Abstract
PURPOSE
To determine safety and feasibility of adjuvant ipilimumab following resection of high-risk melanoma and to identify surrogate markers for benefit.
EXPERIMENTAL DESIGN
In this phase II trial, 75 patients with resected stage IIIc/IV melanoma received the CTLA-4 antibody ipilimumab every 6-8 weeks for one year. Eligible patients received further maintenance treatments. The first 25 patients received 3 mg/kg of ipilimumab, and an additional 50 patients received 10 mg/kg. HLA-A*0201+ patients received multi-peptide immunizations in combination with ipilimumab. Leukapheresis was performed prior to and 6 months after initiation of treatment.
RESULTS
Median overall and relapse-free survivals were not reached after a median follow-up of 29.5 months. Significant immune-related adverse events were observed in 28 of 75 patients and were positively associated with longer relapse-free survival. Antigen-specific T cell responses to vaccine were variable, and vaccine combination was not associated with additional benefit. No effects on T regulatory cells were observed. Higher changes in Th-17 inducible frequency were a surrogate marker of freedom from relapse (p=0.047), and higher baseline C-reactive protein (CRP) levels were associated with freedom from relapse (p=0.035).
CONCLUSIONS
Adjuvant ipilimumab following resection of melanoma at high risk for relapse appeared to be associated with improved outcome compared to historical reports. Significant immune-related adverse events were generally reversible and appeared to be associated with improved relapse-free survival. While vaccination failed to induce a consistent in vitro measurable response, a higher change in Th-17 inducible cells and higher baseline CRP levels were positively associated with freedom from relapse.
Keywords: melanoma, adjuvant, immune responses, antigen
INTRODUCTION
The clinical development of antibodies that abrogate or augment co-stimulatory interactions between T cells and antigen-presenting cells has opened a new field with great promise. Cytotoxic T lymphocyte antigen-4 (CTLA-4) is a checkpoint protein expressed on activated T cells and has been shown to bind to B7-1 and B7-2 molecules expressed on mature, activated dendritic cells and other antigen presenting cells, preventing their interaction with CD28 on T cells (1, 2). The expression of membrane-bound CTLA-4 down-modulates T cell responses. The CTLA-4-B7 interaction serves as a “brake” for T cell responses and leads to decreases in the PI3 kinase pathway (3, 4). A fully human IgG1 antibody, ipilimumab, binds to and inhibits CTLA-4, leading to increases in T cell activation and IL-2 secretion. CTLA-4 antibody has been shown to induce rejection of established tumors and decrease relapses in a rodent model of prostate cancer in which spontaneous metastasis occurs. CTLA-4 blockade augmented the clinical activity of murine tumor cell vaccines against established tumors, and depletion of T regulatory cells further increased the activity of the antibody (5-8). These data led to the testing of CTLA-4 antibody alone or with a peptide vaccine in patients after complete surgical resection of high-risk nodal or distant metastatic melanoma. Ipilimumab at doses of 3 mg/kg or greater, alone or with a peptide vaccine, induces regression of metastatic melanoma and has activity in renal cell cancer and prostate cancer (9-14). A recent phase III clinical trial demonstrated that ipilimumab prolonged overall survival in patients with unresectable stages III and IV melanoma after failing at least one prior therapy (15). Responses after treatment with ipilimumab are often sustained in the absence of further therapy but have been shown to be associated with immune-related adverse events (irAEs) (11-14). In a prior phase I study, we administered increasing doses of the CTLA-4 antibody ipilimumab and demonstrated that irAEs were inversely correlated with the rate of relapse in patients with resected advanced melanoma (13). No clinical parameter has been consistently found to be a surrogate or predictive marker for response to ipilimumab therapy. To further investigate the activity of ipilimumab and potential factors associated with clinical benefit, we performed a pilot phase II adjuvant study of doses of 3 and 10 mg/kg administered at the extended dosing interval of 6-8 weeks. As in our prior phase I trial, a multi-peptide vaccine was also administered to HLA-A*0201-positive patients with frequent injections over 1 year. Primary endpoints of the study were toxicity and tolerability, with immune parameters, relapse and time to relapse or death being secondary endpoints. Patients were also followed for overall survival; however this was outside the scope of the original trial design. Data involving baseline CRP and change in Th17 inducibility of peripheral blood mononuclear cells (PBMC), were obtained and correlated with outcome.
MATERIALS AND METHODS
Patients and trial eligibility
Eligible patients were ≥ 18 years of age with resected stage IIIc or stage IV melanoma according to the 2002 modified American Joint Commission on Cancer staging system and were rendered free of disease surgically. Patients were required to have a magnetic resonance imaging or computed tomographic (CT) scan of the brain and CT imaging of the chest, abdomen, and pelvis performed within 4 weeks of initiation of therapy showing no evidence of disease. Eligibility criteria also included adequate renal, hepatic, and hematologic function. To be eligible for vaccination, patients were required to be HLA-A*0201 positive, and immunohistochemical tumor staining for one or more of MART-1/Melan-A, HMB-45, and tyrosinase on at least 10% of tumor cells was required. Fifty patients were HLA-A*0201 positive by a DNA polymerase chain reaction (PCR) assay performed at the UCLA Immunogenetics or the American Red Cross Laboratories. Ocular and mucosal melanomas were included. Exclusion criteria included active autoimmune disease, steroid dependence, and prior treatment with ipilimumab. Patients were required to comprehend and sign an informed consent form approved by the Cancer Therapy Research Program of the National Cancer Institute (CTEP/NCI) and the Los Angeles County/University of Southern California or University of South Florida Institutional Review Boards.
Treatment
Ipilimumab was administered intravenously at 3 or 10 mg/kg over 90 minutes every 6 to 8 weeks for 12 months. Reductions in dose were not permitted. Consenting patients who were free from relapse and significant toxicity were continued on maintenance ipilimumab at 10 mg/kg administered every three months. For patients who were HLA-A*0201 positive, antibody infusions were accompanied by three separate subcutaneous vaccine injections of 1 mg each of tyrosinase368-376 (370D), gp100209-217 (210M), and MART-126-35 (27L) peptides emulsified in Montanide ISA 51 VG in alternating lower extremities. The peptide vaccines were administered as outpatient therapy as previously described (16). The intervals between injections were every 2 weeks for the first six injections, every 4 weeks for the next four injections, and 12 weeks between the final two injections. Leukapheresis with exchange of 5 to 7 liters to obtain PBMC for immune analyses was performed within 1 week before and at 6 months after the initiation of therapy. Patients were followed until relapse. Overall survival was also determined but was outside the scope of the original trial design.
Clinical grade peptides, adjuvant, and ipilimumab
GMP-grade tyrosinase368-376 (370D) [NSC# 699048], MART-126-35 (27L) [NSC# 709401], and gp100209-217 (210M) [NSC #683472] 9 or 10 amino acid epitope peptides were prepared. Peptides were produced by Ben Venue Laboratories, Inc. (Bedford, OH) and were provided by CTEP/NCI (Bethesda, MD). Montanide ISA-51 VG was manufactured by Seppic, Inc. (Franklin Lakes, NJ).
Ipilimumab (a CTLA4 monoclonal antibody formerly known as MDX-010) is a fully human immunoglobulin (IgG1ĸ) anti-CTLA4 monoclonal antibody (manufactured by Medarex, Inc., Annandale, NJ, and Bristol Myers Squibb, Princeton, NJ).
Preparation of PBMC specimens
Pheresis samples were processed to purify PBMCs and frozen at −168° C as previously described (16).
ELISPOT assay
PBMCs were thawed and cultured overnight and then tested in an ELISPOT assay performed as previously described by our group (16). After processing, ELISPOT plates were read on a KS Elispot reader (Carl Zeiss, Thornwood, NY). Values were normalized to spots per 100,000 CD8+ T cells.
Flow cytometry and data analysis
PBMC from evaluable patients were stained with fluorophore-conjugated anti-CD3, anti-CD8, anti-CD4, anti-CD25, anti-HLA-DR, anti-peptide/HLA-A2.1 tetramers, anti-CD14, anti-CD19, and anti-CD56 antibodies along with a fluorescent viability marker at 4°C for 30 minutes. Cells were analyzed on a FACS LSR II (BD Biosciences, San Jose, CA) as previously described (17). Tumor-specific effector T cells were characterized as viable CD4, 14, 19 and 56 negative, CD3 and CD8 positive using FloJo software (Treestar, San Carlos, CA). The frequency of tetramer expression of effector T cells was determined by gating on the tetramer-high population compared to the negative tetramer control-stained cells. Activated helper and effector T cells were characterized as viable CD3 and HLA-DR positive, CD14, 19 and 56 negative, and CD4 or CD8+, respectively
Immunohistochemical staining for gp100, MART-1, and tyrosinase
Immunohistochemical staining of paraffin-embedded sections for gp100, MART-1, and tyrosinase was performed as previously described (13).
T regulatory cell inhibition of allogeneic mixed lymphocyte reaction
Fresh PBMC derived from leukapheresis were stained with anti-CD4 and CD25 antibodies (BD Biosciences), and T regulatory cells were isolated by performing fluorescence-activated cell sorting with a BD FACS-Aria (BD Biosciences). The recovered T regulatory cells were added in increasing concentrations to a mixed lymphocyte reaction (MLR). The MLR comprised co-incubation of T regulatory cells, and a 10:1 ratio of autologous responder cells and allogeneic dendritic cells pre-treated with 30 Gy of radiation. Cells were incubated in a 96-well round bottom plate at 37°C/5%CO2 for four days, after which 1 μCi of 3H-Thymidine was added per well. Cells were incubated for an additional 16 hours then washed and transferred onto a filter mat using a Filtermate Harvester (Perkin Elmer, Boston MA). Filter mats were assayed for 3H activity by a MicroBeta Trilux Luminescence Counter (Perkin Elmer). All assays were performed in quintuplicate.
Th-17 inducibility
To induce Th-17 cells, available PBMC derived from patients before and 6 months after ipilimumab treatment were cultured with PMA (250 ng/mL) and ionomycin (1 μM) for 2.5 hours, with 4 μl of GolgiStop™ (BD Biosciences) per 6 mL of culture media added after the first 30 minutes. The cells were recovered, stained with a viability dye, and fluorophore-labeled anti-CD4, and anti-CD8 (BD Biosciences). Cells were then permeabilized (BD Cytofix/Perm kit) and stained with fluorophore-labeled anti-IL17 (eBiosciences, San Diego, CA) or matched isotype control. Cells were analyzed on a BD FACS Calibur cytometer, and the data were analyzed with FloJo software (Treestar). Th17 inducible frequency was determined by the frequency of total viable CD4+ CD8- cells that stained positively for IL17. The change in Th17 frequency was calculated by subtracting the pre-treatment frequency from the post-treatment frequency.
Statistical considerations
Primary endpoints of the study were toxicity and tolerability. An immune-related adverse event was considered to be “significant” if the event required initiation of systemic steroids and/or cessation of therapy. Multiple immune parameters, relapse status and time to relapse or death were secondary endpoints. Patients were also followed for overall survival; however, this was outside the scope of the original trial design. Differences in the pre- and post-treatment responses to gp100209-217 (210M), MART-126-35 (27L), and tyrosinase368-376 (370D) peptides (Beckman Coulter, Fullerton, CA) in ELISPOT assays and tetramer expression assayed by flow cytometry were evaluated by the paired Student t test or Wilcoxon signed rank test, whichever was appropriate. For some statistical analyses, a cut point was chosen post hoc to divide patients into roughly equal groups. This facilitated statistical analyses of the association of a given covariate with outcome endpoints. The two-sample t-test (with equal or unequal variances) or Wilcoxon rank-sum test, when appropriate, was used to study the difference of a continuous variable such as change in Th17. Association between a categorical variable and the relapse status was examined using a contingency table and Chi-square or Fisher exact test. The Kaplan-Meier (KM) product limit method was used to estimate the distribution of a time-to-event endpoint such as relapse-free survival (RFS) and overall survival (OS); and the log-rank test was used to informally compare the two KM curves between two groups of patients. Statistical analyses were performed using SAS version 9.1 or later. A p-value of less than 0.05 was considered to be statistically significant. No multiple testing adjustment was made due to the exploratory nature of the study.
RESULTS
Patient characteristics
Seventy-five patients were treated in this phase II clinical trial, including 44 (59%) men and 31 (41%) women with a median age of 56 (range: 21-78) years. Forty-six (61%) patients had resected stage IV disease, and the remaining 29 (39%) had resected stage IIIc disease (Table 1). Forty-four (59%) patients had received prior immunotherapy with a cell vaccine, GM-CSF, high dose IL-2 or interferon-alpha, four had received biochemotherapy, and all patients had undergone surgery (Table 1). Twenty-five (33%) patients received ipilimumab at 3 mg/kg, and 50 (67%) patients received 10 mg/kg.
Table 1.
Patient Demographics and Disease Characteristics (n=75)
| n | Frequency | |
|---|---|---|
| Gender | ||
| Male | 44 | 59% |
| Female | 31 | 41% |
| Stage | ||
| Stage IIIc | 29 | 39% |
| Stage IV | 46 | 61% |
| Ethnicity | ||
| Caucasian | 74 | 99% |
| Hispanic | 1 | 1% |
| Prior Treatment | ||
| None | 26 | 35% |
| Interferon | 28 | 37% |
| IL-2 | 4 | 5% |
| Vaccine Therapy | 9 | 12% |
| Biochemotherapy | 4 | 5% |
| GM-CSF | 3 | 4% |
| Taxol | 3 | 4% |
| Temodar/DTIC | 3 | 4% |
| Thalidomide | 1 | 1% |
| Vorinostat | 1 | 1% |
| Tamoxifen | 1 | 1% |
| Radiation Therapy | 27 | 36% |
| Age | Years | |
| Median | 56 | |
| Mean | 53 | |
| Range | 21-78 |
Toxicities
A total of 28 (37%) of 75 patients on this trial had a “significant” irAE, which was defined as a grade II, III or IV irAE that required cessation of therapy and/or initiation of systemic steroid therapy. Of these, 11 (15%) patients had hypophysitis, 10 (13%) patients had colitis, and two (3%) had dermatitis. In the remaining five (7%) patients, one case of each of the following irAEs was observed: sarcoidosis, hypercalcemia with renal failure, idiopathic thrombocytopenic purpura, hepatitis, and arthritis. Six patients had a grade II irAE, 21 patients had a grade III irAE, and one patient had a grade IV irAE. All 21 patients with grade III irAEs received tapering doses of systemic steroids, and all 12 patients with grade III gastrointestinal and skin toxicities all returned to normal within 3 months of the onset of symptoms. No patients with grade II gastrointestinal irAEs required cessation of therapy or initiation of systemic steroids. By treatment guidelines, these patients were treated symptomatically with loperamide or diphenoxylate and budesonide, a non-absorbable steroid, by mouth. Two patients developed endoscopically-documented colitis that required surgical intervention. One patient required bowel rest with a diverting ileostomy and total parenteral nutrition for 4 months and eventually fully recovered. Another patient developed a bowel perforation requiring partial colectomy and eventually fully recovered to pre-treatment status. Of 11 patients with grade II or III hypophysitis, all required replacement corticosteroids, six required treatment for at least two years, including one patient for five years who remains well without symptoms. Of note, three of the 11 patients with hypophysitis have been successfully weaned from systemic steroids. This indicates that steroid requirement induced by ipilimumab is not necessarily permanent. There were no treatment-related deaths. Toxicities, which were predominantly immune-related, are summarized in Table 2.
Table 2.
Summary of Adverse Events (n=75)†
| Adverse Event | Grade I | Grade II | Grade III | Grade IV |
|---|---|---|---|---|
| Diarrhea | 26 | 13 | 10 | 1 |
| Colitis | 0 | 4 | 10 | 0 |
| Hypopituitarism (low ACTH) |
0 | 5 | 6 | 0 |
| Hepatitis | 2 | 1 | 2 | 0 |
| Idiopathic thrombocytopenia |
0 | 0 | 1 | 0 |
| Hypercalcemia | 0 | 0 | 0 | 1 |
| Arthritis/Arthralgia | 11 | 4 | 2 | 0 |
| Rash | 40 | 16 | 2 | 0 |
| Skin/Subcut Induration |
45 | 45 | 0 | 0 |
| Injection Site Reaction |
33 | 10 | 1 | 0 |
| Vitiligo | 3 | 0 | 0 | 0 |
| Pruritis | 34 | 12 | 1 | 0 |
| Fatigue | 42 | 22 | 1 | 1 |
| Myositis/Myalgia | 13 | 2 | 1 | 0 |
| Chills/Rigors/ Diaphoresis |
59 | 3 | 1 | 0 |
| Fever (non- neutropenic) |
30 | 4 | 1 | 0 |
| Nausea | 20 | 4 | 4 | 0 |
| Mastitis/Mastalgia | 4 | 1 | 1 | 0 |
| Abd Pain/Distension NOS |
29 | 6 | 4 | 0 |
| Headache | 25 | 7 | 0 | 0 |
| Dyspnea | 10 | 1 | 0 | 0 |
| Anorexia | 11 | 2 | 1 | 0 |
| Blurred Vision | 9 | 1 | 0 | 0 |
| Edema | 19 | 1 | 1 | 0 |
| Dehydration | 2 | 2 | 3 | 0 |
| Eye or Extremity Pain |
6 | 2 | 0 | 0 |
| Insomnia | 14 | 0 | 0 | 0 |
| Weight Loss | 9 | 0 | 0 | 0 |
| Cellulitis | 1 | 3 | 0 | 0 |
| Somnolence | 5 | 2 | 0 | 0 |
| Back Pain | 5 | 2 | 1 | 0 |
| Hernia exacerbation | 1 | 1 | 1 | 0 |
| Depression | 4 | 2 | 0 | 0 |
| Paresthesia | 4 | 1 | 0 | 0 |
| Decreased libido/Low testosterone |
3 | 1 | 0 | 0 |
| Weight gain | 2 | 0 | 0 | 0 |
| Cholecystitis | 0 | 0 | 1 | 0 |
| Esophagitis/gastritis/ duodenitis |
1 | 1 | 2 | 0 |
| Constipation | 7 | 0 | 0 | 0 |
| Pulmonary embolism |
0 | 1 | 0 | 1 |
| Non-cardiogenic chest pain |
12 | 0 | 0 | 0 |
| Lightheadedness | 8 | 0 | 0 | 0 |
| Sarcoidosis | 0 | 1 | 0 | 0 |
| Hypertension | 0 | 2 | 0 | 0 |
| Hyponatremia | 3 | 1 | 2 | 0 |
| Hyperkalemia | 2 | 0 | 0 | 0 |
| Conjunctivitis | 4 | 1 | 0 | 0 |
| Emesis | 4 | 1 | 3 | 1 |
| Xeropthalmia | 2 | 0 | 0 | 0 |
| Dysmenorrhea | 2 | 0 | 0 | 0 |
| Elevated lipase | 0 | 1 | 0 | 0 |
| Elevated amylase | 1 | 0 | 0 | 0 |
| Herpes recurrence | 2 | 1 | 0 | 0 |
| Hyperglycemia | 1 | 1 | 1 | 0 |
| Sepsis | 0 | 0 | 1 | 0 |
| Intestinal perforation | 0 | 0 | 2 | 0 |
| Myocardiac infarction |
0 | 0 | 1 | 0 |
| Mouth ulceration | 4 | 0 | 0 | 0 |
| Nasal congestion | 4 | 0 | 0 | 0 |
| Mood swings | 4 | 1 | 0 | 0 |
| Confusion | 3 | 0 | 0 | 0 |
| Hypothyroidism | 1 | 0 | 0 | 0 |
No treatment related death/grade V toxicity.
Clinical results
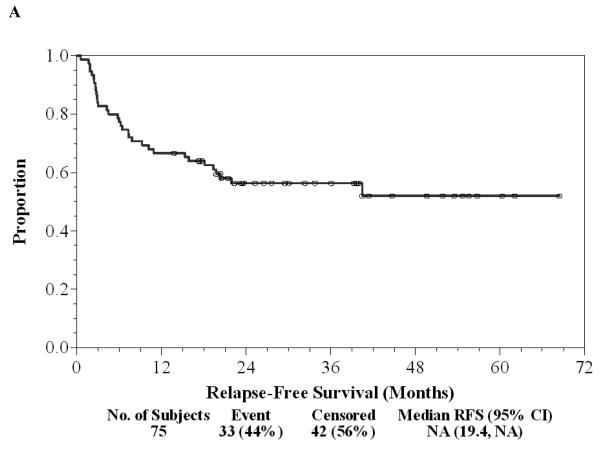
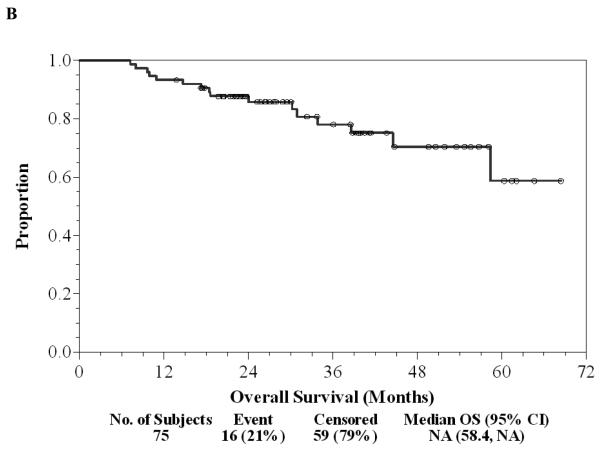
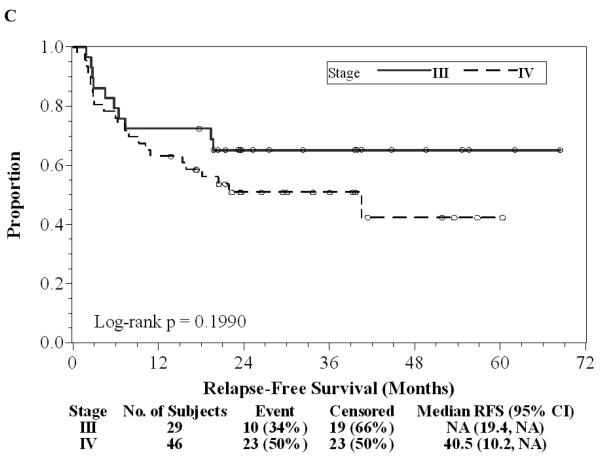
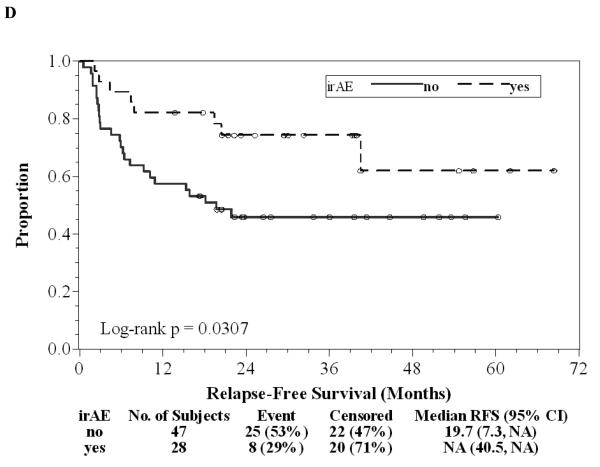
Of the 75 patients, 37 (49%) finished all seven doses of ipilimumab, 17 (23%) stopped treatment early because of dose-limiting toxicity, 20 (27%) patients stopped treatment early due to relapse during the first year of treatment, and one (1%) patient withdrew consent prior to completing seven doses. Twenty (27%) patients continued on maintenance ipilimumab after the initial seven doses, one of whom has continued for 3.5 years without significant toxicity or relapse. There have been 33 (44%) relapses to date and a two-year RFS rate of 56% (95% CI: 44%-67%) with a median follow-up of 29.5 (range: 13.8-68.4) months as of June 1, 2010 (Figure 1A). Of 33 patients who relapsed, nine (12%) are again free of disease after subsequent treatment and eight (11%) are alive with disease. There were 16 (21%) deaths, all attributable to disease, and the two-year OS was 86% (95% CI: 75%-92%, Figure 1B). There was a trend in favor of the stage IIIc patients for longer duration of RFS compared to stage IV patients (Log-rank p=0.20, Figure 1C). Of 33 patients with a relapse, only eight (24%) had significant irAEs; 20 (48%) of 42 without relapse had a significant irAE (OR=0.35, 95% CI: 0.13-0.96, Chi-square p=0.038). The median RFS and OS without a significant irAE were 20 and 58 months, respectively, while the median RFS and OS with a significant irAE have not been reached after a median follow-up of 29.5 months. Development of significant irAEs appeared to be positively associated with a longer duration of RFS (HR=0.43, 95% CI: 0.19-0.91; Log-rank p=0.031, Figure 1D). Further follow-up may help determine whether overall survival is significantly associated with irAE.
Figure 1. Patient outcomes.
A, Kaplan-Meier relapse-free survival curve for all patients treated with ipilimumab. B, Kaplan-Meier overall survival curve for all patients treated with ipilimumab. C, Kaplan-Meier relapse-free survival curves comparing Stage IIIc patients (solid) to Stage IV patients (dashed). D, Kaplan-Meier relapse-free survival curves comparing patients with irAE (dashed) to patients without irAE (solid). “o” indicates censored observations.
Effect of ipilimumab on functional assays of immunity
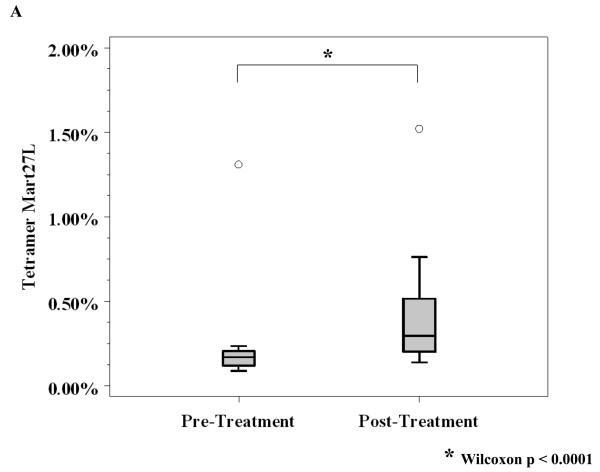
Sixty-one of 75 patients completed leukapheresis both prior to, and 6 months after initiating the trial. For HLA-A*0201 positive patients, peptide-specific immune responses to MART-1, gp100 and tyrosinase were assayed using functional, gamma-interferon ELISPOT assays as well as flow cytometry for tetramer expression on matched PBMC before and 6 months after starting ipilimumab treatment. For ELISPOT assays, 40 patients of 50 with available PBMC were evaluated. Only ten (25%) of the 40 evaluable patients had evidence of an immune response to MART-1 or gp100 (data not shown). There was no clear correlation of ELISPOT reactivity with relapse status or with irAEs, although the limited number of patients precludes making definitive correlations. No significant ELISPOT reactivity to tyrosinase was observed (data not shown). There were twenty-three patients who received vaccine and who had available PBMC evaluable for tetramer expression by flow cytometry. In contrast to the ELISPOT results, there was a significant increase in MART 27L tetramer expression 6 months after treatment compared to baseline (median expression post-treatment was 0.30 vs. median pre-treatment expression of 0.17, Wilcoxon p < 0.0001, Figure 2A).
Figure 2. Assays of immunity.
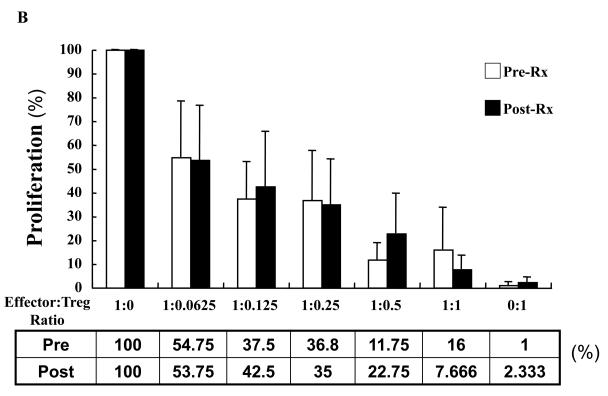
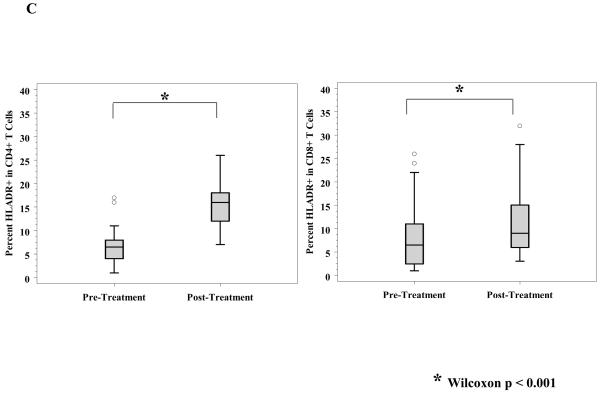
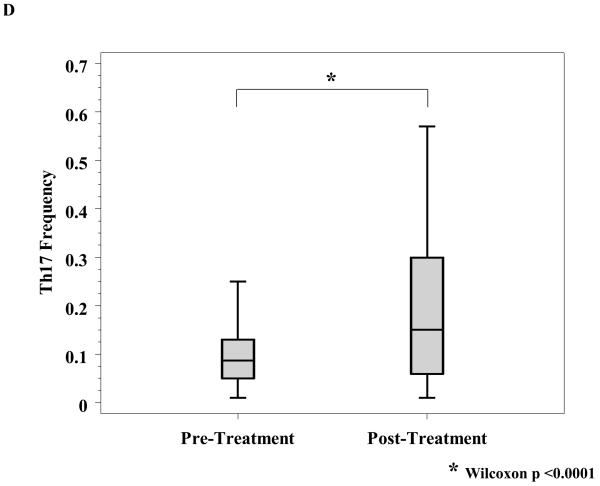
A, Reactivity to MART-27L was assayed by tetramer staining with flow cytometry before and after ipilimumab treatment. B, T regulatory cells (Treg) from patients before and after ipilimumab treatment were isolated, and change in Treg function was assayed by titration into a mixed lymphocyte reaction assay with effector T cells at the indicated ratios. C, T cells from patients before and 71 days after ipilimumab treatment were phenotyped for CD4 and HLADR (left) and CD8 and HLADR (right). D, Th17 inducibility before and 6 months after treatment was determined by flow cytometry.
Since ipilimumab did not consistently increase antigen-specific circulating T cells in vitro, we examined the effect of ipilimumab on suppressive T regulatory cells. T regulatory cells that were CD4+, CD25+ high were measured by flow cytometry, and no difference before treatment compared to 6 month post-treatment initiation (after four doses of CTLA-4 antibody) was observed (data not shown). This is consistent with prior reports in metastatic melanoma (17, and data not shown). Fresh PBMC pre- and 6 months post-treatment from 8 patients were also sorted and recovered by flow cytometry on the basis of CD4 positivity and high levels of CD25 to perform assays measuring T regulatory activity. The sorted cells were titrated into a mixed lymphocyte reaction with irradiated allogeneic dendritic cells and autologous effector cells. Their inhibitory activity was evaluated by suppression of an allogeneic MLR as their ratios to effectors were increased. No differences in MLR reactivity were observed in the pre- versus post-treatment samples as T regulatory cell numbers were increased. These data shown in Figure 2B suggest that administration of ipilimumab did not affect the number or function of natural CD4+ CD25+ high, T regulatory cells.
We assayed the number and activation status of overall circulating immune cells before and after treatment. Flow cytometry analyses were performed on a subset of PBMC specimens at the time of the first, second, and third ipilimumab infusions as above. CD3+, CD4+, CD8+ T cells, CD56+ NK cells, and CD19+ B cells were enumerated. There were no consistent changes over time in those subsets after ipilimumab treatment (data not shown). The activation marker HLA-DR was also measured on T cells. A significant increase over time in both CD4+/HLA-DR+ and CD8+/HLA-DR+ T cells (Wilcoxon p < 0.001) was noted, as shown in Figure 2C, reflecting an increased population of activated T helper and cytolytic cells.
We investigated the effect of ipilimumab on a novel class of helper T cells termed Th17 cells. Th17 cells have been associated with autoimmune and anti-tumor immunity in murine models and in human autoimmune diseases (18-20). A report on a different CTLA-4 blocking antibody, tremelimumab, previously demonstrated a treatment-induced increase in the inducibility of Th17 cells (21). In our patients, ipilimumab treatment significantly increased the inducibility of Th17 cells after stimulation with PMA and ionomycin (Wilcoxon, p < 0.0001, Figure 2D). We subsequently explored the association of Th17 inducibility with relapse, discussed below.
Correlative studies associated with outcome
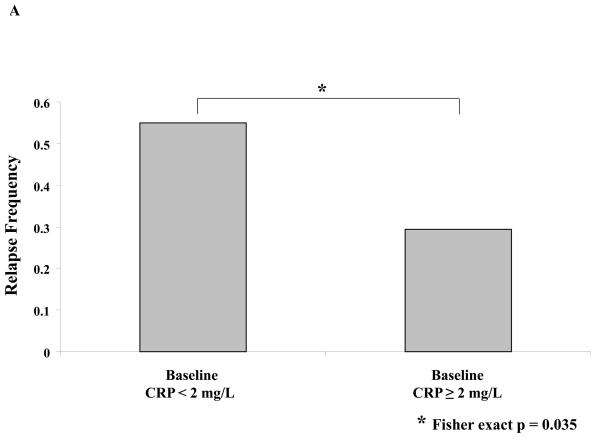
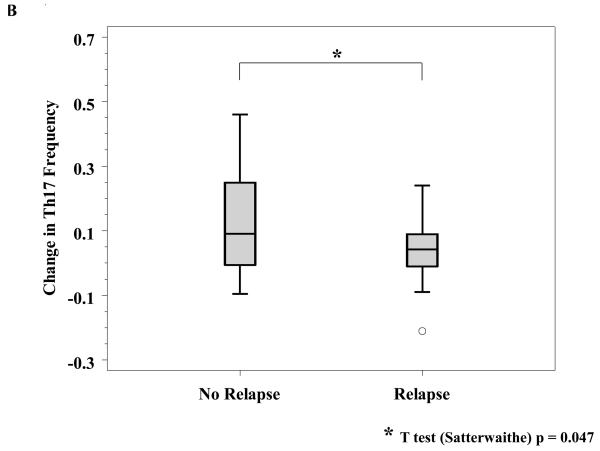
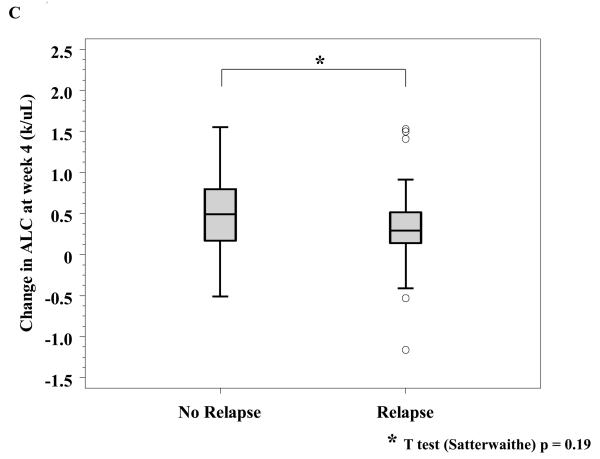
No factors have been consistently associated with clinical benefit from ipilimumab. A number of serologic and hematologic parameters were assessed at baseline and at varying time points after initiating ipilimumab. Patients whose baseline C-reactive protein (CRP) was 2 mg/L or greater had a significantly improved freedom from relapse (OR of relapse comparing <2 versus ≥ 2: 0.34, 95% CI: 0.13-0.90; Fisher exact p = 0.035, Figure 3A) as well as a marginally significantly improved RFS (HR: 0.50; 95% CI: 0.24-1.11, p=0.081). As described above, treatment with ipilimumab was associated with a significant increase in Th-17 inducibility. Th-17 inducibility was also associated with outcome, as a higher change in Th17 inducibility from baseline to 6 months was positively associated with freedom from relapse in evaluable patients (n = 47; median Th17 change: 0.04 and 0.09 for those who never relapsed versus those who did during the study, respectively; Satterwaithe t test p = 0.047, Figure 3B). The small number of deaths precluded an assessment regarding overall survival. There were no significant correlations between any chemistry or hematologic parameter and outcome analyzed, including baseline and change in absolute lymphocyte count (Figure 3C, and data not shown).
Figure 3. Correlative studies related to relapse.
A, Serum C-reactive peptide level (CRP) was determined prior to initiation of treatment and was correlated to relapse. B, Th17 inducibility, before and 6 months after treatment, was quantified by flow cytometry and the difference was correlated to relapse. C, Absolute lymphocyte count (ALC) was drawn prior to and four weeks after initiation of treatment, and the difference was correlated to relapse.
DISCUSSION
In this clinical trial, we treated 75 patients with high-risk resected melanoma with extended dose ipilimumab every 6-8 weeks. HLA-A*0201 patients also received multiple peptide vaccinations. Overall clinical results were favorable, with only 16 (21%) deaths and 33 (44%) relapses in 75 patients. Nine (12%) patients that relapsed were rendered free of disease with subsequent therapy. Median relapse-free and overall survivals for all patients have not yet been reached after a median follow-up of 29.5 months in a population of patients with a poor expected outcome. These results are promising compared to historical reports. Two recent reports, one of resected stage IV melanoma patients and one involving resected stage IIIb, IIIc disease, reported median relapse-free survivals of 7.2 and 8.8 months, respectively (22, 23). In this study, the median relapse-free survival of stage IV patients was 40.5 months and was not reached for the stage IIIc patients after a median of 29.5 months of follow-up. However, larger randomized trials will be required to definitively establish the utility of ipilimumab in the adjuvant setting.
Data derived from patients receiving ipilimumab doses of 3 and 10 mg/kg were pooled. Pooling enhanced the robustness of the statistical analysis due to the small number of patients in the two dosing cohorts. We did not observe a significant difference in toxicity or outcome when comparing the two dosing regimens. Wolchok et al. recently reported a randomized phase II clinical trial involving patients with unresectable advanced melanoma who were randomized to 0.3 mg/kg, 3 mg/kg and 10 mg/kg (24). While there was a favorable difference in response rate in the 10 mg/kg group, consistent with our findings, the authors reported that overall survival and the disease control rate were not significantly different between the 3 and 10 mg/kg groups.
There was an inverse association between the development of significant irAEs and relapse, supporting results from a prior trial (13) and consistent with published reports in patients with metastatic melanoma (14). There were two episodes of grade III colitis requiring surgery. One patient required a diverting loop ileostomy and bowel rest with TPN, the other had a perforation that required colectomy. Both patients eventually recovered fully to pre-treatment status, and both patients are currently free of disease. There were no treatment-related deaths. A 37% rate of significant irAEs was observed, the majority of which were managed with outpatient steroids and cessation of ipilimumab. A potential concern for the use of ipilimumab in the adjuvant setting involves the development of permanent steroid dependence due to hypophysitis that could compromise future immunotherapy in the event of a subsequent relapse. In this report, six (8%) of 75 patients required replacement steroids due to persistent hypophysitis for more than two years. Only one of these patients had a relapse but was rendered free of disease with subsequent resection. Thus, steroid requirement associated with irAE on this trial did not significantly impair subsequent treatment after relapse. Three of 11 patients requiring hormone replacement for hypophysitis were successfully weaned, indicating that hypophysitis induced by ipilimumab is reversible in some patients.
In this study we identified two markers associated with clinical benefit from ipilimumab. A higher frequency of Th17 inducible cells after 6 months of therapy was a surrogate marker for freedom from relapse. A previous study involving a different CTLA-4 blocking antibody, tremelimumab, in unresectable stage IV melanoma patients, reported that low baseline CRP levels were associated with overall survival (25). However, in our report, a higher baseline CRP level appeared to be positively associated with freedom from relapse (odds of having no relapse for those whose baseline CRP < 2.0 was reduced by 66% compared to those with a baseline CRP ≥ 2.0, p=0.035). In contrast to a prior report involving ipilimumab treatment of unresectable stage IV melanoma, we did not observe a correlation of increase in absolute lymphocyte count to clinical benefit (26). The difference in the CRP data could be explained by the use of a different class of CTLA-4 blocking antibody in the first study. More likely, however, the observed differences in both results involving baseline CRP and absolute lymphocyte count could be due to the inherent difference in the biology advanced measurable disease compared to that of resected disease.
Antigen-specific immune assays did not reveal any association with clinical benefit, despite a significant increase in tetramer MART-positive circulating T cells after vaccination. CD4+/HLA-DR and CD8+/HLA-DR+ T cells were increased in number, reflecting a general state of immune activation and a pharmacodynamic marker for the effect of ipilimumab, as shown in past trials (13, 14). Assays for the function of T regulatory cells using sorted CD4+CD25+ high T cells did not reveal any differences after treatment with CTLA-4 antibody. These data confirm prior in vitro findings (27, 28 and Weber et al., unpublished observations) and published experiments from patients with metastatic melanoma (17). However, ipilimumab may influence T regulatory cells in the tumor microenvironment rather than in the circulation. In patients with localized bladder cancer, Liakou et al. observed a consistent decrease in FoxP3+ T regulatory cells in tumor-infiltrating lymphocytes after CTLA-4 blockade (p < 0.05), while the effect on circulating T regulatory cells was inconsistent (29). Because our study was conducted in the adjuvant setting after surgical resection, analysis of the tumor microenvironment was precluded.
Important questions remain about CTLA-4 abrogation as a cancer therapy. By what immune mechanism do CTLA-4 antibodies induce clinical benefit in melanoma? It is possible that CD4 cells may be the effectors that mediate clinical benefit with ipilimumab, and that it is the provision of augmented non-specific T cell help that is responsible for the anti-melanoma immune response. Trials combining CTLA-4 antibody with class I peptide vaccines have not documented increased peptide-specific CD8 immune responses in peripheral blood samples (13, 14). However, if CTLA-4 abrogation does indeed act at the level of the CD8+ cytolytic T cell, the effects may best be evaluated in the draining lymph nodes and in tumor-infiltrating T cells rather than in peripheral blood.
Alternatively, epitope-spreading to antigens not included in the vaccine may occur. The cancer testis antigen, NY-ESO-1, represents a potential candidate. A recent study reported 15 patients with metastatic melanoma treated with ipilimumab, and treatment response was correlated with de novo NY-ESO-1 reactivity without prior NY-ESO-1 immunization. Five of the eight patients with a clinical response to CTLA-4 blockade were sero-positive for NY-ESO-1, while all seven non-responders were sero-negative (30). Finally, the peptide vaccination itself may not be important for the induction of anti-tumor immunity, and CTLA-4 blockade alone may be sufficient to derive clinical benefit.
Important therapeutic issues for CTLA-4 abrogating antibodies are the value of long-term maintenance therapy and the toxicities associated with it. This has been partly answered by the current trial, since 32% of patients exhibited grade III toxicity with a dosing interval of 8 weeks. These toxicity data are similar to a previous study with approximately the same proportion of grade III toxicity when the drug was given with a similar vaccine at the same dose but at a frequency of every 3 weeks to patients with unresectable stage IV melanoma (31). The onset of toxicity was delayed compared to this study, but the eventual proportion of patients with grade III irAEs was similar. The value of long-term maintenance therapy is still unknown, but maintenance therapy is currently being evaluated in trials of patients with stage IV unresectable and stage III resected disease.
STATEMENT OF TRANSLATIONAL SIGNIFICANCE.
In this phase II adjuvant clinical trial, we treated 75 patients with resected stage IIIc and IV melanoma with the CTLA-4 blocking antibody, ipilimumab; HLA-A*0201 positive patients also received a multi-peptide vaccine. The median relapse-free and overall survivals were not reached with a median follow-up of 29.5 months, which compared favorably to historical reports. The development of immune-related adverse events appeared to be positively associated with improved relapse-free survival. Increased frequency of inducible Th-17 cells from baseline to 6 months into therapy and higher baseline C-reactive protein levels were positively associated with freedom from relapse and may represent surrogate markers of clinical benefit with ipilimumab that merit future investigation. The study supports further assessment of ipilimumab for the adjuvant treatment of high-risk resected melanoma.
Acknowledgement
We thank Rasa Hamilton at the Moffitt Cancer Center for editorial assistance, and Jodi Kroeger and Kate Shapland from the Moffitt Flow Cytometry core for their technical advice and assistance.
Footnotes
Clinical trial number: NCT00084656
REFERENCES
- 1.Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol. 2002;3:611–8. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 2.Chikuma S, Bluestone JA. CTLA-4 and tolerance: the biochemical point of view. Immunol Res. 2003;28:241–53. doi: 10.1385/IR:28:3:241. [DOI] [PubMed] [Google Scholar]
- 3.Lee KM, Chuang E, Griffin M, et al. Molecular basis of T cell inactivation by CTLA-4. Science. 1998;282:2263–6. doi: 10.1126/science.282.5397.2263. [DOI] [PubMed] [Google Scholar]
- 4.Leach D, Krummel M, Allison JP. Enhancement of anti-tumor immunity by CTLA-4 blockade. Science. 1996;271:1734–6. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 5.van Elsas A, Sutmuller RP, Hurwitz AA, et al. Elucidating the autoimmune and antitumor effector mechanisms of a treatment based on cytotoxic T lymphocyte antigen-4 blockade in combination with a B16 melanoma vaccine: comparison of prophylaxis and therapy. J Exp Med. 2001;194:427–38. doi: 10.1084/jem.194.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–66. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hurwitz AA, Yu TF, Leach DR, Allison JP. CTLA-4 blockade synergizes with tumor-derived granulocyte-macrophage colony-stimulating factor for treatment of an experimental mammary carcinoma. Proc Natl Acad Sci U S A. 1998;95:10067–71. doi: 10.1073/pnas.95.17.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sotomayor EM, Borrello I, Tubb E, Allison JP, Levitsky HI. In vivo blockade of CTLA-4 enhances the priming of responsive T cells but fails to prevent the induction of tumor antigen-specific tolerance. Proc Natl Acad Sci U S A. 1999;96:11476–81. doi: 10.1073/pnas.96.20.11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodi FS, Mihm MC, Soiffer RJ, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003;100:4712–7. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korman A, Yellin M, Keler T. Tumor immunotherapy: pre-clinical and clinical activity of CTLA-4 antibodies. Curr Opin Invest Drugs. 2005;6:582–91. [PubMed] [Google Scholar]
- 11.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372–7. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber J. Anti-CTLA-4 antibody ipilimumab: Case studies of clinical response and immune-related adverse events (IRAEs) Oncologist. 2007;12:864–72. doi: 10.1634/theoncologist.12-7-864. [DOI] [PubMed] [Google Scholar]
- 13.Sanderson K, Scotland R, Lee P, et al. Autoimmunity in a phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and Montanide ISA 51 for patients with resected stages III and IV melanoma. J Clin Oncol. 2005;23:741–50. doi: 10.1200/JCO.2005.01.128. [DOI] [PubMed] [Google Scholar]
- 14.Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–53. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010 doi: 10.1056/NEJMoa1003466. Epub PMID 20525992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamid O, Solomon JC, Scotland R, et al. Alum with interleukin-12 augments immunity to a melanoma peptide vaccine: correlation with time to relapse in patients with resected high-risk disease. Clin Cancer Res. 2007;13:215–22. doi: 10.1158/1078-0432.CCR-06-1450. [DOI] [PubMed] [Google Scholar]
- 17.Maker AV, Attia P, Rosenberg SA. Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J Immunol. 2005;175:7746–54. doi: 10.4049/jimmunol.175.11.7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofstetter HH, Ibrahim SM, Koczan D, et al. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol. 2005;237:123–130. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Hueber AJ, Asquith DL, Miller AM, et al. Mast cells express IL-17A in rheumatoid arthritis synovium. J Immunol. 2010;340:3336–40. doi: 10.4049/jimmunol.0903566. [DOI] [PubMed] [Google Scholar]
- 20.Crome SQ, Wang AY, Levings MK. Translational mini-review series on Th17 cells: function and regulation of human T helper 17 cells in health and disease. Clin Exp Immunol. 2010;159:109–19. doi: 10.1111/j.1365-2249.2009.04037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Euw E, Chodon T, Attar N, et al. CTLA4 blockade increases Th17 cells in patients with metastatic melanoma. J Transl Med. 2009;20:7–35. doi: 10.1186/1479-5876-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morton DL, Mozzillo N, Thompson JF, et al. An international, randomized, phase III trial of bacillus Calmette-Guerin (BCG) plus allogeneic melanoma vaccine (MCV) or placebo after complete resection of melanoma metastatic to regional or distant sites. J Clin Oncol. 2007;18S:abstr8508. [Google Scholar]
- 23.Lawson DH, Lee SJ, Tarhini AA, et al. E4697: Phase III cooperative group study of yeast-derived granulocyte macrophage colony-stimulating factor (GM-CSF) versus placebo as adjuvant treatment of patients with completely resected stage III-IV melanoma. J Clin Oncol. 2010;28S:abst 8504. [Google Scholar]
- 24.Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, Waterfield W, Schadendorf D, Smylie M, Guthrie T, Jr, Grob JJ, Chesney J, Chin K, Chen K, Hoos A, O'Day SJ, Lebbé C. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–64. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 25.Marshall MA, Ribas A, Huang B. Evaluation of baseline serum C-reactive protein (CRP) and benefit from tremelimumab compared to chemotherapy in first-line melanoma. J Clin Oncol. 2010;28s:abstr 2609. [Google Scholar]
- 26.Berman DM, Wolchok J, Weber J, et al. Association of peripheral blood absolute lymphocyte count (ALC) and clinical activity in patients with advanced melanoma treated with ipilimumab. J Clin Oncol. 2009;27S:abstr 3020. [Google Scholar]
- 27.Tang Q, Boden EK, Henriksen KJ, Bour-Jordan H, Bi M, Bluestone JA. Distinct roles of CTLA-4 and TGF-beta in CD4+CD25+ regulatory T cell function. Eur J Immunol. 2004;34:2996–3005. doi: 10.1002/eji.200425143. [DOI] [PubMed] [Google Scholar]
- 28.Sutmuller RP, van Duivenvoorde LM, van Elsas A, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–32. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liakou CI, Kamat A, Tang DN, et al. CTLA-4 blockade increases IFNg-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci U S A. 2008;105:14987–92. doi: 10.1073/pnas.0806075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan J, Gnjatic S, Li H, et al. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc Natl Acad Sci U S A. 2008;105:20410–5. doi: 10.1073/pnas.0810114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Downey SG, Klapper JA, Smith FO, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13:6681–8. doi: 10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]