Abstract
We examined the impact of fetal programming on the functional responses of renal angiotensin receptors. Fetal sheep were exposed in utero to betamethasone (BMX; 0.17 mg/kg) or control (CON) at 80–81 days gestation with full term delivery. Renal nuclear and plasma membrane fractions were isolated from 1.0–1.5 year old sheep for receptor binding and fluorescence detection of reactive oxygen species (ROS) or nitric oxide (NO). Mean arterial blood pressure and blood pressure variability were significantly higher in the BMX-exposed adult offspring versus control (CON) sheep. The proportion of nuclear AT1 receptors sensitive to losartan (LOS) was 2-fold higher [67 ± 6% vs. 27 ± 9%, p < 0.01] in BMX compared to control. In contrast, the proportion of AT2 sites was only one-third that of controls (BMX: 25 ± 11% vs. CON: 78 ± 4%, p < 0.01) with a similar reduction in sites sensitive to the Ang-(1-7) antagonist D-Ala7-Ang-(1-7) with BMX exposure. Functional studies revealed that Ang II stimulated ROS to a greater extent in BMX than control sheep (16 ± 3% vs. 6 ± 4%; P<0.05); however NO production to Ang II was attenuated in BMX (26 ± 7% vs. 82 ± 14%; P<0.05). BMX-exposure was also associated with a reduction in the Ang-(1-7) NO response [75 ± 8% vs. 131 ± 26%; P<0.05]. We conclude that altered expression of angiotensin receptor subtypes may be one mechanism whereby functional changes in NO- and ROS-dependent signaling pathways may favor the sustained increase in blood pressure evident in fetal programming.
Keywords: Angiotensin, receptors, kidney, fetal programming, hypertension
INTRODUCTION
Glucocorticoids have an important influence on cell maturation and differentiation, particularly in the developing lung1. Indeed, the treatment of mothers in immediate jeopardy of preterm delivery with synthetic glucocorticoids enhances fetal lung maturation and lessens respiratory distress syndrome, as well as substantially reduces neonatal mortality and morbidity; glucocorticoid administration is now standard care for women with preterm labor before 35 weeks gestation. However, a single course of antenatal corticosteroid (ANCS) is associated with higher blood pressure in adolescence and decreased insulin sensitivity and renal function in adulthood2, 3.
Experimental studies demonstrate that exposure of the fetus to elevated glucocorticoids during the critical period of gestation results in the development of elevated blood pressure once the offspring reaches adulthood4. In a well-characterized model of fetal programming, adult offspring of sheep exposed antenatally to the glucocorticoid betamethasone (BMX) exhibit increased mean arterial pressure (MAP) that is associated with reduced baroreflex sensitivity and an attenuated capacity to excrete sodium5–7. Furthermore, steroid-induced programming may selectively influence the enzymatic components of the renin-angiotensin system (RAS) such that the ratio of ACE to ACE2 is increased in the circulation and kidney favoring the Ang II-AT1 receptor axis8, 9. In this regard, sensitivity to Ang II and other stressors is enhanced in the offspring of glucocorticoid-exposed mothers9–13. Indeed, acute treatment with an AT1 receptor antagonist lowers blood pressure in adult exposed sheep at a therapeutic dose that does not influence pressure in the non-exposed adults5.
We recently reported the increased expression of the angiotensin receptor subtype AT1, and a concomitant decrease in the AT2 subtype within the kidney cortex of older (3–5 years) versus younger adult (1.5 years) sheep14. This altered receptor ratio was associated with an increased response in the Ang II-dependent release of reactive oxygen species (ROS) that was abolished by an AT1 receptor antagonist14. In lieu of the functional role of glucocorticoids to influence tissue maturation, we hypothesize that antenatal exposure to glucocorticoids may influence RAS receptors to promote the development and/or progression of high blood pressure associated with fetal programming. The present studies investigated the impact of antenatal BMX administration on the expression and function of angiotensin receptors within the cortical and medullary areas of the adult kidney from control and exposed sheep.
MATERIALS AND METHODS
Animals
Pregnant ewes were administered two intramuscular doses of 0.17 mg/kg betamethasone acetate [BMX] or saline [control] at days 80 and 81 of gestation 24 hours apart5. Offspring were delivered at full term, farm raised and transferred to the Wake Forest University School of Medicine animal facility at 1.0–1.5 years of age. Animals were fed a normal diet with access to water ad libitum, and maintained on a 12:12 hour light-dark cycle. All procedures were approved by the Institutional Animal Care and Use Committee at Wake Forest University School of Medicine.
Blood Pressure Measurements
Sheep were anesthetized with ketamine and isoflurane followed by catheterization into the femoral artery and vein for blood pressure measurements5. After at least 5 days recovery, MAP and heart rate (HR) were recorded in conscious animals and digitized with Acknowledge software (BIOPAC 3.8.1, Santa Barbara, CA). Blood pressure variability (BPV) was measured by power of the spectral density of systolic arterial pressure in the low-frequency range (LFSAP) in normalized units using the Nevrokard BRS software (Nevrokard BRS, Medistar, Ljubljana, Slovenia)5.
Tissue Preparation
The kidneys tissues were obtained from adult mixed - breed sheep anesthetized with ketamine and isoflurane. Kidneys were dissected in ice-cold saline into renal cortex and medulla and either immediately frozen on dry ice and stored at −80°C or processed at 4°C for isolation of nuclei.
Isolation of renal nuclei and plasma membrane
Nuclei and plasma membrane fractions were prepared as described previously14–16. Fresh or frozen tissue (~0.500 gm) was homogenized in buffer containing 25 mmol/L KCl, 5 mmol/L MgCl2, 20 mmol/L Tricine-KOH and 25 mmol/L sucrose (pH 7.8). The homogenate was passed through a 100-μm mesh-filter and centrifuged twice at 1,000 × g (4°C) for 10 min to obtain the nuclear fraction. The resultant supernatant was centrifuged at 25,000 × g for 20 min (4°C), yielding the plasma membrane fraction. The pellet from the nuclear fraction was re-suspended in 20% OptiPrep solution (Accurate Chemical and Scientific, Westbury, NY) and layered on a discontinuous density gradient column of 25%, 30%, and 35% OptiPrep solution. Each gradient was topped with 10% OptiPrep solution and centrifuged at 10,000 × g for 20 min (4°C). The enriched fraction of isolated nuclei was recovered at the 30–35% layer interface.
Angiotensin binding studies
Angiotensin binding sites were determined with the radioligand 125I-[Sar1, Thr8] Ang II (125I-Sarthran) in the presence of losartan, PD123319, D-Ala7-Ang-(1-7) [A779 or D-Ala] or non-labeled Sarthran as previously described14.
Reactive Oxygen Species Production
The production of reactive oxygen species (ROS) was measured in isolated renal cortical nuclei using the fluorescence dye, 5-(and-6)-chloromethy1–2′,7′-dichlorodihydrofluorescein diacetate-acetyl ester (DCF; 20 μg/ml) (Molecular Probes, Eugene OR) as described16. The NADPH oxidase inhibitor DPI (1 mmol/L) abolished the Ang II response.
Nitric Oxide Production
Production of NO in isolated cortical nuclei was assessed with the fluorescence dye, 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF; 5μg/ml; Molecular Probes, Invitrogen)14. The NOS inhibitor L-NAME (1 mmol/L) abolished the Ang II and Ang-(1-7) responses.
Determination of ACE, ACE2 and neprilysin activities
Peptidase activities were determined in isolated cortical nuclei as described15. One unit (U) of activity was defined as one fmol product per mg protein per minute of incubation at 37°C.
Statistical analysis
Data are represented as means ± SEM. Paired or unpaired Student's t-test, one way ANOVA with Tukey's multiple comparison post-hoc and liner regression analysis were performed using GraphPad Prism 5.0 plotting and statistical software.
RESULTS
The MAP was significantly higher in adult sheep exposed antenatally to BMX, as compared to control animals that received saline (98 ± 3 versus 79 ± 2 mmHg; p<0.05; Figure 1A). Although the corresponding HR was not different between the BMX-exposed and control sheep (Figure 1B), blood pressure variability (%LFSAP) was significantly higher in the exposed sheep (Figure 1C). Body weights for the two groups of sheep were not significantly different.
Figure 1.
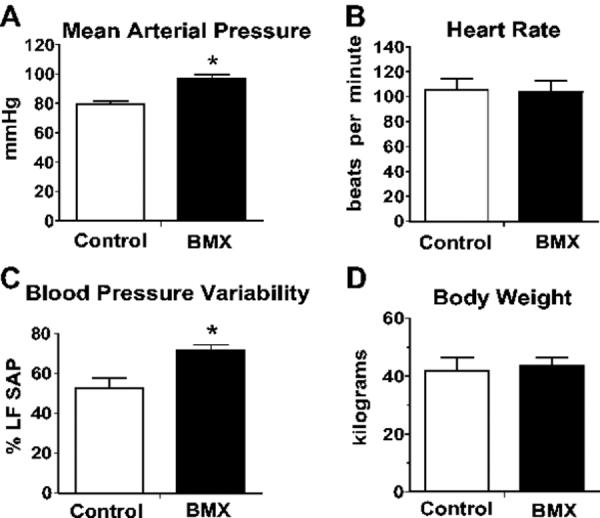
Influence of antenatal betamethasone (BMX) exposure on mean arterial pressure (A); heart rate (B); blood pressure variability (C); and body weight (D) in adult sheep. Blood pressure variability was determined by power of the spectral density of systolic arterial pressure in the low-frequency range (LFSAP) in normalized units (nu) in non-exposed (Control) and BMX-exposed sheep. Data are 6 males and 2 females per group. Values are means ± SEM; *P < 0.05 vs. control.
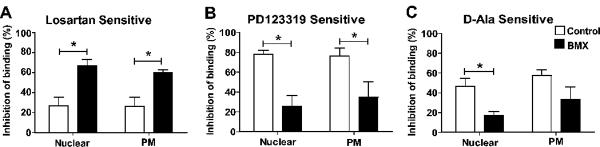
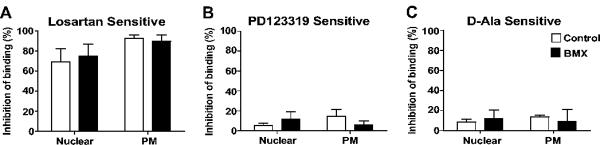
The relative proportions of Ang II receptor subtypes were determined for both nuclear and plasma membrane fractions from the renal cortex and medulla of control and BMX-exposed sheep by competition for 125I-Sarthran binding with selective isotype antagonists. As shown in Figure 2A, the AT1 receptor antagonist losartan competed for a greater percentage of Sarthran binding in both the nuclear and plasma membrane fractions from the BMX-exposed sheep compared to the control animals. Competition by the AT2 antagonist PD123319 in the renal cortex was lower in both nuclear and plasma membrane fractions of BMX-exposed than control animals (Figure 2B). Similar to the PD123319, the Ang-(1-7) antagonist D-Ala competed to a lesser degree in the cortical nuclei of the BMX-exposed sheep (Figure 2C). In contrast, the AT1 receptor was the predominant subtype in either nuclei or plasma membrane fractions of the renal medulla of control sheep (Figure 3A). Moreover, the extent of competition by losartan, PD123319 or D-Ala in renal medullary tissue was not altered in BMX-exposed sheep (Figure 3, panels A–C).
Figure 2.
Angiotensin receptor subtypes in nuclear and plasma membranes (PM) fractions from renal cortex of non-exposed (Control) and betamethasone-exposed (BMX) sheep. Competition binding was performed with 0.5 nmol/L 125I-Sarthran and 10 μmol/L of losartan (A); PD123319 (B); or [D-Ala7]-Ang-(1-7) [C, D-Ala]. Data are expressed as means ± SEM; n = 5 per group (3 males, 2 females) except for D-Ala/PM-BMX (n = 2 males); *p <0.01 vs. Control.
Figure 3.
Angiotensin receptor subtypes in nuclear and plasma membranes (PM) fractions of from renal medulla of non-exposed (Control) and betamethasone-exposed (BMX) sheep. Competition binding was performed with 0.5 nmol/L 125I-Sarthran and 10 μmol/L of losartan (A); PD123319 (B); or [D-Ala7]-Ang-(1-7) [C, D-Ala]. Data are expressed as means ± SEM; n = 5 per group (3 males, 2 females) except for D-Ala/PM-BMX (n = 2 males).
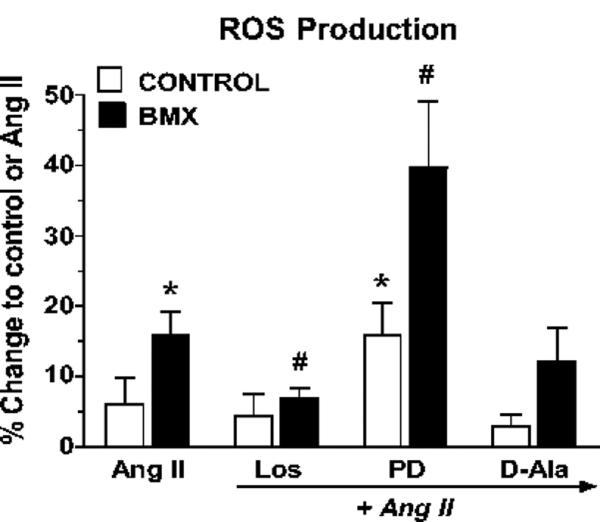
We next determined whether functional differences accompanied the alterations in Ang receptor subtypes in the cortical nuclei from BMX-exposed sheep. Generation of ROS (an AT1- receptor mediated event) was assessed in response to Ang II in freshly isolated nuclei from the renal cortex. Ang II (1 nmol/L) elicited a 2-fold greater increase in ROS in cortical nuclei of BMX-exposed animals than the control animals (Figure 4). The ROS response was significantly reduced in nuclei from BMX-exposed animals pre-incubated with the AT1 receptor antagonist losartan. In contrast, the AT2 receptor antagonist PD123319 more than doubled the ROS response to Ang II in cortical nuclei of the BMX-exposed sheep as compared to Ang II alone (Figure 4). Renal nuclei from control animals that were pre-incubated with losartan showed a small reduction in the low levels of ROS produced following stimulation with Ang II. Similar to BMX-exposed sheep, blockade of the AT2 receptor further increased ROS production in nuclei of control sheep. Treatment with the Ang-(1-7) antagonist D-Ala did not influence the Ang II response in the cortical nuclei from either control or the BMX-exposed sheep (Figure 4). Although not shown, the NADPH oxidase inhibitor DPI abolished the Ang II response consistent with previous data in the kidney of non-exposed sheep and rat16, 17.
Figure 4.
Angiotensin II (Ang II) stimulates reactive oxygen species (ROS) in renal cortical nuclei from non-exposed (Control) and betamethasone-exposed (BMX) sheep. Cortical nuclei were freshly isolated by gradient separation and pre-incubated with the fluorescent dye, dichlorofluorescein (DCF). Nuclei were stimulated with Ang II (1 nmol/L) in the presence (1 μmol/L final concentration) of losartan (Los), PD123319 (PD) or [D-Ala7]-Ang-(1-7) [D-Ala]. Data are the means ± SEM; *p< 0.05 vs. Ang II - Control; #p < 0.05 vs. Ang II - BMX. Control group is n = 11 (8 male, 3 female); BMX group is n = 8 (6 male, 2 female).
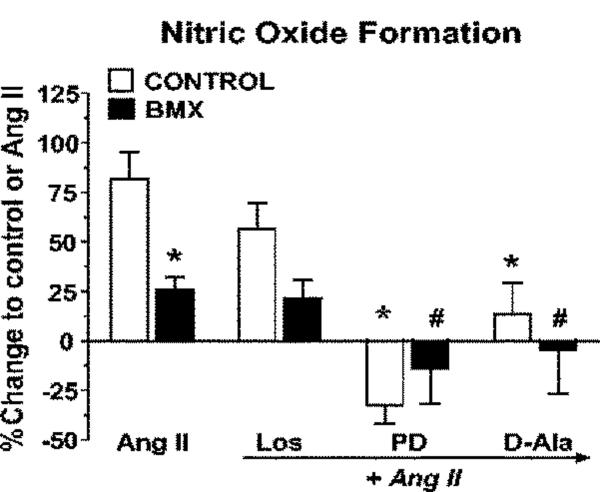
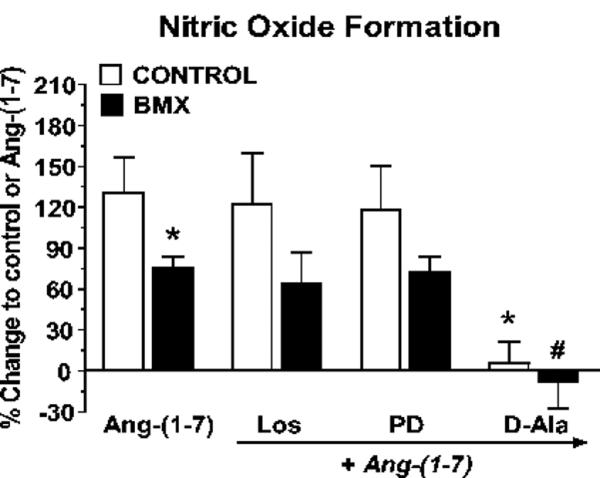
Previous studies in control sheep revealed that both AT2 and Ang-(1-7) receptors are functionally coupled to NO formation14, 15. As shown in Figure 5, NO production in response to Ang II was significantly lower in cortical nuclei from BMX-exposed animals compared to the non-exposed sheep (Control). Blockade of the AT1 receptor with losartan did not significantly influence NO formation in either the control or BMX-exposed animals; however, pretreatment of nuclei with the AT2 antagonist PD123319 abolished Ang II-stimulated NO in both groups. The NOS inhibitor L-NAME abolished the NO response to Ang II (data not shown). In addition, co-incubation with the Ang-(1-7) antagonist DAla significantly attenuated the Ang II response in the Control and BMX-exposed animals (Figure 5). As shown in Figure 6, the NO response to Ang-(1-7) [1 nmol/L] was also attenuated in renal cortical nuclei from the BMX-exposed sheep. The D-Ala antagonist abolished the Ang-(1-7) response in both Control and BMX-treated groups; however, neither losartan nor PD123319 influenced NO formation to Ang-(1-7). The Ang-(1-7) response was abolished by the NOS inhibitor L-NAME (data not shown) consistent with previous results in non-exposed sheep15.
Figure 5.
Angiotensin II (Ang II) stimulates nitric oxide (NO) in renal cortical nuclei from non-exposed (Control) and betamethasone-exposed (BMX) sheep. Cortical nuclei were freshly isolated by gradient separation and pre-incubated with the selective NO fluorescence detector, DAF. Nuclei were stimulated with Ang II (1 nmol/L) in the presence (1 μmol/L final concentration) of losartan (Los), PD123319 (PD) or [D-Ala7]-Ang-(1-7) [D-Ala]. Data are the means ± SEM; *p< 0.05 vs. Ang II - Control; #p < 0.05 vs. Ang II - BMX. Control group is n = 11 (8 male, 3 female); BMX group is n = 8 (6 male, 2 female).
Figure 6.
Angiotensin-(1-7) [Ang-(1-7)] stimulates nitric oxide (NO) in renal cortical nuclei from non-exposed (Control) and betamethasone-exposed (BMX) sheep. Cortical nuclei were freshly isolated by gradient separation and pre-incubated with the selective NO fluorescence detector, DAF. Nuclei were stimulated with Ang II (1 nmol/L) in the presence (1 μmol/L final concentration) of losartan (Los), PD123319 (PD) or [D-Ala7]-Ang-(1-7) [D-Ala]. Data are the means + SEM; *p< 0.05 vs. Ang-(1-7) -Control; #p < 0.05 vs. Ang-(1-7) - BMX. Control group is n = 6 (4 male, 2 female); BMX group is n = 5 (4 male, 1 female).
In lieu of the functional changes for the three Ang receptors following BMX exposure, correlation analyses for the ROS or NO response and changes in mean arterial pressure were performed. As shown in Figure 7A, the ROS response to Ang II was positively correlated to blood pressure (r = 0.7360) among the non-exposed and BMX-exposed sheep. In contrast, the NO responses to both Ang II (r = −0.842) and Ang-(1-7) (r = −0.871) were negatively correlated to blood pressure in the two groups (Figures 7B and 7C, respectively).
Figure 7.
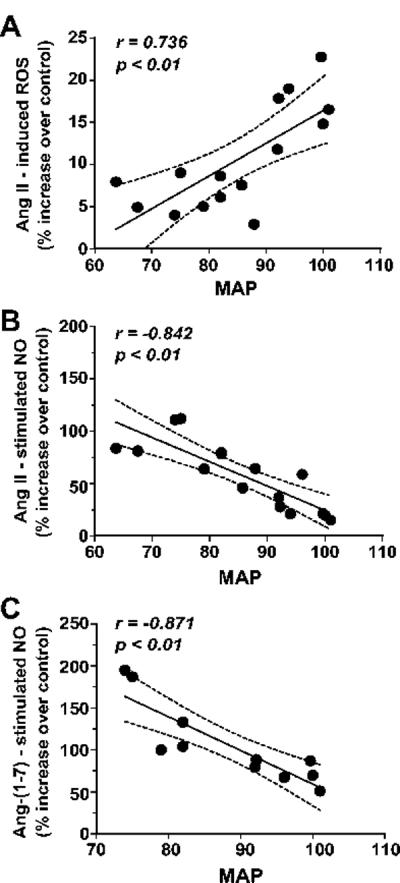
Correlation analyses reveal a positive association of the angiotensin II-reactive oxygen species [Ang II-ROS] response and mean arterial blood pressure (MAP) (A) but negative associations for the Ang II-nitric oxide [Ang II-NO] (B) and angiotensin-(1-7)-NO [Ang-(1-7)-NO] (C) responses with MAP in non-exposed and betamethasone-exposed sheep.
Finally, we determined whether differences in the functional response were attributable to alterations in intracellular peptide metabolism. Although ACE, ACE2 and neprilysin were evident on isolated nuclei, their activity levels were similar between the non-exposed and the BMX-exposed animals for ACE [496 ± 111 vs. 388 ± 70 U], ACE2 [161 ± 54 vs. 172 ± 54 U] and neprilysin [297 ± 90 vs. 245 ± 3 U; n = 3 males per group], respectively. Assessment of non-ACE dependent pathways for Ang II (absence of chymostatin) did not reveal intracellular conversion of Ang I to Ang II in either control or treated animals (data not shown).
DISCUSSION
Glucocorticoids are widely administered to mothers who are at risk for pre-term delivery to facilitate the maturation of pulmonary function of the developing fetus. However, exposure to excess endogenous or exogenous glucocorticoids during the perinatal period may “program” the mal-development of the brain and kidney of the offspring for metabolic and cardiovascular disease in adulthood2–4, 18–20. We characterized the effects of antenatal exposure to the synthetic glucocorticoid BMX during the 80th day of gestation that corresponds to the peak period of nephrogenesis, as well as the time synthetic glucocorticoids are routinely administered to expectant mothers in jeopardy of premature delivery. The present results find a sustained increase in blood pressure in the exposed sheep and confirm previous findings of a ~15% increase in pressure and blood pressure variability of adult sheep exposed antenatally to BMX5,8. The AT1 receptor antagonist candesartan normalized blood pressure in exposed sheep but did not change pressure in the control animals indicating activation of the Ang II-AT1 receptor axis in the adult animals exposed to glucocorticoids5, 8. Indeed, the current results demonstrate an increase in the ratio of AT1 to AT2 receptors within the nuclear and plasma membrane compartments of the renal cortex from the exposed animals. The altered binding profile in the glucocorticoid exposed animals was functionally associated with an enhanced stimulation of ROS by Ang II via AT1 receptors and a reduction in the AT2 dependent generation of NO. Moreover, the generation of NO by Ang-(1-7) was attenuated in the nuclear fraction isolated from the cortex of the BMX exposed sheep. We did not find changes in angiotensin receptor subtypes within the renal medulla nor intracellular alterations in the peptidases ACE, ACE2, or neprilysin of BMX-treated sheep suggesting selective effects of antenatal steroid exposure on cortical angiotensin receptors. The functional responses for Ang II and Ang-(1-7) were correlated to blood pressure among the non-exposed and steroid-exposed sheep.
We previously reported that Ang II stimulates ROS through the AT1 receptor within renal nuclei from both rat and sheep16, 17. Furthermore, this effect was markedly pronounced in nuclei isolated from older adult sheep which exhibited a greater proportion of AT1 sites as compared to young adult sheep16. The current study revealed that the Ang II-dependent ROS response was 2-fold higher in younger adult sheep following antenatal BMX exposure. Our data support earlier findings by Roghair and colleagues that report an enhanced constriction of coronary vessels by Ang II and increased AT1 protein expression, as well as an exaggerated ROS response in glucocorticoid-exposed sheep21, 22. The ROS response to Ang II in the present study was reduced with losartan, but exacerbated by PD123319 suggesting that the AT receptor may counterbalance the actions of an activated Ang II-AT1 receptor pathway23. The enhanced response may reflect a reduction in the AT2-dependent signaling pathways that would normally attenuate Ang II-AT1 receptor-coupled intracellular events23 This does not necessarily imply that the AT1 receptor also exerts an inhibitory influence on AT2 receptor-dependent events within nuclei since losartan did not exacerbate the Ang II-AT2 receptor pathway for NO. Nonetheless, an increased ratio of AT1 to AT2 receptors is consistent with the more pronounced effects of Ang II on sodium reabsorption and renal vascular resistance in the adult sheep following antenatal BMX exposure11. Moreover, the reduction in the proportion and functional response of the AT2 receptor also supports the findings of reduced AT2 mRNA and/or levels protein levels in the offspring of protein-restricted rats, a rodent model of fetal programming that exhibits higher glucocorticoid levels in utero24-26.
In addition to alterations in the AT1 to AT2 receptor ratio, the NO response to Ang-(1-7) was attenuated in the kidney of the exposed sheep. The extent of D-Ala competition for Sarthran binding in the renal cortex was significantly lower in the BMX-exposed group as well. The stimulation of NO by Ang-(1-7) was abolished by D-Ala but not losartan or PD123319. These data suggest that Ang-(1-7)/Mas receptor may also be downregulated by antenatal glucocorticoid exposure. We did not find differences in the intracellular complement of peptidases that influence Ang II and Ang-(1-7) expression; however, it is possible that signaling events downstream from the Ang-(1-7) receptor may be altered in the BMX-exposed sheep. Several reports suggest that Ang-(1-7)-dependent activation of intracellular phosphatases mitigate the actions of the Ang II-AT1 receptor pathway in proximal tubules and other cell types27–29, although the nuclear signaling events in the current study, as well as the overall cellular pathways in fetal programming remained to be elucidated. Nonetheless, a reduction in Ang-(1-7)/NO tone supports previous findings that BMX exposure was associated with an attenuated natriuretic and diuretic response to exogenous Ang-(1-7), as well as the overall natriuretic actions of the peptide on the proximal tubule12,30–32. Indeed, in control sheep, the Mas receptor protein was evident on proximal tubules, thick ascending limb and collecting ducts of the kidney that are key sites for sodium reabsorption15. However, further studies are necessary to determine the cell types within the kidney that reflect the alterations in receptor subtype and response following BMX exposure. We note that the Ang-(1-7) antagonist D-Ala blocked the Ang II-dependent NO response (Figure 4). PD123319 did not inhibit NO synthesis by Ang-(1-7) (Figures 5), therefore, it is unlikely that the Ang II response reflects the conversion to Ang-(1-7) on nuclei by ACE2 and more feasible that the concentration of the D-Ala employed in our studies may interact with the AT2 receptor.
Emerging evidence reveals that the kidney expresses an intracellular system in which receptors localized within distinct cellular organelle mediate the actions of Ang II and Ang-(1-7)14–16, 33–35. There is a high density of intracellular AT1 sites localized to the nuclear fraction in both the cortical and medullary areas of the rat kidney33–35. We also find nuclear AT1 sites in the cortex of sheep kidney; however, functional AT2 and Ang-(1-7)/Mas receptors are evident in cortical nuclei as well14–16. Intracellular alterations in the ratio of AT1 to AT2 and/or Ang-(1-7) receptors may influence gene expression that ultimately contributes to programming mechanisms and a sustained increase in blood pressure following glucocorticoid exposure. Zhuo and colleagues report that Ang II via the AT1receptor directly stimulates mRNA transcripts for the sodium hydrogen exchanger (NHE), MCP-1 and TGF-β in isolated cortical nuclei35. Their preliminary studies also show that intracellular expression of non-secreted Ang II in the proximal tubule increases blood pressure that is abrogated by losartan treatment36. Nattel and colleagues find that Ang II increases the mRNA expression of NFkB to a greater extent in isolated nuclei from cardiomyocytes than in intact cells37. Clearly, the upregulation of sodium transporters such as NHE or the enhanced expression of inflammatory molecules may contribute to renal dysfunction and an elevation in blood pressure; however, whether these mechanisms are operative in this model of programming remains to be established.
Finally, we previously reported that BMX exposure induces a similar increase in blood pressure in female and male sheep at 6 and 36 months of age, as well as a comparable reduction (~25%) in the number of glomeruli12, 38. The existing literature on sex differences in fetal programming is somewhat equivocal regarding the extent that females exhibit cardiovascular protection which may reflect species differences, as well as the timing and nature of the programming event21, 22, 38–42. The current study did not distinguish sex specific alterations in receptor expression or function due to the low number of females in the BMX-exposed group. We have noted sex differences in sodium excretion and natriuretic responses to exogenous Ang-(1-7) following an acute sodium load in control and exposed sheep that vary with age11, 12. It is entirely possible that different, although as of yet undefined mechanisms contribute to the increase in blood pressure in male and female sheep exposed to antenatal glucocorticoids; additional studies are necessary to identify these potential mechanisms. Additional studies are also required to determine whether alterations to intracellular angiotensin receptors precede the changes in blood pressure or contribute to the progression of fetal programmed hypertension. Moreover, at this point it is not evident as to the underlying mechanisms whereby acute fetal BMX exposure alters the relative proportion of the three receptor subtypes within the renal cortex. Finally, although the AT1 site was the predominant subtype in the renal medulla of both control and BMX-exposed sheep, it is possible that BMX exposure may also influence signaling pathways downstream from the AT1 receptor, particularly given the importance of ROS and NO in medullary function43.
PERSPECTIVES
Insults of various types occurring during the critical period of fetal development may have prolonged effects on the offspring in adulthood. Antenatal exposure to excess endogenous or exogenous glucocorticoids, particularly those administered to mothers at risk for pre-term delivery, may predispose the offspring to metabolic and cardiovascular disease including hypertension and renal injury. The effects of steroid exposure on the kidney and kidney development may induce mechanisms that lead to oxidative stress through increased ROS and concomitant decreases in NO. Indeed, steroid-induced programming events may promote a state of accelerated aging that encompasses alterations in multiple angiotensin receptors and their requisite signaling pathways44. Thus, potential therapeutic approaches in “fetal programmed” hypertension should consider targeting all three receptor subtypes to re-set the balance of both the extracellular and intracellular RAS.
Acknowledgments
SOURCES OF FUNDING This work was supported by grants from the National Institutes of Health HD17644, HD47584, HL-56973 and HL51952. Hossam Shaltout is an Assistant Professor in the Department of Pharmacology and Toxicology, School of Pharmacy, University of Alexandria, Alexandria 21526, Egypt.
Footnotes
DISCLOSURES: None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- 1.Bolt RJ, van Weissenbruch MM, Lafeber HN, Delemarre-van de Waal HA. Glucocorticoids and lung development in the fetus and preterm infant. Pediatr Pulmonol. 2001;32:76–91. doi: 10.1002/ppul.1092. [DOI] [PubMed] [Google Scholar]
- 2.Doyle LW, Ford GW, Davis NM, Callanan C. Antenatal corticosteroid therapy and blood pressure at 14 years of age in preterm children. Clin Sci (Lond) 2000;98:137–142. [PubMed] [Google Scholar]
- 3.Seckl JR, Holmes MC. Mechanisms of disease:glucocorticoids, their placental metabolism and fetal `programming' of adult pathophysiology. Nat Clin Pract Endocrinol Metab. 2007;3:479–488. doi: 10.1038/ncpendmet0515. [DOI] [PubMed] [Google Scholar]
- 4.Nuyt AM, Alexander BT. Developmental programming and hypertension. Curr Opin Nephrol Hypertens. 2009;18:144–152. doi: 10.1097/MNH.0b013e328326092c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaltout HA, Rose JC, Figueroa JP, Chappell MC, Diz DI, Averill DB. Acute AT(1)-receptor blockade reverses the hemodynamic and baroreflex impairment in adult sheep exposed to antenatal betamethasone. Am J Physiol Heart Circ Physiol. 2010;299:H541–H547. doi: 10.1152/ajpheart.00100.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kantorowicz L, Valego NK, Tang L, Figueroa JP, Chappell MC, Carey LC, Rose JC. Plasma and renal renin concentrations in adult sheep after prenatal betamethasone exposure. Reprod Sci. 2008;15:831–838. doi: 10.1177/1933719108318599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang L, Carey LC, Bi J, Valego N, Sun X, Deibel P, Perrott J, Figueroa JP, Chappell MC, Rose JC. Gender differences in the effects of antenatal betamethasone exposure on renal function in adult sheep. Am J Physiol Regul Integr Comp Physiol. 2009;296:R309–R317. doi: 10.1152/ajpregu.90645.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaltout HA, Figueroa JP, Rose JC, Diz DI, Chappell MC. Alterations in circulatory and renal angiotensin-converting enzyme and angiotensin-converting enzyme 2 in fetal programmed hypertension. Hypertension. 2009;53:404–408. doi: 10.1161/HYPERTENSIONAHA.108.124339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moritz KM, Johnson K, Douglas-Denton R, Wintour EM, Dodic M. Maternal glucocorticoid treatment programs alterations in the renin-angiotensin system of the ovine fetal kidney. Endocrinology. 2002;143:4455–4463. doi: 10.1210/en.2002-220534. [DOI] [PubMed] [Google Scholar]
- 10.Dodic M, Moritz K, Wintour EM. Prenatal exposure to glucocorticoids and adult disease. Arch Physiol Biochem. 2003;111:61–69. doi: 10.1076/apab.111.1.61.15144. [DOI] [PubMed] [Google Scholar]
- 11.Contag SA, Bi J, Chappell MC, Rose JC. Developmental effect of antenatal exposure to betamethasone on renal angiotensin II activity in the young adult sheep. Am J Physiol Renal Physiol. 2010;298:F847–F856. doi: 10.1152/ajprenal.00497.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang L, Bi J, Valego NK, Carey LC, Figueroa JP, Chappell MC, Rose JC. Prenatal betamethasone exposure alters renal function in immature sheep: Sex differences in effects. Am J Physiol Regul Integr Comp Physiol. 2009;296:R309–R317. doi: 10.1152/ajpregu.90645.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sahajpal V, Ashton N. Renal function and angiotensin AT1 receptor expression in young rats following intrauterine exposure to a maternal low-protein diet. Clin Sci (Lond) 2003;104:607–614. doi: 10.1042/CS20020355. [DOI] [PubMed] [Google Scholar]
- 14.Gwathmey TM, Shaltout HA, Pendergrass KD, Pirro NT, Figueroa JP, Rose JC, Diz DI, Chappell MC. Nuclear angiotensin II type 2 (AT2) receptors are functionally linked to nitric oxide production. Am J Physiol Renal Physiol. 2009;296:F1484–F1493. doi: 10.1152/ajprenal.90766.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gwathmey TM, Westwood BM, Pirro NT, Tang L, Rose JC, Diz DI, Chappell MC. The nuclear Angiotensin-(1-7) receptor is functionally coupled to the formation of nitric oxide. Am J Physiol Renal Physiol. 2010;299:F983–F990. doi: 10.1152/ajprenal.00371.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gwathmey TM, Pendergrass KD, Reid SD, Rose JC, Diz DI, Chappell MC. Angiotensin-(1-7)-angiotensin-converting enzyme 2 attenuates reactive oxygen species formation to angiotensin II within the cell nucleus. Hypertension. 2010;55:166–171. doi: 10.1161/HYPERTENSIONAHA.109.141622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pendergrass KD, Gwathmey TM, Michalek RD, Grayson JM, Chappell MC. The angiotensin II-AT1 receptor stimulates reactive oxygen species within the cell nucleus. Biochem Biophys Res Commun. 2009;384:149–154. doi: 10.1016/j.bbrc.2009.04.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fowden AL, Forhead AJ. Endocrine mechanisms of intrauterine programming. Reproduction. 2004;127:515–526. doi: 10.1530/rep.1.00033. [DOI] [PubMed] [Google Scholar]
- 19.Matthews SG, Owen D, Kalabis G, Banjanin S, Setiawan EB, Dunn EA, Andrews MH. Fetal glucocorticoid exposure and hypothalamo-pituitary-adrenal (HPA) function after birth. Endocr Res. 2004;30:827–836. doi: 10.1081/erc-200044091. [DOI] [PubMed] [Google Scholar]
- 20.Owen D, Andrews MH, Matthews SG. Maternal adversity, glucocorticoids and programming of neuroendocrine function and behaviour. Neurosci Biobehav Rev. 2005;29:209–226. doi: 10.1016/j.neubiorev.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Roghair RD, Lamb FS, Bedell KA, Smith OM, Scholz TD, Segard JL. Late-gestation betamethasone enhances coronary artery responsiveness to angiotensin II in fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2004;286:R80–R88. doi: 10.1152/ajpregu.00421.2003. [DOI] [PubMed] [Google Scholar]
- 22.Roghair RD, Miller FJ, Scholz TD, Lamb FS, Segard JL. Coronary constriction to angiotensin II is enhanced by endothelial superoxide production in sheep programmed by dexamethasone. Pediatr Res. 2008;63:370–374. doi: 10.1203/PDR.0b013e3181659bfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carey RM. Cardiovascular and renal regulation by the angiotensin type 2 receptor. The AT2 receptor comes of age. Hypertension. 2005;45:840–844. doi: 10.1161/01.HYP.0000159192.93968.8f. [DOI] [PubMed] [Google Scholar]
- 24.Sahajpal V, Ashton N. Increased glomerular angiotensin II binding in rats exposed to a maternal low protein diet in utero. J Physiol. 2005;563:193–201. doi: 10.1113/jphysiol.2004.078642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vehaskari VM, Stewart T, Lafont D, Soyez C, Seth D, Manning J. Kidney angiotensin and angiotensin receptor expression in prenatally programmed hypertension. Am J Physiol Renal Physiol. 2004;287:F262–F267. doi: 10.1152/ajprenal.00055.2004. [DOI] [PubMed] [Google Scholar]
- 26.McMullen S, Gardner DS, Langley-Evans SC. Prenatal programming of angiotensin II type 2 receptor expression in the rat. Br J Nutr. 2004;91:133–140. doi: 10.1079/bjn20031029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su Z, Zimpelmann J, Burns KD. Angiotensin-(1-7) inhibitis angiotensin II-stimulated phosphorylation of MAP kinases in proximal tubular cells. Kidney Int. 2006;69:2212–2218. doi: 10.1038/sj.ki.5001509. [DOI] [PubMed] [Google Scholar]
- 28.Gallagher PE, Ferrario CM, Tallant EA. MAP kinase/phosphatase pathway mediates the regulation of ACE2 by angiotensin peptides. Am J Physiol Cell Physiol. 2008;295:C1169–C1174. doi: 10.1152/ajpcell.00145.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sampaio WO, Henrique de CC, Santos RA, Schiffrin EL, Touyz RM. Angiotensin-(1-7) counterregulates angiotensin II signaling in human endothelial cells. Hypertension. 2007;50:1093–1098. doi: 10.1161/HYPERTENSIONAHA.106.084848. [DOI] [PubMed] [Google Scholar]
- 30.Dilauro M, Burns KD. Angiotensin-(1-7) and its effects in the kidney. Scientific World Journal. 2009;9:522–535. doi: 10.1100/tsw.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chappell MC. Emerging evidence for a functional angiotesin-converting enzyme 2-angiotensin-(1-7) mas receptor axis; more than regulation of blood pressure? Hypertension. 2007;50:596–599. doi: 10.1161/HYPERTENSIONAHA.106.076216. [DOI] [PubMed] [Google Scholar]
- 32.Santos RA, Ferreira AJ, Simoes e Silva AC. Recent advances in the angiotensin-converting enzyme 2-angiotensin(1-7)-Mas axis. Exp Physiol. 2008;93:519–527. doi: 10.1113/expphysiol.2008.042002. [DOI] [PubMed] [Google Scholar]
- 33.Pendergrass KD, Averill DB, Ferrario CM, Diz DI, Chappell MC. Differential expression of nuclear AT1 receptors and angiotensin II within the kidney of the male congenic mRen2.Lewis rat. Am J Physiol Renal Physiol. 2006;290:F1497–F1506. doi: 10.1152/ajprenal.00317.2005. [DOI] [PubMed] [Google Scholar]
- 34.Zhuo JL, Li XC, Garvin JL, Navar LG, Carretero OA. Intracellular ANG II induces cytosolic Ca2+ mobilization by stimulating intracellular AT1 receptors in proximal tubule cells. Am J Physiol Renal Physiol. 2006;290:F1382–F1390. doi: 10.1152/ajprenal.00269.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li XC, Zhuo JL. Intracellular angiotensin II induces in vitro transcription of TGF-β1, MCP-1 and NHE3 mRNAs in rat renal cortical nuclei via activation of nuclear AT1 receptors. Am J Physiol Cell Physiol. 2008;294:C1034–C1045. doi: 10.1152/ajpcell.00432.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li XC, Cook JL, Rubera I, Zhang F, Tauc M, Zhuo JL. Proximal Tubule Cell-Specific Adenoviral transfer of an intracellular angiotensin II fusion protein elevates blood pressure by activating AT1 receptors. Hypertension. 2009;54:E74, P214. [Abstract] [Google Scholar]
- 37.Tadevosyan A, Maguy A, Villeneuve LR, Babin J, Bonnefoy A, Allen BG, Nattel S. Nuclear-delimited angiotensin receptor-mediated signaling regulates cardiomyocyte gene expression. J Biol Chem. 2010;285:22338–22349. doi: 10.1074/jbc.M110.121749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J, Massmann GA, Rose JC, Figueroa JP. Differential effects of clinical doses of antenatal betamethasone on nephron endowment and glomerular filtration rate in adult sheep. Reprod Sci. 2010;17:186–95. doi: 10.1177/1933719109351098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woods LL, Weeks DA. Naturally occurring intrauteirne growth retardaton and adult pressure in rats. Pediatric Res. 2004;56:460–467. doi: 10.1203/01.PDR.0000142589.08246.77. [DOI] [PubMed] [Google Scholar]
- 40.Wintour EM, Moritz KM, Johnson K, Ricardo S, Samuel CS, Dodic M. Reduced nephron number in adult sheep, hypertensive as a result of prenatal glucocorticoid treatment. J Physiol. 2003;549:929–935. doi: 10.1113/jphysiol.2003.042408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roghair RD, Segar JL, Volk KA, Chapleau MW, Dallas LM, Soresnon AR, Scholz TD, Lamb FS. Vascular nitric oxide and superoxide anion contriubte to sex-specific programmed caridovsuclar physiology in mice. Am J Physiol Regul Integr Comp Physiol. 2008;296:R651–R662. doi: 10.1152/ajpregu.90756.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ojeda NB, Grigore D, Robertson EB, Alexander BT. Estrogen protects against increased blood pressure in postpubertal female growth restricted offspring. Hypertension. 2007;50:679–85. doi: 10.1161/HYPERTENSIONAHA.107.091785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol Cell Physiol. 2008;294:C1034–C1045. doi: 10.1152/ajpregu.00250.2005. [DOI] [PubMed] [Google Scholar]
- 44.Benigni A, Corna D, Zoja C, Sonzogni A, Latini R, Salio M, Conti S, Rottoli D, Longaretti L, Cassis P, Morigi M, Coffman TM, Remuzzi G. Disruption of the Ang II type 1 receptor promotes longevity in mice. J Clin Invest. 2009;119:524–530. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]