cells by a vitamin D metabolite. Nature. 1983 Dec 1;306(5942):492–494. doi: 10.1038/306492a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sachs L. Constitutive uncoupling of pathways of gene expression that control growth and differentiation in myeloid leukemia: a model for the origin and progression of malignancy. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6152–6156. doi: 10.1073/pnas.77.10.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeland E., Godal T., Ruud E., Beiske K., Funderud S., Clark E. A., Pfeifer-Ohlsson S., Ohlsson R. The specific induction of myc protooncogene expression in normal human B cells is not a sufficient event for acquisition of competence to proliferate. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6255–6259. doi: 10.1073/pnas.82.18.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenlund A., Zabielski J., Ahola H., Moreno-Lopez J., Pettersson U. Messenger RNAs from the transforming region of bovine papilloma virus type I. J Mol Biol. 1985 Apr 20;182(4):541–554. doi: 10.1016/0022-2836(85)90240-2. [DOI] [PubMed] [Google Scholar]
- Tötterman T. H., Nilsson K., Sundström C. Phorbol ester-induced differentiation of chronic lymphocytic leukaemia cells. Nature. 1980 Nov 13;288(5787):176–178. doi: 10.1038/288176a0. [DOI] [PubMed] [Google Scholar]
- Westin E. H., Wong-Staal F., Gelmann E. P., Dalla-Favera R., Papas T. S., Lautenberger J. A., Eva A., Reddy E. P., Tronick S. R., Aaronson S. A. Expression of cellular homologues of retroviral onc genes in human hematopoietic cells. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2490–2494. doi: 10.1073/pnas.79.8.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Straaten F., Müller R., Curran T., Van Beveren C., Verma I. M. Complete nucleotide sequence of a human c-onc gene: deduced amino acid sequence of the human c-fos protein. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3183–3187. doi: 10.1073/pnas.80.11.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]