Abstract
Background
Chronic HCV is one of the major causes of morbidity and mortality in the present day world. The assessment of disease progression not only provides useful information for diagnosis and therapeutic supervision judgment but also for monitoring disease. Different invasive and non invasive methods are applied to diagnose the disease from initial to end stage (mild fibrosis to cirrhosis). Although, liver biopsy is still considered as gold standard to identify liver histological stages, an assessment of the disease development based on non-invasive clinical findings is also emerging and this may replace the need of biopsy in near future. This review gives brief insight on non-invasive methods currently available for predicting liver fibrosis in HCV with their current pros and cons to make easier for a clinician to choose better marker to assess liver fibrosis in HCV infected patients.
Methods
More than 200 studies regarding invasive and noninvasive markers available for HCV liver disease diagnosis were thoroughly reviewed. We examined year wise results of these markers based on their sensitivity, specificity, PPV, NPV and AUROCs.
Results
We found that in all non-invasive serum markers for HCV, FibroTest, Forn's Index, Fibrometer and HepaScore have high five-year predictive value but with low AUROCs (0.60~0.85) and are not comparable to liver biopsy (AUROC = 0.97). Even though from its beginning, Fibroscan is proved to be best with high AUROCs (> 0.90) in all studies, no single noninvasive marker is able to differentiate all fibrosis stages from end stage cirrhosis. Meanwhile, specific genetic markers may not only discriminate fibrotic and cirrhotic liver but also differentiate individual fibrosis stages.
Conclusions
There is a need of marker which accurately determines the stage based on simplest routine laboratory test. Genetic marker in combination of imaging technique may be the better non invasive diagnostic method in future.
1. Introduction
Chronic Hepatitis C (HCV) is one of the major causes of liver fibrosis, with distortion of the hepatic architecture, and ultimate progression to cirrhosis. Approximately more than 3% of the total world population is chronically infected with HCV and due to gradual increase in the prevalence of HCV; future burden of chronic HCV is predicted to raise at least 3 fold by the year 2020. Common causes of liver fibrosis are viral hepatitis and steato hepatitis with alcohol or obesity. Fibrosis caused by excessive deposition of extracellular matrix (ECM) by histological and molecular reshuffling of various components like collagens, glycoproteins, proteoglycans, matrix proteins and matrix bound growth factors. These changes can lead to metabolic and synthesis impairment to hepatocytes, epithelial cells and hepatic stellate cells (HSC). HSC activation the main step leading to fibrosis, involves several changes in liver like fibrogenesis, proliferation, contractility, chemotaxis, matrix degradation and cytokine release. Fibrosis can be defined as net result of the balance between ECM production and degradation. As ECM tissues not only involve matrix production but also matrix degradation leading to ECM remodeling, fibrosis is potentially a reversible process in early stages (advance stages in some cases) [1-6].
Fibrosis stages information not only indicate treatment response but also reflect/indicate cirrhosis development disaster. We can evaluate fibrosis in HCV infected patients invasively or non-invasively. Liver biopsy an invasive method is used for histological scoring and still used as reference test for fibrosis staging. With the increasing knowledge of molecular biology, genetics and availability of modern imaging techniques, many clinicians and related scientists developed several non-invasive methods to assess liver fibrosis and cirrhosis. These markers need to be more precise, reproducible and non-invasive to evaluate liver fibrosis in HCV infected patients. Therefore, an assessment of the disease development based on clinical findings is still critical for patients infected with HCV. The accuracy of a serological test either individually or in combination is given as the area under the curve (AUC) of the receiver operator characteristic (ROC) of specific serum diagnosis test. In the meantime, genetic marker should reflect differential expression in different fibrosis stages [4,7-13]. This article will focus on the technologies that can be used to assess hepatic fibrosis in HCV infected patients with unequal values. Figure 1 shows an outline of possible methods used for fibrosis evaluation in HCV infected patients.
Figure 1.
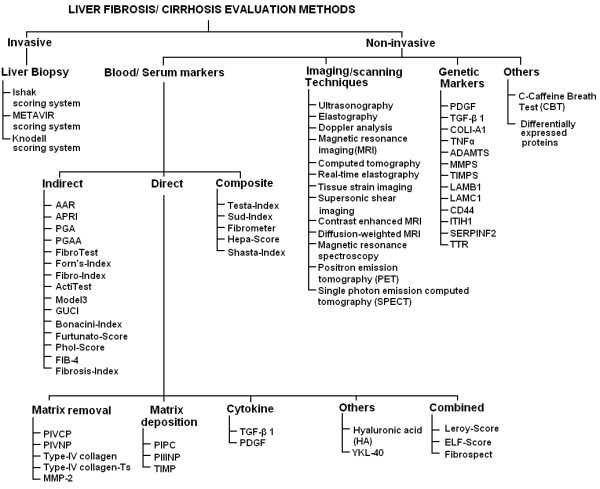
Schematic diagram of noninvasive methods used to assess liver fibrosis and cirrhosis in HCV or co-infected patients.
2. Invasive Method
In clinical practice, grading and staging involve semi-quantitative scoring systems, and elementary lesion expressed as a numerical value [14,15]. Three scoring systems, Knodell, Ishak and Metavir are extensively used to assess fibrosis [16-18]. In Metavir system, one of the most clinically validated systems; F0-F1 is considered none to mild, F2-F3 moderate to advance fibrosis and F4 as cirrhosis. Liver biopsy, an invasive method is considered the gold standard to identify liver fibrosis. Unfortunately, procedure of liver biopsy is invasive, expensive with severe side effects leading to death and not suitable for all patients. Other limitations of liver biopsy comprises sampling error, intra and inter observer variation and somehow static, not accurately predict disease progression [19,20].
3. Non-invasive Methods
Non-invasive methods can be classified as serum, genetic and imaging techniques. These markers are addressed below in detail.
4. Serum markers
Serological markers refer to the measurement of one or more molecules within blood or serum correlating to hepatic fibrosis [21-23]. There are several proposed serological markers or combinations of serum markers for hepatic fibrosis measurement. Their levels vary by changes in their clearance, metabolism, and excretion, and their significant contribution from non-hepatic sources, such as, bones, joints, lungs, kidneys and skin [24,25]. Proposed hepatic fibrosis serological markers can be divided in three categories as direct, indirect or composite. Combinations of both direct and indirect, markers are taking place as an emerging and promising alternative to liver biopsy [26-29]. Figure 1 gives a brief idea about the non-invasive methods used for fibrosis and cirrhosis prediction in HCV infected patients.
4.1. Direct serum markers
Direct serum markers reflect ECM turnover, balance between hepatic fibrogenesis and fibrolysis, and in the deposition and removal of ECM. Levels of direct serum markers are elevated during disease progression and an independent association between stage of fibrosis and direct markers was observed [30-32]. Some of the markers reported are discussed below.
4.1.1. Matrix deposition and removal markers
These may be classified into following
Procollagen I carboxy terminal peptide (PICP), Procollagen III amino-terminal peptide (PIIINP) and Type IV collagen
PICP and PIIINP released into the serum during matrix removal and deposition. PIIINP reflects the stage of fibrosis and known to be elevated in chronic liver disease. PIIINP is a good inflammatory score predictor as compared to fibrosis. PICP usually indicates cirrhosis and used for quantifying disease severity. However, it reflects alcohol etiology better than diagnosis of chronic liver disease. Type IV serum collagen reflects matrix degradation and increased in chronic liver disease. Murawaki et al (1996) established the cutoff value of 110 ng/mL for stages greater than F2 and 130 ng/mL for F3 fibrosis stage [33-37].
Matrix metalloproteinase (MMP's) and tissue inhibitor of metalloproteinases (TIMPs)
MMP's enzymes produced intracellularly and secreted in a pro-enzyme form that requires cleavage by cell surface mechanisms control matrix degradation. Although these proteins act both to degrade and deposition of ECM, also involve in activation of growth factor, effect on cell proliferation and inhibition of apoptosis; their association with liver fibrosis is not clear [4,23]. TIMPs also increased during HCV infection, while a decrease is reported after interferon therapy. These have high diagnostic ability to detect cirrhosis [38].
Cytokines
Two types of cytokines TGF-β 1 (transforming growth factors β 1) and PDGF (platelet derived growth factor) are mainly used to assess the fibrosis progression. TGF-β 1 is the dominant stimulus for producing extracellular matrix and it showed a significant correlation with degree of hepatic fibrosis. A significant association was found between TGF-β 1 serum levels and fibrosis progression. Serum level of PDGF has also showed high ability as serum marker for fibrosis progression [39-41].
4.1.2.Combined direct markers
FibroSpect
FibroSpect assay is a combination of three parameters: HA, TIMP-1 and alpha-2-macroglobulin and can differentiate between no/mild and moderate/severe fibrosis [42,43]. Maximum sensitivity and specificity of this assay was observed at two extreme stages (F0 and F4). This assay was further developed by adding YKL-40 serum marker for assessing Ishak stages and digital quantification of fibrosis [23].
ELF
European liver fibrosis group (ELF) developed an algorithm consisted of HA, PIIINP, TIMP-1 and age. However this assay showed low performance while predicting fibrosis in chronic HCV patients [44].Leroy Score
This score was developed by Leroy et al and contains PIIINP and MMP-1 as basic components. It can differentiate between mild and significant fibrosis [45].
4.1.3.Others
Hyaluronic acid (HA)
HA is best validated, an essential component of extracellular matrix of body tissues. HA levels increases with the fibrosis progression and correlate with the degree of fibrosis and inflammation in chronic HCV patients. The diagnostic accuracy of HA is better than that of PIIINP [32,35,46-49].
Chondrex, human cartilage glycoprotein (YKL-40)
In liver fibrosis, YKL-40 plays role in tissue degradation and extracellular matrix remodeling. YKL-40 level is observed to decrease after interferon therapy. In a combination of different direct serum markers, HA and YKL-40 were more useful for monitoring fibrosis progression with 80% PPV of predicting stage specific fibrosis. A significant association of HA with liver fibrosis was observed when compared with TGF-β1 [50-53].
Table 1 briefly describes a year wise overview of the AUROCs, PPV, NPV, sensitivity and specificity of direct serum markers used in various studies to predict fibrosis and cirrhosis in HCV infected patients. Direct serum markers; HA, YKL-40 and ELF were able to predict significant fibrosis as well as cirrhosis with AUROC 0.70-0.85. However, these markers showed low sensitivity and NPV for predicting fibrosis and high efficiency to detect cirrhosis.
Table 1.
Diagnostic accuracies of direct serum markers
| Markers | Study | Year | Prognosis | Sen | Spe | PPV | NVP | AUC |
|---|---|---|---|---|---|---|---|---|
| ELF Score | Rosenberg [44] | 2004 | Fibrosis | 90 | 41 | 99 | 92 | 0.80 |
| Cirrhosis | - | - | - | - | 0.89 | |||
| Cales [79] | 2005 | Fibrosis | - | - | - | - | 0.88 | |
| Parkes [12] | 2006 | Fibrosis | - | - | - | - | 0.78 | |
| Lee [107] | 2010 | Cirrhosis | 0.70 | |||||
| FibroSpect | Patel [42] | 2004 | Fibrosis | 77 | 73 | 74 | 75 | 0.83 |
| Cales [79] | 2005 | Fibrosis | - | - | - | - | 0.87 | |
| Zaman [43] | 2007 | Fibrosis | 72 | 74 | 61 | 82 | 0.82 | |
| HA | Guechot [34] | 1996 | Fibrosis | 64 | 91 | - | - | 0.86 |
| Cirrhosis | 79 | 89 | - | - | 0.92 | |||
| Murawaki [47] | 2001 | Fibrosis | 75 | 80 | 77 | 78 | 0.86 | |
| Cirrhosis | 50 | 79 | 42 | 84 | 0.92 | |||
| Halfon [49] | 2005 | Fibrosis | 14 | 99 | 94 | 57 | 0.75 | |
| Cirrhosis | 31 | 99 | 57 | 96 | 0.89 | |||
| Suzuki [48] | 2005 | Fibrosis | 85 | 80 | 51 | 96 | 0.89 | |
| Cirrhosis | - | - | - | - | 0.92 | |||
| Saitou [51] | 2005 | Fibrosis | 80 | 80 | 80 | 80 | 0.92 | |
| Parise [81] | 2006 | Fibrosis | 85 | 71 | - | - | 0.88 | |
| Cirrhosis | 91 | 82 | - | - | 0.91 | |||
| Leroy Score | Leroy [45] | 2004 | Fibrosis | 43 | 64 | 45 | 40 | - |
| PIIINP | Guechot [34] | 1996 | Fibrosis | 70 | 63 | - | - | 0.69 |
| Cirrhosis | 60 | 74 | - | - | 0.73 | |||
| Murawaki [47] | 2001 | Fibrosis | 74 | 75 | 75 | 92 | - | |
| Cirrhosis | 64 | 59 | 33 | 84 | - | |||
| Saitou [51] | 2005 | Fibrosis | 78 | 75 | 76 | 77 | 0.75 | |
| Cirrhosis | 77 | 66 | 69 | 67 | 0.79 | |||
| PIVNP | Murawaki [47] | 2001 | Fibrosis | 70 | 73 | 71 | 72 | - |
| Cirrhosis | 63 | 73 | 41 | 87 | - | |||
| TIMP | Murawaki [47] | 2001 | Fibrosis | 79 | 56 | 63 | 73 | - |
| Cirrhosis | 82 | 54 | 34 | 94 | - | |||
| Boeker [38] | 2002 | Fibrosis | 52 | 88 | - | - | 0.71 | |
| Cirrhosis | 100 | 75 | - | - | 0.90 | |||
| YKL-40 | Saitou [51] | 2005 | Fibrosis | 78 | 81 | 80 | 79 | 0.81 |
| Cirrhosis | 80 | 71 | 73 | 78 | 0.80 |
5. Indirect serum fibrosis markers
The other category of serum marker is indirect markers that are based on the disturbance of hepatic function or structure.
5.1. Serum ALT, AST and AFP levels
Serum ALT released from liver tissue into the circulation in proportion to the degree of hepatocellular damage due to viral infections and toxic substances [54,55]. ALT is thought as one of the more sensitive marker of liver injury and disease progression [56-58]. However, ALT enzymatic activity may not always reflect the degree of hepatic damage as about 26% patients have persistently normal ALT levels but have a histological score greater than A1F1 [59]. Serum AST levels are most important predictor of histological activity than ALT [60-62]. Serum AFP is alpha-1-globulin secreted by fetal hepatocytes and fetal gastrointestinal tract. Elevated serum AFP levels are associated with acute and chronic HCV, toxic liver injury concentrations and correlate with tumor size and decrease or normalize after tumor removal. Elevated AFP levels are observed in cirrhotic patients [63-66].
5.2. Platelet count (PLT)
Decreased production of thrombopoietin by hepatocytes and reduced platelet production is associated with fibrosis progression. Platelet count (< 150 × 109/L < 100) can differentiate cirrhotic (F4) from fibrosis (F1-F3) in 75-80% chronic HCV patients [67-70].
5.3. Prothrombin time (PT)
PT reflects the synthesis capacity of the liver and essential mechanism of blood coagulation. Its clinical reference range is usually around 12-15 seconds. Prolonged PT is associated with esophageal varices and is one of the earliest indicators of liver cirrhosis [71-73].
5.4. AST/ALT ratio (AAR)
Sheth et al. reported an AST/ALT ratio ≥ 1 having 100% PPV for the presence of cirrhosis in chronic HCV patients [74]. Reedy et al. observed that AAR failed to predict cirrhosis accurately in HCV patients [75], while Giannini et al. reported high diagnostic accuracy of the AAR for prediction of cirrhosis in HCV infected patients [76]. However, many authors could not able to find high accuracy of this marker [4,70,77].
5.5. AST to platelet ratio Index (APRI)
APRI was the simplest and accurate test for significant liver fibrosis and cirrhosis [28]. Several authors verified this marker for fibrosis and cirrhosis and found it better than AAR. However, APRI was unable to identify individual stages of fibrosis [77-86].
5.6. PGA and PGAA Index
PGA was known to be the original index of hepatic fibrosis in 1990 s and combines gamma glutamyl transferase (γGT), apolipoprotein A1 (PGA) and prothrombin index. PGAA index is modified form of PGA index by the addition of alpha-2-macroglobulin, resulted in its improved version. The diagnostic accuracy of the PGA and PGAA for detecting cirrhosis reported between 66-72% and 80%, respectively [87-92].
5.7. FibroTest/FibroSure
FibroTest is the combination of five markers: alpha-2-macroglobulin, haptoglobin, apolipoprotein A1, GGT and total bilirubin [26,80]. This marker has 75% sensitivity and 85% specificity with reproducibility for fibrosis diagnosis [83-85]. However, Rossi et al. reported low AUROC (0.739) for significant fibrosis with NPV and PPV 85% and 78%, respectively. Meanwhile, FibroTest is validated and suggested as an alternative to liver biopsy in chronic HCV patients [93-105].
5.8. Fibro Index
It combines three markers; AST, platelet count and gamma globulin. AUROC for prediction of significant fibrosis was 0.83 [106].
5.9. Forns Index
This index is based on four available variables; age, GGT, platelet count and cholesterol levels in a study on HCV patients, included both test and validation cohorts [27]. The limitation of this index was the identification of advance liver disease with minimal fibrosis [79,80,106,107].
5.10. ActiTest
ActiTest reflects both necroinflamatory activity and liver cirrhosis. It is modified form of Fibrotest with addition of ALT level (26). Fibrotest and ActiTest were found to be potential non-invasive assays for the assessment of hepatic fibrosis and necro-inflammatory activity in pediatric patients with chronic HCV in comparison with liver biopsy [90,91,108].
5.11. SteatoTest
It incorporates the FibroTest, ALT, body mass index, serum cholesterol, triglycerides and glucose adjusted for age and gender. It has 63% PPV for steatosis prevalence with 93% NPV [109].
5.12. Model 3
This model is based on AST, platelet count and prothrombin time expressed as international normalized ration (INR). Patients with liver cirrhosis can be excluded at cutoff value of < 0.20 with 99% NPV [110,111].
5.13. Goteborg University Cirrhosis Index (GUCI)
Islam et al. found strong association between AST, prothrombin-INR and platelet count. By using a cutoff value 1.0, the sensitivity and specificity for the diagnosis of cirrhosis was 80% and 78% respectively, while the NPV and PPV were 97% and 31%, respectively [112].
5.14. Fibrosis Index
This index comprises of platelet count and albumin contents. It can differentiate significant fibrosis and cirrhosis from mild fibrosis [113].
5.15. Phol Score
This index comprises of AST, ALT and platelet count. It showed great accuracy for discriminating significant fibrosis and cirrhosis with high PPV and NPV. However, it showed limited ability to predict fibrosis in later study [114,115].
5.16. Bonacini Index
This index incorporates ALT/AST ratio, INR and platelet count. It showed 94% specificity for predicting significant fibrosis in initial cohort [116].
Table 2 represents the diagnostic accuracies of indirect serum markers. Indirect serum markers are easily available and routinely used. These markers have the ability to differentiate fibrosis and cirrhosis but lesser extent to direct serum markers. APRI and FibroTest are most validated serum markers with AUROC range between 0.60-0.85 for predicting fibrosis and cirrhosis.
Table 2.
Diagnostic accuracies of indirect serum markers
| Markers | Study | Year | Prognosis | Sen | Spe | PPV | NVP | AUC |
|---|---|---|---|---|---|---|---|---|
| AAR | Sheth [74] | 1998 | Cirrhosis | 53 | 100 | 100 | 81 | 0.85 |
| Afdhal [4] | 2004 | Fibrosis | 47 | - | - | 88 | - | |
| Cirrhosis | - | 96 | 74 | - | - | |||
| Lackner [70] | 2005 | Fibrosis | 53 | 100 | - | - | 0.57 | |
| Cirrhosis | 36 | 90 | 41 | 87 | 0.73 | |||
| Fuji [77] | 2009 | Fibrosis | - | - | - | - | 0.56 | |
| ActiTest | Imbert-Bismut [26] | 2001 | Fibrosis | 91 | 42 | - | - | 0.79 |
| Halfon [100] | 2008 | Fibrosis | 90 | 38 | - | - | 0.75 | |
| APRI | Wai [28] | 2003 | Fibrosis | 41 | 95 | 64 | 90 | 0.88 |
| Cirrhosis | - | - | 57 | - | 0.94 | |||
| Cales [79] | 2005 | Fibrosis | - | - | - | - | 0.79 | |
| Bourliere [80] | 2006 | Fibrosis | 22 | 95 | 63 | 76 | 0.71 | |
| Cirrhosis | 38 | 96 | 96 | 40 | 0.81 | |||
| Parise [81] | 2006 | Fibrosis | 85 | 66 | - | - | 0.82 | |
| Cirrhosis | 73 | 81 | - | - | 0.84 | |||
| De Ledinghen [82] | 2006 | Cirrhosis | - | - | - | - | 0.73 | |
| Halfon [83] | 2007 | Fibrosis | 77 | 66 | 61 | 80 | 0.76 | |
| Cirrhosis | 100 | 83 | 18 | 100 | 0.92 | |||
| Leroy [84] | 2008 | Fibrosis | 39 | 95 | 88 | 62 | 0.79 | |
| Cales [85] | 2008 | Fibrosis | 62 | 83 | 80 | 67 | 0.78 | |
| Cirrhosis | - | - | - | - | 0.84 | |||
| Kamphues [86] | 2010 | Fibrosis | 70 | 63 | 80 | 80 | 0.68 | |
| Cirrhosis | 89 | 44 | 14 | 97 | 0.63 | |||
| Fuji [77] | 2009 | Cirrhosis | - | - | - | - | 0.76 | |
| Fibro Index | Koda [106] | 2007 | Fibrosis | 36 | 97 | 94 | 59 | 0.83 |
| Fibrosis Index | Ohta [113] | 2006 | Fibrosis | 68 | 71 | 75 | 81 | 0.85 |
| FibroTest | Imbert-Bismut [26] | 2001 | Fibrosis | 87 | 59 | 63 | 85 | 0.87 |
| Cirrhosis | ||||||||
| Bedosa [102] | 2003 | Fibrosis | 27 | 97 | 90 | 55 | - | |
| Myers [101] | 2003 | Fibrosis | - | 95 | 88 | - | 0.83 | |
| Poynard [90] | 2003 | Fibrosis | - | - | - | - | 0.73 | |
| Rossi [97] | 2003 | Fibrosis | 83 | 52 | 52 | 83 | 0.74 | |
| Colletta [103] | 2005 | Fibrosis | 64 | 31 | 33 | 62 | - | |
| Bourliere [80] | 2006 | Fibrosis | 55 | 90 | 73 | 79 | 0.82 | |
| De Ledinghen [82] | 2006 | Cirrhosis | - | - | - | - | 0.73 | |
| Halfon [83] | 2007 | Fibrosis | 67 | 80 | 70 | 78 | 0.79 | |
| Cirrhosis | 85 | 74 | 11 | 99 | 0.86 | |||
| Leroy [84] | 2008 | Fibrosis | 57 | 85 | 78 | 68 | 0.80 | |
| Cales [85] | 2008 | Fibrosis | 67 | 82 | 80 | 70 | 0.81 | |
| Shaheen [104] | 2008 | Fibrosis | 47 | 90 | - | - | 0.81 | |
| Cirrhosis | - | - | - | - | 0.90 | |||
| Cales [105] | 2010 | Fibrosis | - | - | - | - | 0.81 | |
| Cirrhosis | - | - | - | - | 0.88 | |||
| Forn's Index | Forn [27] | 2002 | Fibrosis | 94 | 51 | 40 | 96 | 0.78 |
| Cales [79] | 2005 | Fibrosis | - | - | - | - | 0.82 | |
| Bourliere [80] | 2006 | Fibrosis | 30 | 96 | 65 | 83 | 0.76 | |
| Koda [106] | 2007 | Fibrosis | - | - | - | - | 0.79 | |
| Model 3 | Lok [110] | 2005 | Cirrhosis | 10 | 100 | 100 | 86 | 0.78 |
| PGA | Teare [87] | 1993 | Fibrosis | 94 | 81 | - | - | - |
| Cirrhosis | - | - | 86 | - | - | |||
| Poynard [90] | 2003 | Fibrosis | 91 | 81 | - | - | - | |
| Poynard [91] | 2004 | Fibrosis | 79 | 89 | - | - | - | |
| PGAA | Naveau [92] | 2005 | Cirrhosis | 89 | 79 | - | - | 0.93 |
| Phol Score | Pohl [114] | 2001 | Fibrosis | 41 | 99 | 93 | 85 | - |
| Cheung [115] | 2008 | Fibrosis | - | - | - | - | 0.53 |
6. Composite fibrosis markers
6.1. FibroMeter
FibroMeter can differentiate fibrosis progression in viral disease consist of combination of HA, AST, platelet count, prothrombin index, alpha-2-macroglobulin, urea and age of the patients [105].
6.2. Hepascore
Hepascore is a model consisting of bilirubin, GGT, HA, alpha-2-macroglobulin, gender and age. AUROC of this test is 0.85, 0.96 and 0.94 for significant fibrosis, advanced fibrosis and cirrhosis, respectively [117-120].
6.3. Shasta Index
It combines HA, AST and albumin. Optimal results of this assay are observed in extreme conditions. This assay showed similar accuracy with FibroTest [121].
6.4. Apricot (FIB-4)
This assay combines four markers: AST, ALT, platelet count and age. This index can predict significant fibrosis in patients infected with HIV/HCV [122]. Later studies validated this index not only in co-infected patients but also in HCV infected patients [85,123,124].
6.5. Sud Index
This assay is also known as FPI comprises of age, AST, cholesterol, insulin resistance and alcohol intake. This index showed high specificity and PPV for detecting advance fibrosis [125].
6.6. Testa Index
This index relate platelet count and spleen diameter. This ratio showed 78% concordance with the histological score [126].
6.7. Fortunato score
This model contains fibronectin, prothrombin time, PCHE, ALT, Mn-SOD and β-NAG as essential components. It has ability to classify cirrhotic from chronic patients with high accuracy in initial and validation cohort [127].
Table 3 gives an idea about the prediction levels of combined serum markers. These markers showed high AUROCs (0.80-0.90) for predicting fibrosis and cirrhosis in HCV infected patients. FIB-4, Fibrometer and Hepascore are most precise and validated serum markers. Combined serum markers are easily available and most preferable non invasive serum markers now a day.
Table 3.
Prognosis accuracies of combined serum markers
| Markers | Study | Year | Prognosis | Sen | Spe | PPV | NVP | AUC |
|---|---|---|---|---|---|---|---|---|
| FIB-4 | Sterling [122] | 2006 | Fibrosis | 70 | 74 | 42 | 71 | 0.80 |
| Cirrhosis | ||||||||
| De Ledingh [82] | 2006 | Cirrhosis | - | - | - | - | 0.73 | |
| Vallet-Pichard [123] | 2007 | Fibrosis | 74 | 80 | 82 | 95 | 0.85 | |
| Cales [85] | 2008 | Fibrosis | 74 | 72 | 74 | 71 | 0.80 | |
| Cirrhosis | - | - | - | - | 0.87 | |||
| Mallet [124] | 2009 | Fibrosis | 71 | 73 | 52 | 86 | 0.81 | |
| Cirrhosis | - | - | - | - | 0.87 | |||
| Lee [107] | 2010 | Cirrhosis | - | - | - | - | 0.71 | |
| Fibrometer | Halfon [83] | 2007 | Fibrosis | 92 | 87 | 21 | 100 | 0.94 |
| Cirrhosis | 62 | 87 | 21 | 100 | 0.94 | |||
| Cales [85] | 2008 | Fibrosis | - | - | - | - | 0.90 | |
| Cirrhosis | - | - | - | - | 0.90 | |||
| Cales [105] | 2010 | Fibrosis | - | - | - | - | 0.88 | |
| Cirrhosis | - | - | - | - | 0.88 | |||
| Fortunato Score | Fortunato [127] | 2001 | Fibrosis | - | 94 | - | - | - |
| HepaScore | Adams [117] | 2005 | Fibrosis | 63 | 89 | 88 | 90 | 0.82 |
| Cirrhosis | 71 | 89 | - | - | 0.90 | |||
| Bourliere [80] | 2006 | Fibrosis | - | - | - | - | 0.82 | |
| Cirrhosis | - | - | - | - | 0.90 | |||
| Halfon [83] | 2007 | Fibrosis | 77 | 63 | 59 | 80 | 0.76 | |
| Cirrhosis | 92 | 72 | 11 | 100 | 0.89 | |||
| Leroy [118] | 2007 | Fibrosis | 54 | 84 | 78 | 64 | 0.79 | |
| Leroy [84] | 2008 | Fibrosis | 63 | 80 | 75 | 70 | 0.78 | |
| Cales [85] | 2008 | Fibrosis | 66 | 79 | 77 | 68 | 0.78 | |
| Cirrhosis | - | - | - | - | 0.90 | |||
| Becker [119] | 2009 | Fibrosis | 82 | 65 | 70 | 78 | 0.81 | |
| Cirrhosis | - | - | - | - | 0.88 | |||
| Cales [105] | 2010 | Fibrosis | - | - | - | - | 0.78 | |
| Cirrhosis | - | - | - | - | 0.89 | |||
| Guechot [120] | 2010 | Fibrosis | 77 | 70 | 71 | 77 | 0.81 | |
| Cirrhosis | 86 | 74 | 37 | 97 | 0.88 | |||
| Shasta Index | Kelleher [121] | 2005 | Fibrosis | 88 | 72 | 55 | 94 | 0.87 |
| Sud Index | Sud [125] | 2004 | Fibrosis | 42 | 98 | 97 | 54 | 0.84 |
| Testa Index | Testa [126] | 2006 | Fibrosis | 78 | 79 | - | - | 0.80 |
7. Imaging/scanning techniques
Imaging techniques are rational noninvasive approach to assess liver fibrosis. Imaging techniques are not only capable to detect changes in the hepatic parenchyma, these can differentiate between moderate and severe fibrosis. However, high cost and lack of validation of concerning studies remains controversial. Brief detail of these techniques is given under, while there limitations are addressed in Table 4.
Table 4.
Summarized imaging techniques with their limitations
| Method | Technique | Limitations |
|---|---|---|
| Ultrasonography | Identification of portal hypertension | Limited capability to measure mild or moderate fibrosis and cirrhosis, contradictory results |
| Elastography | Liver stiffness | Vulnerable measurements due to narrow intercostals spaces, ascites or obesity |
| Doppler Analysis | Measures velocity of blood flow, hemodynamic variations | Limited data, lack of reproducibility, contradictory results |
| Magnetic Resonance Imaging | Observe changes in hepatic parenchyma | High cost, lack of research support |
| Computed Tomography | Identifies micro vascular permeability changes | Recent technique, not much literature is available, can not performed in renal failure and contrast agent allergic patients |
7.1. Ultrasonography (US)
Ultrasonography detects changes appear in liver echogenicity, nodularity and signs of portal hypertension. A number of studies proposed the role of ultrasonography as a non-invasive diagnostic marker of liver fibrosis and revealed a great sensitivity of ultrasonography to detect late stages of progressive hepatic fibrosis, but a limited capability to measure mild or moderate fibrosis. Ultrasound can identify cirrhosis in patients with sensitivity of 84% and specificity of 100% and diagnose accurately 94%. Shen et al. observed that the echo pattern of the hepatic surface contributed to diagnostic accuracy, which was also confirmed in a separate study. However, Oberti found ultrasonography as weak diagnostic marker when compared it with other clinical and biochemical examinations [128-133].
7.2. Transient Elastography (FibroScan): an applicable alternative to liver biopsy
Transient elastography measures tissue stiffness. It can measure liver sample size 100 times greater than a standard biopsy sample size, as liver biopsy size strongly effects the grading of chronic viral hepatitis [134-137]. FibroScan results reported 100% sensitivity and specificity for PPV & NPV respectively (103). In a study of 935 patients Fibroscan was found to be 97% successful in grading chronic HCV infection [138]. In another study on 711 patients, liver stiffness measurements (LSM) were also found closely related to fibrosis stage [139]. Vizzutti et al. has also reported a good correlation between liver stiffness measurement and HVPG (hepatic venous pressure gradient) in cirrhotic patients. Success rate depends on patient body mass index, observer expertise and inter-coastal spaces with 5% failure chances. Several authors assess the performance of elastography and configure it best for the diagnosis of fibrosis [13,86,103,140-149]. A combination of FibroScan with FibroTest also gives a better understanding to detect fibrosis and cirrhosis with high AUC [104]. Table 5 briefly describes the diagnostic accuracy of FibroScan with or without combination with FibroTest. In all studies, FibroScan showed highest AUROC (> 0.90) but not more than liver biopsy (AUROC > 0.970).
Table 5.
Diagnostic accuracy of Fibroscan with and without FibroTest
| Markers | Study | Year | Prognosis | Sen | Spe | PPV | NVP | AUC |
|---|---|---|---|---|---|---|---|---|
| Fibro Scan | Ziol [13] | 2005 | Fibrosis | 56 | 91 | 88 | 56 | 0.79 |
| Cirrhosis | 86 | 96 | 78 | 97 | 0.97 | |||
| Colletta [103] | 2005 | Fibrosis | 100 | 100 | 100 | 100 | 1.00 | |
| Foucher [139] | 2006 | Fibrosis | 64 | 85 | 90 | 52 | 0.80 | |
| Cirrhosis | 77 | 97 | 91 | 92 | 0.96 | |||
| Corpechot [145] | 2006 | Fibrosis | - | - | - | - | 0.95 | |
| Ganne-Carrie [146] | 2006 | Cirrhosis | 79 | 95 | 74 | 96 | 0.95 | |
| Kettaneh [138] | 2007 | Fibrosis | - | - | - | - | 0.79 | |
| Cirrhosis | - | - | - | - | 0.91 | |||
| Shaheen [147] | 2007 | Fibrosis | 64 | 87 | - | - | 0.83 | |
| Cirrhosis | - | - | - | - | 0.95 | |||
| Friedrich-Rust [148] | 2009 | Fibrosis | - | - | - | - | 0.84 | |
| Cirrhosis | - | - | - | - | 0.94 | |||
| Kamphues [86] | 2010 | Fibrosis | 72 | 83 | 96 | 58 | 0.81 | |
| Cirrhosis | 100 | 65 | 23 | 100 | 0.87 | |||
| Sanchez-Conde [149] | 2010 | Fibrosis | 76 | 75 | 70 | 81 | 0.93 | |
| Cirrhosis | 100 | 94 | 57 | 100 | 0.99 | |||
| Fibro Scan + FibroTest | Castera [160] | 2005 | Fibrosis | - | - | - | - | 0.88 |
| Cirrhosis | - | - | - | - | 0.95 | |||
| Shaheen [104] | 2008 | Fibrosis | 47 | 90 | - | - | - |
7.3. Doppler analysis
Doppler measures the velocity of blood flow hemodynamic variations in hepatic vasculature, as sever fibrosis causes irregularities and abnormalities in hepatic blood vessels. Recent data indicate a close correlation between arterioportal ratio and degree of fibrosis, higher ratio indicates severe fibrosis (F3-F4) and low ratio shows moderate fibrosis (F1-F2) [150-153].
7.4. Magnetic resonance imaging (MRI)
MRI observes changes in hepatic parenchyma. Non-invasive prognosis of liver cirrhosis is proposed by using double contrast material-enhanced MR imaging. This can detect cirrhosis with great sensitivity and specificity of 90%. Combining Doppler ultrasonography with MRI can give a good picture of liver fibrosis in patients suffering with chronic HCV [154-156].
7.5. Computed tomography (CT)
CT identifies microvascular permeability changes in a model of liver fibrosis. In a latest study, the severity of liver fibrosis was predicted by heterogeneous enhancement of the liver; hepatic parameters. Perfusion calculated with a dynamic contrast-enhanced single-section CT, linked with the severity of chronic liver disease. However, no well characterized study has specifically evaluated the worth of CT in diagnosing degree of fibrosis. Therefore, currently its role in diagnosis of liver fibrosis is still lacking [157-159].
7.6. Fibroscan + Fibrotest
The combination of two useful noninvasive methods, fibroscan and fibrotest showed high AUROC for predicting cirrhosis [104,160].
7.7. Modified imaging techniques
Imaging techniques with modification like Real-time elastography, Tissue strain imaging, Supersonic shear imaging, Contrast enhanced MRI, Diffusion-weighted MRI, Magnetic resonance spectroscopy, Positron emission tomography (PET), Single photon emission computed tomography (SPECT) are also in use to evaluate liver fibrosis and cirrhosis with considerable limitations like, lack of data and expertise, high cost, radiation exposure and short half-life of the tracer in PET and SPECT.
8. Genetic markers for liver fibrosis evaluation
ECM metabolism is very dynamic process and required an intricate balance between ECM deposition and removal. Several genetic polymorphisms influenced by factors/cytokines and affect fibrosis progression [98]. Genome-wide analysis of abnormal gene expression showed transcript deregulations during HCC development with identification of novel serum markers differentiating between normal, mild and severe fibrosis. Advantage of genetic markers over liver biopsy is intrinsic and long life while liver biopsy represents only one time point [161-163].
Huang and colleagues developed an assay known as cirrhosis risk score (CRS), a set of seven marker genes to predict cirrhosis risk in HCV infected patients. Of the seven genes, AZIN1 and TLR4 have an identified role in hepatic fibrosis, while the identification of functional mechanism of the other 5 genes is under process. The authors suggested that fibrosis risk can be identified by host genetic factors like single nucleotide polymorphism (SNP's) [164,165].
A strong association between CXCR3-associated chemokines CXCL9 and CXCL10 with liver fibrosis suggested that they may have promise as new non-invasive markers of liver fibrosis in HCV infected patients [166,167].
CTGF expression is significantly correlated with fibrosis stages and remarkably increased in advanced stages in HCV patients. The AUROC of CTGF to discriminate between mild and advanced fibrosis is 0.842 for HCV infected patients [168].
Sharma et al. reported the significant association and elevated interleukin-18 (IL-18) levels in fibrotic and cirrhotic liver stages, severity of disease and necrosis in HCV patients [169].
A recent study by Caillot et al. used microarray technique and found a significant association of ITIH1, SERPINF2 and TTR genes expression and their related proteins with all fibrosis stages. Expression of these genes and related proteins gradually decreased during the fibrosis development to its end stage cirrhosis [170].
A review by Gutierrez-Reyes et al. briefly described role and selection of appropriate genes for fibrosis indication. They briefly explain the role of various genes like PDGF, TGF-β1, collagens COL1-A1, TNFα, interlukin, ADAMTS, MMPs, TIMPs, LAMB1, LAMC1, Cadherin, CD44, ICAM1, ITGA, APO and CYP2C8 [171]. Figure 2 represents gene clustering according to fibrosis progression on available data.
Figure 2.
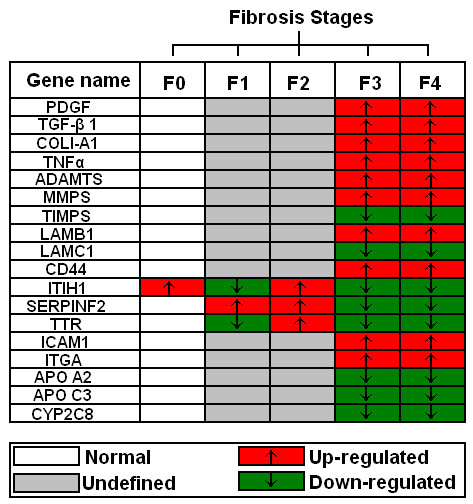
Genes expressed differentially between different fibrosis stages (F0-F3) including cirrhosis (F4).
9. Others markers for liver fibrosis evaluation
9.1. C-Caffeine Breath Test (CBT)
Caffeine has high oral bioavailability and undergoes hepatic metabolism and can be use as quantitative test for liver function [172]. Park et al. performed caffeine breath test (CBT) and observed the correlation of orally administrated caffeine with plasma caffeine clearance and degree of liver dysfunction. Chronic patients showed significantly reduced CBT values when compared with controls [173].
9.2.Differentially expressed proteins
Differentially expressed proteins were identified by mass spectroscopy among different degrees of fibrosis (F0-F4) and between early (F0-F1) and late (F2-F4) fibrosis. Mac-2-binding protein, alpha-2-macroglobulin and hemopexin levels were found increased while A-1-antitrypsin, leucine-rich alpha-2-glycoprotein and fetuin-A were decreased in advanced fibrosis F4 as compared to early fibrosis F0/F1 [115].
10. Clinical utilization and future of non-invasive markers
Non-invasive markers should be able to differentiate between different fibrosis stages but also reflect the treatment outcome. Even though the invasive liver biopsies considered as gold standard for final assessment of liver fibrosis, non-invasive markers are risk free, reflect the liver status and may offer an attractive alternative to liver biopsy in future. However, none of currently available serum markers completely fulfill these criteria. The outcome of non-invasive markers in several studies is not same. Due to this, non-invasive markers are used in parallel to liver biopsy and not in position to completely replace liver biopsy till date.
Poynard et al. reported the effect of interferon plus ribavirin before and after therapy with respect to FibroTest and Actitest scores. A substantial reduction in FibroTest and Actitest was observed in patients who had showed a sustained virological response [81,90,115]. Several other studies reported the down level of serum markers like HA, YKL-40, TIMP-1 and PIIINP after interferon therapy. In these studies, level of serum markers continue to fall following treatment but most often return to permanent levels with biochemical and virological relapse. These findings suggest that these assays may be useful for initial staging of disease progression as well as histological response to therapy [174-177]. Fibroscan showed positive correlation with fibrosis stages. However, it is reported that AUROC value of Fibroscan and FibroTest must be improved as their values fall in treated patients irrespective of their virological response [178,179]. Furthermore, HCV clearance is associated with a significant reduction in non-invasive fibrosis serological markers like FibroTest, Forns Index, age-platelet ratio index, Shasta, FIB-4, Hepascore and FibroMeter [180]. Patel et al. compared two commercially available serum marker panels Fibrosure and Fibrospect-II in HCV patients during interferon-based therapy. Both assays showed comparable performance for differentiating mild fibrosis from moderate-severe stage [181]. Imaging techniques also have some technical limitations. These are very expensive and are not easy to handle. Their presence in each hospital or laboratory is not possible especially in poor countries. On the other hand genetic markers showed a great variability for detecting cirrhosis and fibrosis. They are also able to differentiate among fibrosis stages. But a lot of work is needed for them to become an integral part of hepatic analysis.
11. Conclusions
Our study showed that there are only three to four markers or set of marker that are used continuously based on their precision and accuracy in various studies for fibrosis and cirrhosis prediction. In serum non-invasive markers, FibroTest, Forn's Index, Fibrometer and HeapaScore have a high five-year prognostic value but not compared to liver biopsy (AUROC = 0.97), while Fibroscan showed maximum accuracy nearer to liver biopsy (AUROC > 0.90). Recently, genetic markers showed differential gene expression in different fibrosis stages, but these are not frequently available in all labs. Imaging techniques like ultrasound and elastography not only used to diagnose liver fibrosis but also monitor disease progression. However, genetic markers showed high ability to distinguish not only mild and advance stages of liver fibrosis but also differentiate between intermediate fibrosis stages. Although present published literature do not provide any evidence for non-invasive markers to become an integrated part of the complete assessment of liver fibrosis in HCV patients, a combination of two or more serum markers with imaging techniques may improve the accuracy of diagnosis.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AW, IB, GS, AS and HS designed the study, analyze the data and wrote paper. JS, KS, SMT, KH, SA and SS checked the revised manuscript thoroughly and confirmed all the data given in manuscript. All work was performed under supervision of HS. All authors read and approved the final manuscript.
Authors' information
Shah Jahan, Saba Khaliq and Samrin A (PhD in Molecular biology), Bushra Ijaz (M Phil Molecular Biology), Waqar Ahmad (M Phil Chemistry) and Gull S (MSc Biochemistry) are Research Officer; Sawar MT and Shahid I are Phd scholars, Asad S is MPhil scholars, while Sajida Hassan (PhD Molecular Biology) is Principal Investigator at CEMB, University of the Punjab, Lahore
Contributor Information
Waqar Ahmad, Email: waqarchemist@hotmail.com.
Bushra Ijaz, Email: bijaz_009@yahoo.com.
Sana Gull, Email: sanagull_ibb@hotmail.com.
Sultan Asad, Email: sa_ghouri2010@yahoo.com.
Saba Khaliq, Email: sabahat711@yahoo.com.
Shah Jahan, Email: captainmalik@hotmail.com.
Muhammad T Sarwar, Email: mts_bdkm@yahoo.com.
Humera Kausar, Email: humeratariq@yahoo.com.
Aleena Sumrin, Email: asumrin@yahoo.com.
Imran Shahid, Email: imran_shahid34@yahoo.com.
Sajida Hassan, Email: sajihassan2004@yahoo.com.
Acknowledgements
Financial support by Higher Education Commission (Grant # 863) is highly acknowledged.
References
- Memon MI, Memon MA. Hepatitis C: an epidemiological review. J Viral Hepat. 2002;9:84–100. doi: 10.1046/j.1365-2893.2002.00329.x. [DOI] [PubMed] [Google Scholar]
- WHO. Global distribution of hepatitis A, B and C, 2001. Weakly Epidimiological Records. 2002;77 41,48. [Google Scholar]
- Marcellin P, Asselah T, Boyer N. Fibrosis and disease progression in hepatitis C. Hepatology. 2002;36:S47–56. doi: 10.1002/hep.1840360707. [DOI] [PubMed] [Google Scholar]
- Afdhal NH, Nunes D. Evaluation of liver fibrosis: a concise review. Am J Gastroenterol. 2004;99:1160–1174. doi: 10.1111/j.1572-0241.2004.30110.x. [DOI] [PubMed] [Google Scholar]
- Dienstag JL, McHutchison JG. American Gastroenterological Association technical review on the management of hepatitis C. Gastroenterology. 2006;130:231–264. doi: 10.1053/j.gastro.2005.11.010. quiz 214-237. [DOI] [PubMed] [Google Scholar]
- Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40(Suppl 1):S5–10. doi: 10.1097/01.mcg.0000168638.84840.ff. [DOI] [PubMed] [Google Scholar]
- Harbin WP, Robert NJ, Ferrucci JT Jr. Diagnosis of cirrhosis based on regional changes in hepatic morphology: a radiological and pathological analysis. Radiology. 1980;135:273–283. doi: 10.1148/radiology.135.2.7367613. [DOI] [PubMed] [Google Scholar]
- Gressner AM. The cell biology of liver fibrogenesis - an imbalance of proliferation, growth arrest and apoptosis of myofibroblasts. Cell Tissue Res. 1998;292:447–452. doi: 10.1007/s004410051073. [DOI] [PubMed] [Google Scholar]
- Arthur MJ. Reversibility of liver fibrosis and cirrhosis following treatment for hepatitis C. Gastroenterology. 2002;122:1525–1528. doi: 10.1053/gast.2002.33367. [DOI] [PubMed] [Google Scholar]
- Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33:1358–1364. doi: 10.1053/jhep.2001.24432. [DOI] [PubMed] [Google Scholar]
- El-Serag HB. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology. 2002;36:S74–83. doi: 10.1002/hep.1840360710. [DOI] [PubMed] [Google Scholar]
- Parkes J, Guha IN, Roderick P, Rosenberg W. Performance of serum marker panels for liver fibrosis in chronic hepatitis C. J Hepatol. 2006;44:462–474. doi: 10.1016/j.jhep.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Ledinghen V, Marcellin P, Dhumeaux D, Trinchet JC, Beaugrand M. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48–54. doi: 10.1002/hep.20506. [DOI] [PubMed] [Google Scholar]
- Saadeh S, Cammell G, Carey WD, Younossi Z, Barnes D, Easley K. The role of liver biopsy in chronic hepatitis C. Hepatology. 2001;33:196–200. doi: 10.1053/jhep.2001.20534. [DOI] [PubMed] [Google Scholar]
- Booth JC, O'Grady J, Neuberger J. Clinical guidelines on the management of hepatitis C. Gut. 2001;49(Suppl 1):I1–21. doi: 10.1136/gut.49.suppl_1.I1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–435. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- group TFMcs. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994;20:15–20. [PubMed] [Google Scholar]
- Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- Goldin RD, Goldin JG, Burt AD, Dhillon PA, Hubscher S, Wyatt J, Patel N. Intra-observer and inter-observer variation in the histopathological assessment of chronic viral hepatitis. J Hepatol. 1996;25:649–654. doi: 10.1016/S0168-8278(96)80234-0. [DOI] [PubMed] [Google Scholar]
- Westin J, Lagging LM, Wejstal R, Norkrans G, Dhillon AP. Interobserver study of liver histopathology using the Ishak score in patients with chronic hepatitis C virus infection. Liver. 1999;19:183–187. doi: 10.1111/j.1478-3231.1999.tb00033.x. [DOI] [PubMed] [Google Scholar]
- Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38(Suppl 1):S38–53. doi: 10.1016/S0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Kelleher TB, Afdhal N. Noninvasive assessment of liver fibrosis. Clin Liver Dis. 2005;9:667–683. doi: 10.1016/j.cld.2005.08.002. vii. [DOI] [PubMed] [Google Scholar]
- Idobe Y, Murawaki Y, Ikuta Y, Koda M, Kawasaki H. Post-prandial serum hyaluronan concentration in patients with chronic liver disease. Intern Med. 1998;37:568–575. doi: 10.2169/internalmedicine.37.568. [DOI] [PubMed] [Google Scholar]
- Saif MW, Alexander D, Wicox CM. Serum Alkaline Phosphatase Level as a Prognostic Tool in Colorectal Cancer: A Study of 105 patients. J Appl Res. 2005;5:88–95. [PMC free article] [PubMed] [Google Scholar]
- Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069–1075. doi: 10.1016/S0140-6736(00)04258-6. [DOI] [PubMed] [Google Scholar]
- Forns X, Ampurdanes S, Llovet JM, Aponte J, Quinto L, Martinez-Bauer E, Bruguera M, Sanchez-Tapias JM, Rodes J. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36:986–992. doi: 10.1053/jhep.2002.36128. [DOI] [PubMed] [Google Scholar]
- Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- Thabut D, Simon M, Myers RP, Messous D, Thibault V, Imbert-Bismut F, Poynard T. Noninvasive prediction of fibrosis in patients with chronic hepatitis C. Hepatology. 2003;37:1220–1221. doi: 10.1053/jhep.2003.50109. author reply 1221. [DOI] [PubMed] [Google Scholar]
- Guechot J, Loria A, Serfaty L, Giral P, Giboudeau J, Poupon R. Serum hyaluronan as a marker of liver fibrosis in chronic viral hepatitis C: effect of alpha-interferon therapy. J Hepatol. 1995;22:22–26. doi: 10.1016/0168-8278(95)80255-X. [DOI] [PubMed] [Google Scholar]
- Pares A, Deulofeu R, Gimenez A, Caballeria L, Bruguera M, Caballeria J, Ballesta AM, Rodes J. Serum hyaluronate reflects hepatic fibrogenesis in alcoholic liver disease and is useful as a marker of fibrosis. Hepatology. 1996;24:1399–1403. doi: 10.1002/hep.510240615. [DOI] [PubMed] [Google Scholar]
- McHutchison JG, Blatt LM, de Medina M, Craig JR, Conrad A, Schiff ER, Tong MJ. Measurement of serum hyaluronic acid in patients with chronic hepatitis C and its relationship to liver histology. Consensus Interferon Study Group. J Gastroenterol Hepatol. 2000;15:945–951. doi: 10.1046/j.1440-1746.2000.02233.x. [DOI] [PubMed] [Google Scholar]
- Hayasaka A, Saisho H. Serum markers as tools to monitor liver fibrosis. Digestion. 1998;59:381–384. doi: 10.1159/000007493. [DOI] [PubMed] [Google Scholar]
- Guechot J, Laudat A, Loria A, Serfaty L, Poupon R, Giboudeau J. Diagnostic accuracy of hyaluronan and type III procollagen amino-terminal peptide serum assays as markers of liver fibrosis in chronic viral hepatitis C evaluated by ROC curve analysis. Clin Chem. 1996;42:558–563. [PubMed] [Google Scholar]
- Fabris C, Falleti E, Federico E, Toniutto P, Pirisi M. A comparison of four serum markers of fibrosis in the diagnosis of cirrhosis. Ann Clin Biochem. 1997;34(Pt 2):151–155. doi: 10.1177/000456329703400203. [DOI] [PubMed] [Google Scholar]
- George DK, Ramm GA, Walker NI, Powell LW, Crawford DH. Elevated serum type IV collagen: a sensitive indicator of the presence of cirrhosis in haemochromatosis. J Hepatol. 1999;31:47–52. doi: 10.1016/S0168-8278(99)80162-7. [DOI] [PubMed] [Google Scholar]
- Murawaki Y, Ikuta Y, Koda M, Yamada S, Kawasaki H. Comparison of serum 7 S fragment of type IV collagen and serum central triple-helix of type IV collagen for assessment of liver fibrosis in patients with chronic viral liver disease. J Hepatol. 1996;24:148–154. doi: 10.1016/S0168-8278(96)80023-7. [DOI] [PubMed] [Google Scholar]
- Boeker KH, Haberkorn CI, Michels D, Flemming P, Manns MP, Lichtinghagen R. Diagnostic potential of circulating TIMP-1 and MMP-2 as markers of liver fibrosis in patients with chronic hepatitis C. Clin Chim Acta. 2002;316:71–81. doi: 10.1016/S0009-8981(01)00730-6. [DOI] [PubMed] [Google Scholar]
- Zhang BB, Cai WM, Weng HL, Hu ZR, Lu J, Zheng M, Liu RH. Diagnostic value of platelet derived growth factor-BB, transforming growth factor-beta1, matrix metalloproteinase-1, and tissue inhibitor of matrix metalloproteinase-1 in serum and peripheral blood mononuclear cells for hepatic fibrosis. World J Gastroenterol. 2003;9:2490–2496. doi: 10.3748/wjg.v9.i11.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JS, Christoffersen P, Moller S, Price PA, Henriksen JH, Garbarsch C, Bendtsen F. Serum YKL-40 is increased in patients with hepatic fibrosis. J Hepatol. 2000;32:911–920. doi: 10.1016/S0168-8278(00)80095-1. [DOI] [PubMed] [Google Scholar]
- Kanzler S, Baumann M, Schirmacher P, Dries V, Bayer E, Gerken G, Dienes HP, Lohse AW. Prediction of progressive liver fibrosis in hepatitis C infection by serum and tissue levels of transforming growth factor-beta. J Viral Hepat. 2001;8:430–437. doi: 10.1046/j.1365-2893.2001.00314.x. [DOI] [PubMed] [Google Scholar]
- Patel K, Gordon SC, Jacobson I, Hezode C, Oh E, Smith KM, Pawlotsky JM, McHutchison JG. Evaluation of a panel of non-invasive serum markers to differentiate mild from moderate-to-advanced liver fibrosis in chronic hepatitis C patients. J Hepatol. 2004;41:935–942. doi: 10.1016/j.jhep.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Zaman A, Rosen HR, Ingram K, Corless CL, Oh E, Smith K. Assessment of FIBROSpect II to detect hepatic fibrosis in chronic hepatitis C patients. Am J Med. 2007;120:280. doi: 10.1016/j.amjmed.2006.06.044. e289-214. [DOI] [PubMed] [Google Scholar]
- Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, Hubscher S, Roskams T, Pinzani M, Arthur MJ. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004;127:1704–1713. doi: 10.1053/j.gastro.2004.08.052. [DOI] [PubMed] [Google Scholar]
- Leroy V, Monier F, Bottari S, Trocme C, Sturm N, Hilleret MN, Morel F, Zarski JP. Circulating matrix metalloproteinases 1, 2, 9 and their inhibitors TIMP-1 and TIMP-2 as serum markers of liver fibrosis in patients with chronic hepatitis C: comparison with PIIINP and hyaluronic acid. Am J Gastroenterol. 2004;99:271–279. doi: 10.1111/j.1572-0241.2004.04055.x. [DOI] [PubMed] [Google Scholar]
- Murawaki Y, Ikuta Y, Nishimura Y, Koda M, Kawasaki H. Serum markers for connective tissue turnover in patients with chronic hepatitis B and chronic hepatitis C: a comparative analysis. J Hepatol. 1995;23:145–152. doi: 10.1016/0168-8278(95)80328-9. [DOI] [PubMed] [Google Scholar]
- Murawaki Y, Ikuta Y, Okamoto K, Koda M, Kawasaki H. Diagnostic value of serum markers of connective tissue turnover for predicting histological staging and grading in patients with chronic hepatitis C. J Gastroenterol. 2001;36:399–406. doi: 10.1007/s005350170084. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Angulo P, Lymp J, Li D, Satomura S, Lindor K. Hyaluronic acid, an accurate serum marker for severe hepatic fibrosis in patients with non-alcoholic fatty liver disease. Liver Int. 2005;25:779–786. doi: 10.1111/j.1478-3231.2005.01064.x. [DOI] [PubMed] [Google Scholar]
- Halfon P, Bourliere M, Penaranda G, Deydier R, Renou C, Botta-Fridlund D, Tran A, Portal I, Allemand I, Rosenthal-Allieri A, Ouzan D. Accuracy of hyaluronic acid level for predicting liver fibrosis stages in patients with hepatitis C virus. Comp Hepatol. 2005;4:6. doi: 10.1186/1476-5926-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakala BE, White C, Recklies AD. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J Biol Chem. 1993;268:25803–25810. [PubMed] [Google Scholar]
- Saitou Y, Shiraki K, Yamanaka Y, Yamaguchi Y, Kawakita T, Yamamoto N, Sugimoto K, Murata K, Nakano T. Noninvasive estimation of liver fibrosis and response to interferon therapy by a serum fibrogenesis marker, YKL-40, in patients with HCV-associated liver disease. World J Gastroenterol. 2005;11:476–481. doi: 10.3748/wjg.v11.i4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanvisens A, Serra I, Tural C, Tor J, Ojanguren I, Barluenga E, Rey-Joly C, Clotet B, Muga R. Hyaluronic acid, transforming growth factor-beta1 and hepatic fibrosis in patients with chronic hepatitis C virus and human immunodeficiency virus co-infection. J Viral Hepat. 2009;16:513–518. doi: 10.1111/j.1365-2893.2009.01103.x. [DOI] [PubMed] [Google Scholar]
- Schiavon LL, Carvalho-Filho RJ, Narciso-Schiavon JL, Medina-Pestana JO, Lanzoni VP, Ferraz ML, Silva AE. YKL-40 and hyaluronic acid (HA) as noninvasive markers of liver fibrosis in kidney transplant patients with HCV chronic infection. Scand J Gastroenterol. 2010;45:615–622. doi: 10.3109/00365521003637203. [DOI] [PubMed] [Google Scholar]
- Felig P. The glucose-alanine cycle. Metabolism. 1973;22:179–207. doi: 10.1016/0026-0495(73)90269-2. [DOI] [PubMed] [Google Scholar]
- Daxboeck F, Gattringer R, Mustafa S, Bauer C, Assadian O. Elevated serum alanine aminotransferase (ALT) levels in patients with serologically verified Mycoplasma pneumoniae pneumonia. Clin Microbiol Infect. 2005;11:507–510. doi: 10.1111/j.1469-0691.2005.01154.x. [DOI] [PubMed] [Google Scholar]
- Sherman KE. Alanine aminotransferase in clinical practice. A review. Arch Intern Med. 1991;151:260–265. doi: 10.1001/archinte.151.2.260. [DOI] [PubMed] [Google Scholar]
- Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB. Diagnosis and monitoring of hepatic injury. I. Performance characteristics of laboratory tests. Clin Chem. 2000;46:2027–2049. [PubMed] [Google Scholar]
- Akkaya O, Kiyici M, Yilmaz Y, Ulukaya E, Yerci O. Clinical significance of activity of ALT enzyme in patients with hepatitis C virus. World J Gastroenterol. 2007;13:5481–5485. doi: 10.3748/wjg.v13.i41.5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Oh SW, Kim DJ, Choi EY. Abundance of immunologically active alanine aminotransferase in sera of liver cirrhosis and hepatocellular carcinoma patients. Clin Chem. 2009;55:1022–1025. doi: 10.1373/clinchem.2008.102996. [DOI] [PubMed] [Google Scholar]
- Shiffman ML, Diago M, Tran A, Pockros P, Reindollar R, Prati D, Rodriguez-Torres M, Lardelli P, Blotner S, Zeuzem S. Chronic hepatitis C in patients with persistently normal alanine transaminase levels. Clin Gastroenterol Hepatol. 2006;4:645–652. doi: 10.1016/j.cgh.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Okuda M, Li K, Beard MR, Showalter LA, Scholle F, Lemon SM, Weinman SA. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002;122:366–375. doi: 10.1053/gast.2002.30983. [DOI] [PubMed] [Google Scholar]
- Zechini B, Pasquazzi C, Aceti A. Correlation of serum aminotransferases with HCV RNA levels and histological findings in patients with chronic hepatitis C: the role of serum aspartate transaminase in the evaluation of disease progression. Eur J Gastroenterol Hepatol. 2004;16:891–896. doi: 10.1097/00042737-200409000-00013. [DOI] [PubMed] [Google Scholar]
- Cedrone A, Covino M, Caturelli E, Pompili M, Lorenzelli G, Villani MR, Valle D, Sperandeo M, Rapaccini GL, Gasbarrini G. Utility of alpha-fetoprotein (AFP) in the screening of patients with virus-related chronic liver disease: does different viral etiology influence AFP levels in HCC? A study in 350 western patients. Hepatogastroenterology. 2000;47:1654–1658. [PubMed] [Google Scholar]
- Chu CW, Hwang SJ, Luo JC, Lai CR, Tsay SH, Li CP, Wu JC, Chang FY, Lee SD. Clinical, virologic, and pathologic significance of elevated serum alpha-fetoprotein levels in patients with chronic hepatitis C. J Clin Gastroenterol. 2001;32:240–244. doi: 10.1097/00004836-200103000-00014. [DOI] [PubMed] [Google Scholar]
- Chen TM, Huang PT, Tsai MH, Lin LF, Liu CC, Ho KS, Siauw CP, Chao PL, Tung JN. Predictors of alpha-fetoprotein elevation in patients with chronic hepatitis C, but not hepatocellular carcinoma, and its normalization after pegylated interferon alfa 2a-ribavirin combination therapy. J Gastroenterol Hepatol. 2007;22:669–675. doi: 10.1111/j.1440-1746.2007.04898.x. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Yamagiwa S, Aoki Y, Kurita S, Suda T, Ohkoshi S, Nomoto M, Aoyagi Y. Serum alpha-fetoprotein levels during and after interferon therapy and the development of hepatocellular carcinoma in patients with chronic hepatitis C. Dig Dis Sci. 2009;54:2530–2537. doi: 10.1007/s10620-008-0642-y. [DOI] [PubMed] [Google Scholar]
- Aster RH. Pooling of platelets in the spleen: role in the pathogenesis of "hypersplenic" thrombocytopenia. J Clin Invest. 1966;45:645–657. doi: 10.1172/JCI105380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T, Takeshita A, Souda K, Kobayashi Y, Kikuyama M, Suzuki F, Kageyama F, Sasada Y, Shimizu E, Murohisa G. et al. Serum thrombopoietin levels in patients with chronic hepatitis and liver cirrhosis. Am J Gastroenterol. 1999;94:1918–1922. doi: 10.1111/j.1572-0241.1999.01231.x. [DOI] [PubMed] [Google Scholar]
- Adinolfi LE, Giordano MG, Andreana A, Tripodi MF, Utili R, Cesaro G, Ragone E, Durante Mangoni E, Ruggiero G. Hepatic fibrosis plays a central role in the pathogenesis of thrombocytopenia in patients with chronic viral hepatitis. Br J Haematol. 2001;113:590–595. doi: 10.1046/j.1365-2141.2001.02824.x. [DOI] [PubMed] [Google Scholar]
- Lackner C, Struber G, Liegl B, Leibl S, Ofner P, Bankuti C, Bauer B, Stauber RE. Comparison and validation of simple noninvasive tests for prediction of fibrosis in chronic hepatitis C. Hepatology. 2005;41:1376–1382. doi: 10.1002/hep.20717. [DOI] [PubMed] [Google Scholar]
- Croquet V, Vuillemin E, Ternisien C, Pilette C, Oberti F, Gallois Y, Trossaert M, Rousselet MC, Chappard D, Cales P. Prothrombin index is an indirect marker of severe liver fibrosis. Eur J Gastroenterol Hepatol. 2002;14:1133–1141. doi: 10.1097/00042737-200210000-00015. [DOI] [PubMed] [Google Scholar]
- Pilette C, Oberti F, Aube C, Rousselet MC, Bedossa P, Gallois Y, Rifflet H, Cales P. Non-invasive diagnosis of esophageal varices in chronic liver diseases. J Hepatol. 1999;31:867–873. doi: 10.1016/S0168-8278(99)80288-8. [DOI] [PubMed] [Google Scholar]
- Craxi A, Camma C, Giunta M. Clinical aspects of bleeding complications in cirrhotic patients. Blood Coagul Fibrinolysis. 2000;11(Suppl 1):S75–79. doi: 10.1097/00001721-200004001-00015. [DOI] [PubMed] [Google Scholar]
- Sheth SG, Flamm SL, Gordon FD, Chopra S. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1998;93:44–48. doi: 10.1111/j.1572-0241.1998.044_c.x. [DOI] [PubMed] [Google Scholar]
- Reedy DW, Loo AT, Levine RA. AST/ALT ratio > or = 1 is not diagnostic of cirrhosis in patients with chronic hepatitis C. Dig Dis Sci. 1998;43:2156–2159. doi: 10.1023/A:1018888021118. [DOI] [PubMed] [Google Scholar]
- Giannini E, Risso D, Botta F, Chiarbonello B, Fasoli A, Malfatti F, Romagnoli P, Testa E, Ceppa P, Testa R. Validity and clinical utility of the aspartate aminotransferase-alanine aminotransferase ratio in assessing disease severity and prognosis in patients with hepatitis C virus-related chronic liver disease. Arch Intern Med. 2003;163:218–224. doi: 10.1001/archinte.163.2.218. [DOI] [PubMed] [Google Scholar]
- Fujii H, Enomoto M, Fukushima W, Ohfuji S, Mori M, Kobayashi S, Iwai S, Morikawa H, Tamori A, Sakaguchi H. et al. Noninvasive laboratory tests proposed for predicting cirrhosis in patients with chronic hepatitis C are also useful in patients with non-alcoholic steatohepatitis. J Gastroenterol. 2009;44:608–614. doi: 10.1007/s00535-009-0046-6. [DOI] [PubMed] [Google Scholar]
- Poynard T, Morra R, Halfon P, Castera L, Ratziu V, Imbert-Bismut F, Naveau S, Thabut D, Lebrec D, Zoulim F. et al. Meta-analyses of FibroTest diagnostic value in chronic liver disease. BMC Gastroenterol. 2007;7:40. doi: 10.1186/1471-230X-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cales P, Oberti F, Michalak S, Hubert-Fouchard I, Rousselet MC, Konate A, Gallois Y, Ternisien C, Chevailler A, Lunel F. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology. 2005;42:1373–1381. doi: 10.1002/hep.20935. [DOI] [PubMed] [Google Scholar]
- Bourliere M, Penaranda G, Renou C, Botta-Fridlund D, Tran A, Portal I, Lecomte L, Castellani P, Rosenthal-Allieri MA, Gerolami R. et al. Validation and comparison of indexes for fibrosis and cirrhosis prediction in chronic hepatitis C patients: proposal for a pragmatic approach classification without liver biopsies. J Viral Hepat. 2006;13:659–670. doi: 10.1111/j.1365-2893.2006.00736.x. [DOI] [PubMed] [Google Scholar]
- Parise ER, Oliveira AC, Figueiredo-Mendes C, Lanzoni V, Martins J, Nader H, Ferraz ML. Noninvasive serum markers in the diagnosis of structural liver damage in chronic hepatitis C virus infection. Liver Int. 2006;26:1095–1099. doi: 10.1111/j.1478-3231.2006.01356.x. [DOI] [PubMed] [Google Scholar]
- de Ledinghen V, Douvin C, Kettaneh A, Ziol M, Roulot D, Marcellin P, Dhumeaux D, Beaugrand M. Diagnosis of hepatic fibrosis and cirrhosis by transient elastography in HIV/hepatitis C virus-coinfected patients. J Acquir Immune Defic Syndr. 2006;41:175–179. doi: 10.1097/01.qai.0000194238.15831.c7. [DOI] [PubMed] [Google Scholar]
- Halfon P, Bacq Y, De Muret A, Penaranda G, Bourliere M, Ouzan D, Tran A, Botta D, Renou C, Brechot MC. et al. Comparison of test performance profile for blood tests of liver fibrosis in chronic hepatitis C. J Hepatol. 2007;46:395–402. doi: 10.1016/j.jhep.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Leroy V, Halfon P, Bacq Y, Boursier J, Rousselet MC, Bourliere M, de Muret A, Sturm N, Hunault G, Penaranda G. et al. Diagnostic accuracy, reproducibility and robustness of fibrosis blood tests in chronic hepatitis C: a meta-analysis with individual data. Clin Biochem. 2008;41:1368–1376. doi: 10.1016/j.clinbiochem.2008.06.020. [DOI] [PubMed] [Google Scholar]
- Cales P, de Ledinghen V, Halfon P, Bacq Y, Leroy V, Boursier J, Foucher J, Bourliere M, de Muret A, Sturm N. et al. Evaluating the accuracy and increasing the reliable diagnosis rate of blood tests for liver fibrosis in chronic hepatitis C. Liver Int. 2008;28:1352–1362. doi: 10.1111/j.1478-3231.2008.01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphues C, Lotz K, Rocken C, Berg T, Eurich D, Pratschke J, Neuhaus P, Neumann UP. Chances and limitations of non-invasive tests in the assessment of liver fibrosis in liver transplant patients. Clin Transplant. 2010;24:652–659. doi: 10.1111/j.1399-0012.2009.01152.x. [DOI] [PubMed] [Google Scholar]
- Teare JP, Sherman D, Greenfield SM, Simpson J, Bray G, Catterall AP, Murray-Lyon IM, Peters TJ, Williams R, Thompson RP. Comparison of serum procollagen III peptide concentrations and PGA index for assessment of hepatic fibrosis. Lancet. 1993;342:895–898. doi: 10.1016/0140-6736(93)91946-J. [DOI] [PubMed] [Google Scholar]
- Naveau S, Poynard T, Benattar C, Bedossa P, Chaput JC. Alpha-2-macroglobulin and hepatic fibrosis. Diagnostic interest. Dig Dis Sci. 1994;39:2426–2432. doi: 10.1007/BF02087661. [DOI] [PubMed] [Google Scholar]
- Oberti F, Valsesia E, Pilette C, Rousselet MC, Bedossa P, Aube C, Gallois Y, Rifflet H, Maiga MY, Penneau-Fontbonne D, Cales P. Noninvasive diagnosis of hepatic fibrosis or cirrhosis. Gastroenterology. 1997;113:1609–1616. doi: 10.1053/gast.1997.v113.pm9352863. [DOI] [PubMed] [Google Scholar]
- Poynard T, McHutchison J, Manns M, Myers RP, Albrecht J. Biochemical surrogate markers of liver fibrosis and activity in a randomized trial of peginterferon alfa-2b and ribavirin. Hepatology. 2003;38:481–492. doi: 10.1053/jhep.2003.50319. [DOI] [PubMed] [Google Scholar]
- Poynard T, Munteanu M, Imbert-Bismut F, Charlotte F, Thabut D, Le Calvez S, Messous D, Thibault V, Benhamou Y, Moussalli J, Ratziu V. Prospective analysis of discordant results between biochemical markers and biopsy in patients with chronic hepatitis C. Clin Chem. 2004;50:1344–1355. doi: 10.1373/clinchem.2004.032227. [DOI] [PubMed] [Google Scholar]
- Naveau S, Raynard B, Ratziu V, Abella A, Imbert-Bismut F, Messous D, Beuzen F, Capron F, Thabut D, Munteanu M. et al. Biomarkers for the prediction of liver fibrosis in patients with chronic alcoholic liver disease. Clin Gastroenterol Hepatol. 2005;3:167–174. doi: 10.1016/S1542-3565(04)00625-1. [DOI] [PubMed] [Google Scholar]
- Sebastiani G, Alberti A. Non invasive fibrosis biomarkers reduce but not substitute the need for liver biopsy. World J Gastroenterol. 2006;12:3682–3694. doi: 10.3748/wjg.v12.i23.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich-Rust M, Rosenberg W, Parkes J, Herrmann E, Zeuzem S, Sarrazin C. Comparison of ELF, FibroTest and FibroScan for the non-invasive assessment of liver fibrosis. BMC Gastroenterol. 2010;10:103. doi: 10.1186/1471-230X-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfon P, Imbert-Bismut F, Messous D, Antoniotti G, Benchetrit D, Cart-Lamy P, Delaporte G, Doutheau D, Klump T, Sala M. et al. A prospective assessment of the inter-laboratory variability of biochemical markers of fibrosis (FibroTest) and activity (ActiTest) in patients with chronic liver disease. Comp Hepatol. 2002;1:3. doi: 10.1186/1476-5926-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poynard T, Imbert-Bismut F, Ratziu V, Chevret S, Jardel C, Moussalli J, Messous D, Degos F. Biochemical markers of liver fibrosis in patients infected by hepatitis C virus: longitudinal validation in a randomized trial. J Viral Hepat. 2002;9:128–133. doi: 10.1046/j.1365-2893.2002.00341.x. [DOI] [PubMed] [Google Scholar]
- Rossi E, Adams L, Prins A, Bulsara M, de Boer B, Garas G, MacQuillan G, Speers D, Jeffrey G. Validation of the FibroTest biochemical markers score in assessing liver fibrosis in hepatitis C patients. Clin Chem. 2003;49:450–454. doi: 10.1373/49.3.450. [DOI] [PubMed] [Google Scholar]
- Ngo Y, Munteanu M, Messous D, Charlotte F, Imbert-Bismut F, Thabut D, Lebray P, Thibault V, Benhamou Y, Moussalli J. et al. A prospective analysis of the prognostic value of biomarkers (FibroTest) in patients with chronic hepatitis C. Clin Chem. 2006;52:1887–1896. doi: 10.1373/clinchem.2006.070961. [DOI] [PubMed] [Google Scholar]
- Halfon P, Bourliere M, Deydier R, Botta-Fridlund D, Renou C, Tran A, Portal I, Allemand I, Bertrand JJ, Rosenthal-Allieri A. et al. Independent prospective multicenter validation of biochemical markers (fibrotest-actitest) for the prediction of liver fibrosis and activity in patients with chronic hepatitis C: the fibropaca study. Am J Gastroenterol. 2006;101:547–555. doi: 10.1111/j.1572-0241.2006.00411.x. [DOI] [PubMed] [Google Scholar]
- Halfon P, Munteanu M, Poynard T. FibroTest-ActiTest as a non-invasive marker of liver fibrosis. Gastroenterol Clin Biol. 2008;32:22–39. doi: 10.1016/S0399-8320(08)73991-5. [DOI] [PubMed] [Google Scholar]
- Myers RP, De Torres M, Imbert-Bismut F, Ratziu V, Charlotte F, Poynard T. Biochemical markers of fibrosis in patients with chronic hepatitis C: a comparison with prothrombin time, platelet count, and age-platelet index. Dig Dis Sci. 2003;48:146–153. doi: 10.1023/A:1021702902681. [DOI] [PubMed] [Google Scholar]
- Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–1457. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- Colletta C, Smirne C, Fabris C, Toniutto P, Rapetti R, Minisini R, Pirisi M. Value of two noninvasive methods to detect progression of fibrosis among HCV carriers with normal aminotransferases. Hepatology. 2005;42:838–845. doi: 10.1002/hep.20814. [DOI] [PubMed] [Google Scholar]
- Shaheen AA, Myers RP. Systematic review and meta-analysis of the diagnostic accuracy of fibrosis marker panels in patients with HIV/hepatitis C coinfection. HIV Clin Trials. 2008;9:43–51. doi: 10.1310/hct0901-43. [DOI] [PubMed] [Google Scholar]
- Cales P, Boursier J, Bertrais S, Oberti F, Gallois Y, Fouchard-Hubert I, Dib N, Zarski JP, Rousselet MC. Optimization and robustness of blood tests for liver fibrosis and cirrhosis. Clin Biochem. 2010;43:1315–1322. doi: 10.1016/j.clinbiochem.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Koda M, Matunaga Y, Kawakami M, Kishimoto Y, Suou T, Murawaki Y. FibroIndex, a practical index for predicting significant fibrosis in patients with chronic hepatitis C. Hepatology. 2007;45:297–306. doi: 10.1002/hep.21520. [DOI] [PubMed] [Google Scholar]
- Lee MH, Cheong JY, Um SH, Seo YS, Kim DJ, Hwang SG, Yang JM, Han KH, Cho SW. Comparison of surrogate serum markers and transient elastography (Fibroscan) for assessing cirrhosis in patients with chronic viral hepatitis. Dig Dis Sci. 2010;55:3552–3560. doi: 10.1007/s10620-010-1219-0. [DOI] [PubMed] [Google Scholar]
- El-Shabrawi MH, Mohsen NA, Sherif MM, El-Karaksy HM, Abou-Yosef H, El-Sayed HM, Riad H, Bahaa N, Isa M, El-Hennawy A. Noninvasive assessment of hepatic fibrosis and necroinflammatory activity in Egyptian children with chronic hepatitis C virus infection using FibroTest and ActiTest. Eur J Gastroenterol Hepatol. 2010;22:946–951. doi: 10.1097/MEG.0b013e328336ec84. [DOI] [PubMed] [Google Scholar]
- Poynard T, Ratziu V, Naveau S, Thabut D, Charlotte F, Messous D, Capron D, Abella A, Massard J, Ngo Y. et al. The diagnostic value of biomarkers (SteatoTest) for the prediction of liver steatosis. Comp Hepatol. 2005;4:10. doi: 10.1186/1476-5926-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok AS, Ghany MG, Goodman ZD, Wright EC, Everson GT, Sterling RK, Everhart JE, Lindsay KL, Bonkovsky HL, Di Bisceglie AM. et al. Predicting cirrhosis in patients with hepatitis C based on standard laboratory tests: results of the HALT-C cohort. Hepatology. 2005;42:282–292. doi: 10.1002/hep.20772. [DOI] [PubMed] [Google Scholar]
- Cheong JY, Um SH, Seo YS, Kim DJ, Hwang SG, Lee YJ, Cho M, Yang JM, Kim YB, Park YN, Cho SW. Non-Invasive Index for Predicting Significant Liver Fibrosis: Comparison of Diagnostic Performances in Patients with Chronic Hepatitis B and C. Dig Dis Sci. 2010;56:555–563. doi: 10.1007/s10620-010-1305-3. [DOI] [PubMed] [Google Scholar]
- Islam S, Antonsson L, Westin J, Lagging M. Cirrhosis in hepatitis C virus-infected patients can be excluded using an index of standard biochemical serum markers. Scand J Gastroenterol. 2005;40:867–872. doi: 10.1080/00365520510015674. [DOI] [PubMed] [Google Scholar]
- Ohta T, Sakaguchi K, Fujiwara A, Fujioka S, Iwasaki Y, Makino Y, Araki Y, Shiratori Y. Simple surrogate index of the fibrosis stage in chronic hepatitis C patients using platelet count and serum albumin level. Acta Med Okayama. 2006;60:77–84. doi: 10.18926/AMO/30729. [DOI] [PubMed] [Google Scholar]
- Pohl A, Behling C, Oliver D, Kilani M, Monson P, Hassanein T. Serum aminotransferase levels and platelet counts as predictors of degree of fibrosis in chronic hepatitis C virus infection. Am J Gastroenterol. 2001;96:3142–3146. doi: 10.1111/j.1572-0241.2001.05268.x. [DOI] [PubMed] [Google Scholar]
- Cheung RC, Currie S, Shen H, Bini EJ, Ho SB, Anand BS, Hu KQ, Wright TL, Morgan TR. Can we predict the degree of fibrosis in chronic hepatitis C patients using routine blood tests in our daily practice? J Clin Gastroenterol. 2008;42:827–834. doi: 10.1097/MCG.0b013e318046ea9a. [DOI] [PubMed] [Google Scholar]
- Bonacini M, Hadi G, Govindarajan S, Lindsay KL. Utility of a discriminant score for diagnosing advanced fibrosis or cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1997;92:1302–1304. [PubMed] [Google Scholar]
- Adams LA, Bulsara M, Rossi E, DeBoer B, Speers D, George J, Kench J, Farrell G, McCaughan GW, Jeffrey GP. Hepascore: an accurate validated predictor of liver fibrosis in chronic hepatitis C infection. Clin Chem. 2005;51:1867–1873. doi: 10.1373/clinchem.2005.048389. [DOI] [PubMed] [Google Scholar]
- Leroy V, Hilleret MN, Sturm N, Trocme C, Renversez JC, Faure P, Morel F, Zarski JP. Prospective comparison of six non-invasive scores for the diagnosis of liver fibrosis in chronic hepatitis C. J Hepatol. 2007;46:775–782. doi: 10.1016/j.jhep.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Becker L, Salameh W, Sferruzza A, Zhang K, ng Chen R, Malik R, Reitz R, Nasser I, Afdhal NH. Validation of hepascore, compared with simple indices of fibrosis, in patients with chronic hepatitis C virus infection in United States. Clin Gastroenterol Hepatol. 2009;7:696–701. doi: 10.1016/j.cgh.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Guechot J, Lasnier E, Sturm N, Paris A, Zarski JP. Automation of the Hepascore and validation as a biochemical index of liver fibrosis in patients with chronic hepatitis C from the ANRS HC EP 23 Fibrostar cohort. Clin Chim Acta. 2010;411:86–91. doi: 10.1016/j.cca.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Kelleher TB, Mehta SH, Bhaskar R, Sulkowski M, Astemborski J, Thomas DL, Moore RE, Afdhal NH. Prediction of hepatic fibrosis in HIV/HCV co-infected patients using serum fibrosis markers: the SHASTA index. J Hepatol. 2005;43:78–84. doi: 10.1016/j.jhep.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, M SS, Torriani FJ, Dieterich DT, Thomas DL. et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, Pol S. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- Mallet V, Dhalluin-Venier V, Roussin C, Bourliere M, Pettinelli ME, Giry C, Vallet-Pichard A, Fontaine H, Pol S. The accuracy of the FIB-4 index for the diagnosis of mild fibrosis in chronic hepatitis B. Aliment Pharmacol Ther. 2009;29:409–415. doi: 10.1111/j.1365-2036.2008.03895.x. [DOI] [PubMed] [Google Scholar]
- Sud A, Hui JM, Farrell GC, Bandara P, Kench JG, Fung C, Lin R, Samarasinghe D, Liddle C, McCaughan GW, George J. Improved prediction of fibrosis in chronic hepatitis C using measures of insulin resistance in a probability index. Hepatology. 2004;39:1239–1247. doi: 10.1002/hep.20207. [DOI] [PubMed] [Google Scholar]
- Testa R, Testa E, Giannini E, Borro P, Milazzo S, Isola L, Ceppa P, Lantieri PB, Risso D. Noninvasive ratio indexes to evaluate fibrosis staging in chronic hepatitis C: role of platelet count/spleen diameter ratio index. J Intern Med. 2006;260:142–150. doi: 10.1111/j.1365-2796.2006.01673.x. [DOI] [PubMed] [Google Scholar]
- Fortunato G, Castaldo G, Oriani G, Cerini R, Intrieri M, Molinaro E, Gentile I, Borgia G, Piazza M, Salvatore F, Sacchetti L. Multivariate discriminant function based on six biochemical markers in blood can predict the cirrhotic evolution of chronic hepatitis. Clin Chem. 2001;47:1696–1700. [PubMed] [Google Scholar]
- Aube C, Oberti F, Korali N, Namour MA, Loisel D, Tanguy JY, Valsesia E, Pilette C, Rousselet MC, Bedossa P. et al. Ultrasonographic diagnosis of hepatic fibrosis or cirrhosis. J Hepatol. 1999;30:472–478. doi: 10.1016/S0168-8278(99)80107-X. [DOI] [PubMed] [Google Scholar]
- Mathiesen UL, Franzen LE, Aselius H, Resjo M, Jacobsson L, Foberg U, Fryden A, Bodemar G. Increased liver echogenicity at ultrasound examination reflects degree of steatosis but not of fibrosis in asymptomatic patients with mild/moderate abnormalities of liver transaminases. Dig Liver Dis. 2002;34:516–522. doi: 10.1016/S1590-8658(02)80111-6. [DOI] [PubMed] [Google Scholar]
- Colli A, Fraquelli M, Andreoletti M, Marino B, Zuccoli E, Conte D. Severe liver fibrosis or cirrhosis: accuracy of US for detection--analysis of 300 cases. Radiology. 2003;227:89–94. doi: 10.1148/radiol.2272020193. [DOI] [PubMed] [Google Scholar]
- Zheng RQ, Wang QH, Lu MD, Xie SB, Ren J, Su ZZ, Cai YK, Yao JL. Liver fibrosis in chronic viral hepatitis: an ultrasonographic study. World J Gastroenterol. 2003;9:2484–2489. doi: 10.3748/wjg.v9.i11.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colli A, Colucci A, Paggi S, Fraquelli M, Massironi S, Andreoletti M, Michela V, Conte D. Accuracy of a predictive model for severe hepatic fibrosis or cirrhosis in chronic hepatitis C. World J Gastroenterol. 2005;11:7318–7322. doi: 10.3748/wjg.v11.i46.7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Li JQ, Zeng MD, Lu LG, Fan ST, Bao H. Correlation between ultrasonographic and pathologic diagnosis of liver fibrosis due to chronic virus hepatitis. World J Gastroenterol. 2006;12:1292–1295. doi: 10.3748/wjg.v12.i8.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrin L, Tanter M, Gennisson JL, Catheline S, Fink M. Shear elasticity probe for soft tissues with 1-D transient elastography. IEEE Trans Ultrason Ferroelectr Freq Control. 2002;49:436–446. doi: 10.1109/58.996561. [DOI] [PubMed] [Google Scholar]
- Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F. et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–1713. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol. 2003;39:239–244. doi: 10.1016/S0168-8278(03)00191-0. [DOI] [PubMed] [Google Scholar]
- Cobbold JF, Morin S, Taylor-Robinson SD. Transient elastography for the assessment of chronic liver disease: ready for the clinic? World J Gastroenterol. 2007;13:4791–4797. doi: 10.3748/wjg.v13.i36.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettaneh A, Marcellin P, Douvin C, Poupon R, Ziol M, Beaugrand M, de Ledinghen V. Features associated with success rate and performance of FibroScan measurements for the diagnosis of cirrhosis in HCV patients: a prospective study of 935 patients. J Hepatol. 2007;46:628–634. doi: 10.1016/j.jhep.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Foucher J, Chanteloup E, Vergniol J, Castera L, Le Bail B, Adhoute X, Bertet J, Couzigou P, de Ledinghen V. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403–408. doi: 10.1136/gut.2005.069153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maor Y, Halfon P, Bashari D, Penaranda G, Morali G, Klar R, Bar-Meir S, Martinowitz U, Oren R. Fibrotest or Fibroscan for evaluation of liver fibrosis in haemophilia patients infected with hepatitis C. Haemophilia. 2010;16:148–154. doi: 10.1111/j.1365-2516.2009.02092.x. [DOI] [PubMed] [Google Scholar]
- Friedrich-Rust M, Ong MF, Herrmann E, Dries V, Samaras P, Zeuzem S, Sarrazin C. Real-time elastography for noninvasive assessment of liver fibrosis in chronic viral hepatitis. AJR Am J Roentgenol. 2007;188:758–764. doi: 10.2214/AJR.06.0322. [DOI] [PubMed] [Google Scholar]
- Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G, Colombo M. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968–973. doi: 10.1136/gut.2006.111302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizzutti F, Arena U, Romanelli RG, Rega L, Foschi M, Colagrande S, Petrarca A, Moscarella S, Belli G, Zignego AL. et al. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology. 2007;45:1290–1297. doi: 10.1002/hep.21665. [DOI] [PubMed] [Google Scholar]
- de Ledinghen V, Vergniol J. Transient elastography for the diagnosis of liver fibrosis. Expert Rev Med Devices. 2010;7:811–823. doi: 10.1586/erd.10.46. [DOI] [PubMed] [Google Scholar]
- Corpechot C, El Naggar A, Poujol-Robert A, Ziol M, Wendum D, Chazouilleres O, de Ledinghen V, Dhumeaux D, Marcellin P, Beaugrand M, Poupon R. Assessment of biliary fibrosis by transient elastography in patients with PBC and PSC. Hepatology. 2006;43:1118–1124. doi: 10.1002/hep.21151. [DOI] [PubMed] [Google Scholar]
- Ganne-Carrie N, Ziol M, de Ledinghen V, Douvin C, Marcellin P, Castera L, Dhumeaux D, Trinchet JC, Beaugrand M. Accuracy of liver stiffness measurement for the diagnosis of cirrhosis in patients with chronic liver diseases. Hepatology. 2006;44:1511–1517. doi: 10.1002/hep.21420. [DOI] [PubMed] [Google Scholar]
- Shaheen AA, Wan AF, Myers RP. FibroTest and FibroScan for the prediction of hepatitis C-related fibrosis: a systematic review of diagnostic test accuracy. Am J Gastroenterol. 2007;102:2589–2600. doi: 10.1111/j.1572-0241.2007.01466.x. [DOI] [PubMed] [Google Scholar]
- Friedrich-Rust M, Wunder K, Kriener S, Sotoudeh F, Richter S, Bojunga J, Herrmann E, Poynard T, Dietrich CF, Vermehren J. et al. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology. 2009;252:595–604. doi: 10.1148/radiol.2523081928. [DOI] [PubMed] [Google Scholar]
- Sanchez-Conde M, Montes-Ramirez ML, Miralles P, Alvarez JM, Bellon JM, Ramirez M, Arribas JR, Gutierrez I, Lopez JC, Cosin J. et al. Comparison of transient elastography and liver biopsy for the assessment of liver fibrosis in HIV/hepatitis C virus-coinfected patients and correlation with noninvasive serum markers. J Viral Hepat. 2010;17:280–286. doi: 10.1111/j.1365-2893.2009.01180.x. [DOI] [PubMed] [Google Scholar]
- Abu-Yousef MM. Duplex Doppler sonography of the hepatic vein in tricuspid regurgitation. AJR Am J Roentgenol. 1991;156:79–83. doi: 10.2214/ajr.156.1.1898574. [DOI] [PubMed] [Google Scholar]
- Albrecht T, Blomley MJ, Cosgrove DO, Taylor-Robinson SD, Jayaram V, Eckersley R, Urbank A, Butler-Barnes J, Patel N. Non-invasive diagnosis of hepatic cirrhosis by transit-time analysis of an ultrasound contrast agent. Lancet. 1999;353:1579–1583. doi: 10.1016/S0140-6736(98)06373-9. [DOI] [PubMed] [Google Scholar]
- Hirata M, Akbar SM, Horiike N, Onji M. Noninvasive diagnosis of the degree of hepatic fibrosis using ultrasonography in patients with chronic liver disease due to hepatitis C virus. Eur J Clin Invest. 2001;31:528–535. doi: 10.1046/j.1365-2362.2001.00840.x. [DOI] [PubMed] [Google Scholar]
- Bonekamp S, Kamel I, Solga S, Clark J. Can imaging modalities diagnose and stage hepatic fibrosis and cirrhosis accurately? J Hepatol. 2009;50:17–35. doi: 10.1016/j.jhep.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Lucidarme O, Baleston F, Cadi M, Bellin MF, Charlotte F, Ratziu V, Grenier PA. Non-invasive detection of liver fibrosis: Is superparamagnetic iron oxide particle-enhanced MR imaging a contributive technique? Eur Radiol. 2003;13:467–474. doi: 10.1007/s00330-002-1667-9. [DOI] [PubMed] [Google Scholar]
- Numminen K, Tervahartiala P, Halavaara J, Isoniemi H, Hockerstedt K. Non-invasive diagnosis of liver cirrhosis: magnetic resonance imaging presents special features. Scand J Gastroenterol. 2005;40:76–82. doi: 10.1080/00365520410009384. [DOI] [PubMed] [Google Scholar]
- Aguirre DA, Behling CA, Alpert E, Hassanein TI, Sirlin CB. Liver fibrosis: noninvasive diagnosis with double contrast material-enhanced MR imaging. Radiology. 2006;239:425–437. doi: 10.1148/radiol.2392050505. [DOI] [PubMed] [Google Scholar]
- Taura T, Nakamura K, Takashima S, Kaminou T, Yamada R, Shuto T, Wakasa K. Heterogeneity of hepatic parenchymal enhancement on computed tomography during arterial portography: quantitative analysis of correlation with severity of hepatic fibrosis. Hepatol Res. 2001;20:182–192. doi: 10.1016/S1386-6346(01)00074-2. [DOI] [PubMed] [Google Scholar]
- Materne R, Annet L, Dechambre S, Sempoux C, Smith AM, Corot C, Horsmans Y, Van Beers BE. Dynamic computed tomography with low- and high-molecular-mass contrast agents to assess microvascular permeability modifications in a model of liver fibrosis. Clin Sci (Lond) 2002;103:213–216. doi: 10.1042/CS20020095. [DOI] [PubMed] [Google Scholar]
- Wang S, Fu D, Xu M, Hu D. Advanced fuzzy cellular neural network: application to CT liver images. Artif Intell Med. 2007;39:65–77. doi: 10.1016/j.artmed.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Castera L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Ledinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Asselah T, Bieche I, Laurendeau I, Paradis V, Vidaud D, Degott C, Martinot M, Bedossa P, Valla D, Vidaud M, Marcellin P. Liver gene expression signature of mild fibrosis in patients with chronic hepatitis C. Gastroenterology. 2005;129:2064–2075. doi: 10.1053/j.gastro.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Smith MW, Walters KA, Korth MJ, Fitzgibbon M, Proll S, Thompson JC, Yeh MM, Shuhart MC, Furlong JC, Cox PP. et al. Gene expression patterns that correlate with hepatitis C and early progression to fibrosis in liver transplant recipients. Gastroenterology. 2006;130:179–187. doi: 10.1053/j.gastro.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Huang H, Shiffman ML, Cheung RC, Layden TJ, Friedman S, Abar OT, Yee L, Chokkalingam AP, Schrodi SJ, Chan J. et al. Identification of two gene variants associated with risk of advanced fibrosis in patients with chronic hepatitis C. Gastroenterology. 2006;130:1679–1687. doi: 10.1053/j.gastro.2006.02.032. [DOI] [PubMed] [Google Scholar]
- Machida K, Cheng KT, Sung VM, Levine AM, Foung S, Lai MM. Hepatitis C virus induces toll-like receptor 4 expression, leading to enhanced production of beta interferon and interleukin-6. J Virol. 2006;80:866–874. doi: 10.1128/JVI.80.2.866-874.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Shiffman ML, Friedman S, Venkatesh R, Bzowej N, Abar OT, Rowland CM, Catanese JJ, Leong DU, Sninsky JJ. et al. A 7 gene signature identifies the risk of developing cirrhosis in patients with chronic hepatitis C. Hepatology. 2007;46:297–306. doi: 10.1002/hep.21695. [DOI] [PubMed] [Google Scholar]
- Zeremski M, Petrovic LM, Chiriboga L, Brown QB, Yee HT, Kinkhabwala M, Jacobson IM, Dimova R, Markatou M, Talal AH. Intrahepatic levels of CXCR3-associated chemokines correlate with liver inflammation and fibrosis in chronic hepatitis C. Hepatology. 2008;48:1440–1450. doi: 10.1002/hep.22500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeremski M, Dimova R, Brown Q, Jacobson IM, Markatou M, Talal AH. Peripheral CXCR3-associated chemokines as biomarkers of fibrosis in chronic hepatitis C virus infection. J Infect Dis. 2009;200:1774–1780. doi: 10.1086/646614. [DOI] [PubMed] [Google Scholar]
- Kovalenko E, Tacke F, Gressner OA, Zimmermann HW, Lahme B, Janetzko A, Wiederholt T, Berg T, Muller T, Trautwein C. et al. Validation of connective tissue growth factor (CTGF/CCN2) and its gene polymorphisms as noninvasive biomarkers for the assessment of liver fibrosis. J Viral Hepat. 2009;16:612–620. doi: 10.1111/j.1365-2893.2009.01110.x. [DOI] [PubMed] [Google Scholar]
- Sharma A, Chakraborti A, Das A, Dhiman RK, Chawla Y. Elevation of interleukin-18 in chronic hepatitis C: implications for hepatitis C virus pathogenesis. Immunology. 2009;128:e514–522. doi: 10.1111/j.1365-2567.2008.03021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillot F, Hiron M, Goria O, Gueudin M, Francois A, Scotte M, Daveau M, Salier JP. Novel serum markers of fibrosis progression for the follow-up of hepatitis C virus-infected patients. Am J Pathol. 2009;175:46–53. doi: 10.2353/ajpath.2009.080850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Reyes G, Gutierrez-Ruiz MC, Kershenobich D. Liver fibrosis and chronic viral hepatitis. Arch Med Res. 2007;38:644–651. doi: 10.1016/j.arcmed.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Desmond PV, Patwardhan RV, Johnson RF, Schenker S. Impaired elimination of caffeine in cirrhosis. Dig Dis Sci. 1980;25:193–197. doi: 10.1007/BF01308138. [DOI] [PubMed] [Google Scholar]
- Park GJ, Katelaris PH, Jones DB, Seow F, Le Couteur DG, Ngu MC. Validity of the 13C-caffeine breath test as a noninvasive, quantitative test of liver function. Hepatology. 2003;38:1227–1236. doi: 10.1053/jhep.2003.50475. [DOI] [PubMed] [Google Scholar]
- Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/S0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- Kumar D, Farrell GC, Fung C, George J. Hepatitis C virus genotype 3 is cytopathic to hepatocytes: Reversal of hepatic steatosis after sustained therapeutic response. Hepatology. 2002;36:1266–1272. doi: 10.1053/jhep.2002.36370. [DOI] [PubMed] [Google Scholar]
- Abe S, Tabaru A, Ono M, Tai M, Narita R, Moriyama A, Otsuki M. High-dose interferon-alpha therapy lowers the levels of serum fibrogenesis markers over 5 years in chronic hepatitis C. Hepatol Res. 2003;25:22–31. doi: 10.1016/S1386-6346(02)00203-6. [DOI] [PubMed] [Google Scholar]
- Derbala MF, Al Kaabi SR, El Dweik NZ, Pasic F, Butt MT, Yakoob R, Al-Marri A, Amer AM, Morad N, Bener A. Treatment of hepatitis C virus genotype 4 with peginterferon alfa-2a: impact of bilharziasis and fibrosis stage. World J Gastroenterol. 2006;12:5692–5698. doi: 10.3748/wjg.v12.i35.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto R, Nakamuta M, Aoyagi Y, Fujino T, Yasutake K, Koga K, Yoshimoto T, Miyahara T, Fukuizumi K, Wada Y. et al. Validity of FibroScan values for predicting hepatic fibrosis stage in patients with chronic HCV infection. J Dig Dis. 2009;10:145–148. doi: 10.1111/j.1751-2980.2009.00377.x. [DOI] [PubMed] [Google Scholar]
- Vergniol J, Foucher J, Castera L, Bernard PH, Tournan R, Terrebonne E, Chanteloup E, Merrouche W, Couzigou P, de Ledinghen V. Changes of non-invasive markers and FibroScan values during HCV treatment. J Viral Hepat. 2009;16:132–140. doi: 10.1111/j.1365-2893.2008.01055.x. [DOI] [PubMed] [Google Scholar]
- Halfon P, Carrat F, Bedossa P, Lambert J, Penaranda G, Perronne C, Pol S, Cacoub P. Effect of antiviral treatment on serum markers of liver fibrosis in HIV-hepatitis C virus-coinfected patients: the Fibrovic 2 Study - ANRS HC02. Antivir Ther. 2009;14:211–219. [PubMed] [Google Scholar]
- Patel K, Benhamou Y, Yoshida EM, Kaita KD, Zeuzem S, Torbenson M, Pulkstenis E, Subramanian GM, McHutchison JG. An independent and prospective comparison of two commercial fibrosis marker panels (HCV FibroSURE and FIBROSpect II) during albinterferon alfa-2b combination therapy for chronic hepatitis C. J Viral Hepat. 2009;16:178–186. doi: 10.1111/j.1365-2893.2008.01062.x. [DOI] [PubMed] [Google Scholar]


