Abstract
Background
α2-adrenergic receptors (ARs) mediate many cellular actions of epinephrine and norepinephrine and inhibit their secretion from adrenal chromaffin cells. Like many other G-protein coupled receptors (GPCRs), they undergo agonist-dependent phopshorylation and desensitization by GPCR Kinases (GRKs), a phenomenon recently shown to play a major role in the sympathetic overdrive that accompanies and aggravates chronic heart failure. A deletion polymorphism in the human α2B-AR gene (Glu301-303) causes impaired agonist-promoted receptor phosphorylation and desensitization in heterologous cell lines. Given the importance of α2-ARs in regulation of catecholamine secretion from chromaffin cells, we sought to investigate, in the present study, the desensitization properties and the sympatho-inhibitory activity of this variant in a chromaffin cell line. For this purpose, we expressed this variant and its wild type counterpart in the well-established chromaffin cell line PC12, and performed receptor phosphorylation and desensitization studies, as well as in vitro catecholamine secretion assays.
Results
Both the agonist-induced phosphorylation and agonist-dependent desensitization of the human Glu301-303 deletion polymorphic α2B-AR are significantly impaired in PC12 cells, resulting in enhanced signaling to inhibition of cholinergic-induced catecholamine secretion in vitro.
Conclusion
This α2B-AR gene polymorphism (Glu301-303 deletion) might confer better protection against conditions characterized and aggravated by sympathetic/catecholaminergic overstimulation in vivo.
Background
Three distinct α2-adrenergic receptor (α2-AR) subtypes (α2A, α2B, α2C) that mediate many of the physiological actions of the catecholamines (CAs) epinephrine (Epi) and norepinephrine (NE) have been described [1]. They belong to the family of G-protein coupled or seven transmembrane-spanning receptors (GPCRs or 7TMRs) and they are linked to the inhibitory Gi/o proteins [1]. The α2B-AR is critically involved in cardiovascular regulation, as its gene disruption in mice affects blood pressure responses to α2-adrenoceptor agonists (e.g. clonidine) [2]. Its role in the Central Nervous System (CNS) remains largely elusive. It may be important in developmental processes, since homozygous α2B-KO mice do not breed well [2].
Like many other GPCRs, the α2B-AR undergoes agonist promoted desensitization [3] initiated by the phosphorylation of the receptor in its third intracellular loop by a well-characterized family of serine/threonine kinases termed G protein-coupled receptor kinases (GRKs), the most prominent member of which is the ubiquitously expressed GRK2 [4]. The phosphorylated receptor then interacts with a certain family of proteins termed arrestins, which physically uncouple the receptor from G proteins, thus terminating receptor signaling [4].
α2-ARs play a very important role in autocrine feedback regulation of catecholamine secretion from the chromaffin cells of the adrenal medulla [5]. By coupling to the Gi/o proteins, they inhibit further CA release upon their stimulation by the secreted CA, thereby participating in an autocrine negative feedback loop controlling adrenal CA secretion [5,6]. A number of recent studies by our group and others have documented the (patho)physiological importance of this α2-AR-mediated control of adrenal CA secretion, as deregulation of this signaling system in the adrenal chromaffin cells has been shown to underlie excessive sympathetic outflow and circulating CA levels that accompany and aggravate chronic heart failure [7-9]. More specifically, up-regulated GRK2 has been found to desensitize and down-regulate chromaffin cell α2-ARs extensively in HF mouse and rat adrenal glands, thus rendering these receptors nonfunctional in HF. This allows for unopposed, continuous CA secretion, which contributes to the enhanced CA levels in heart failure [7].
A common genetic variant of the α2B-AR subtype consisting of a deletion of three glutamic acid residues (residues 301-303) displays impaired agonist-promoted receptor phosphorylation and desensitization in various transfected cell lines [10,11], and, very recently, it was shown to be resistant to desensitization (in terms of inducing vasoconstriction) in vivo, as well [12]. Given the important role of α2-ARs in chromaffin cell physiology with respect to CA secretion regulation, in the present study we sought to investigate the impact of this α2B-AR polymorphism on desensitization and sympatho-inhibitory function of this receptor in these cells. To this end, we utilized the PC12 cell line, a rat pheochromocytoma-derived chromaffin cell line [13], which we transfected with the wild-type α2B-AR (WT α2B-AR) or the polymorphic α2B-AR (Del α2B-AR) cDNA constructs in order to express and compare head-to-head these two receptor variants in chromaffin cells. We found that desensitization of the Del α2B-AR is impaired also in PC12 cells, which results in enhanced inhibition of CA secretion.
Results
Impaired agonist-promoted phosphorylation of the Del α2B-AR in PC12 cells
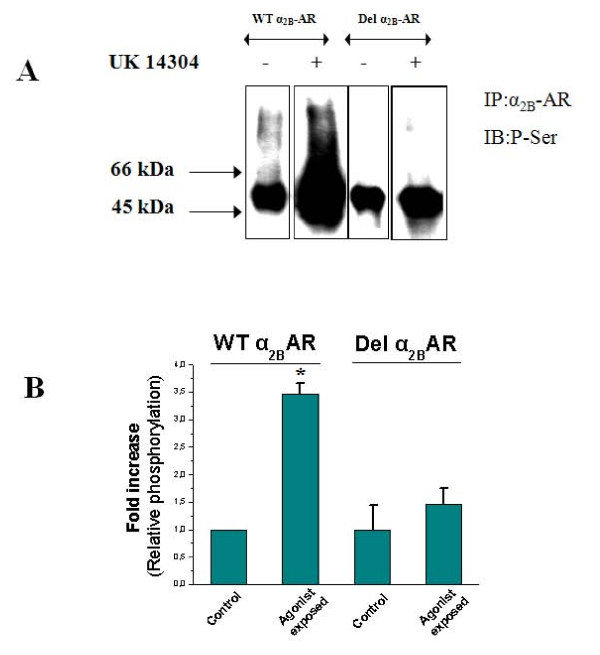
After verifying that PC12 cells express substantial endogenous levels of both GRK2 and GRK3, more than sufficient to phosphorylate α2-ARs (data not shown), we performed whole cell phosphorylation studies of the two receptors in PC12 cells in response to 10 min treatments with 10 μM UK14304, an α2-AR-specific full agonist, or vehicle. Since GRKs are serine/threonine kinases, we used a specific polyclonal anti-phosphoserine antibody (anti-P-Ser) to examine the receptors' agonist-induced phosphorylation. As shown in Figure 1A, the Del α2B-AR displays indeed a dramatically decreased agonist-promoted phosphorylation compared to the WT α2B-AR. More specifically, WT α2B-AR displays ~3.5-fold increase in phosphorylation in response to agonist, whereas agonist-induced phosphorylation of the Del α2B-AR is virtually abolished (Figure 1B). Of note, GRK2 levels were equal between the two α2B-AR-expressing PC12 lines, indicating that Del α2B-AR transfection and overexpression did not have any effects on endogenous GRK2 levels in PC12 cells (data not shown).
Figure 1.
The Del α2B-AR displays impaired agonist-promoted phosphorylation in PC12 cells. A. Transfected PC12 cells were exposed to 10 μΜ UK 14304 for 10 min and then immunoprecipitation (IP) of the α2B-ARs was performed, followed by immunoblotting (IB) to measure their phosphoserine (P-Ser) content. Equal amounts of receptor were loaded on each lane, as determined from ligand binding data and protein determination by the Bradford method. Shown is a representative blot from five independent experiments. B. Densitometric analysis of the immunoblots of Panel A, showing agonist-induced receptor phosphorylation as fold increase of the basal phosphorylation of WT α2B-AR. *, p < 0.05, vs. all other conditions, n = 5.
Impaired agonist-promoted desensitization of the Del α2B-AR in PC12 cells
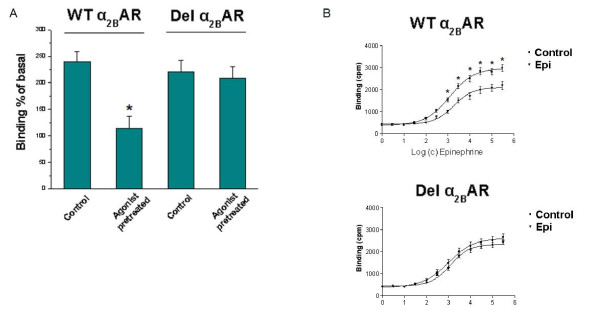
We next examined the impact of this impaired phosphorylation on the functional desensitization, i.e. on the agonist-induced G-protein coupling, of the Del α2B-AR with the [35S] GTPγS binding assay. This assay is widely used to measure the G-protein coupling efficiency of GPCRs [14]. Again, we treated the PC12 cells with 10 μM UK 14304 for 30 minutes or with vehicle, and then membranes were prepared from these cells and the [35S] GTPγS binding assay was performed on these membranes using again 10 μM UK 14304 as agonist. As shown in Table 1 and in Figure 2A, the two non-agonist-pretreated receptors (Controls) exhibit about the same agonist-stimulated [35S] GTPγS binding (240 ± 13% of basal for the WT α2B-AR and 221 ± 15% of basal for the Del α2B-AR). However, the agonist-pretreated WT α2B-AR displays a significantly decreased agonist-stimulated [35S] GTPγS binding (114 ± 18% of basal), compared to the Control WT α2B-AR, which reflects a significant functional desensitization of this receptor (~53%). In contrast, the agonist-pretreated Del α2B-AR shows almost the same agonist-stimulated [35S] GTPγS binding (208 ± 20% of basal) as the Control Del α2B-AR, indicating that the Del α2B-AR fails to undergo desensitization in PC12 cells.
Table 1.
Agonist-stimulated [35S] GTPγS binding measurements for the WT and Del α2B-ARs expressed in PC12 cells
| Basal | Agonist-stimulated | Des. | ||
|---|---|---|---|---|
| Control | UK14304 | |||
| Cpm/min/15 μg | % | % | ||
| WT α2B-AR | 2983,15 ± 09,74 | 240,01 ± 13,02 | 113,74 ± 18,40a | 53 |
| Del α2B-AR | 5717,39 ± 13,02b | 221,15 ± 14,94 | 208,47 ± 19,93b | 6 |
Agonist-stimulated [35S]GTPγS binding was determined in response to 10 μM UK14304 (agonist) in agonist-pretreated (UK14304) and in non-pretreated (Control) membranes. Des. = Desensitization.
a p < 0.05 compared with control, b p < 0.05 compared with WT, n = 6 independent experiments.
Figure 2.
[35S] GTPγS binding for the two α2B-ARs in PC12 cells. A. PC12 cells were exposed to vehicle (Control) or 10 μM UK14304 (Agonist-pretreated) and agonist-stimulated [35S] GTPγS binding was determined. Results are expressed as % of basal binding, indicating a ~ 53% desensitization of the WT α2B-AR (see also Table 1). *, p < 0.05, vs. Control WT α2B-AR, n = 6 independent experiments. B. Membranes from control (Control) and 10 μM epinephrine-pretreated (Epi) cells were incubated with increasing concentrations (c) of epinephrine. Only the Epi-pretreated WT α2B-AR displays decreased [35S] GTPγS binding (i.e. desensitization) in response to Epi, whereas the Del α2B-AR fails to do so. *, p < 0.05, vs. Epi, n = 6 independent experiments.
In addition, GTPγS binding dose-response curves of Epi-pretreated cells revealed a significant increase (1.8-fold, p = 0.012) in the EC50 of Epi to stimulate GTPγS binding through the WT α2B-AR, whereas Epi pretreatment did not induce significant changes on the EC50 for the Del α2B-AR (Figure 2B). This result provides additional strong evidence that the Del α2B-AR, in contrast to its wild type counterpart which undergoes agonist-induced desensitization normally as expected, fails to desensitize in PC12 cells. This impaired desensitization of the Del α2B-AR comes as a consequence of its impaired agonist-induced phosphorylation in PC12 cells.
Enhanced inhibition of catecholamine secretion by the Del α2B-AR in PC12 cells
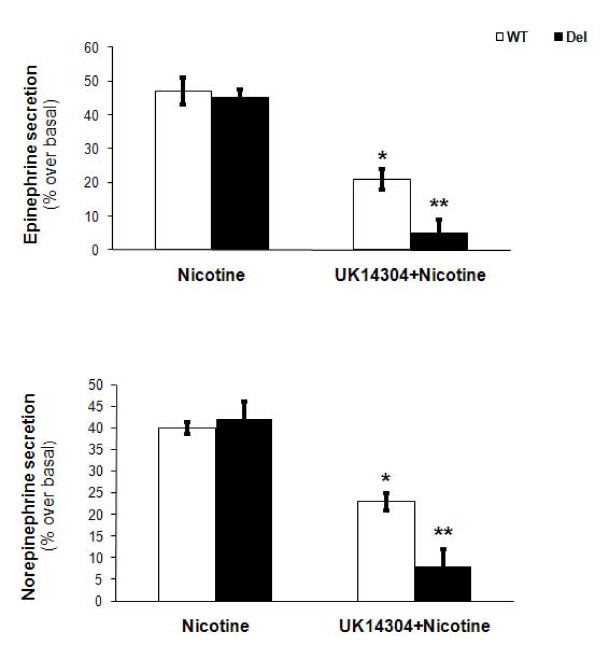
Finally, we examined the functional impact of this impaired phosphorylation/desensitization of the Del α2B-AR in PC12 cells, and, more specifically, the impact on the ability of the receptor to inhibit CA secretion. Physiologically in chromaffin cells, CA secretion is tonically stimulated by acetylcholine acting on nicotinic cholinergic receptors endogenously expressed in chromaffin cell membranes [5]. Thus, we performed in vitro CA secretion assays in the PC12 cells using nicotine (which also stimulates nicotinic cholinergic receptors) as the stimulus for the CA secretion and UK14304 as the agonist for the α2B-ARs. As shown in Figure 3 nicotine induced comparable amounts of CA secretion from the two transfected and UK14304-pretreated PC12 cell lines. Of note, when no challenge with UK14304 preceded nicotine exposure, CA secretion between the two cell lines was similar (data not shown). However, when the cells were pretreated with UK14304 to induce desensitization of the α2B-ARs, secretion of both Epi and NE from the UK14304-pretreated PC12α2BDel cells was significantly reduced compared to agonist-pretreated PC12α2BWT cells, indicating that the impaired desensitization of the Del α2B-AR allows it to be a stronger inhibitor of CA secretion from PC12 cells, compared to its wild-type counterpart (Figure 3).
Figure 3.
Enhanced inhibition of nicotine-induced catecholamine secretion by the Del α2B-AR in PC12 cells. In vitro catecholamine secretion from PC12 cells, expressing the WT α2B-AR (WT) or the Del α2B-AR (Del) and pretreated with 10 μM UK14304, in response to either 50 μM nicotine alone (Nicotine) or to 50 μM nicotine plus another challenge of 10 μM UK14304 (UK14304 + Nicotine). No differences between the two non-pretreated cell lines were observed (data not shown). *, p < 0.05, vs. WT/Nicotine, **, p < 0.05, vs. WT/UK14304 + Nicotine, n = 3 independent experiments.
Notably, and because UK14304 is largely reported in the literature to be a partial agonist at the α2B-AR subtype, we repeated these experiments with another, full α2B-AR agonist, oxymetazoline, and the results were identical (data not shown). Thus, diminished desensitization of the Del α2B-AR mutant seems to translate into enhanced inhibitory function of the receptor on CA secretion from PC12 cells, regardless of the structure or the individual pharmacological properties of the activating agonist.
Discussion
A polymorphic variant of the human α2B-adrenoceptor, which consists of a deletion of three glutamic acids (residues 301-303) in the third intracellular loop (Del α2B-AR) has been identified [15] and characterized in transfected CHO-K1 cells [10]. Given the important role of α2-ARs in chromaffin cell physiology with respect to CA secretion regulation [2,7] in the present study we sought to analyze the properties of this polymorphic receptor in PC12 cells, a very well established and widely used chromaffin cell line [13]. In particular, we wanted to see whether the phenotype of the impaired phosphorylation and desensitization of the Del α2B-AR also applies in chromaffin cells, and, if so, what consequences this might have on the CA secretion inhibitory (sympatho-inhibitory) function of the receptor in these cells. We found that the agonist-promoted phosphorylation and subsequent desensitization of the Del α2B-AR is indeed dramatically impaired in PC12 cells, as well. In particular, it appears that the agonist-induced (i.e. GRK-mediated) phosphorylation and desensitization of the Del α2B-AR are deficient, since both of these receptor properties were studied in response to agonist stimulation (UK14304). This is entirely consistent with what has been observed for this receptor variant in another heterologous (but physiologically irrelevant) cell line (COS-7) and in neuronal cells [10,11].
Importantly, in the present study, we report that this impaired phosphorylation and desensitization of the Del α2B-AR in PC12 cells leads to enhanced inhibition of Epi and NE secretion by the receptor in this chromaffin cell line. This novel phenotypic/functional finding for this polymorphism is therefore now added to the ever-expanding, over the past several years, list of its (genetic) associations with low basal metabolic rate in some obese populations [15], with acute coronary events [16] and increased risk of sudden cardiac death [17], with impaired endothelial function as assessed by flow-mediated dilatation of the brachial artery [18], with elevated blood pressure in conjunction with stressful work environment [19], with risk reduction for incident diabetes after dietary changes [20], with impaired first-phase insulin secretion that may predict impaired glucose tolerance [21], with reduced autonomic responsiveness by altering cardiac sympathetic and vagal function during sustained handgrip exercise in normotensive obese women [22], and with depressed general autonomic tone and impaired vagal activity in non-diabetic men, which is accentuated by central obesity [23].
Given that α2-ARs of the sympathetic nerves and adrenal glands are crucial regulators of SNS activity/outflow and of circulating CA levels in heart failure and in other diseases characterized and aggravated by sympathetic overactivity, by virtue of inhibiting CA secretion and NE release from sympathetic nerve terminals [5], the finding of enhanced inhibition of CA secretion from chromaffin cells by the Del α2B-AR reported in the present study is of obvious physiological importance. It strongly implies that carriers of this α2B-AR polymorphism might have lower levels of sympathetic outflow, since this polymorphic variant displays enhanced sympatho-inhibitory function due to its impaired desensitization (i.e. termination of signaling). In fact, its previously reported associations with reduced autonomic responsiveness by altering cardiac sympathetic and vagal function and with depressed general autonomic tone and impaired vagal activity [22,23] are entirely consistent with the above postulated effect of this polymorphic α2B-AR on sympathetic outflow in vivo.
It is worth noting that whether the α2B-AR subtype is endogenously expressed in adrenal chromaffin cells, and in particular in human adrenal chromaffin cells, and participates therein in regulation of CA secretion, is somewhat a matter of debate [5], making the present findings difficult to interpret physiologically. There is however one reported function of α2B-AR when expressed in PC12 cells, and that is stimulation of neuronal differentiation of these cells upon chronic epinephrine treatment [24,25]. Thus, in that context, and based on the findings of the present study, this polymorphic Del α2B-AR would be expected to stimulate neuronal differentiation of PC12 cells to an even higher extent than its wild-type counterpart.
There appears to be a species-dependent variation in the particular adrenal α2-AR subtypes expressed and some contradictory data have been reported in the literature, even for the same species [5]. In fact, our previous study has indicated that only the α2A subtype is present endogenously in rat adrenal chromaffin cells (the species that PC12 cells also originate from), and this appears to be the case also in human adrenal glands [7]. Making matters even more complex, PC12 cells do not express any adrenoceptors at appreciable levels endogenously [24,26]. Nevertheless, based on our present findings, it is entirely legitimate to speculate that this polymorphic α2B-AR will exert enhanced feedback inhibition of CA release, if present in human adrenal chromaffin cells or wherever in the CNS and in peripheral sympathetic nerve terminals it is expressed in humans in vivo. Therefore, carriers of this α2B-AR gene polymorphism might be better protected and experience less severe symptoms from pathological conditions and diseases characterized and aggravated by sympathetic/catecholaminergic overstimulation in vivo, including (but not limited to) heart failure, hypertension, and hyperthyroidism.
Conclusions
A deletion polymorphism in the human α2B-AR gene confers impaired agonist-dependent receptor phosphorylation and desensitization also in the adrenal chromaffin cell line PC12, resulting in enhanced inhibitory function against cholinergic-induced catecholamine secretion in vitro. Thus, this α2B-AR gene polymorphism might confer better protection against conditions characterized and aggravated by sympathetic/catecholaminergic overstimulation in vivo, such as heart failure, hypertension, and hyperthyroidism.
Methods
Materials
Guanosine diphosphate (GDP), Guanosine 5'-O-(3-thiotriphosphate) (GTPγS), nicotine, epinephrine, 5-bromo-6-(2-imidazoline-2-ylamino)-quinoxaline (UK14304), and oxymetazoline were from Sigma-Aldrich (St. Louis, MO). The anti-phosphoserine (anti-P-Ser) rabbit polyclonal antibody was from Chemicon (Temecula, CA). The anti-α2B-AR and anti-GRK2/3 antibodies were from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). [35S] Guanosine 5'-O-(3-thiotriphosphate), [35S] GTPγS was from Amersham Pharmacia Biotech (Buckinghamshire, UK).
Cell lines, transfection and cell culture
The cDNA of the human α2B-AR (Missouri S&T cDNA Resource Center, Rolla, MO, USA) was modified (Del322-325) using polymerase chain reaction-mediated mutagenesis (Stratagene Cloning Systems, La Jolla, CA). PC12 cells were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) and transfected with the constructs of interest (i.e. wild type or Del α2B-AR) via the Lipofectamine method (Invitrogen, Carlsbad, CA, USA). For comparison of the properties of the two receptors, cell lines with comparable expression levels were chosen, as determined by ligand binding with the α2AR-specific antagonist [3H]-rauwolscine (3.1 pmol/mg of protein for PC12α2BWT, 2.95 pmol/mg of protein for PC12α2BDel). The culture of PC12 cells was performed, as described previously [13].
Receptor phosphorylation studies
After a 10 min stimulation with UK14304 or vehicle PC12 cells were washed three times with ice-cold phosphate-buffered saline and solubilized in 1 ml of a buffer containing 1% Triton X-100, 0.1% SDS, 20 mM Tris-Cl pH 7.5, 125 mM NaCl, 1 mM MgCl2 and 1 mM CaCl2, protease and phosphatase inhibitors. The α2B-ARs were immunoprecipitated with a specific anti-α2B-AR antibody and subsequently, the proteins in the supernatant were fractionated on a 12% SDS-polyacrylamide gel, followed by western blotting with an anti-phosphoserine (anti-P-Ser) specific antibody. Equal amounts of protein were loaded in each lane.
[35S] GTPγS binding assay
Cell membranes were prepared by centrifugation and agonist-induced stimulation of [35S] GTPγS binding was measured as described previously [14]. Briefly, membrane pellets were resuspended in hypotonic lysis buffer. The reaction was started by adding an aliquot of membrane suspension (15 μg of membrane protein per tube) to reaction buffer (25 mM Tris-HCl, pH 7.4, 5 mM MgCl2, 1 mM EDTA, 1 mM dithiothreitol, 100 mM NaCl, 1 μM GDP, and 2 nM [35S] GTPγS) with or without agonist (10 μM UK 14304) in a total volume of 100 μl. The incubation was terminated by dilution with 4 volumes of ice-cold 10 mM Tris-HCl buffer, pH 7.4, and vacuum filtration through Whatman GF/B glass fiber filters, which were then placed in scintillation vials for counting in a liquid scintillation counter (Beckman counter). Non-specific binding was measured in the presence of 10 μM GTPγS. For desensitization experiments, cells were pretreated with 10 μM UK 14304 or 10 μM epinephrine for 30 min at 37°C or with vehicle, placed on ice, and washed three times with ice-cold phosphate-buffered saline prior to membrane preparation.
In vitro catecholamine secretion assay
In vitro epinephrine and norepinephrine secretion in response to various treatments was measured in the supernatant of transfected PC12 cells by ELISA, as described previously [8]. Cells were pretreated with UK14304 (or oxymetazoline) for 30 min prior to the nicotine challenge, and supernatant was collected at 20 min post-nicotine application.
Statistical analysis
Data are summarized as mean ± SEM. Comparisons were made using t tests or ANOVA as appropriate. A Bonferroni correction was applied to the probability values whenever multiple comparisons arose. Values of p < 0.05 were considered significant.
List of Abbreviations
α2-AR: α2-adrenergic receptor; GPCR: G-protein coupled receptor; GRK: G-protein coupled receptor kinase; KO: knockout; MAPK: Mitogen activated protein kinase; NE: norepinephrine; Epi: epinephrine; PC12: rat pheochromocytoma cell line: [35S] GTPγS: [35S] Guanosine 5'-O-(3-thiotriphosphate)
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
KN carried out the [35S] GTPγS binding and in vitro catecholamine secretion assays, and helped to draft the manuscript. TK participated in the receptor phosphorylation experiments. AL conceived of the study, designed it, performed the transfections to establish the PC12 clones and the receptor phosphorylation experiments, and assisted in drafting and editing the manuscript. All authors read and approved the final manuscript.
Contributor Information
Kristy Nguyen, Email: nkristy@nova.edu.
Theodoros Kassimatis, Email: tkassimatis@yahoo.gr.
Anastasios Lymperopoulos, Email: al806@nova.edu.
Acknowledgements
This study was supported (in part) by a Scientist Development Grant from the American Heart Association to A.L. (AHA #09SDG2010138, National Center).
References
- Bylund DB, Eikenberg DC, Hieble JP, Langer SZ, Lefkowitz JR, Minneman KP, Molinoff PB, Ruffolo RR, Trendelenburg U. IV International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol Rev. 1994;46:121–136. [PubMed] [Google Scholar]
- Philipp M, Hein L. Adrenergic receptor knockout mice: distinct functions of 9 receptor subtypes. Pharmacol Ther. 2004;101:65–74. doi: 10.1016/j.pharmthera.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Eason MG, Liggett SB. Subtype-selective desensitization of alpha 2-adrenergic receptors. Different mechanisms control short and long term agonist-promoted desensitization of alpha 2C10, alpha 2C4, and alpha 2C2. J Biol Chem. 1992;267:25473–25479. [PubMed] [Google Scholar]
- Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Ann Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- Lymperopoulos A, Rengo G, Koch WJ. Adrenal adrenoceptors in heart failure: Fine-tuning cardiac stimulation. Tr Mol Med. 2007;13:503–511. doi: 10.1016/j.molmed.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Brede M, Nagy G, Philipp M, Sorensen JB, Lohse MJ, Hein L. Differential Control of Adrenal and Sympathetic Catecholamine Release by α2-Adrenoceptor Subtypes. Mol Endocrinol. 2003;17:1640–1646. doi: 10.1210/me.2003-0035. [DOI] [PubMed] [Google Scholar]
- Lymperopoulos A, Rengo G, Funakoshi H, Eckhart AD, Koch WJ. Adrenal GRK2 upregulation mediates sympathetic overdrive in heart failure. Nat Med. 2007;13:315–323. doi: 10.1038/nm1553. [DOI] [PubMed] [Google Scholar]
- Lymperopoulos A, Rengo G, Gao E, Ebert SN, Dorn GW, Koch WJ. Reduction of sympathetic activity via adrenal-targeted GRK2 gene deletion attenuates heart failure progression and improves cardiac function after myocardial infarction. J Biol Chem. 2010;285:16378–16386. doi: 10.1074/jbc.M109.077859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymperopoulos A, Rengo G, Zincarelli C, Soltys S, Koch WJ. Modulation of adrenal catecholamine secretion by in vivo gene transfer and manipulation of G protein-coupled receptor kinase-2 activity. Mol Ther. 2008;16:302–307. doi: 10.1038/sj.mt.6300371. [DOI] [PubMed] [Google Scholar]
- Small KM, Brown KM, Forbes SL, Liggett SB. Polymorphic deletion of three intracellular acidic residues of the alpha 2B-adrenergic receptor decreases G protein-coupled receptor kinase-mediated phosphorylation and desensitization. J Biol Chem. 2001;276:4917–4922. doi: 10.1074/jbc.M008118200. [DOI] [PubMed] [Google Scholar]
- Salim S, Desai AN, Taneja M, Eikenburg DC. Chronic adrenaline treatment fails to down-regulate the Del301-303-alpha2B-adrenoceptor in neuronal cell. Br J Pharmacol. 2009;158:314–327. doi: 10.1111/j.1476-5381.2009.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muszkat M, Kurnik D, Sofowora GG, Solus J, Xie HG, Harris PA, Williams SM, Wood AJ, Stein CM. Desensitization of vascular response in vivo: contribution of genetic variation in the [alpha]2B-adrenergic receptor subtype. J Hypertension. 2010;28:278–284. doi: 10.1097/HJH.0b013e328333d212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells, which respond to nerve growth factor. Proc Natl Acad Sci USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltonen JM, Pihlavisto M, Scheinin M. Subtype-specific stimulation of [35S] GTPgammaS binding by recombinant alpha2-adrenoceptors. Eur J Pharmacol. 1998;355:275–279. doi: 10.1016/S0014-2999(98)00518-4. [DOI] [PubMed] [Google Scholar]
- Heinonen P, Koulu M, Pesonen U, Karvonen MK, Rissanen A, Laakso M, Valve R, Uusitupa M, Scheinin M. Identification of a three-amino acid deletion in the alpha2B-adrenergic receptor that is associated with reduced basal metabolic rate in obese subjects. J Clin Endocrinol Metabol. 1999;84:2429–2433. doi: 10.1210/jc.84.7.2429. [DOI] [PubMed] [Google Scholar]
- Snapir A, Heinonen P, Tuomainen TP, Alhopuro P, Karvonen MK, Lakka TΑ, Nyyssonen K, Salonen R, Kauhanen J, Valkonen VP, Pesonen U, Koulu M, Scheinin M, Salonen JT. An insertion/deletion polymorphism in the alpha2B-adrenergic receptor gene is a novel genetic risk factor for acute coronary events. J Am Coll Cardiol. 2001;37:1516–1522. doi: 10.1016/S0735-1097(01)01201-3. [DOI] [PubMed] [Google Scholar]
- Snapir A, Mikkelsson J, Perola M, Penttila A, Scheinin M, Karhunen PJ. Variation in the alpha2B-adrenoceptor gene as a risk factor for prehospital fatal myocardial infarction and sudden cardiac death. J Am Coll Cardiol. 2003;41:190–194. doi: 10.1016/S0735-1097(02)02702-X. [DOI] [PubMed] [Google Scholar]
- Heinonen P, Jartti L, Jarvisalo MJ, Pesonen U, Kaprio JA, Ronnemaa T, Raitakari OT, Scheinin M. Deletion polymorphism in the alpha2B-adrenergic receptor gene is associated with flow-mediated dilatation of the brachial artery. Clin Sci. 2002;103:517–524. doi: 10.1042/CS20020097. [DOI] [PubMed] [Google Scholar]
- Ohlin B, Berglund G, Nilsson PM, Melander O. Job strain, decision latitude and alpha2B-adrenergic receptor polymorphism significantly interact, and associate with high blood pressures in men. J Hypertension. 2007;25:1613–1619. doi: 10.1097/HJH.0b013e3281ab6c7d. [DOI] [PubMed] [Google Scholar]
- Laaksonen DE, Siitonen N, Lindstrom J, Eriksson JG, Reunanen P, Tuomilehto J, Uusitupa M. Physical activity, diet and incident diabetes in relation to an ADRA2B Polymorphism. Med Sci Sports Exerc. 2007;39:2227–232. doi: 10.1249/01.mss.0000246998.02095.bf. [DOI] [PubMed] [Google Scholar]
- Siitonen N, Lindstom J, Eriksson J, Valle TT, Hamalainen H, IIanne-Parikka P, Keinanen-Kiukaanniemi S, Tuomilehto J, Laakso M, Uusitupa M. Association between a deletion/insertion polymorphism in the alpha2B-adrenergic receptor gene and insulin secretion and type 2 diabetes. The Finnish Diabetes Prevention Study. Diabetologia. 2004;47:1416–1424. doi: 10.1007/s00125-004-1462-z. [DOI] [PubMed] [Google Scholar]
- Ueno LM, Frazzatto ES, Batalha LT, Trombetta IC, do Socorro Brasileiro M, Irigoyen C, Brum PC, Villares SM, Negrao CE. Alpha2B-adrenergic receptor deletion polymorphism and cardiac autonomic nervous system responses to exercise in obese women. Intl J Obes. 2006;30:214–220. doi: 10.1038/sj.ijo.0803140. [DOI] [PubMed] [Google Scholar]
- Sivenius K, Niskanen L, Laakso M, Uusitupa M. A deletion in the alpha2B-adrenergic receptor gene and automatic nervous function in central obesity. Obes Res. 2003;11:962–970. doi: 10.1038/oby.2003.133. [DOI] [PubMed] [Google Scholar]
- Lymperopoulos A, Karkoulias G, Koch WJ, Flordellis CS. Alpha(2)-Adrenergic receptor subtype-specific activation of NF-kappaB in PC12 cells. NeurosciLett. 2006;402:210–215. doi: 10.1016/j.neulet.2006.03.066. [DOI] [PubMed] [Google Scholar]
- Taraviras S, Olli-Lahdesmaki T, Lymperopoulos A, Charitonidou D, Mavroidis E, Kallio J, Scheinin M, Flordellis C. Subtype-specific neuronal differentiation of PC12 cells transfected with α2-adrenergic receptors. Eur J Cell Biol. 2002;81:363–374. doi: 10.1078/0171-9335-00250. [DOI] [PubMed] [Google Scholar]
- Duzic E, Lanier SM. Factors determining the specificity of signal transduction by guanine nucleotide-binding protein-coupled receptors. III. Coupling of alpha 2-adrenergic receptor subtypes in a cell type-specific manner. J Biol Chem. 1992;267:24045–24052. [PubMed] [Google Scholar]