Abstract
Innate and adaptive immune system cells play a major role in regulating the growth of cancer. Although it is commonly thought that an immune response localized to the tumor will inhibit cancer growth, it is clear that some types of inflammation induced in a tumor may also lead to cancer proliferation, invasion, and dissemination. Recent evidence suggests, however, that some patients with cancer can mount an antitumor immune response that has the potential to control or eliminate cancer. Indeed, a so-called “immune response” signature has been described in malignancy that is associated with improved outcomes in several tumor types. Moreover, the presence of specific subsets of T cells, which have the capability to penetrate tumor stroma and infiltrate deep into the parenchyma, identifies patients with an improved prognosis. Immune-based therapies have the potential to modulate the tumor microenvironment by eliciting immune system cells that will initiate acute inflammation that leads to tissue destruction.
INTRODUCTION
Cancer is an inflammatory disease. The types of immune system cells that are found infiltrating human malignancy are varied and consist of cells of the innate immune system (eg, macrophage, neutrophils) as well as cells associated with an adaptive immune response (eg, T and B cells). Innate immunity represents the body's “gut reaction” to an abnormality, such as cancer, and does not involve specific recognition of immunogenic proteins, which are called antigens. Adaptive immunity is a specific response to a particular tumor-associated antigen. Both innate and adaptive immune cells orchestrate an inflammatory environment that may function to either stimulate or inhibit cancer growth (Table 1). It is suggested that the inflammatory response found in many cancers is one of chronic inflammation, resulting in an environment rich in innate immune cells, which secrete substances that promote angiogenesis and cell proliferation. The growth-enhancing properties of this type of inflammatory response have been likened to inflammation consistent with wound healing.1 Increasing evidence, however, indicates that some patients with cancer mount an adaptive immune response specifically directed against antigenic proteins expressed in their tumors. T cells that secrete cytokines such as interferon gamma (IFN-γ) generate acute inflammation that results in expansion of cytotoxic T cells (CTLs), tissue destruction, and the potential control or even elimination of cancer.2 The tissue-destructive properties of this type of inflammatory response are consistent with what would be operative in acute allograft rejection. The clinical application of immune-based cancer therapeutics requires strategies that will elicit a tumor-specific acute inflammatory response that induces tumor rejection. The cancer must appear dangerous enough for the immune system to initiate a tissue- destructive response.3
Table 1.
Innate and Adaptive Immune Cells Involved in Regulating Tumor Growth
| Stimulate Cancer Growth | Inhibit Cancer Growth |
|---|---|
| Innate immune cells | |
| Neutrophils | Dendritic cells* |
| Macrophage (M2) | Macrophage (M1) |
| Myeloid-derived suppressor cells | |
| Adaptive immune cells | |
| Th2 CD4+ T cell | Cytotoxic CD8+ T cell |
| CD4+ T regulatory cell | Th1 CD4+ T cell |
| B lymphocytes* | Th17 CD4+ T cell |
Abbreviation: Th, T-helper.
Have been associated with both stimulation and inhibition.
CHRONIC INFLAMMATION IN CANCER CAN ELICIT TUMOR CELL PROLIFERATION AND ENHANCED INVASION
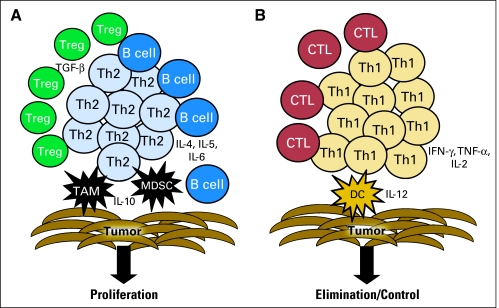
In recent years, the role of inflammatory cells in facilitating the progression of cancer is becoming better understood. Most cancers contain some evidence of inflammatory infiltrate, and many of these cells are components of the innate immune system. Innate immune system cells are involved in tissue repair and remodeling; thus, the factors secreted by these cells operate to enhance rather than inhibit tumor growth.1 There are several types of innate immune system cells that are implicated in cancer progression. Macrophages are a primary source of secreted proinflammatory cytokines. These cells can be generally categorized as type 1 (M1) or type 2 (M2). M1s secrete cytokines such as interleukin 12 (IL-12) and can actually aid in the generation of T-helper 1 (Th1) adaptive immunity and impart a direct cytotoxic effect to tumor cells.4 M2s secrete immunosuppressive cytokines and promote tumor cell growth. Tumor-associated macrophages (TAMs) are, in general, of the M2 phenotype, and infiltration by these cells has been shown to be an independent predictor of poor prognosis in multivariate analysis in many malignancies such as lymphoma, non–small-cell lung cancer, and hepatocellular carcinoma.5–7 TAMs secrete proteases that enhance invasion and metastases, cytokines that can inhibit an adaptive tumor-specific immune response, and angiogenic factors that increase neovascularization (Fig 1).8 Myeloid-derived suppressor cells (MDSCs) are another innate immune cell that promotes cancer growth. MDSCs are elicited during chronic inflammatory states when the differentiation of immature myeloid precursors into mature myeloid cells is blocked. MDSCs inhibit the adaptive immune response via multiple mechanisms: direct secretion of substances that effect T cell function as well as the induction of adaptive T regulatory (Treg) cells.9 Tregs are CD4+ T cells that have upregulated the transcription factor Foxp3, which enables the cell to suppress inflammation as well as specific immune responses.10 Induction of Foxp3 can be mediated by T cell receptor stimulation via antigen recognition; IL-2 secretion; and the presence of tumor growth factor β (TGF-β), which is abundant in tumors.11 Furthermore, it has recently been shown that MDSCs play a role in skewing adaptive tumor-specific immunity to a T-helper 2 (Th2) response (Fig 1).12 Other innate immune system cells have also been shown to create a proliferative tumor microenvironment, including neutrophils, mast cells, and eosinophils.1 Even dendritic cells (DCs), antigen-presenting cells (APCs) that are critical to the stimulation of effective antitumor adaptive immune responses, can become defective in the tumor microenvironment and aid in tumor immune evasion by failing to stimulate T cells.13 Cell types associated with adaptive immunity may also facilitate tumor growth. Indeed, it has been postulated that B cells play a significant role in recruiting innate inflammatory cells to tumors.14 There have been several recent excellent reviews of the proliferative effect of innate immune cells in cancer inflammation, as well as their role in inhibiting T cell effector function; therefore, information provided here will focus on the role of adaptive immunity in regulating cancer progression.15–18
Fig 1.
T-helper (Th) 2 and Th1 tumor-specific T cell immunity. (A) Th2 T cells are stimulated by tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs). Together these cells types generate a tumor environment rich in interleukin (IL) -10, IL-4, IL-5, and IL-6. A B-cell response predominates. Regulatory T cells (Tregs) are elicited via these antigen-presenting cells presenting self-antigens, and the resultant secretion of tumor growth factor (TGF) -ß by Treg inhibits the generation of cytotoxic T lymphocytes (CTLs). The result is potential proliferation of tumor cells. (B) Th1 T cells are stimulated by type I dendritic cells (DCs). Together these cell types generate a tumor environment rich in IL-12, interferon (IFN) -γ, tumor necrosis factor (TNF) -α, and IL-2. A CTL response predominates. The result is potential elimination or control of tumor cell growth.
ACUTE INFLAMMATION IN CANCER CAN ELICIT TUMOR CELL DESTRUCTION
An adaptive T cell response, which requires antigen recognition, is composed of both cytotoxic CD8+ T cells and CD4+ T cells. CD4+ T cells can secrete cytokines involved in the regulation and propagation of the acute inflammatory response, and thus are referred to as T-helper (Th) cells. Animal models have demonstrated that in vivo eradication of tumors is, for the most part, mediated by CTL. However, there has been an increased understanding over the last several years of the importance of the CD4+ Th cell in enhancing or limiting CTL. Th manifests several phenotypes: Th1 cells secrete cytokines such as interferon-gamma (IFN-γ), tumor necrosis factor-α, and IL-2 and support CTL and tissue destruction (Fig 1); Th2 cells secrete cytokines such as IL-10, IL-4, and IL-5 and limit CTL proliferation (Fig 1); Th17 cells secrete IL-17 and are operative in pathologic autoimmune disease; and Tregs secrete IL-10 and TGF-ß, which dampen immune responses.19 The type of protein presented to the immune system may affect the type of immune response elicited. Malignancies associated with viruses have clearly demonstrated that viral antigens are excellent immunological targets for cancer control.20 Viral antigens provide a foreign danger signal that can elicit a tissue-destructive response. Many of the antigens that have been identified in common cancers, however, are nonmutated self-proteins with an altered expression that may make them immunogenic.21 The National Cancer Institute has made an effort to identify those tumor antigens with the greatest potential clinical importance by asking a panel of experts to evaluate a number of immunogenic cancer-associated proteins on the basis of specific characteristics.22 Of the 75 tumor antigens assessed using a variety of clinical and laboratory-based criteria, 46 were associated with some level of evidence of inducing immunogenicity in clinical trials, and 20 demonstrated some evidence of being associated with clinical efficacy based on the opinions of the experts.22 Of those 20 antigens that may have been associated with some clinical impact, 80% would be considered self-proteins (Table 2). Immunotherapies that target self-antigens and are associated with a clinical response must, in some way, subvert the normal self-regulatory mechanisms that prevent the development of autoimmunity.
Table 2.
Tumor Antigens That May Be Associated With Improved Clinical Outcomes
| Foreign or Mutated Tumor Antigens | Self-Tumor Antigens |
|---|---|
| LMP2 | WT1 |
| Human papilloma virus E6, E7 | MUC1 |
| EGFR vIII | HER-2/neu |
| Idiotype | MAGE A3 |
| NY-ESO-1 | |
| PSMA | |
| GD2 | |
| CEA | |
| Melan A/MART-1 | |
| gp100 | |
| Proteinase 3 (PR1) | |
| Tyrosinase | |
| PSA | |
| PAP | |
| NA17 | |
| PSCA |
Abbreviations: LMP2, latency membrane protein 2; WT1, Wilms tumor 1; MUC1, mucine 1; EGFR, epidermal growth factor receptor; HER-2, human epidermal growth factor receptor-2; MAGE, melanoma antigen A3; PSMA, prostate-specific membrane antigen; CEA, carcinoembryonic antigen; MART, melanoma antigen recognized by T cells; PSA, prostate-specific antigen; PAP, prostatic acid phosphatase; PSCA, prostate stem cell antigen.
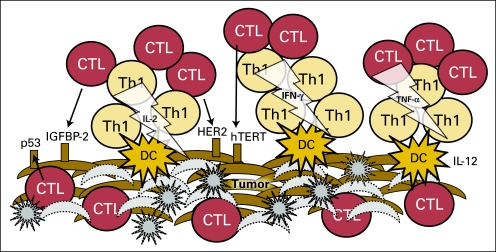
A clinically effective antitumor immune response would be one that is similar to that seen in acute allograft rejection, in which specific adaptive immunity against alloantigens results in tissue destruction and cell death. Multiple studies evaluating gene expression changes induced during acute renal allograft rejection have demonstrated significant upregulation of gene pathways involved in adaptive immunity, for example, antigen presentation, IFN-γ signaling presumably mediated via CD4+ T cells, T cell receptor signaling, cell adhesion, and chemotaxis.23 Recent retrospective analyses of tumors derived from patients with cancer across multiple tissue types have demonstrated that elements of immune rejection can be observed in some individuals and may be associated with improved clinical outcomes. These investigations have suggested that prognosis in patients with cancer is positively affected by (1) the presence of a tumor gene signature consistent with a type I adaptive immune response (ie, increased antigen presentation, IFN-γ signaling, and T cell receptor signaling), and (2) the presence of T cells that have penetrated through tumor stroma and are found infiltrating deeply into the parenchyma; intratumoral T cells (ie, cell adhesion and chemotaxis; Fig 2).
Fig 2.
Elements of imunity-induced tumor rejection. Acute inflammation induced by T-helper (Th) 1, stimulated by dendritic cells (DCs), results in a tumor environment that supports cytotoxic T lymphocytes (CTLs). Cytokines such as interleukin (IL) -12, interferon (IFN) -γ, tumor necrosis factor (TNF) -α, and IL-2 support the proliferation of CTLs, the upregulation of immune receptor molecules on local antigen-presenting cells (APC) and potentially the tumor itself, and the presentation of a variety of tumor-associated antigens such as p53, IFGBP-2, HER2, and hTERT. T cells begin to penetrate through the tumor stroma and kill tumor cells. Gray star shapes represent lysed tumor cells, gray crescent shapes depict dead tumor cells.
Gene expression studies in multiple solid tumor types have indicated that an immune response signature, particularly one that demonstrates upregulation of IFN-γ, may be associated with an improved survival or prognosis. One of the first investigations to demonstrate this association was performed in approximately 70 patients with colon cancer.24 The patients were heterogenic in stage (range, I-IV) but had well-annotated clinical follow-up of a sufficient duration to assess outcome. Investigators identified a cluster of genes that, when upregulated, were inversely correlated with tumor recurrence (P < .05). These seven genes, encoding T-box transcription factor 21, IFN regulatory factor 1, IFN-γ, CD3, CD8, granulysin, and granzyme B, are associated with Th1 immunity and CTL generation. Subsequently, an IFN-based gene signature has been identified in patients with triple-negative breast cancer who are more likely to remain metastasis free, as well as in early stage lung cancer, independently predicting improved relapse-free survival (RFS) and overall survival (OS).25,26 A tumor environment rich in type I cytokines would result in increased antigen presentation and generation of cytotoxic T cells, and would favor tissue destruction.
The second key feature of a potentially effective immune response is the ability of T cells to travel to the site of the tumor and actually infiltrate deep into the tumor parenchyma. One of the first studies to identify the location of infiltrating adaptive immune cells as important evaluated T cell infiltrations in primary cutaneous melanoma.27 After evaluation of 285 cases, in multivariate analysis, tumor thickness and T cell infiltrates were found to be significant independent histologic prognostic factors. The importance of intratumoral T cells in prognosis was further underscored in a study that evaluated immune infiltrates in patients with ovarian cancer.28 One of the first studies to identify the location of infiltrating T cells in relation to the tumor as important, its results demonstrated that patients with intratumoral T cells had superior progression-free survival and OS compared to patients with T cells surrounding but not invading the tumor (P < .001 for both). The presence of intratumoral T cells was an independent predictor of improved survival in multivariate analysis. The presence of the invading cells was also associated with expression of IFN-γ and IL-2, two type I cytokines. A similar study, performed in colon cancer, confirmed and extended these observations by further phenotyping the intratumoral T cells.29 Investigators demonstrated that high levels of intratumoral CD45RO+ (memory) T cells correlated with better survival. Tumors derived from the same patient population underwent the gene expression analysis as described above, demonstrating the Th1 signature that presumably facilitated the effector memory T cell infiltration.24 Intratumoral T cell infiltration has now been shown to be associated with survival benefit in non–small-cell lung cancer, hepatocellular carcinoma, and uroepithelial cancers, to name a few reports.30–32 Indeed, a recent study in non–small-cell lung cancer demonstrated an intratumoral immune response so vigorous that tertiary lymphoid structures had developed in the tumor, complete with both T cells and DCs.30
The ability to generate a rejection-type inflammation signal and the ability of T cells to overcome stromal barriers may make a clinical impact in a minority of patients, but clearly cancer still does develop in these individuals. One of the most important mechanisms that limits the ability to eradicate cancer via tumor-specific immunity is not global immunosuppression induced by bulky tumor growth, but rather a natural regulatory response of the immune system that prevents a perceived autoimmune reaction.
SELF-REGULATORY MECHANISMS THAT UNDERMINE THE CANCER-SPECIFIC IMMUNE RESPONSE
There have been several demonstrations of a link between the induction of autoimmunity with immune-based cancer therapies and an antitumor response. Autoimmunity and tumor regression have occurred after the use of a variety of immune-based treatments. The development of vitiligo and uveitis has been reported after tumor regressions were induced with the adoptive transfer of T cells (adoptive T cell therapy) expanded ex vivo from tumor-infiltrating lymphocytes in patients with metastatic melanoma.33 In a small phase I trial in 13 patients, both the antitumor responses and the autoimmune sequelae were attributed to the expansion of MART-1–specific T cells in vivo. Treatment of melanoma patients with IFN, in the adjuvant setting, has been associated with improved RFS and OS.34 In a study of 200 patients with stage IIB-C or III melanoma undergoing high-dose IFN-α-2B adjuvant treatment, it was observed that 26% developed autoantibodies and clinical symptoms consistent with autoimmunity.35 The median RFS in those patients who did not develop autoimmunity was 16 months, and in those patients with autoimmunity the RFS had not been reached (median follow-up of 45.6 months). Autoimmunity, in this study, was demonstrated to be an independent predictor of both RFS and OS (P < .001). Of note, an analysis of a similar population by other investigators found no such association.36
Because most tumor antigens are self-proteins, antitumor immunity is autoimmunity. There are several pathways that are operative in preventing the development of the autoimmune response against cancer. Self-regulation of the adaptive immune response can be accomplished in several ways. Initial immunity developing against cancer can be of a phenotype that does not support tissue destruction. For example, investigations of the Th response to peptide epitopes derived from the MAGE-6 protein, a tumor antigen in patients with renal cell carcinoma, demonstrated that T cell immunity was highly skewed to Th2 rather than Th1 in these patients.37 Although the MAGE-6 response was dominated by Th2, patients retained Th1 immunity to viral antigens, indicating that this skewing was tumor antigen–specific. Th2 skewing has also been demonstrated in patients with bladder, pancreatic, breast, and lung cancer.38–40 Antigen-specific Th2 can impair tissue destruction via direct suppression of CTL generation, as well as by the modulation of other inflammatory cell types (Fig 1).41 Data generated in a transgenic model of breast cancer suggest that Th2 IL-4–secreting CD4+ cells regulate tumor-associated macrophage to an M2 phenotype.42 The M2 phenotype promotes increased tumor cell growth and invasion.
Self–tumor antigens may also induce the preferential proliferation of CD4+CD25+FOXP3+ Treg cells. Tregs function to maintain immune homeostasis and limit acute inflammation.43 These cells interfere with T cell priming and can affect the antitumor function of effector cells via secretion of TGF-ß and IL-10.44 Several investigations have suggested that Tregs infiltrating the tumor may adversely affect prognosis. One of the first studies to demonstrate the clinical importance of Treg cells evaluated CD4+CD25+ T cells in the ascities of patients with ovarian cancer and showed that high levels of Tregs were associated with decreased survival.45 Subsequently, increased tumoral Tregs have been shown to be an independent predictor of poor prognosis or an increased chance of relapse in some subtypes of breast cancer, early-stage non–small-cell lung cancer, and hepatocellular carcinomas.31,46,47 The adverse effect of Tregs on the immune response is underscored by the demonstration that depletion of Tregs can enhance tumor-specific immunity after vaccination. Clinical trials of Treg depletion or inactivation via CD25 targeting, along with antigen-specific vaccination, suggested that an increase in the induced tumor-specific immune response occurred with the reduction in Treg brought about by using either a carcinoembryonic antigen or a tumor RNA–based vaccine.48,49 Increased levels of Treg cells, however, have been associated with a favorable prognosis in follicular lymphoma, head and neck cancers, and colon cancer.50–52 The mechanisms of this paradoxical effect, which appears to depend on tissue type, are not well understood. Some have theorized that Tregs may suppress inflammation induced by growth-promoting innate immune system cells in these cancers. It is also important to note that the Th phenotype is not static during the evolving immune response. It has recently been reported that Th1 cells, once tissue destruction occurs, begin to self-regulate by cosecreting IL-10, thus turning off the “rejection” signal.41,53
There are several “immune checkpoints” that control evolving adaptive immunity in an attempt to limit the damage induced by inflammation. All of these play a role in limiting tumor-specific immunity. As an example, programmed death-1 (PD-1) is a receptor that is found on the surface of T cells after encounter with an antigen.54 When PD-1 is bound by ligands PD-L1 (expressed on cells of multiple lineages) or PD-L2 (expressed on macrophages and DCs), the function of T cells is inhibited. Overexpression of PD-L1 has been reported in several tumor types and is associated with poor prognosis, presumably because of the inhibitory effect ligation would have on the antitumor immune response.55,56 Indeed, PD-1 has been suggested to regulate tumor-specific T cell expansion in patients with melanoma.57 Monoclonal antibodies designed to block PD-L1 are currently in clinical trials.
CTL antigen 4 (CTLA-4) inhibits activated T cells. Anti–CTLA-4 antibodies prevent such a blockade and are thought to enhance immunity, especially responses to self-antigens. Recently, the development of autoimmunity was correlated with an antitumor response induced in patients with metastatic melanoma after anti–CTLA-4 monoclonal antibody therapy.58 A 13% response rate was seen in 56 patients treated, including two complete responders. A quarter of the patients experienced significant grade 3 or 4 autoimmunity as a result of treatment. In those patients with significant autoimmune toxicity, 36% had evidence of a clinical response compared with only 5% of those who did not develop such toxicity (P = .008). Only a minority of patients achieved an antitumor response. The low response rates observed are most likely due to the natural tendency of the immune system to prevent the initiation of tissue-destructive immunity against self-antigens via numerous anti-inflammatory mechanisms.
EVIDENCE OF CLINICAL EFFICACY OF IMMUNITY-BASED THERAPY FOR CANCER
Despite the self-regulatory obstacles described above, immune-based cancer therapies can be clinically effective, and there are some immune-based approaches that are the standard of care in the treatment of cancer. Intravesicular bacillus Calmette-Guérin, as a treatment for superficial bladder cancer, presumably controls disease via immune-mediated mechanisms.59 Imiquimod, a Toll-like receptor 7 agonist, is widely used in the treatment of superficial skin cancers.60 Topical imiquimod treatment induces upregulation of genes associated with tissue rejection in these cancers.61 Donor lymphocyte infusions have become a standard treatment for relapse after allogeneic transplant for hematologic malignancies.62 The clinical efficacy of monoclonal antibody therapy with agents such as trastuzumab and rituxan is likely due, in part, to immune-mediated mechanisms.63 A common theme relevant to the success of these approaches is that they are generally used when malignancies are at a low burden of disease and/or noninvasive. Furthermore, monoclonal antibody therapy is often given concurrently with cytoreductive chemotherapy, thereby controlling further cancer growth. Immunotherapy has had greatest success in the treatment of infectious disease. In vaccination against an infection, however, immunity is generated before exposure to the pathogen. Once an infection has become established, it is often difficult to overcome a rapidly proliferating pathogen with a modality such as active immunization. Similarly, immunotherapy may be difficult to apply to established malignancy. Clinical trials performed at the National Cancer Institute have shown that adoptive T cell therapy, infusion of tumor-competent T cells that have been expanded to great numbers outside the body and then reinfused, can elicit a 50% response rate in patients with refractory advanced stage melanoma. T cells derived from melanoma lesions were isolated, expanded in culture, and infused into patients with disease refractory to both chemotherapy and immunotherapy (IL-2).64 Although a significant response rate was achieved, and some patients had prolonged stabilization of disease and occasional complete responders have been reported, in the majority of patients the immune response provided did not result in complete tumor eradication. These types of studies, encouraging but not completely effective, have led to more extensive study of immune-based cancer treatments in the adjuvant setting. In many solid tumors, unlike infectious disease, the growth of the cancer is slow, which may allow an immune response to proliferate and eradicate the tumor before becoming overwhelmed.65 Although there are many promising immune-based cancer therapies under development, acting via a variety of different mechanisms, not all can be reviewed here. The clinical application of cancer vaccines to the treatment of human malignancy in the last several years is an excellent representation of the rapid evolution of cancer immunotherapy.
Cancer can be prevented with active immunization, and there are many different types of cancer vaccine formulations in use today, each designed to optimize the ability to immunize against self-proteins (Table 3). Studies have shown that vaccination against human papillomavirus (HPV) type 16 is highly effective in preventing the development of cervical cancer.66 These investigations have been extended to demonstrate that a quadrivalent vaccine targeting HPV types 6, 11, 16, and 18 can prevent a variety of cancers associated with HPV infection.67 In these randomized controlled clinical trials, women received vaccine before the development of infection, so these trials are not analogous to the immunization of cancer-bearing individuals. However, a recent early trial suggests that HPV vaccines can affect existing disease. Investigators immunized 20 women with HPV 16–positive, high-grade vulvar intraepithelial neoplasia with a mixture of peptide epitopes derived from the viral oncoproteins E6 and E7.20 These peptides, small fragments of the HPV protein, are potentially capable of interacting with immune receptor molecules or being processed by APCs. All patients developed IFN-γ–secreting T cell immunity. Three months after the last vaccination, 60% of patients manifested a clinical response. However, at 12 months after vaccinations had ended, 79% of patients demonstrated a response, with 47% complete responses. Clinical responders appeared to have more vigorous antigen-specific type I immunity than nonresponders.20
Table 3.
Characteristics of Common Vaccine Formulations
| Vaccine Construct | Benefits | Drawbacks |
|---|---|---|
| Peptides | Simple formulation and construction | May not include all immunogenic epitopes |
| Avoid epitopes that induce CD4+ regulatory T cells | HLA restricted in some cases | |
| Tumor lysates | Contain the entire tumor antigenic repertoire | Complex to reproducibly formulate |
| If allogenic, provides a “foreign” signal | May include antigens that strongly induce tolerance | |
| Dendritic cell based | Provide the APC most capable of stimulating T cells appropriately | Complex to generate reproducibly |
| Adapted to present any antigen or complex of antigens (RNA, DNA, protein, peptide) | In most cases must be tailor-made for each patient | |
| Plasmid based | Simple inexpensive formulation, stable over long periods | Poorly immunogenic without additional adjuvants |
| Adaptable to multiantigen formulations | ||
| Viral/bacterial vector | Provides strong foreign signal along with self antigen | Complex to generate |
| Enhanced antigen presentation | Concerns in vaccinating immunosuppressed patients |
Additional early-phase clinical trials of cancer vaccines have provided evidence that immune-based therapies may actively modulate the immunosuppressive tumor microenvironment to support a Th1 immune response. As examples, a clinical trial of an autologous DC vaccine pulsed with melanoma tumor cell lysates demonstrated a memory T cell response as measured by delayed type hypersensitivity in the majority of melanoma patients vaccinated.68 Those patients who developed delayed type hypersensitivity responses to lysate-based antigens also demonstrated significant decreases in circulating Tregs as compared with patients who did not mount a cellular immune response (P < .001). A trial of a peptide-based vaccine targeting HER-2/neu in patients with advanced-stage breast cancer demonstrated that active immunization could produce substantial levels of Th1 tumor antigen–specific T cells.69 Furthermore, patients displayed evidence of epitope spreading, which is a broadening of immunity to other tumor antigens expressed in their cancers. Investigators showed that the greater the magnitude of the IFN-γ–secreting T cell response associated with epitope spreading, the greater the declines of serum TGF-ß in these patients with advanced disease. Theoretically, T cells that travel to the tumor location and secrete IFN-γ at high levels in the local tumor environment would active APCs, resulting in cross-priming (Fig 2). As tumor cells often downregulate the receptors needed for immune recognition, the primary method by which the immune system responds to cancer is via tumor antigen uptake by local APCs and presentation of processed antigen to T cells (cross-priming). Cross-priming leads to the development of polyantigen tumor-associated immunity (Fig 2). In these clinical trials, patients experienced an unusual response rate or OS compared with historical controls. These data suggest that the “rejection signal” may be elicited with active immunization.
In 2009, preliminary data from three large randomized clinical trials suggested that cancer vaccines may be clinically useful in a variety of human malignancies: prostate cancer, follicular lymphoma, and melanoma. In the first example, patients with metastatic androgen-independent prostate cancer were immunized with a vaccine targeting prostatic acid phosphatase.70 Antigen was loaded on autologous peripheral blood mononuclear cells, which presumably act as a source of APCs. Five hundred twelve patients were randomly assigned to receive vaccine or placebo. The primary end point of the study was OS. The median survival of patients (n = 341) in the vaccinated arm was 25.8 months compared with 21.7 months for patients (n = 171) in the placebo arm (P = .032; hazard ratio = 0.775; 95% CI, 0.614 to 0.979).71 Of note, this vaccine recently became the first therapeutic cancer vaccine targeting a self-antigen to be approved for clinical use in the United States. The second vaccine study, performed in follicular lymphoma, utilized an idiotype (Id) vaccine unique to individual patients.72,73 The phase III trial enrolled patients with advanced stage follicular lymphoma that had been previously untreated. After standard therapy with prednisone, doxorubicin, cyclophosphamide, and etoposide, patients with a complete response were stratified according to International Prognostic Index risk group and randomly assigned to receive either vaccination with Id-KLH and granulocyte macrophage-colony stimulating factor (GM-CSF) or a placebo that consisted of the immune stimulatory agents alone (KLH/GM-CSF). Eventually, 76 patients received the vaccine and 41 patients received the placebo. At a median follow-up of 56 months, the median time to relapse in the vaccinated arm was 44.2 months compared with 30.6 months in the control arm (P = .045; hazard ratio = 1.6).74 The final vaccine trial was conducted in patients with stage IV and locally advanced stage III cutaneous melanoma. Patients were randomly assigned to receive high-dose IL-2 with or without a peptide vaccine targeting the gp100 protein, a tumor antigen in melanoma. The primary outcome measure was improved response rate. Patients who received the vaccine had a greater overall response rate (22% v 9%; P = .0223) and progression-free survival (2.9 months v 1.6 months; P = .0101) compared with controls. The difference in median OS, however, was not statistically significant between the two groups (P = .0964).75 Although the clinical benefit of each of the vaccines was moderate, these represent some of the first phase III cancer vaccine studies to demonstrate statistically significant benefits. Of note, the prostate and melanoma studies were conducted in patients with established disease, making the results all the more encouraging.
CONCLUSION
The delineation of the function and interaction of innate and adaptive immune cells, the definition of tumor antigens, and the molecular profiling of multiple cancer types have all led to an improved understanding of the methods by which the immune system modulates tumor growth. New agents developed to stimulate tumor-specific adaptive immunity are now being designed to counterbalance mechanisms of immune suppression. For the first time, clinical trials of immune-based therapies are demonstrating antitumor efficacy in numbers of patients with cancer, not just one or two unique individuals. The challenge before us is to determine not only how to initiate the “rejection” pathway in human malignancy but also how to sustain that rejection. This will require a better understanding of the host factors that may prevent an effective immune response, the development of combinatorial approaches that disable multiple mechanisms of tumor immune escape, and the integration of immune-based treatments into the standard therapeutic pathway of most patients with cancer.
Acknowledgment
I thank Molly Boettcher for providing outstanding assistance in manuscript preparation.
Glossary Terms
- Adaptive immunity:
An immune response that is usually directed against a specific antigen. This type of immune response results in immunologic memory.
- Adoptive T cell therapy:
The culture and expansion of T lymphocytes outside the body and then the infusion of those lymphocytes into patients for therapeutic purposes.
- Cross-priming:
The mechanism by which T cells are primed at the site of the tumor. Antigen-presenting cells capture environmental antigens and present them to T cells to stimulate antigen-specific immunity.
- Epitope spreading:
The spreading of an adaptive immune response to multiple antigens beyond the initial antigen recognized by the immune system. Epitope spreading has been described for both T cell and B cell responses.
- Innate immunity:
A nonspecific immune response the body has to any foreign stimulus. This type of immunity is not specific to any particular antigen, and no immunologic memory is generated.
- Myeloid-derived suppressor cell:
Immature myeloid cells that are triggered to proliferate by chronic inflammation. These cells can suppress both innate and adaptive immune responses.
- Tertiary lymphoid structure:
Lymph node–like formations that contain T cells, B cells, and antigen-presenting cells in an organized structure. These formations occur in nonlymphatic sites.
- T-helper 1:
CD4 T cells that secrete cytokines such as interferon-gamma, tumor necrosis factor-alpha, and interleukin-2. These cells are immune system activators and support the growth and function of cytotoxic CD8 T cells.
- T-helper 2:
CD4 T cells that secrete cytokines such as IL-4, IL-10, and IL-6. These cells support the proliferation of B cells and dampen the function of cytotoxic CD8 T cells.
- Toll-like receptor:
A class of receptors that are present on many cells in the body and are responsible for initiating innate immune responses. They are particularly effective at triggering dendritic cells.
Footnotes
Supported by the Gateway Foundation; the Susan G. Komen Foundation; and National Institutes of Health Grants No. R01 CA098761, CA101190, and CA 129517.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Author's disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Mary L. Disis, VentiRx (C) Stock Ownership: None Honoraria: None Research Funding: Mary L. Disis, GlaxoSmithKline, Hemispherx Expert Testimony: None Other Remuneration: None
REFERENCES
- 1.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 3.Matzinger P. Friendly and dangerous signals: Is the tissue in control? Nat Immunol. 2007;8:11–13. doi: 10.1038/ni0107-11. [DOI] [PubMed] [Google Scholar]
- 4.Sica A, Larghi P, Mancino A, et al. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18:349–355. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Ho CC, Liao WY, Wang CY, et al. TREM-1 expression in tumor-associated macrophages and clinical outcome in lung cancer. Am J Respir Crit Care Med. 2008;177:763–770. doi: 10.1164/rccm.200704-641OC. [DOI] [PubMed] [Google Scholar]
- 6.Kelley T, Beck R, Absi A, et al. Biologic predictors in follicular lymphoma: Importance of markers of immune response. Leuk Lymphoma. 2007;48:2403–2411. doi: 10.1080/10428190701665954. [DOI] [PubMed] [Google Scholar]
- 7.Zhu XD, Zhang JB, Zhuang PY, et al. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol. 2008;26:2707–2716. doi: 10.1200/JCO.2007.15.6521. [DOI] [PubMed] [Google Scholar]
- 8.Allavena P, Sica A, Solinas G, et al. The inflammatory micro-environment in tumor progression: The role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66:1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: Linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: More of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Disis ML. Enhancing cancer vaccine efficacy via modulation of the tumor microenvironment. Clin Cancer Res. 2009;15:6476–6478. doi: 10.1158/1078-0432.CCR-09-2256. [DOI] [PubMed] [Google Scholar]
- 12.Sinha P, Clements VK, Bunt SK, et al. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 13.Chaput N, Conforti R, Viaud S, et al. The Janus face of dendritic cells in cancer. Oncogene. 2008;27:5920–5931. doi: 10.1038/onc.2008.270. [DOI] [PubMed] [Google Scholar]
- 14.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansson M, Denardo DG, Coussens LM. Polarized immune responses differentially regulate cancer development. Immunol Rev. 2008;222:145–154. doi: 10.1111/j.1600-065X.2008.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y, Zhou BP. Inflammation: A driving force speeds cancer metastasis. Cell Cycle. 2009;8:3267–3273. doi: 10.4161/cc.8.20.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sallusto F, Lanzavecchia A. Heterogeneity of CD4+ memory T cells: Functional modules for tailored immunity. Eur J Immunol. 2009;39:2076–2082. doi: 10.1002/eji.200939722. [DOI] [PubMed] [Google Scholar]
- 20.Kenter GG, Welters MJ, Valentijn AR, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 21.Goodell V, Waisman J, Salazar LG, et al. Level of HER-2/neu protein expression in breast cancer may affect the development of endogenous HER-2/neu-specific immunity. Mol Cancer Ther. 2008;7:449–454. doi: 10.1158/1535-7163.MCT-07-0386. [DOI] [PubMed] [Google Scholar]
- 22.Cheever MA, Allison JP, Ferris AS, et al. The prioritization of cancer antigens: A National Cancer Institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saint-Mezard P, Berthier CC, Zhang H, et al. Analysis of independent microarray datasets of renal biopsies identifies a robust transcript signature of acute allograft rejection. Transpl Int. 2009;22:293–302. doi: 10.1111/j.1432-2277.2008.00790.x. [DOI] [PubMed] [Google Scholar]
- 24.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 25.Kreike B, Hart G, Bartelink H, et al. Analysis of breast cancer related gene expression using natural splines and the Cox proportional hazard model to identify prognostic associations. Breast Cancer Res Treat. doi: 10.1007/s10549-009-0588-6. epub ahead of print on October 27, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Roepman P, Jassem J, Smit EF, et al. An immune response enriched 72-gene prognostic profile for early-stage non-small-cell lung cancer. Clin Cancer Res. 2009;15:284–290. doi: 10.1158/1078-0432.CCR-08-1258. [DOI] [PubMed] [Google Scholar]
- 27.Clemente CG, Mihm MC, Jr, Bufalino R, et al. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303–1310. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 29.Pagès F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 30.Dieu-Nosjean MC, Antoine M, Danel C, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26:4410–4417. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 31.Gao Q, Qiu SJ, Fan J, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 32.Sharma P, Shen Y, Wen S, et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci U S A. 2007;104:3967–3972. doi: 10.1073/pnas.0611618104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirkwood JM, Strawderman MH, Ernstoff MS, et al. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: The Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14:7–17. doi: 10.1200/JCO.1996.14.1.7. [DOI] [PubMed] [Google Scholar]
- 35.Gogas H, Ioannovich J, Dafni U, et al. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med. 2006;354:709–718. doi: 10.1056/NEJMoa053007. [DOI] [PubMed] [Google Scholar]
- 36.Bouwhuis MG, Suciu S, Collette S, et al. Autoimmune antibodies and recurrence-free interval in melanoma patients treated with adjuvant interferon. J Natl Cancer Inst. 2009;101:869–877. doi: 10.1093/jnci/djp132. [DOI] [PubMed] [Google Scholar]
- 37.Tatsumi T, Kierstead LS, Ranieri E, et al. Disease-associated bias in T helper type 1 (Th1)/Th2 CD4(+) T cell responses against MAGE-6 in HLA-DRB10401(+) patients with renal cell carcinoma or melanoma. J Exp Med. 2002;196:619–628. doi: 10.1084/jem.20012142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caras I, Grigorescu A, Stavaru C, et al. Evidence for immune defects in breast and lung cancer patients. Cancer Immunol Immunother. 2004;53:1146–1152. doi: 10.1007/s00262-004-0556-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Satyam A, Singh P, Badjatia N, et al. A disproportion of T(H)1/T(H)2 cytokines with predominance of T(H)2, in urothelial carcinoma of bladder. Urol Oncol. doi: 10.1016/j.urolonc.2009.06.002. epub ahead of print on October 16, 2009. [DOI] [PubMed] [Google Scholar]
- 40.Tassi E, Braga M, Longhi R, et al. Non-redundant role for IL-12 and IL-27 in modulating Th2 polarization of carcinoembryonic antigen specific CD4 T cells from pancreatic cancer patients. PLoS One. 2009;4:e7234. doi: 10.1371/journal.pone.0007234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu XS, Leerberg J, MacDonald K, et al. IFN-gamma promotes generation of IL-10 secreting CD4+ T cells that suppress generation of CD8 responses in an antigen-experienced host. J Immunol. 2009;183:51–58. doi: 10.4049/jimmunol.0802047. [DOI] [PubMed] [Google Scholar]
- 42.DeNardo DG, Barreto JB, Andreu P, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knutson KL, Disis ML, Salazar LG. CD4 regulatory T cells in human cancer pathogenesis. Cancer Immunol Immunother. 2007;56:271–285. doi: 10.1007/s00262-006-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li MO, Flavell RA. Contextual regulation of inflammation: A duet by transforming growth factor-beta and interleukin-10. Immunity. 2008;28:468–476. doi: 10.1016/j.immuni.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 46.Bates GJ, Fox SB, Han C, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24:5373–5380. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 47.Petersen RP, Campa MJ, Sperlazza J, et al. Tumor infiltrating Foxp3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer. 2006;107:2866–2872. doi: 10.1002/cncr.22282. [DOI] [PubMed] [Google Scholar]
- 48.Dannull J, Su Z, Rizzieri D, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morse MA, Hobeika AC, Osada T, et al. Depletion of human regulatory T cells specifically enhances antigen-specific immune responses to cancer vaccines. Blood. 2008;112:610–618. doi: 10.1182/blood-2008-01-135319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Badoual C, Hans S, Rodriguez J, et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12:465–472. doi: 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- 51.Salama P, Phillips M, Grieu F, et al. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–192. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 52.Carreras J, Lopez-Guillermo A, Fox BC, et al. High numbers of tumor-infiltrating FOXP3-positive regulatory T cells are associated with improved overall survival in follicular lymphoma. Blood. 2006;108:2957–2964. doi: 10.1182/blood-2006-04-018218. [DOI] [PubMed] [Google Scholar]
- 53.Gabrysova L, Nicolson KS, Streeter HB, et al. Negative feedback control of the autoimmune response through antigen-induced differentiation of IL-10-secreting Th1 cells. J Exp Med. 2009;206:1755–1767. doi: 10.1084/jem.20082118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saibil SD, Deenick EK, Ohashi PS. The sound of silence: Modulating anergy in T lymphocytes. Curr Opin Immunol. 2007;19:658–664. doi: 10.1016/j.coi.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 55.Gao Q, Wang XY, Qiu SJ, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971–979. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 56.Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fourcade J, Kudela P, Sun Z, et al. PD-1 is a regulator of NY-ESO-1-specific CD8+ T cell expansion in melanoma patients. J Immunol. 2009;182:5240–5249. doi: 10.4049/jimmunol.0803245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patard JJ, Rodriguez A, Lobel B. The current status of intravesical therapy for superficial bladder cancer. Curr Opin Urol. 2003;13:357–362. doi: 10.1097/00042307-200309000-00002. [DOI] [PubMed] [Google Scholar]
- 60.Gaspari AA. Mechanism of action and other potential roles of an immune response modifier. Cutis. 2007;79:36–45. [PubMed] [Google Scholar]
- 61.Panelli MC, Stashower ME, Slade HB, et al. Sequential gene profiling of basal cell carcinomas treated with imiquimod in a placebo-controlled study defines the requirements for tissue rejection. Genome Biol. 2007;8:R8. doi: 10.1186/gb-2007-8-1-r8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kolb HJ, Mittermuller J, Clemm C, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76:2462–2465. [PubMed] [Google Scholar]
- 63.Clynes RA, Towers TL, Presta LG, et al. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 64.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Finn OJ. Cancer vaccines: Between the idea and the reality. Nat Rev Immunol. 2003;3:630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 66.Koutsky LA, Ault KA, Wheeler CM, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347:1645–1651. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- 67.Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 68.López MN, Pereda C, Segal G, et al. Prolonged survival of dendritic cell-vaccinated melanoma patients correlates with tumor-specific delayed type IV hypersensitivity response and reduction of tumor growth factor beta-expressing T cells. J Clin Oncol. 2009;27:945–952. doi: 10.1200/JCO.2008.18.0794. [DOI] [PubMed] [Google Scholar]
- 69.Disis ML, Wallace DR, Gooley TA, et al. Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer. J Clin Oncol. 2009;27:4685–4692. doi: 10.1200/JCO.2008.20.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 71.Schellhammer PF HC, Berger ER, Shore N, Small E, Penson D, Ferrari A, Sims R, Yuh L, Frohlich M, Kantoff P for the IMPACT Study Investigators. A randomized, double-blind, placebo-controlled, multi-center, phase III trial of sipuleucel-T in men with metastatic, androgen independent prostatic adenocarcinoma (AIPC) American Urological Association Annual Meeting. 2009 [Google Scholar]
- 72.Flowers CR. BiovaxID idiotype vaccination: Active immunotherapy for follicular lymphoma. Expert Rev Vaccines. 2007;6:307–317. doi: 10.1586/14760584.6.3.307. [DOI] [PubMed] [Google Scholar]
- 73.Bendandi M, Gocke CD, Kobrin CB, et al. Complete molecular remissions induced by patient-specific vaccination plus granulocyte-monocyte colony-stimulating factor against lymphoma. Nat Med. 1999;5:1171–1177. doi: 10.1038/13928. [DOI] [PubMed] [Google Scholar]
- 74.Schuster SJ, Neelapu SS, Gause BL, et al. Idiotype vaccine therapy (BiovaxID) in follicular lymphoma in first complete remission: Phase III clinical trial results. J Clin Oncol. 2009;27(suppl):5s. abstr 2. [Google Scholar]
- 75.Schwartzentruber DJ, Lawson J, Richards RM, et al. A phase III multi-institutional randomized study of immunization with the gp100: 209-217(210M) peptide followed by high-dose IL-2 compared with high-dose IL-2 alone in patients with metastatic melanoma. J Clin Oncol. 2009;27(suppl):463s. abstr CRA9011. [Google Scholar]