Abstract
Background
Patients with non-ischemic left ventricular cardiomyopathy (LVCM) and ventricular tachycardia (VT) have complex three-dimensional substrate with variable involvement of the endocardium (ENDO) and epicardium (EPI). The purpose of this study was to determine if ENDO unipolar (UNI) mapping with a larger electrical field of view could identify EPI low bipolar (BIP) voltage regions in patients with LVCM undergoing VT ablation.
Methods and Results
The reference value for normal ENDO unipolar voltage was determined from 6 patients without structural heart disease. Consecutive patients undergoing VT ablation over an eight-year period with detailed (>100 point) LV ENDO and EPI mapping and normal LV ENDO BIP voltage were identified. From this cohort, we compared patients with structurally normal hearts and normal EPI BIP voltage (EPI-, Group 1) to patients with LVCM and low LV EPI BIP voltage regions present (EPI+, Group 2). Confluent regions of ENDO UNI and EPI BIP low voltage (> 2 cm2) were measured. The normal signal amplitude was >8.27mV for LV ENDO UNI electrograms. Detailed LV ENDO-EPI maps in 5 EPI- patients were compared to 11 EPI+ patients. Confluent ENDO UNI low voltage regions were seen in 9/11 (82%) of the EPI+ (Group 2) patients compared to 0/5 EPI- (Group 1) patients (P <0.001). In all 9 patients with ENDO UNI low voltage, the ENDO UNI low voltage regions were directly opposite to an area of EPI BIP low voltage (61% ENDO UNI-EPI BIP low voltage area overlap).
Conclusions
EPI arrhythmia substrate can be reliably identified in most patients with LVCM using ENDO UNI voltage mapping in the absence of ENDO BIP abnormalities.
Keywords: catheter ablation, ventricular tachycardia, electrophysiology mapping, cardiomyopathy
The value of bipolar (BIP) electroanatomical mapping in patients with non-ischemic LV cardiomyopathy (LVCM) and scar-related ventricular tachycardia (VT) has been well established. 1–4 The heterogeneous and often complex substrate distribution present in patients creates unique challenges for catheter-based VT therapy.
Although the true prevalence of epicardial substrate in the LVCM population remains unknown, many LVCM patients have more sizeable bipolar low voltage regions on the epicardium (EPI) than the endocardium (ENDO). 5 We have also found a limited number of LVCM patients presenting with VT of EPI origin to have completely normal ENDO BIP voltage characteristics. Limitations in the field-of-view of BIP EGMs in the setting of complex, non-transmural substrate may be responsible for the inability to detect these EPI abnormalities with BIP ENDO mapping. The utility of unipolar (UNI) ENDO electrogram recordings, which have a larger field of view to identify an epicardial substrate, has not been assessed in this population.
The purpose of this study was to evaluate whether ENDO UNI mapping can identify the presence and location of EPI low BIP voltage regions in patients with LVCM undergoing VT ablation who do not demonstrate endocardial bipolar voltage abnormalities.
Methods
Study Population
We examined consecutive patients undergoing VT ablation at the University of Pennsylvania from June 2002 – June 2010. All procedures were performed following the institutional guidelines of the University of Pennsylvania Health System and all patients provided written informed consent. Patients undergoing detailed (>100 point) ENDO and EPI electroanatomical mapping with complete sampling of all LV segments were included. The decision for an epicardial approach was made based upon either: 1) the characteristics of the VT on the surface 12-lead ECG; 2) the presence of epicardial substrate on imaging studies (CT, MR, intracardiac echocardiography); and/or 3) the failure of prior endocardial ablation procedure. 6
From the total cohort, we examined patients with normal LV ENDO BIP voltage. We then identified two study groups from the initial cohort: (1) patients with structurally normal hearts and normal EPI BIP voltage (EPI-, Group 1); and (2) patients with LVCM and LV EPI BIP low voltage regions present (EPI+, Group 2).
Structural heart disease was excluded in the EPI- patients with transthoracic echocardiography and stress testing (if >30 years old). The diagnosis of LVCM was established by the absence of significant (>70% stenosis) coronary artery disease, documented prior myocardial infarction or significant primary valvular abnormalities. Other likely causes of dilated cardiomyopathy were also excluded including: arrhythmogenic right ventricular dysplasia/cardiomyopathy, cardiac sarcoidosis, and alcoholic cardiomyopathy. All study patients had a previous history of spontaneous sustained monomorphic VT documented either by surface ECG or stored intracardiac electrograms from an implanted cardioverter-defibrillator (ICD).
Sinus Rhythm Electroanatomical Mapping
Electroanatomical mapping of the endocardium and the epicardium during the baseline rhythm was performed during the same procedure using the CARTOTM system (Biosense Webster Inc., Diamond Bar, California). Either a 4 mm distal-tip/2-mm ring electrode ablation catheter (NaviStar®, Biosense Webster Inc., Diamond Bar, California) or a 3.5 mm distal tip irrigated catheter (Navistar Thermocool®, Biosense Webster Inc., Diamond Bar, California) was used as the mapping catheter. Bipolar signals were recorded between the distal electrode pair and were filtered at 30 to 400 Hz and displayed at 200 mm/s. Unipolar signals were recorded between the distal tip of the ablation catheter (cathode) and Wilson’s central terminal and were filtered at 1–240 Hz and displayed at 200 mm/s. All electrograms were visually reviewed for the presence of noise or pacing artifact. Electroanatomical points that were clearly internal to the 3D surface were excluded. All quantitative electroanatomical data were acquired using a fill threshold of 20 on the CARTOTM mapping system.
A retrograde, transaortic approach was used to access the LV ENDO in all cases. Access to the pericardial space and epicardium was obtained using the technique described by Sosa and colleagues. 7 Briefly, under general anesthesia a Tuohy needle was introduced via a subxiphoid approach to gain access for sheath and ablation catheter placement. A value of 1.5mV defined normal LV endocardial bipolar electrogram amplitude. Our recent work defined normal LV EPI BIP signal amplitude to be greater than 1.0 mV (after excluding the regions within 1.5 cm of the coronary vasculature). 5
Reference Values for Voltage Abnormality with Electroanatomical Mapping
We determined the reference value for endocardial LV UNI electrogram voltage by examining the voltage characteristics in a separate cohort of 6 patients without structural heart disease who underwent electrophysiologic testing for symptomatic PVCs. Detailed (>100 point) electroanatomical mapping was performed in each patient. Structural heart disease was excluded in these patients with transthoracic echocardiography and stress testing (if >30 years old).
A 4 mm distal-tip/2-mm ring electrode ablation catheter (NaviStarR, Biosense Webster Inc.) was used as the mapping catheter. Unipolar signals were recorded between the tip of the ablation catheter (cathode) and Wilson’s central terminal and were filtered at 1–240 Hz and displayed at 200 mm/s. All electrograms were visually reviewed for the presence of noise or pacing artifact. Electroanatomical points that were clearly internal to the three dimensional surface were excluded. Catheter contact at each site sampled was verified using a combination of fluoroscopic assessment as well as temporal stability of the recorded electrograms. Normal LV ENDO UNI signal amplitude was defined as that exceeded by 95% of all electrograms.
Quantitative Assessment of Confluent Low Voltage Regions
Confluent regions of ENDO UNI and EPI BIP low voltage (> 2 cm2) were measured using the standard surface area measurement tool on the CARTO system (software version 9.0.34). When multiple areas of confluent low voltage were present, the aggregate area from individual regions of interest was calculated. We also measured the surface area of direct spatial overlap between the ENDO UNI and EPI BIP low voltage areas using the mesh feature on the CARTO software, and reported a percentage overlap (ENDO UNI and EPI BIP overlap area/total ENDO UNI area).
The low amplitude EPI BIP regions were considered abnormal if confluent signal amplitude was <1.0mV and >20% of sites also demonstrated any of the following abnormal EGM characteristics: (1) wide - >80ms in duration; (2) split- two or more distinct components with >20 ms isoelectric segment between peaks of individual components; or (3) late- distinct EGM with onset after the end of the QRS complex. Areas within 1.5 cm of the major coronary arteries were excluded from the low voltage assessment. 5
When assessing confluent ENDO UNI low voltage regions, areas within 1 cm of the mitral and aortic valve annuli were excluded from the measurement. This was done to prevent overestimation of the UNI low voltage area around the valve, which may record low voltage because of the absence of myocardium within the area of the valve annulus.
Measurement of Endocardial to Epicardial Distance
In order to prevent potential confounding of voltage measurements related to variable distances between the ENDO and EPI (ENDO-EPI) electroanatomical mapping surfaces, the ENDO-EPI distance was calculated using the standard distance measurement tool on the CARTO software. The perpendicular ENDO-EPI distances were measured in each patient at two separate sites (typically the basal and mid lateral LV segments) adjacent to the low voltage region; a mean distance value was reported.
Statistical Analysis
All electroanatomical measurements were tested using the one-sample Kolmogorov-Smirnov test against a normal distribution. Continuous data are expressed as a mean ± SD or a range, as appropriate. When comparing continuous variables, a Student’s t-test was used for normally distributed data; the Mann-Whitney U test was used for data which was not normally distributed. McNemar’s test was used to compare dichotomous variables. A p value ≤ 0.05 was considered statistically significant.
Results
Reference Values for Voltage Abnormality with Electroanatomical Mapping
The reference population consisted of 6 patients (5 males, 1 female) with a mean age of 36 ± 18 years. A total of 683 LV electrograms were analyzed (range 100–168 points per patient). Ninety five percent of LV ENDO unipolar signals had an amplitude > 8.27mV (mean 19.6 ± 6.9 mV), defined as the value of normal LV ENDO UNI signal amplitude.
Patient Characteristics
The clinical characteristics of the study patients are displayed in Table 1. Of the 1517 patients undergoing ablation for VT at our institution between June 2002 and June 2010, 168 had a combined ENDO-EPI procedure. Of these patients, 16 had detailed mapping of the ENDO and EPI with normal ENDO BIP voltage. From this group of 16 patients, 5 had structurally normal hearts and normal BIP EPI voltage (EPI-) and underwent mapping and ablation of idiopathic VT/VPDs. Eleven additional patients had LVCM and VT with confirmed epicardial origin with a confluent region (> 2cm2)of EPI BIP low voltage present (EPI+).
Table 1.
Baseline Patient Characteristics
| No | Age (yrs) | Sex | LVEF,% | AAD Before Procedure | # Prior Procedures | Clinical Arrhythmia | ICD Present |
|---|---|---|---|---|---|---|---|
| EPI-(Group 1) | |||||||
| 1 | 26 | M | 55 | Flecainide | 1 | SMVT | No |
| 2 | 31 | M | 55 | None | 0 | SMVT | No |
| 3 | 19 | F | 65 | None | 2 | SMVT | No |
| 4 | 47 | M | 50 | Sotalol | 0 | PVC | No |
| 5 | 53 | F | 65 | None | 1 | PVC | No |
| EPI+ (Group 2) | |||||||
| 1 | 51 | F | 30 | Lido+Proc | 0 | SMVT | Yes |
| 2 | 48 | M | 45 | Amiodarone | 0 | SMVT | Yes |
| 3 | 53 | M | 50 | Sotalol+Mex | 0 | SMVT | Yes |
| 4 | 48 | M | 45 | Propafenone | 3 | SMVT | Yes |
| 5 | 73 | M | 25 | Amiodarone | 1 | SMVT | Yes |
| 6 | 26 | M | 60 | None | 1 | SMVT | No |
| 7 | 51 | M | 30 | Amiodarone | 1 | SMVT | Yes |
| 8 | 27 | F | 20 | Dofet+Mex | 0 | SMVT | Yes |
| 9 | 39 | M | 55 | Amiodarone | 1 | SMVT | Yes |
| 10 | 52 | M | 50 | Amiodarone | 1 | SMVT | Yes |
| 11 | 58 | M | 50 | Amio+Quin | 0 | SMVT | No |
AAD: antiarrhythmic drugs EF: ejection fraction; F: female; M: male; PVC: premature ventricular complex; SMVT: sustained monomorphic ventricular tachycardia.
Electroanatomical Mapping
The characteristics of the electroanatomical maps from the EPI+ patients are shown in Table 2. The mean number of electroanatomical points on the ENDO and EPI maps was similar between EPI+ and EPI- patients (ENDO maps: 188±57 vs. 166±67, p=0.3; EPI maps: 491±178 vs. 361±206, p=0.1). The mean ENDO UNI voltage was significantly lower in the EPI+ vs. EPI-groups (10.5±3.2 vs. 14.7±2.6 mV, p=0.04). In contrast, there was no difference in ENDO BIP voltage between the EPI+ and EPI- patients (4.1±0.6 vs. 4.3±0.8 mV; p=0.9).
Table 2.
Electroanatomical Mapping Findings In Group 2 Patients (EPI+)
| No | Map (Endo=UNI; Epi=BIP) | No. of Points Mapped | Low Voltage Area (cm2) | % Direct Area Overlap1 | Low Voltage Location | Endo-Epi Distance (mm) |
|---|---|---|---|---|---|---|
| 1 | Endo | 171 | 45.7 | 63 | B-M Ant Lat, B Inf Lat, Apical | 18 |
| Epi | 372 | 28.6 | B-M Inf Lat | |||
| 2 | Endo | 154 | 22.8 | 100 | B-M Lat, B Inf Lat, B Inf Sep | 17 |
| Epi | 594 | 154.8 | B-A Ant Lat-Inf Sep | |||
| 3 | Endo | 190 | 5.4 | 100 | B Inf Lat | 15 |
| Epi | 605 | 50.7 | B Inf Lat, M Lat | |||
| 4 | Endo | 154 | 1.1 | 100 | M Inf Lat | 10 |
| Epi | 445 | 12.4 | M Inf Lat | |||
| 5 | Endo | 155 | 94.6 | 45 | B-A Ant Lat-Inf Sept | 16 |
| Epi | 655 | 62.4 | B-M Ant Lat-Inf Lat | |||
| 6 | Endo | 204 | 9.8 | 49 | B-M Lat | 15 |
| Epi | 250 | 40.9 | M Lat-Inf Lat | |||
| 7 | Endo | 248 | 8.1 | 100 | B-M Lat, B Inf Lat, Apical | 17 |
| Epi | 698 | 58.2 | B-M Lat-Inf Lat, Apical | |||
| 8 | Endo | 319 | 3.3 | 100 | B Inf Sept, M Ant, B Inf Lat | 15 |
| Epi | 501 | 20 | M Ant, B Inf Lat | |||
| 9 | Endo | 102 | 5.4 | 100 | B Inf Lat | 18 |
| Epi | 221 | 45.8 | B-M Inf Lat | |||
| 10 | Endo | 181 | 0 | - | - | 17 |
| Epi | 338 | 24.4 | M Inf Lat | |||
| 11 | Endo | 187 | 15.2 | 39 | B-M Lat-Inf Lat | 21 |
| Epi | 724 | 42.3 | B Inf Lat |
ENDO UNI-EPI BIP Overlap area as a fraction of total UNI Area
Confluent ENDO UNI low voltage regions (> 2 cm2) were seen in 9/11 (82%) of the EPI+ patients compared to 0/5 of the EPI- patients (Figure 1). In the EPI+ group, the mean EPI BIP low voltage area was significantly larger than the corresponding ENDO UNI area (49.1 ± 38.4 cm2 vs. 19.2 ± 28.2, p=0.02). In all 9 patients with ENDO UNI low voltage regions, at least one of the ENDO UNI regions was directly opposite to an area of EPI BIP low voltage (Figure 2). Of the total ENDO UNI low voltage area, 61% directly overlapped EPI BIP low voltage regions. The mean LV ENDO UNI voltage within the low voltage areas in the EPI+ patient cohort was 5.5±1.7 mV.
Figure 1.
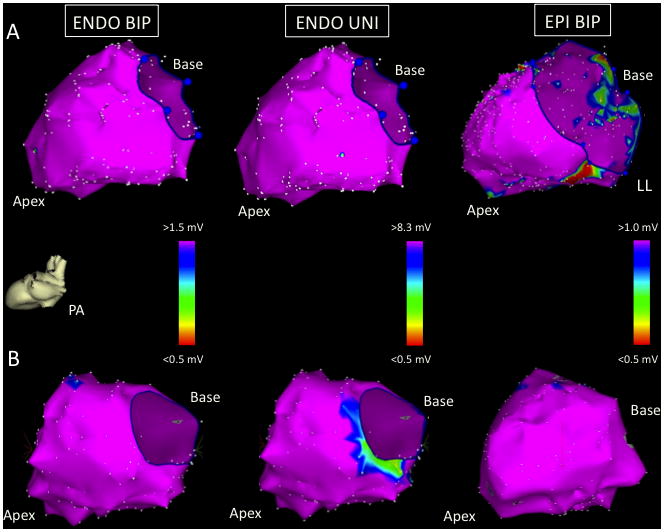
Images taken from two EPI- patients in the posterior-anterior (PA) projection: patient 1 (panel A) and patient 2 (panel B). The LV ENDO BIP (left), ENDO UNI (middle), and EPI BIP (right) voltage maps are normal.
Figure 2.
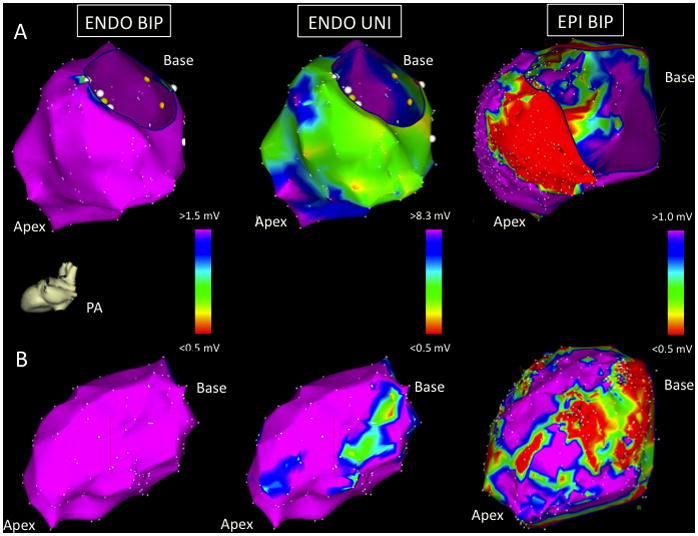
Images taken from two EPI+ patients in the posterior-anterior (PA) projection: patient 5 (panel A) and patient 7 (panel B). The LV ENDO BIP voltage maps (left) are normal in both patients. Patient 5 has extensive LV ENDO UNI low voltage involving the entire lateral and inferior LV walls (A, middle). There is a large region of corresponding LV EPI BIP low voltage seen (A, right) corresponding spatially with the ENDO UNI abnormality. Patient 7 has two confluent low UNI voltage regions at the basal-mid lateral and apical LV segments (panel B, middle). The corresponding LV EPI BIP map shows two corresponding low voltage areas (panel B, right). See text for further discussion.
Endocardial to Epicardial Distance
The mean overall distance from ENDO-EPI as assessed by electroanatomic mapping was 15.7±4.0 mm. There was no difference in the ENDO-EPI distance in the EPI+ vs. EPI- patients (16.0±3.0 vs. 15.0±5.4 mm, p=0.5).
Discussion
This study describes the detection of EPI substrate in LVCM using ENDO electroanatomical mapping in patients with normal endocardial bipolar voltage maps. Our data reveal regions of low LV ENDO UNI voltage in 82% of patients with confirmed epicardial scar as indexed by confluent area of low bipolar voltage with fractionated and late electrograms. Furthermore, the findings appeared to be specific in that the 5 patients with idiopathic VPDs or VT and normal EPI and ENDO bipolar voltage maps did not demonstrate any ENDO unipolar electrogram abnormalities. The close spatial correlation of the ENDO UNI and EPI BIP low voltage regions in the absence of ENDO BIP attenuation suggests that the UNI ENDO signals characterize tissue more remote from the ENDO.
Four of the eleven EPI+ patients had confluent regions of ENDO UNI low voltage that did not correlate with EPI BIP abnormalities. In fact, two EPI+ patients had larger ENDO UNI low voltage areas compared to the corresponding EPI BIP voltage. Cardiac magnetic resonance (MR) imaging was available in two of these four patients; both scans confirmed regions of mid-myocardial delayed enhancement directly adjacent to the ENDO UNI abnormality (Figure 3). Thus, the most likely explanation for the discordant ENDO UNI and EPI BIP low voltage is the presence of mid-myocardial substrate, which is detected better with ENDO UNI mapping than with either ENDO or EPI BIP mapping.
Figure 3.
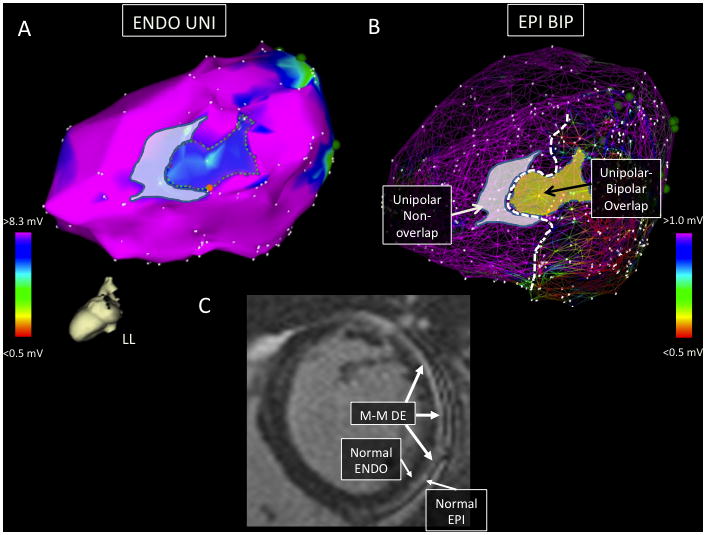
Images are taken from EPI+ patient 6. Panel A shows the LV ENDO UNI map in the left lateral (LL) projection with a confluent low voltage region involving the basal-mid lateral LV segment. The dotted region in panel A surrounds low UNI voltage directly overlapping a region of EPI BIP scar. The remaining low UNI voltage region in panel A (grey shading) did not have a corresponding area of BIP scar on the EPI map. Panel B shows a mesh overlay of the ENDO and EPI voltage maps with the ENDO UNI low voltage regions displayed (ENDO UNI and EPI BIP overlap region now shaded yellow). The dotted white line demarcates the apical extent of the EPI BIP voltage abnormality. Panel C shows a basal, short-axis image from the patient’s preoperative cardiac MR scan. There is a large region of predominantly mid-myocardial (M-M) delayed enhancement (DE) involving the anterolateral to the inferolateral LV and extending from the base to the mid-cavity. In this slice, normal ENDO and EPI enhancement (white arrows) are seen adjacent to the mid-myocardial scar. The shaded (non-overlapping) region of low ENDO UNI voltage likely represents detection of mid-myocardial scar with UNI mapping.
When we compared the mean ENDO UNI and BIP voltages in patients without structural heart disease to those with LVCM, only the UNI voltages differed significantly between the EPI+ and EPI- groups. It is likely that the UNI electrograms provide a larger “antenna” to detail a more complex three-dimensional substrate pattern commonly present in the patients with NICM who have VT. It also suggests that the UNI voltage difference is more than simply a threshold effect of the applied cutoff voltage.
Use of Unipolar Mapping in Post-Infarction Substrate
Several reports have examined the UNI voltage characteristics in chronic animal infarction models. 8–10 Based upon this previous work, the threshold values of unipolar voltage required to differentiate infarcted from normal tissue ranged between 6.2–10 mV. Our UNI voltage cut-off of 8.27 mV was determined in human subjects by examining voltage characteristics in normal hearts.
Field-of-View of Bipolar Voltage Mapping
Several publications as well as clinical experience have called into question the “field-of-view” of bipolar electrograms. One publication described that bipolar and unipolar EGM amplitude reduction (<1.5 and <6.5 mV, respectively) were predictive of the presence of delayed enhancement on MR in patients with infarct-related VT; however neither was useful in predicting the degree of infarct transmurality. 11 The same study found a >20% underestimation of the DE region on MR from the bipolar voltage map in one-third of cases.
A separate report noted significant variability in BIP EGM amplitude in post-infarction patients when the LV activation wavefront was altered using differential pacing maneuvers. 12 Bogun and colleagues demonstrated enhanced representation of DE regions on MR with combined EPI and ENDO bipolar mapping in LV cardiomyopathy patients. 13 These observations reinforce the importance of using adjunctive imaging modalities in characterizing nonischemic VT substrate.
Previous reports describing EPI substrate have focused on local BIP voltage characteristics that necessarily require epicardial access. Although epicardial mapping is often required when ablating LVCM-related VT, most operators do not empirically obtain percutaneous pericardial access in all cases. The UNI ENDO electrogram recording information may provide a valuable clue suggesting the presence of a probable epicardial substrate and the need to pursue epicardial mapping and ablation.
Limitations
This study includes a limited number of patients. We did not include patients with incomplete LV voltage maps both to avoid underestimation of low voltage regions and to represent all LV segments equally. This sampling technique has been well-established clinically for bipolar voltage mapping for more than a decade and has been correlated histopathologically with VT arrhythmia substrate. 14
The distinction between normal and abnormal ENDO UNI voltages is less dramatic than seen with ENDO BIP electrograms; this may represent a composite three-dimensional assessment of scar burden reflected by the unipolar electrogram. Additional investigation is warranted to correlate UNI electrogram characteristics with MR imaging in this patient population.
As indicated, an abnormal unipolar voltage map in the setting of normal bipolar voltage map does not guarantee the presence of an abnormal epicardial substrate. The presence of isolated mid-myocardial scar would be anticipated to also create lower unipolar ENDO voltage.
Conclusions
Our results suggest that UNI ENDO voltage can provide an indication of epicardial VT substrate in patients with LVCM with normal bipolar endocardial voltage.
Acknowledgments
Sources of Funding
Benoit Desjardins MD, PhD is supported by NIH grant K23 EB006481.
List of abbreviations
- VT
ventricular tachycardia
- LVCM
left ventricular cardiomyopathy
- BIP
bipolar
- UNI
unipolar
- ECG
electrocardiogram
- ENDO
endocardium
- EPI
epicardium
- PVC
premature ventricular contraction
- Hz
Hertz
- mV
millivolt
- ICD
implanted cardioverter-defibrillator
- cm
centimeter
Footnotes
Disclosures
Mathew D. Hutchinson, MD, Edward P. Gerstenfeld, MD, Rupa Bala, MD, Michael P. Riley, MD, Fermin C. Garcia, MD, Sanjay Dixit, MD, David Lin, MD, Wendy S. Tzou, MD, Joshua M. Cooper, MD, Ralph J. Verdino, MD, David J. Callans, MD, and Francis E. Marchlinski, MD receive research support from Biosense Webster unrelated to the content of this manuscript.
References
- 1.Hsia HH, Callans DJ, Marchlinski FE. Characterization of endocardial electrophysiological substrate in patients with nonischemic cardiomyopathy and monomorphic ventricular tachycardia. Circulation. 2003;108:704–710. doi: 10.1161/01.CIR.0000083725.72693.EA. [DOI] [PubMed] [Google Scholar]
- 2.Soejima K, Stevenson WG, Sapp JL, Selwyn AP, Couper G, Epstein LM. Endocardial and epicardial radiofrequency ablation of ventricular tachycardia associated with dilated cardiomyopathy: the importance of low-voltage scars. J Am Coll Cardiol. 2004;43:1834–1842. doi: 10.1016/j.jacc.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 3.Marchlinski FE, Callans DJ, Gottlieb CD, Zado E. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation. 2000;101:1288–1296. doi: 10.1161/01.cir.101.11.1288. [DOI] [PubMed] [Google Scholar]
- 4.Delacretaz E, Stevenson WG, Ellison KE, Maisel WH, Friedman PL. Mapping and radiofrequency catheter ablation of the three types of sustained monomorphic ventricular tachycardia in nonischemic heart disease. J Cardiovasc Electrophysiol. 2000;11:11–17. doi: 10.1111/j.1540-8167.2000.tb00728.x. [DOI] [PubMed] [Google Scholar]
- 5.Cano O, Hutchinson M, Lin D, Garcia F, Zado E, Bala R, Riley M, Cooper J, Dixit S, Gerstenfeld E, Callans D, Marchlinski FE. Electroanatomic substrate and ablation outcome for suspected epicardial ventricular tachycardia in left ventricular nonischemic cardiomyopathy. J Am Coll Cardiol. 2009;54:799–808. doi: 10.1016/j.jacc.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 6.Valles E, Bazan V, Marchlinski FE. ECG criteria to identify epicardial ventricular tachycardia in nonischemic cardiomyopathy. Circ Arrhythm Electrophysiol. 2010;3:63–71. doi: 10.1161/CIRCEP.109.859942. [DOI] [PubMed] [Google Scholar]
- 7.Sosa E, Scanavacca M, d’Avila A, Pilleggi F. A new technique to perform epicardial mapping in the electrophysiology laboratory. J Cardiovasc Electrophysiol. 1996;7:531–536. doi: 10.1111/j.1540-8167.1996.tb00559.x. [DOI] [PubMed] [Google Scholar]
- 8.Wolf T, Gepstein L, Dror U, Hayam G, Shofti R, Zaretzky A, Uretzky G, Oron U, Ben-Haim SA. Detailed endocardial mapping accurately predicts the transmural extent of myocardial infarction. J Am Coll Cardiol. 2001;37:1590–1597. doi: 10.1016/s0735-1097(01)01209-8. [DOI] [PubMed] [Google Scholar]
- 9.Kornowski R, Hong MK, Gepstein L, Goldstein S, Ellahham S, Ben-Haim SA, Leon MB. Preliminary animal and clinical experiences using an electromechanical endocardial mapping procedure to distinguish infarcted from healthy myocardium. Circulation. 1998;98:1116–1124. doi: 10.1161/01.cir.98.11.1116. [DOI] [PubMed] [Google Scholar]
- 10.Zheng Y, Fernandes MR, Silva GV, Cardoso CO, Canales J, Gahramenpour A, Baimbridge F, da Graca Cabreira-Hansen M, Perin EC. Histopathological validation of electromechanical mapping in assessing myocardial viability in a porcine model of chronic ischemia. Exp Clin Cardiol. 2008;13:198–203. [PMC free article] [PubMed] [Google Scholar]
- 11.Codreanu A, Odille F, Aliot E, Marie PY, Magnin-Poull I, Andronache M, Mandry D, Djaballah W, Regent D, Felblinger J, de Chillou C. Electroanatomic characterization of post-infarct scars comparison with 3-dimensional myocardial scar reconstruction based on magnetic resonance imaging. J Am Coll Cardiol. 2008;52:839–842. doi: 10.1016/j.jacc.2008.05.038. [DOI] [PubMed] [Google Scholar]
- 12.Brunckhorst CB, Delacretaz E, Soejima K, Maisel WH, Friedman PL, Stevenson WG. Impact of changing activation sequence on bipolar electrogram amplitude for voltage mapping of left ventricular infarcts causing ventricular tachycardia. J Interv Card Electrophysiol. 2005;12:137–141. doi: 10.1007/s10840-005-6549-z. [DOI] [PubMed] [Google Scholar]
- 13.Bogun FM, Desjardins B, Good E, Gupta S, Crawford T, Oral H, Ebinger M, Pelosi F, Chugh A, Jongnarangsin K, Morady F. Delayed-enhanced magnetic resonance imaging in nonischemic cardiomyopathy: utility for identifying the ventricular arrhythmia substrate. J Am Coll Cardiol. 2009;53:1138–1145. doi: 10.1016/j.jacc.2008.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deneke T, Muller KM, Lemke B, Lawo T, Calcum B, Helwing M, Mugge A, Grewe PH. Human histopathology of electroanatomic mapping after cooled-tip radiofrequency ablation to treat ventricular tachycardia in remote myocardial infarction. J Cardiovasc Electrophysiol. 2005;16:1246–1251. doi: 10.1111/j.1540-8167.2005.40826.x. [DOI] [PubMed] [Google Scholar]


