Abstract
Growth anomalies (GAs) are common, tumor-like diseases that can cause significant morbidity and decreased fecundity in the major Indo-Pacific reef-building coral genera, Acropora and Porites. GAs are unusually tractable for testing hypotheses about drivers of coral disease because of their pan-Pacific distributions, relatively high occurrence, and unambiguous ease of identification. We modeled multiple disease-environment associations that may underlie the prevalence of Acropora growth anomalies (AGA) (n = 304 surveys) and Porites growth anomalies (PGA) (n = 602 surveys) from across the Indo-Pacific. Nine predictor variables were modeled, including coral host abundance, human population size, and sea surface temperature and ultra-violet radiation anomalies. Prevalence of both AGAs and PGAs were strongly host density-dependent. PGAs additionally showed strong positive associations with human population size. Although this association has been widely posited, this is one of the first broad-scale studies unambiguously linking a coral disease with human population size. These results emphasize that individual coral diseases can show relatively distinct patterns of association with environmental predictors, even in similar diseases (growth anomalies) found on different host genera (Acropora vs. Porites). As human densities and environmental degradation increase globally, the prevalence of coral diseases like PGAs could increase accordingly, halted only perhaps by declines in host density below thresholds required for disease establishment.
Introduction
Coral reefs represent some of the most biologically diverse ecosystems on the planet, but these important habitats are declining worldwide due to human overexploitation, land-based pollution, global climate change, and disease outbreaks [1]–[6]. While the situation is most severe in the Caribbean, coral reefs are also in decline across the Indo-Pacific, where an annual loss in coral cover of approximately 1% has occurred over the last 20 years, increasing to 2% between 1997 and 2003 [7]. Coral diseases contribute to this decline by causing a loss of live coral cover [8]–[10] that, under extreme circumstances, can lead to complete community phase-shifts (e.g. from coral-dominated to alga-dominated) [11]. The causes of most coral diseases are unknown. However, understanding how coral disease prevalence relates to changes in reef environmental quality may provide clues to disease etiology. Coral disease increases are associated with local anthropogenic stressors such as poor water quality [12]–[17], as well as global stressors such as sea-surface temperature anomalies [18] and resultant coral bleaching events [19]–[22]. Effects of environmental co-factors may vary between disease types [23] but few efforts have been made to model individual coral diseases with multiple, possibly interacting, environmental cofactors, but see [17], [18], [22].
As a step towards understanding disease dynamics, statistical modeling techniques have recently been used over small spatial scales (individual reefs) to examine multiple coral disease-environment associations [17]. In the present study we used statistical modeling to examine the prevalence of two coral diseases, Acropora growth anomalies (AGAs) and Porites growth anomalies (PGAs) (Fig. 1) across the Indo-Pacific region. Growth anomalies appear as distinctive protuberant masses on corals and thus are easily distinguished in the field. These lesions do not suffer from confounding interpretations, as do lesions involving tissue loss (e.g. white syndrome), which may be confused with predation or vice versa. Growth anomalies have been reported to affect a variety of coral genera from both the Caribbean and the Indo-Pacific [24], [25] and have been relatively well characterized at the gross and microscopic levels [26]–[34]. Although the causes of GAs in corals are unknown, they are associated with reduced colony growth [26], [27], partial colony mortality [28], [33] and decreased reproduction [30], [33], and therefore could negatively impact the fitness of host populations. Acroporids appear to be the most susceptible to GAs; they have been recorded in over 17 species [25], [28], [33], [34]. Porites GAs are less common and have been described from seven species [22], [25], [32], [34], [35].
Figure 1. Picture of Porites growth anomaly (top) and Acropora growth anomaly (bottom).
Our objective was to model the prevalence of growth anomalies in Porites spp. and Acropora spp. in relation to a range of environmental parameters at several hundred sites across the Indo-Pacific Ocean. Disease data were collected from reefs in regions that ranged from heavily populated (and therefore potentially more intensely impacted by local stressors), such as the main Hawaiian Islands [36] and Central Philippines [37], to relatively pristine remote reefs with minimal direct human impact, although still vulnerable to global stressors, such as Palmyra Atoll National Wildlife Refuge in the northern Line Islands [38], [39]. This enabled comparative analyses of disease prevalence across multiple gradients for each of our predictors of interest: biological factors (coral host abundance); anthropogenic factors (human population size); and environmental factors (thermal stress events, surface ultra-violet radiation). Our overall aim was to determine the environmental conditions associated with the prevalence of AGAs and PGAs across the Indo-Pacific, while accounting for confounding effects such as variations in survey effort and timing of the disease surveys.
Methods
Prevalence of Acropora and Porites growth anomalies, and potential biological, environmental and anthropogenic predictors
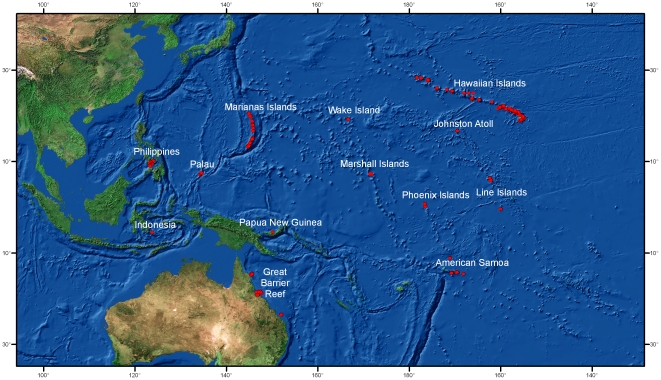
Our analyses were based upon 937 quantitative coral disease surveys from 13 regions from across the Indo-Pacific between 2002 and 2008 (Fig. 2; Table 1; Table S1). Our response variable was disease prevalence (proportion of colonies surveyed affected by GAs) within the survey areas. Biological predictors were host (Porites spp. or Acropora spp.) density and percent cover.
Figure 2. Map showing survey sites across the Indo-Pacific used in the analyses.
Table 1. Numbers of disease surveys conducted at each region by year.
| Survey region | 2002 | 2004 | 2005 | 2006 | 2007 | 2008 | Total |
| Great Barrier Reef | 38 | 42 | 36 | 6 | 12 | 134 | |
| Papua New Guinea | 4 | 4 | |||||
| Indonesia | 5 | 5 | 10 | ||||
| Philippines | 22 | 11 | 33 | ||||
| American Samoa | 11 | 19 | 57 | 58 | 145 | ||
| Palau | 6 | 19 | 25 | ||||
| Marshall Islands | 4 | 4 | |||||
| Marianas | 7 | 66 | 73 | ||||
| Line Islands | 36 | 46 | 82 | ||||
| Phoenix Islands | 12 | 8 | 20 | ||||
| Johnston Atoll | 12 | 25 | 6 | 43 | |||
| Wake | 12 | 12 | |||||
| Hawaiian Islands | 57 | 82 | 100 | 113 | 352 | ||
| Total | 937 |
Belt transects were used to quantify disease and biological predictors, but the number, length and width of transects differed among regions and researchers. Hence, both survey effort (area of reef surveyed (m2)) and timing of the surveys (year) were included as predictors in the models. Global environmental predictors included frequency of weekly sea surface temperature anomalies (WSSTA) and frequency of erythemal surface ultraviolet (UV) radiation anomalies, while human population size served as a proxy for the impact of anthropogenic effects. Coral disease survey locations were imported as geo-referenced points into the GIS and predictor values were extracted for each survey. Human population counts were raster data of 2.5 arc-minutes resolution adjusted to match UN totals for 2005 [40]. Human population size was summed within circular buffers of 1 and 100 km around each survey site. Data were included for all grid cells that intersected a buffer. The mean annual WSSTA values for the four years prior to the year of the survey were extracted for each coral survey location. The frequency of weekly sea surface temperature anomalies (WSSTA) was defined as the number of times over the previous 52 weeks that the weekly sea surface temperature (SST) minus the weekly climatological SST, equaled or exceeded 1°C [41]. SSTA data were approximately 4 km resolution Pathfinder AVHRR raster data on a weekly time scale from 1985 through 2005. The frequency of erythemal surface ultraviolet (UV) radiation anomalies were the number of times between 2000 and 2004 that the monthly average exceeded the climatological mean plus one standard deviation [42]. These values were summed across the 12 months to provide a single value, ranging from 0–19, representing the number of anomalous values for each coral survey location over the entire 5 years. The erythemal surface UV data were measured as part of the GSFC TOMS EP/TOMS satellite program at NASA [43]. These data were processed by NASA to isolate the amount of erythemal ultraviolet (UV) light that reaches Earth's surface. Data were reported as the average Joules (J) per m2 per month at one-degree cell (∼110 km by 110 km) resolution. Figure S1 shows how GIS data were used in the analyses for the main Hawaiian Islands, as an example. These data were prepared and geoprocessed with ArcGIS 9.2 and Matlab 7.1.
Statistical analyses
To investigate associations between prevalence (proportion of colonies affected by GAs) of AGAs and PGAs with each of the predictor variables (Table 2), we used a permutational distance-based multiple regression technique (DISTLM) [44]. DISTLM is robust to zero-inflated data sets, such as ours, and makes no assumptions about the distribution of the response variable (i.e. normality does not have to be satisfied). No two predictors exceeded the recommended cut-off inter-correlation value of 0.95 [45]. In fact, the highest Pearson's correlations between predictors did not exceed 0.65 and 0.44 for AGA and PGA, respectively. Predictors were normalized and were fitted conditionally in a step-wise manner, with tests based on 9999 permutations of the residuals under the reduced model [44], [45]. Model selection (to obtain the best-fit model while maintaining model parsimony) was based on a Bayesian Information Criterion (BIC) [46]. BIC is similar to the more commonly used Akaike's Information Criterion (AIC), however BIC includes a more severe penalty for the inclusion of extraneous predictor variables [45]. To visualize each best-fit model, distance-based redundancy plots (dbRDA) [44] were created based on the prevalence patterns between independent observations. The optimal predictor variable vector(s) (model base variables) was then overlaid as a bi-plot [45]. DISTLM cannot handle missing values within the predictor variable data sets, therefore disease surveys with missing data points for any of the nine predictor variables had to be deleted from the analyses, leaving 304 and 602 surveys for AGA and PGA prevalence, respectively. All prevalence modeling analyses were based on zero-adjusted Bray-Curtis similarity matrices [47] and conducted in PRIMER v6 [48] and PERMANOVA+ [45].
Table 2. Response and predictor variables used in the analyses with their codes and units.
| Variable | Code | Description and units | Min | Max |
| Response | ||||
| Acropora GA | AGA | prevalence | 0 | 9.38 |
| Porites GA | PGA | prevalence | 0 | 16.67 |
| Predictor | ||||
| Acropora cover | AcropCov | % cover | 0.40 | 75.1 |
| Acropora density | AcropDen | # colonies/m2 | 0.01 | 37.8 |
| Porites cover | PorCov | % cover | 0.2 | 90.8 |
| Porites density | PorDen | # colonies/m2 | 0.03 | 41.1 |
| Depth | Depth | m | 0.5 | 18.3 |
| WSSTA during prior 4 years | WSSTA | mean number | 1.5 | 20 |
| Human numbers within 1 km | HumPop1 | number of people | 0 | 50,362 |
| Human numbers within 100 km | HumPop100 | number of people | 0 | 7,705,440 |
| UV input | UV | rating scale | 0 | 15 |
| Year | Year | year of survey | 2002 | 2008 |
| Survey effort | Area | m2 of reef | 60 | 1200 |
Min/Max, minimum and maximum predictor values between independent observations across the entire data set. GA, growth anomaly. WSSTA, weekly sea-surface temperature anomaly. UV, ultraviolet radiation.
Results
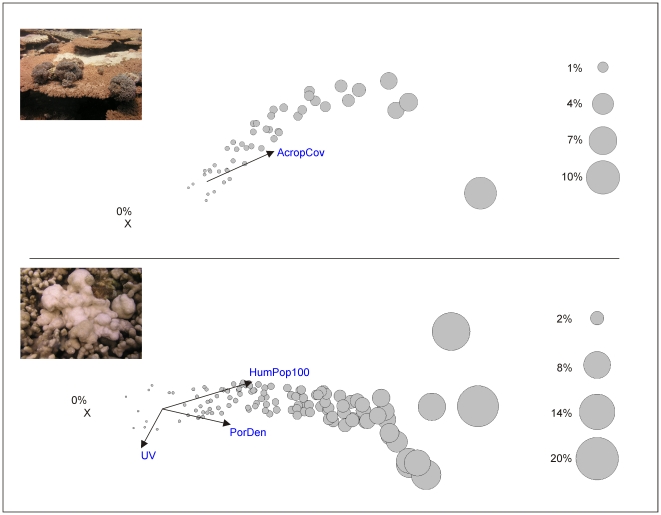
Between 2002 and 2008, AGAs were recorded within approximately 16% of the surveys (n = 534) and PGAs were recorded within 18% of the surveys (n = 855) (Table S2). Prevalence of AGAs (all years and surveys combined) ranged from 0 to 9.4% (Avg. = 0.14%, SD±0.6) and the prevalence of PGAs ranged from 0 to 16.7% (Avg. = 0.2% SD±1.1) (Table S3). AGA prevalence was positively associated with Acropora cover, which explained 16.6% of the variability in disease prevalence (Table 3). No other predictor explained a significant proportion of the variability in AGA prevalence (Table 3, Fig. 3). PGA prevalence was positively associated with higher regional (100 km) human population sizes and with higher Porites colony densities, with the two predictors significantly explaining 28.8% of the variability in disease prevalence. UV input also significantly explained 12.4% of the variability in disease prevalence and increased levels of UV were associated with lower levels of PGA prevalence (Table 3, Fig. 3). The nine predictors explained a greater proportion of the variability in PGA prevalence than in AGA prevalence, with total explained variability equaling 41.2% and 16.6%, respectively (Table 3).
Table 3. Summary results of a distance-based permutational multiple regression analysis for the association of the prevalence of two coral diseases (Acropora and Porites growth anomalies) with 9 predictor variables across surveys (304 and 602, respectively) throughout the Indo-Pacific Ocean.
| Disease | n | Predictor | BIC | Pseudo-F | P value | % variability | % total |
| Acropora GA | 304 | AcropCov | 1925.5 | 21.18 | 0.0001 | 16.6 | 16.6 |
| Porites GA | 602 | HumPop100 | 4349.2 | 36.88 | 0.0001 | 15.8 | |
| PorDen | 4335.9 | 19.98 | 0.0001 | 13.0 | |||
| UV | 4325.8 | 16.57 | 0.0002 | 12.4 | 41.2 |
The optimal predictors of each disease and the proportion of variability (%) in the data set they explained are shown. Predictor variable codes and units are as per Table 2. Model development was based on step-wise selection and a Bayesian Information Criterion (BIC), with the total variation (r2) explained by each best-fit model shown (% total). Analyses based on 9999 permutations of the residuals under a reduced model.
Figure 3. Distance-based multiple regression analyses relating Acropora (top) and Porites (bottom) growth anomaly prevalence to 9 predictor variables across surveys throughout the Indo-Pacific.
Number of surveys where data for all predictor variables was obtained equals 304 and 602 for Acropora GAs and Porites GAs, respectively. Graphs modified from distance-based redundancy plots. The bubbles represent the proportion of corals displaying signs of the disease (% of the population affected) at each survey site. The overlaid bi-plot shows the correlation of the disease prevalence with the optimal predictor(s) forming the best-fit model. The vector line indicates the direction of the relationship with disease prevalence. The length of vector line indicates the relative importance of the predictor. X represents a cluster of sites where the disease prevalence equaled zero. Predictor variable codes and units are as per Table 2.
Discussion
Growth anomalies (GAs) in Acropora (AGAs) and Porites (PGAs) were widespread across the Indo-Pacific occurring in eleven of the thirteen survey regions. GAs were relatively common with the overall frequency of occurrence (percentage of surveys containing GAs) across the Indo-Pacific being 16% for AGAs and 18% for PGAs. Some regions had an even higher disease occurrence, such as the Philippines where PGAs were found in 58% of the surveys (n = 33) and in Palau where AGAs were found in 32% (n = 25). In contrast to the Indo-Pacific, GAs are much less frequent within the Caribbean. For example, no GAs were reported from 160 stations surveyed across the Florida Keys [49], 13 reef areas off the coast of Colombia [50] and 23 sites off Mexico [51]. In fact, there have only been two published reports of AGAs from the Caribbean [27], [28] with no published reports of PGAs.
Although both diseases (AGA and PGA) were widespread on reefs throughout the Indo-Pacific, their average total prevalence was low (<1%). These values are consistent with reports of other diseases within the Indo-Pacific. For example, mean black band disease prevalence at 19 reefs across the GBR equaled 0.1% [52] and white syndrome and GA prevalence in southeast Sulawesi, Indonesia equaled 0.42% and 0.15%, respectively [53]. In Guam, total GA prevalence averaged 1.4% and that of skeletal eroding band, 1.2% [54], in American Samoa, the prevalence of 12 coral diseases was each less than 1% [55], and finally at Palmyra Atoll overall disease prevalence equaled less than 0.4% [56]. However, on some reefs within the Indo-Pacific coral diseases can be quite prevalent. Prevalence of skeletal eroding band from the reefs of Aqaba, Red Sea, ranged from 4 – 41% [57] and the average prevalence of Porites ulcerative white spot disease in the Philippines was 22% [58]. In Guam, white syndrome is, by far, the most prevalent disease (8.9%) [54] and this has remained consistent for several years (Raymundo and Kim unpubl. data). However, while these comparisons provide a snapshot view of regional variability, they do not take into account the possibility that some of these high values may represent seasonal outbreak conditions at surveyed sites and differences in the amount of reef area surveyed.
The emergence of coral disease occurs from a complex interplay between the host, causative agent and environment [23]. Hence, one would expect high variability between sites, as found in this and other studies of coral disease [8], [14], [17], [37], [38], [54], [59]–[61]. The prevalence of AGAs and PGAs varied greatly among survey sites and survey regions. The reefs within the regions we examined represented a range of environmental conditions, differing in water temperature, exposure to ultraviolet radiation, coral host abundance and human population sizes. Using statistical modeling, we found relatively distinct environmental associations between the prevalence of AGAs and PGAs throughout the Indo-Pacific. To sum, the prevalence of AGAs was most positively associated with host abundance, while PGA prevalence showed strong positive association with both increased human population sizes and host abundance. In addition, low prevalence of PGAs on reefs (as opposed to zero prevalence) was associated with increased frequencies of ultraviolet radiation anomalies. These results emphasize that individual coral diseases can show relatively distinct patterns of association with environmental predictors [17] even in the case of similar diseases (GAs) found on different host genera (Acropora vs. Porites). Therefore, future efforts to predict impacts and manage coral diseases on reefs should consider this finding and treat analyses separately for each disease, rather than combining all diseases into a single response variable.
Model performance was good for PGAs, with 41.1% of the variability in prevalence explained. Therefore, we predict that within the Indo-Pacific one would encounter PGAs on reefs with higher Porites cover near high human population centers. In contrast, less variability (16.6%) was explained by modeling AGAs, suggesting that additional variables we did not test may be implicated in driving prevalence patterns. For our analyses, disease data were collected at the genus level, which does not take into account potential species specific differences in susceptibility to GAs. For example, across the Indo-Pacific, the genus Acropora is very species-rich (>160 species) [62]. If species within the genus were differentially susceptible to AGAs then this could partially explain the poor model fit, as our taxonomic resolution did not account for host density differences below the genus level. The prevalence of AGAs in American Samoa, NWHI and Johnston Atoll was higher in plating Acropora sp. (n = 29) as compared to branching (n = 8), encrusting (n = 2) and corymbose (n = 15) morphologies, suggesting that plating colonies may be more prone to GA formation [33]. Many coral species are difficult to identify in the field but including information such as morphological types within genera during surveys may provide more resolution and better explain prevalence patterns.
While it is likely the performance of our models would be improved with species level data, we still found that generic host abundance was an important explanatory variable for the prevalence of both AGAs and PGAs. A positive association between a disease and its host is consistent with disease ecology theory [63], and often reflects the increased horizontal transmission of a disease throughout a population as the population increases in size and distance between individuals decreases [64], [65]. Many examples of relationships between host abundance and disease prevalence exist throughout a wide range of ecosystems and taxa, governed by both density-dependent and frequency-dependent processes [66]–[71]. Host abundance thresholds occur for other coral diseases; for example white syndrome outbreaks along the GBR require, in part, host cover values in excess of 50% [18]. On reefs in Guam and Palau, total disease prevalence was significantly positively associated with coral host abundance or cover [54] and, in Hawaii, Porites trematodiasis and Montipora white syndrome prevalence are both strongly associated with coral host cover [17], [72], [73]. Thus, diseases causing significant mortality and reduced fecundity are likely to have major effects on community structure, as spatially-dominant species will be more impacted by disease.
Only PGA prevalence, and not AGA, showed strong positive associations with human population size suggesting that they are related, directly or indirectly, to some environmental co-factor associated with increased human population size at regional spatial scales. Human activities can result in increased disease levels within wildlife populations, as a result of human-induced environmental degradation caused by pollution, eutrophication, habitat fragmentation, and direct introduction of novel pathogens into ecosystems [74]–[79]. For example, the Hawaiian green sea turtle showed elevated rates of a tumor disease in watersheds with a high nitrogen-footprint reflective of coastal eutrophication [80]. Diseases of corals in tropical ecosystems are proving no exception, with human impacts suggested to affect disease prevalence [81]. If we are to conserve our coral reef resources, it is critical that we determine which components of human impacts may be affecting disease levels. Increased nutrients and reduced water quality have been linked to increased prevalence and severity of coral diseases such as black band disease (caused by a microbial consortium) [13]–[15], [82], and aspergillosis, a sea fan disease caused by the terrestrial soil-borne fungus Aspergillus sydowii [12], [15], [16], [83], [84]. Direct influx of potential pathogens into the marine environment (e.g. through sewage effluent disposal), has been suggested as a causal mechanism for white pox which affects elkhorn Acropora corals in the Caribbean [85]. Although not well-studied, viruses have also been proposed as potential agents of coral disease [86] and marine virus-like particles (VLPs) have been found in increased abundance with proximity to populated coastal areas [87].
While our understanding of coral disease etiology has advanced considerably in recent years [88]–[93], the cause of coral GAs remains largely unknown [33]. For AGAs, damage to cells from ultraviolet (UV) radiation [29] and stressors such as high levels of sedimentation, turbidity and seasonal temperature extremes [28] have been suggested as playing a role in triggering GA formation. Our analyses suggest a link between PGA prevalence and ultraviolet radiation anomalies in areas where human population sizes are lower, however, no such associations were found for AGAs. The link between PGA development and ultraviolet radiation was not supported manipulatively on Porites compressa in Hawaii [94] and no explanations have yet been presented regarding the etiology of PGAs, but one study did find them to be transmissible suggesting an infectious agent [35]. Viruses have been found associated with tumor formation in other animals such as turtles [95], [96] and fish [97]. Given the known positive association between human numbers and densities of marine viruses [87], [98], the common association of viruses with the coral holobiont [99], [100] and the strong association we found between PGAs and human population size, investigations into a potential viral etiology of PGAs would seem a logical next step.
Increases in temperature, like other stressors such as poor water quality, can alter host susceptibility to disease or pathogen virulence [6], [101], [102], ultimately shifting the balance in favor of one or the other [103]. Many coral diseases show positive associations with temperature, for example black band disease in the Caribbean, the Florida Keys and the GBR [104]–[107], Porites tissue loss syndrome in Hawaii [17], and white syndromes along the GBR [18]. However, we found that host abundance and human population size were the optimal predictors for variations in prevalence of AGAs and PGAs, respectively, with WSSTAs showing no such association. It may be that chronic diseases, such as GAs, are less influenced by short-term changes in temperature as compared to the tissue loss diseases, many of which are caused by pathogenic bacteria with virulence factors that may be enhanced at higher temperatures [85], [90], [91], [93]. Many bacteria thrive in warm temperatures and so bacterial diseases could be influenced more by temperature [108]. Understanding the disease-specific responses to environmental and anthropogenic stressors is critical if we are to protect and conserve our reefs from the inevitable threat of future environmental change.
In summary, AGAs and PGAs showed relatively distinct patterns with the predictors tested throughout the Indo-Pacific. While GAs in both genera showed positive associations with host abundance, PGAs additionally showed strong positive associations with human population size. GAs are often progressive and can result in host mortality [33] and so represent a threat to coral reef health worldwide. As human densities and environmental degradation continue to increase across the globe [78], the prevalence of diseases such as PGAs that are associated with these factors may similarly increase throughout the Indo-Pacific, halted only perhaps by declines in host density below thresholds required for disease establishment. Increases in coral disease prevalence and outbreaks, in combination with mass coral bleaching events and other disturbances associated with climate change, pose a great threat to the future survival of coral reef environments on our planet. Future efforts should focus on determining the etiology of AGAs and PGAs so that the environmental associations identified in the present study are put into a better ecological context, thus increasing our understanding of their ecology and ultimately granting us the knowledge to mitigate an increase in their prevalence.
Supporting Information
Example of GIS data used in the analyses. Shown are data for the sites included from the main Hawaiian Islands.
(TIF)
Islands surveyed for Acropora and Porites growth anomalies within each of the regions analyzed.
(DOC)
Frequency of occurrence (FOC) of Acropora growth anomalies (AGAs) and Porites growth anomalies (PGAs) across the Indo-Pacific.
(DOC)
Average prevalence of Acropora growth anomalies (AGAs) and Porites growth anomalies (PGAs) across the Indo-Pacific.
(DOC)
Acknowledgments
The Coral Reef Temperature Anomaly Database (CoRTAD) was developed by the NOAA National Oceanographic Data Center and the University of North Carolina, Chapel Hill. It was provided by the NOAA National Oceanographic Data Center website (accessed 2008) http://www.nodc.noaa.gov/SatelliteData/Cortad/. Human population data was provided by the Center for International Earth Science Information Network (CIESIN), Columbia University; United Nations Food and Agriculture Programme (FAO); and Centro Internacional de Agricultura Tropical (CIAT). We thank the U.S. Fish and Wildlife Service for granting access to all U.S. National Wildlife Refuges. BVA wishes to thank the officers and crew of the NOAA Ship, Hi'ialakai and B Wheeler, J Kenyon, and J Helyer for additional data collection. GW wishes to thank I Knapp for field assistance. LR wishes to thank R Myers and P Rojas for field assistance. Thanks to C Runyon for numerous hours of data processing.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Human population data was provided by the Center for International Earth Science Information Network (CIESIN), Columbia University; United Nations Food and Agriculture Programme (FAO); and Centro Internacional de Agricultura Tropical (CIAT) with funding from the National Aeronautics and Space Administration under Contract NAS5-03117 for the Continued Operation of the Socioeconomic Data and Applications Center (SEDAC). GA gratefully acknowledges funding from the Office of National Marine Sanctuaries (NWHICRER MOA-2005-008/6882). BW acknowledges funding from the Australian Research Council and the Global Environment Fund (GEF)/World Bank CRTR Program Coral Disease Working Group. BVA's work was supported by the NOAA Coral Reef Conservation Program and the Pacific Islands Fisheries Science Center's Coral Reef Ecosystem Division. GW was supported by a Victoria University of Wellington (VUW) Vice-Chancellor's Strategic Research Scholarship and a New Zealand International Doctoral Research Scholarship. GW was additionally funded by grants from the National Geographic Society and the VUW Research Fund awarded to SD. LR was supported by the GEF/CRTR and the NOAA Coral Reef Initiative. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bellwood DR, Hughes TP, Folke C, Nystrom M. Confronting the coral reef crisis. Nature. 2004;429:827–833. doi: 10.1038/nature02691. [DOI] [PubMed] [Google Scholar]
- 2.Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, et al. Climate change, human impacts, and the resilience of coral reefs. Science. 2003;301:929–933. doi: 10.1126/science.1085046. [DOI] [PubMed] [Google Scholar]
- 3.Hoegh-Guldberg O. Climate change, coral bleaching and the future of the world's coral reefs. Marine and Freshwater Research. 1999;50:839–866. [Google Scholar]
- 4.De'ath G, Lough JM, Fabricius KE. Declining Coral Calcification on the Great Barrier Reef. Science. 2009;323:116–119. doi: 10.1126/science.1165283. [DOI] [PubMed] [Google Scholar]
- 5.Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, et al. Coral reefs under rapid climate change and ocean acidification. Science. 2007;318:1737–1742. doi: 10.1126/science.1152509. [DOI] [PubMed] [Google Scholar]
- 6.Harvell CD, Mitchell CE, Ward JR, Altizer S, Dobson AP, et al. Ecology - Climate warming and disease risks for terrestrial and marine biota. Science. 2002;296:2158–2162. doi: 10.1126/science.1063699. [DOI] [PubMed] [Google Scholar]
- 7.Bruno JF, Selig ER. Regional Decline of Coral Cover in the Indo-Pacific: Timing, Extent, and Subregional Comparisons. PLoS ONE. 2007;2:e711. doi: 10.1371/journal.pone.0000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nugues MM. Impact of a coral disease outbreak on coral communities in St. Lucia: What and how much has been lost? Marine Ecology-Progress Series. 2002;229:61–71. [Google Scholar]
- 9.Richardson LL, Voss JD. Changes in a coral population on reefs of the northern Florida Keys following a coral disease epizootic. Marine Ecology-Progress Series. 2005;297:147–156. [Google Scholar]
- 10.Bruckner AW, Hill RL. Ten years of change to coral communities off Mona and Desecheo Islands, Puerto Rico, from disease and bleaching. Diseases of Aquatic Organisms. 2009;87:19–31. doi: 10.3354/dao02120. [DOI] [PubMed] [Google Scholar]
- 11.Aronson RB, Precht WF. White-band disease and the changing face of Caribbean coral reefs. Hydrobiologia. 2001;460:25–38. [Google Scholar]
- 12.Kim K, Harvell CD. Aspergillosis of sea fan corals: dynamics in the Florida Keys. In: Porter JW, Porter KG, editors. The Everglades, Florida Bay, and coral reefs of the Florida Keys: an ecosystem sourcebook. Boca Raton: CRC; 2002. pp. 813–824. [Google Scholar]
- 13.Kuta KG, Richardson LL. Ecological aspects of black band disease of corals: relationships between disease incidence and environmental factors. Coral Reefs. 2002;21:393–398. [Google Scholar]
- 14.Bruno JF, Petes LE, Harvell CD, Hettinger A. Nutrient enrichment can increase the severity of coral diseases. Ecology Letters. 2003;6:1056–1061. [Google Scholar]
- 15.Voss JD, Richardson LL. Nutrient enrichment enhances black band disease progression in corals. Coral Reefs. 2006;25:569–576. [Google Scholar]
- 16.Baker DM, MacAvoy SE, Kim K. Relationship between water quality, delta N-15, and aspergillosis of Caribbean sea fan corals. Marine Ecology-Progress Series. 2007;343:123–130. [Google Scholar]
- 17.Williams GJ, Aeby GS, Cowie ROM, Davy SK. Predictive Modeling of Coral Disease Distribution within a Reef System. PLoS ONE. 2010;5:e9264. doi: 10.1371/journal.pone.0009264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruno JF, Selig ER, Casey KS, Page CA, Willis BL, et al. Thermal stress and coral cover as drivers of coral disease outbreaks. PloS Biology. 2007;5:1220–1227. doi: 10.1371/journal.pbio.0050124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harvell D, Kim K, Quirolo C, Weir J, Smith G. Coral bleaching and disease: contributors to 1998 mass mortality in Briareum asbestinum (Octocorallia, Gorgonacea). Hydrobiologia. 2001;460:97–104. [Google Scholar]
- 20.Muller EM, Rogers CS, Spitzack AS, van Woesik R. Bleaching increases likelihood of disease on Acropora palmata (Lamarck) in Hawksnest Bay, St John, US Virgin Islands. Coral Reefs. 2008;27:191–195. [Google Scholar]
- 21.Brandt ME, McManus JW. Disease incidence is related to bleaching extent in reef-building corals. Ecology. 2009;90:2859–2867. doi: 10.1890/08-0445.1. [DOI] [PubMed] [Google Scholar]
- 22.McClanahan TR, Weil E, Maina J. Strong relationship between coral bleaching and growth anomalies in massive Porites. Global Change Biology. 2009;15:1804–1816. [Google Scholar]
- 23.Work TM, Richardson LL, Reynolds TL, Willis BL. Biomedical and veterinary science can increase our understanding of coral disease. Journal of Experimental Marine Biology and Ecology. 2008;362:63–70. [Google Scholar]
- 24.Peters EC, Gassman NJ, Firman JC, Richmond RH, Power EA. Ecotoxicology of tropical marine ecosystems. Environmental Toxicology and Chemistry. 1997;16:12–40. [Google Scholar]
- 25.Sutherland KP, Porter JW, Torres C. Disease and immunity in Caribbean and Indo-Pacific zooxanthellate corals. Marine Ecology-Progress Series. 2004;266:273–302. [Google Scholar]
- 26.Cheney DP. Hard tissue tumors of scleractinian corals. Adv Exp Med Biol. 1975;64:77–87. doi: 10.1007/978-1-4684-3261-9_9. [DOI] [PubMed] [Google Scholar]
- 27.Bak RPM. Neoplasia, regeneration and growth in the reef-building coral Acropora palmata. Marine Biology. 1983;77:221–227. [Google Scholar]
- 28.Peters EC, Halas JC, McCarty HB. Calicoblastic neoplasms in Acropora palmata, with a review of reports on anomalies of growth and form in corals. Journal of the National Cancer Institute. 1986;76:895–912. [PubMed] [Google Scholar]
- 29.Coles SL, Seapy DG. Ultraviolet absorbing compounds and tumorous growths on acroporid corals from Bandar Khayran, Gulf of Oman, Indian Ocean. Coral Reefs. 1998;17:195–198. [Google Scholar]
- 30.Yamashiro H, Yamamoto M, van Woesik R. Tumor formation on the coral Montipora informis. Diseases of Aquatic Organisms. 2000;41:211–217. doi: 10.3354/dao041211. [DOI] [PubMed] [Google Scholar]
- 31.Gateno D, Leon A, Barki Y, Cortes J, Rinkevich B. Skeletal tumor formations in the massive coral Pavona clavus. Marine Ecology-Progress Series. 2003;258:97–108. [Google Scholar]
- 32.Domart-Coulon IJ, Traylor-Knowles N, Peters E, Elbert D, Downs CA, et al. Comprehensive characterization of skeletal tissue growth anomalies of the finger coral Porites compressa. Coral Reefs. 2006;25:531–543. [Google Scholar]
- 33.Work TM, Aeby GS, Coles SL. Distribution and morphology of growth anomalies in Acropora from the Indo-Pacific. Diseases of Aquatic Organisms. 2008;78:255–264. doi: 10.3354/dao01881. [DOI] [PubMed] [Google Scholar]
- 34.Williams GJ, Work TM, Aeby GS, Knapp IS, Davy SK. Gross and microscopic morphology of lesions in Cnidaria from Palmyra Atoll, Central Pacific. Journal of Invertebrate Pathology. 2010;106(2):165–173. doi: 10.1016/j.jip.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Kaczmarsky L, Richardson LL. Transmission of growth anomalies between Indo-Pacific Porites corals. Journal of Invertebrate Pathology. 2007;94:218–221. doi: 10.1016/j.jip.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Friedlander AM, DeMartini EE. Contrasts in density, size, and biomass of reef fishes between the northwestern and the main Hawaiian islands: the effects of fishing down apex predators. Marine Ecology-Progress Series. 2002;230:253–264. [Google Scholar]
- 37.Raymundo LJ, Rosell KB, Reboton CT, Kaczmarsky L. Coral diseases on Philippine reefs: genus Porites is a dominant host. Diseases of Aquatic Organisms. 2005;64:181–191. doi: 10.3354/dao064181. [DOI] [PubMed] [Google Scholar]
- 38.Sandin SA, Smith JE, DeMartini EE, Dinsdale EA, Donner SD, et al. Baselines and degradation of coral reefs in the northern Line Islands. PLoS ONE. 2008;3:e1548. doi: 10.1371/journal.pone.0001548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams GJ, Knapp IS, Maragos JE, Davy SK. Modeling patterns of coral bleaching at a remote Central Pacific atoll. Marine Pollution Bulletin. 2010;60:1467–1476. doi: 10.1016/j.marpolbul.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 40.CIESIN. 2005. Center for International Earth Science Information Network Gridded Population of the World Version 3 (GPWv3): Population Grids (accessed 2008) http://sedac.ciesin.columbia.edu/gpw.
- 41.Selig ER, Casey KS, Bruno JF. New insights into global patterns of ocean temperature anomalies: implications for coral reef health and management. Global Ecology and Biogeography. 2010;19:397–411. [Google Scholar]
- 42.Halpern BS, Walbridge S, Selkoe KA, Kappel CV, Micheli F, et al. A global map of human impact on marine ecosystems. Science. 2008;319:948–952. doi: 10.1126/science.1149345. [DOI] [PubMed] [Google Scholar]
- 43.McPeters R, Barthia PA, Krueger AJ, Herman JR, Wellemeyer CG, et al. 1998. Earth Probe Total Ozone Mapping Spectrometer (TOMS) Data Products User's Guide, NASA/TP-1998-206895.
- 44.McArdle BH, Anderson MJ. Fitting multivariate models to community data: A comment on distance-based redundancy analysis. Ecology. 2001;82:290–297. [Google Scholar]
- 45.Anderson MJ, Gorley RN, Clarke KR. Plymouth, UK: PRIMER-E; 2008. PERMANOVA+ for PRIMER: Guide to software and statistical methods. [Google Scholar]
- 46.Schwarz G. Estimating the dimension of a model. Annals of Statistics. 1978;6:461–464. [Google Scholar]
- 47.Clarke KR, Somerfield PJ, Chapman MG. On resemblance measures for ecological studies, including taxonomic dissimilarities and a zero-adjusted Bray-Curtis coefficient for denuded assemblages. Journal of Experimental Marine Biology and Ecology. 2006;330:55–80. [Google Scholar]
- 48.Clarke KR, Gorley RN. Plymouth, UK: PRIMER-E; 2006. PRIMER v6: User manual/Tutorial. [Google Scholar]
- 49.Porter JW, Dustan P, Jaap WC, Patterson KL, Kosmynin V, et al. Patterns of spread of coral disease in the Florida Keys. Hydrobiologia. 2001;460:1–24. [Google Scholar]
- 50.Garzon-Ferreira J, Gil-Agudelo DL, Barrios LM, Zea S. Stony coral diseases observed in southwestern Caribbean reefs. Hydrobiologia. 2001;460:65–69. [Google Scholar]
- 51.Jordan-Dahlgren E, Rodriguez-Martinez E. Coral diseases in Gulf of Mexico reefs. In: Rosenberg E, Loya Y, editors. Coral Health and Disease. Berlin: Springer-Verlag; 2004. pp. 105–118. [Google Scholar]
- 52.Page C, Willis B. Distribution, host range and large-scale spatial variability in black band disease prevalence on the Great Barrier Reef, Australia. Diseases of Aquatic Organisms. 2006;69:41–51. doi: 10.3354/dao069041. [DOI] [PubMed] [Google Scholar]
- 53.Haapkyla J, Seymour AS, Trebilco J, Smith D. Coral disease prevalence and coral health in the Wakatobi Marine Park, south-east Sulawesi, Indonesia. Journal of the Marine Biological Association of the United Kingdom. 2007;87:403–414. [Google Scholar]
- 54.Myers RL, Raymundo LJ. Coral disease in Micronesian reefs: a link between disease prevalence and host abundance. Diseases of Aquatic Organisms. 2009;87:97–104. doi: 10.3354/dao02139. [DOI] [PubMed] [Google Scholar]
- 55.Aeby GS, Work TM, Fenner D, Didonato E. Ft. Lauderdale, Florida: Proceedings of the 11th International Coral Reef Symposium; 2008. Coral and crustose coralline algae disease on the reefs of American Samoa. pp. 197–201. [Google Scholar]
- 56.Williams GJ, Aeby GS, Davy SK. Coral disease at Palmyra Atoll, a remote reef system in the Central Pacific. Coral Reefs. 2008;27:207. [Google Scholar]
- 57.Winkler R, Antonius A, Renegar DA. The skeleton eroding band disease on coral reefs of Aqaba, Red Sea. Marine Ecology. 2004;25(2):129–144. [Google Scholar]
- 58.Raymundo LJH, Harvell CD, Reynolds TL. Porites ulcerative white spot disease: description, prevalence, and host range of a new coral disease affecting Indo-Pacific reefs. Diseases of Aquatic Organisms. 2003;56:95–104. doi: 10.3354/dao056095. [DOI] [PubMed] [Google Scholar]
- 59.Aeby GS. Atoll Research Bulletin; 2006. Baseline levels of coral disease in the Northwestern Hawaiian Islands. pp. 471–488. [Google Scholar]
- 60.Vargas-Angel B. Coral health and disease assessment in the US Pacific Remote Island Areas. Bulletin of Marine Science. 2009;84:211–227. [Google Scholar]
- 61.Santavy DL, Mueller E, Peters EC, MacLaughlin L, Porter JW, et al. Quantitative assessment of coral diseases in the Florida Keys: strategy and methodology. Hydrobiologia. 2001;460:39–52. [Google Scholar]
- 62.Veron J. Townsville, Australia: Australian Institute of Marine Science and CPR Qld Pyt Ltd; 2000. Corals of the world (Vol 1-3). [Google Scholar]
- 63.Lloyd-Smith JO, Cross PC, Briggs CJ, Daugherty M, Getz WM, et al. Should we expect population thresholds for wildlife disease? Trends in Ecology & Evolution. 2005;20:511–519. doi: 10.1016/j.tree.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 64.Altizer SM, Augustine DJ. Interactions between frequency-dependent and vertical transmission in host-parasite systems. Proceedings of the Royal Society of London Series B-Biological Sciences. 1997;264:807–814. doi: 10.1098/rspb.1997.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McCallum H, Barlow N, Hone J. How should pathogen transmission be modelled? Trends in Ecology & Evolution. 2001;16:295–300. doi: 10.1016/s0169-5347(01)02144-9. [DOI] [PubMed] [Google Scholar]
- 66.Brown CR, Brown MB. Empirical measurement of parasite transmission between groups in a colonial bird. Ecology. 2004;85:1619–1626. [Google Scholar]
- 67.Ramsey D, Spencer N, Caley P, Efford M, Hansen K, et al. The effects of reducing population density on contact rates between brushtail possums: implications for transmission of bovine tuberculosis. Journal of Applied Ecology. 2002;39:806–818. [Google Scholar]
- 68.Berthier K, Langlais M, Auger P, Pontier D. Dynamics of a feline virus with two transmission modes within exponentially growing host populations. Proceedings of the Royal Society of London Series B-Biological Sciences. 2000;267:2049–2056. doi: 10.1098/rspb.2000.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bjornstad ON, Finkenstadt BF, Grenfell BT. Dynamics of measles epidemics: Estimating scaling of transmission rates using a Time series SIR model. Ecological Monographs. 2002;72:169–184. [Google Scholar]
- 70.Begon M, Feore SM, Brown K, Chantrey J, Jones T, et al. Population and transmission dynamics of cowpox in bank voles: testing fundamental assumptions. Ecology Letters. 1998;1:82–86. [Google Scholar]
- 71.Begon M, Hazel SM, Baxby D, Bown K, Cavanagh R, et al. Transmission dynamics of a zoonotic pathogen within and between wildlife host species. Proceedings of the Royal Society of London Series B-Biological Sciences. 1999;266:1939–1945. doi: 10.1098/rspb.1999.0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aeby GS. Spatial and temporal patterns of Porites trematodiasis on the reefs of Kaneohe Bay, Oahu, Hawaii. Bulletin of Marine Science. 2007;80:209–218. [Google Scholar]
- 73.Aeby GS, Ross M, Williams GJ, Lewis TD, Work TM. Disease dynamics of Montipora white syndrome within Kaneohe Bay, Oahu, Hawaii: distribution, seasonality, virulence, and transmissibility. Diseases of Aquatic Organisms. 2010;91:1–8. doi: 10.3354/dao02247. [DOI] [PubMed] [Google Scholar]
- 74.Daszak P, Cunningham AA, Hyatt AD. Anthropogenic environmental change and the emergence of infectious diseases in wildlife. Acta Tropica. 2001;78:103–116. doi: 10.1016/s0001-706x(00)00179-0. [DOI] [PubMed] [Google Scholar]
- 75.Dobson A, Foufopoulos J. Emerging infectious pathogens of wildlife. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 2001;356:1001–1012. doi: 10.1098/rstb.2001.0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coyner DF, Spalding MG, Forrester DJ. Epizootiology of Eustrongylides ignotus in Florida: Distribution, density, and natural infections in intermediate hosts. Journal of Wildlife Diseases. 2002;38:483–499. doi: 10.7589/0090-3558-38.3.483. [DOI] [PubMed] [Google Scholar]
- 77.Anderson PK, Cunningham AA, Patel NG, Morales FJ, Epstein PR, et al. Emerging infectious diseases of plants: pathogen pollution, climate change and agrotechnology drivers. Trends in Ecology & Evolution. 2004;19:535–544. doi: 10.1016/j.tree.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 78.Bradley CA, Altizer S. Urbanization and the ecology of wildlife diseases. Trends in Ecology & Evolution. 2007;22:95–102. doi: 10.1016/j.tree.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aguirre AA, Tabor GM. Global Factors Driving Emerging Infectious Diseases Impact on Wildlife Populations. Animal Biodiversity and Emerging Diseases: Prediction and Prevention. 2008;1149:1–3. doi: 10.1196/annals.1428.052. [DOI] [PubMed] [Google Scholar]
- 80.Van Houtan KS, Hargrove SK, Balazs GH. Land Use, Macroalgae, and a Tumor-Forming Disease in Marine Turtles. PLoS ONE. 2010;5:e12900. doi: 10.1371/journal.pone.0012900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harvell CD, Kim K, Burkholder JM, Colwell RR, Epstein PR, et al. Review: Marine ecology - Emerging marine diseases - Climate links and anthropogenic factors. Science. 1999;285:1505–1510. doi: 10.1126/science.285.5433.1505. [DOI] [PubMed] [Google Scholar]
- 82.Richardson LL, Miller AW, Broderick E, Kaczmarsky L, Gantar M, et al. Sulfide, microcystin, and the etiology of black band disease. Diseases of Aquatic Organisms. 2009;87:79–90. doi: 10.3354/dao02083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Smith GW, Ives LD, Nagelkerken IA, Ritchie KB. Caribbean sea-fan mortalities. Nature. 1996;383:487–487. [Google Scholar]
- 84.Geiser DM, Taylor JW, Ritchie KB, Smith GW. Cause of sea fan death in the West Indies. Nature. 1998;394:137–138. [Google Scholar]
- 85.Patterson KL, Porter JW, Ritchie KE, Polson SW, Mueller E, et al. The etiology of white pox, a lethal disease of the Caribbean elkhorn coral, Acropora palmata. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:8725–8730. doi: 10.1073/pnas.092260099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Davy SK, Burchett SG, Dale AL, Davies P, Davy JE, et al. Viruses: agents of coral disease? Diseases of Aquatic Organisms. 2006;69:101–110. doi: 10.3354/dao069101. [DOI] [PubMed] [Google Scholar]
- 87.Wetz JJ, Lipp EK, Griffin DW, Lukasik J, Wait D, et al. Presence, infectivity, and stability of enteric viruses in seawater: relationship to marine water quality in the Florida Keys. Marine Pollution Bulletin. 2004;48:698–704. doi: 10.1016/j.marpolbul.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 88.Aeby GS. A digenean metacercaria from the reef coral, Porites compressa, experimentally identified as Podocotyloides stenometra. Journal of Parasitology. 1998;84:1259–1261. [PubMed] [Google Scholar]
- 89.Rosenberg E, Ben-Haim Y, Toren A, Banin E, Kushmaro A, et al. Effect of temperature on bacterial bleaching of corals. In: Rosenberg E, editor. Microbial ecology and infectious disease. Washington DC: American Society for Microbiology; 1999. pp. 242–254. [Google Scholar]
- 90.Denner EBM, Smith GW, Busse HJ, Schumann P, Narzt T, et al. Aurantimonas coralicida gen. nov., sp nov., the causative agent of white plague type II on Caribbean scleractinian corals. International Journal of Systematic and Evolutionary Microbiology. 2003;53:1115–1122. doi: 10.1099/ijs.0.02359-0. [DOI] [PubMed] [Google Scholar]
- 91.Richardson LL. Black band disease. In: Rosenberg E, Loya Y, editors. Coral Health and Disease. Berlin: Springer-Verlag; 2004. pp. 325–336. [Google Scholar]
- 92.Cervino JM, Thompson FL, Gomez-Gil B, Lorence EA, Goreau TJ, et al. The Vibrio core group induces yellow band disease in Caribbean and Indo-Pacific reef-building corals. Journal of Applied Microbiology. 2008;105:1658–1671. doi: 10.1111/j.1365-2672.2008.03871.x. [DOI] [PubMed] [Google Scholar]
- 93.Sussman M, Willis BL, Victor S, Bourne DG. Coral pathogens identified for white syndrome (WS) epizootics in the Indo-Pacific. PLoS ONE. 2008;3:e2393. doi: 10.1371/journal.pone.0002393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stimson J. Coral Reefs; Ecological characterization of coral growth anomalies on Porites compressa in Hawai'i. In Press. [Google Scholar]
- 95.Work TM, Balazs GH, Rameyer RA, Morris RA. Retrospective pathology survey of green turtles Chelonia mydas with fibropapillomatosis in the Hawaiian Islands, 1993-2003. Diseases of Aquatic Organisms. 2004;62:163–176. doi: 10.3354/dao062163. [DOI] [PubMed] [Google Scholar]
- 96.Work TM, Rameyer RA, Balazs GH, Cray C, Chang SP. Immune status of free-ranging green turtles with fibropapillomatosis from Hawaii. Journal of Wildlife Diseases. 2001;37:574–581. doi: 10.7589/0090-3558-37.3.574. [DOI] [PubMed] [Google Scholar]
- 97.Anders K, Yoshimizu M. Role of viruses in the induction of skin tumors and tumor-like proliferations of fish. Diseases of Aquatic Organisms. 1994;19:215–232. [Google Scholar]
- 98.Dinsdale EA, Pantos O, Smriga S, Edwards RA, Angly F, et al. Microbial Ecology of Four Coral Atolls in the Northern Line Islands. PLoS ONE. 2008;3:e1584. doi: 10.1371/journal.pone.0001584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vega Thurber RL, Barott KL, Hall D, Liu H, Rodriguez-Mueller B, et al. Metagenomic analysis indicates that stressors induce production of herpes-like viruses in the coral Porites compressa. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18413–18418. doi: 10.1073/pnas.0808985105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vega Thurber R, Willner-Hall D, Rodriguez-Mueller B, Desnues C, Edwards RA, et al. Metagenomic analysis of stressed coral holobionts. Environ Microbiol. 2009;11:2148–2163. doi: 10.1111/j.1462-2920.2009.01935.x. [DOI] [PubMed] [Google Scholar]
- 101.Fitt WK, Brown BE, Warner ME, Dunne RP. Coral bleaching: interpretation of thermal tolerance limits and thermal thresholds in tropical corals. Coral Reefs. 2001;20:51–65. [Google Scholar]
- 102.Ward JR, Kim K, Harvell CD. Temperature affects coral disease resistance and pathogen growth. Marine Ecology-Progress Series. 2007;329:115–121. [Google Scholar]
- 103.Blanford S, Thomas MB, Pugh C, Pell JK. Temperature checks the Red Queen? Resistance and virulence in a fluctuating environment. Ecology Letters. 2003;6:2–5. [Google Scholar]
- 104.Edmunds PJ. Extent and effect of Black Band Disease on a Caribbean reef. Coral Reefs. 1991;10:161–165. [Google Scholar]
- 105.Bruckner AW, Bruckner RJ. The persistence of black band disease in Jamaica: impact on community structure. Proceedings of the 8th International Coral Reef Symposium, Smithsonian Tropical Research Institute, Panama. 1997;1:601–606. [Google Scholar]
- 106.Kuta KG, Richardson LL. Abundance and distribution of black band disease on coral reefs in the northern Florida Keys. Coral Reefs. 1996;15:219–223. [Google Scholar]
- 107.Boyett HV, Bourne DG, Willis BL. Elevated temperature and light enhance progression and spread of black band disease on staghorn corals of the Great Barrier Reef. Marine Biology. 2007;151:1711–1720. [Google Scholar]
- 108.Bourne DG, Garren M, Work TM, Rosenberg E, Smith GW, et al. Microbial disease and the coral holobiont. Trends in Microbiology. 2009;17:554–562. doi: 10.1016/j.tim.2009.09.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Example of GIS data used in the analyses. Shown are data for the sites included from the main Hawaiian Islands.
(TIF)
Islands surveyed for Acropora and Porites growth anomalies within each of the regions analyzed.
(DOC)
Frequency of occurrence (FOC) of Acropora growth anomalies (AGAs) and Porites growth anomalies (PGAs) across the Indo-Pacific.
(DOC)
Average prevalence of Acropora growth anomalies (AGAs) and Porites growth anomalies (PGAs) across the Indo-Pacific.
(DOC)