Abstract
The ability to predict a particular meal is achieved in part by learned associations with stimuli that predict nutrient availability. Ghrelin is an orexigenic peptide produced by both the gut and brain that rises before anticipated meals and it has been suggested that pre-prandial ghrelin increases may act as a signal to predict meal delivery. Here, we used wild type and ghrelin receptor deficient mice to test the hypothesis that ghrelin signaling is necessary for the processing of emotionally relevant stimuli, spatial learning and habituated feeding responses. We tested spatial and fear-related memory with the morris water maze and step through passive avoidance tests, respectively and utilized food anticipatory activity to monitor habituated feeding responses following two weeks of a meal feeding paradigm. Our results indicate that ghrelin signaling modulates spatial memory performance and is necessary for the development of food anticipatory activity. Collectively, these results suggest that ghrelin receptor signaling is necessary for adaptations in the anticipatory responses that accompany restricted feeding.
Keywords: Ghrelin Signaling, Meal Anticipation, Hippocampus, Learning
1.Introduction
An organism's ability to predict the onset of a meal is a vital function modified by nutrient status and environmental stimuli. The ability to predict a particular meal is achieved in part by learned associations with stimuli that predict nutrient availability (Petrovich et al., 2007, Sclafani et al., 1997, Woods et al., 2009) thus ensuring adequate restoration of energy homeostasis. In some situations, environmental cues associated with palatable foods induce feeding in calorically replete animals (Holland and Petrovich 2005, Petrovich and Gallagher 2007) referred to herein as “non-homeostatic” feeding. Taken together, these observations underscore the impact of learning on both homeostatic and non-homeostatic feeding behavior.
Restricted feeding schedules, in which food is present for only a few hours per day, are often used to induce food anticipatory activity (FAA) in rodents. Restricted feeding increases stress hormones (Poulin and Timofeeva 2008) and palatable foods can induce FAA in the absence of caloric need (Mendoza et al., 2005); suggesting that restricted feeding can be an emotionally relevant experience. Environmental cues associated with the delivery of a meal induce neuronal activation within both hypothalamic and extra-hypothalamic brain regions including the hippocampus, amygdala and frontal cortex (Holland and Gallagher 2004, Mendoza et al., 2005, Poulin and Timofeeva 2008) suggesting that meal anticipation may be influenced by cognitive processing.
Ghrelin is a 28 amino acid peptide that increases food intake (Tschop et al., 2000, Nakazato et al., 2001, Tolle et al., 2002) and gut motility (Masuda et al., 2000). Plasma ghrelin levels peak before expected meals in both humans and animals (Cummings et al., 2001, Drazen et al., 2006, Sugino et al., 2002) and drop postprandially (Tschop et al., 2001), suggesting that ghrelin may act as a meal initiation signal. Endogenous ghrelin signals through a seven transmembrane G coupled protein receptor (GHSR) that is constitutively active (Holst et al., 2003) and present in many brain regions including the hypothalamus, hippocampus, thalamus, and ventral tegmental area (VTA) (Guan et al., 1997, Zigman et al., 2006). Interestingly, ghrelin cell bodies have been identified within the hypothalamus (Cowely et al., 2003) and projections from these neurons are found within the septum and amygdala. In addition, ghrelin signaling within the hippocampus has been reported to modulate spine density and spatial learning (Diano et al., 2006). Thus, it is possible that ghrelin may initiate feeding through its actions on circuits which mediate cognition and reward.
In the current manuscript we tested the hypothesis that ghrelin signaling is necessary for the processing of emotionally relevant stimuli, and hippocampal-dependent learning. We further hypothesized that ghrelin mediates habituated feeding responses. This was achieved by investigating the performance of wild type or ghrelin receptor deficient (GHSR −/−) mice in step through passive avoidance and morris water maze paradigms. In addition, we utilized FAA to measure habituated feeding responses in wild type and GHSR −/− mice. Our results suggest that ghrelin receptor signaling is necessary for hippocampal dependent learning and habituated responding for food.
2.General Methods
2.1 Subjects
Ghrelin receptor null mice (GHSR −/−) and their wild type littermates (n=8/group) weighing 25–30 g were housed individually in a vivarium with a 12:12 light/dark schedule. The temperature of the room was maintained at 25° C. All animals had ad libitum access to standard chow diet (Teklad, 3.41 kcal/gm, 0.51 kcal/gm from fat) and water throughout the study unless otherwise noted. The GHSR −/− mice used in this study were a gift from Jeffery Zigman and Joel Elmquist at University of Texas Southwestern Medical Center in Dallas, TX and were generated as previously described (Zigman et al., 2005). All procedures were approved by the Institutional Animal Care and Use Committee at the University of Cincinnati and are in accordance with the guidelines set forth by the American Psychological Association.
2.2 Body Composition Analysis
Body composition was evaluated using a whole body NMR instrument (Echo-MRI, Waco, TX). Body composition analysis was obtained by placing each mouse into a clear Plexiglas tube and subsequently scanning them for 45 seconds. All mice were measured prior to the meal feeding protocol to determine baseline composition.
Method
2.3 Fear Potentiated Learning
One-trial learning, step-through passive avoidance behavior was measured according to Adler et al. (Adler et al., 1972). Briefly, mice were placed into a two sided chamber, one side was illuminated and the other side was dark. Upon being placed into the illuminated chamber mice were allowed to enter into the dark compartment. Since mice prefer dark to light, they normally entered within 5 s. Two additional trials were delivered on the following day. After the second trial, unavoidable mild electric footshocks (0.75 mA, 2 s) were delivered through the grid floor. After entering into the dark side of the chamber, the mice could not escape the footshock. After this single trial, the mice were immediately removed from the apparatus and placed into their home cage. The consolidation of passive avoidance behavior was tested 24 h later. In that testing session each mouse was placed into the illuminated chamber and the latency to enter the dark compartment was measured up to a maximum of 900 s.
2.4 Spatial Learning
Morris Water Maze: The MWM consisted of a circular fiberglass pool (122 cm diameter, 75 cm height; Rowland Fiberglass Inc., Ingleside, TX) filled with water (17–19 °C, 43 cm deep). A clear glass platform (10.5 cm×10.5 cm; square) was submerged 1 cm below the water surface. The pool was situated in a room that contained extramaze cues visible to the mice during testing (42 cm×76 cm posters printed with contrasting patterns and shapes). Latency to escape the water was calculated for each trial by overhanging digital video camera and computer controlled TopScan software (Cleversystem Inc., Reston, VA) and used as index of spatial learning and memory ability.
Fixed position platform
At the onset of each trial, an individual mouse (Wild type or GHS-R −/−) was placed into the water at one of four possible starting points (N, S, W, and E). The starting location for each trial was varied and all start locations were used in a given day. A trial was terminated and the latency was recorded when the mouse found and climbed onto the platform for 5 s If the mouse did not reach the platform within 1min, the trial was terminated, and the mouse was placed on the platform for 5 s. Each mouse received three trials per day, 30 min apart, for four consecutive days. Training started each day 1 h into the dark phase and was performed in a well-lit room. Each trial was digitally recorded for subsequent path analysis utilizing Cleversystem TopScan software (Reston, VA). On the 4th day a probe trial was performed where the hidden escape platform was removed from the pool and each animal was allowed to swim for 60 s. The amount of time spent in the quadrant of the pool where the platform had been located was quantified.
2.5 Conditioned locomotor activity
To determine if GHSR −/− mice were able to acquire feeding-induced increases in locomotor activity GHSR −/− mice, and their wild type littermates underwent a meal feeding regimen in which each animal was given chow plus water from 1200–1600h and water alone for the remainder of the day. Food intake was monitored daily in meal-fed mice and after 14 d, the meal-fed mice consumed the same amount of chow during the 4h access period as they did in 24h prior to testing. The point being that the mice had learned to adjust their intakes to account for 24h of calories in four hours. On test day (day 14) feeding responses were measured every thirty minutes for two hours of the four hour feeding session to observe differences in the initiation of feeding responses in each group. Each mouse had a home cage fitted with a locomotor activity monitoring unit (Lafayette Instruments, Lafayette, IN). Total activity for each animal was collected over a 24 hour period on days 1 and 14 beginning at 1000h. Conditioned locomotor activity was monitored between 1000–1200h on day 1 and day 14.
2.6 Statistical analysis
Data were analyzed using STATISTICA version 6.0 for PC's. All data were analyzed using analysis of variance (ANOVA) and LSD post-hoc comparisons were used to asses the source of significant main effects.
3.Results
3.1 Body Weight
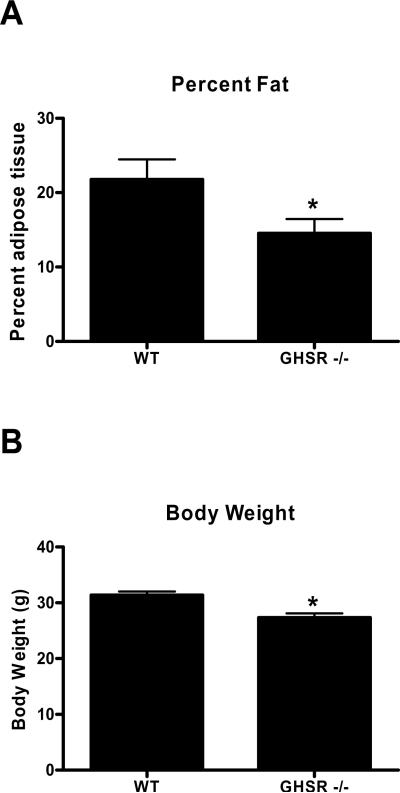
Mice lacking a functional ghrelin receptor (GHSR −/−) were leaner (F(1,13)=9.43, p<0.008) and weighed significantly less (F(1,12)=15.34, p<0.002) (Fig 1A–B) compared to their wild type littermates, suggesting that deletion of functional GHSRs has long term metabolic consequences.
Figure 1.
A) Body composition and B) body weight in wild type and GHSR −/− mice after 16 weeks on standard chow diet *=p<0.05.
3.2 Fear Potentiated Learning
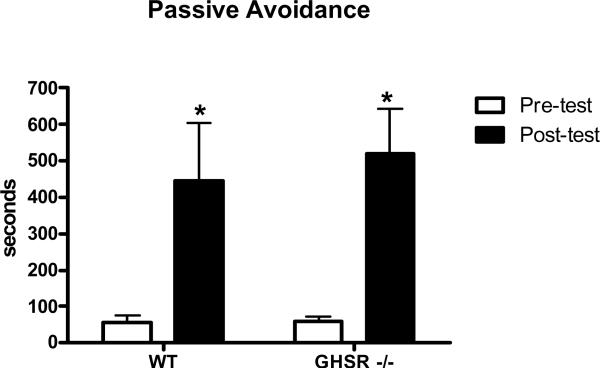
Next, we assessed the ability of GHSR −/− and wild type mice to acquire fear potentiated learning utilizing a step through passive avoidance procedure. Both GHSR −/− and wild type mice displayed significant delays in step through latencies (F(3,20)=15.22, p<0.01) Figure 2, suggesting that both groups learned to avoid the foot shock contingency.
Figure 2.
Passive avoidance learning represented by average step through latencies in wild type and GHSR −/− mice after 4 days of spatial learning training *=p<0.01.
3.3 Hippocampal Dependent Learning
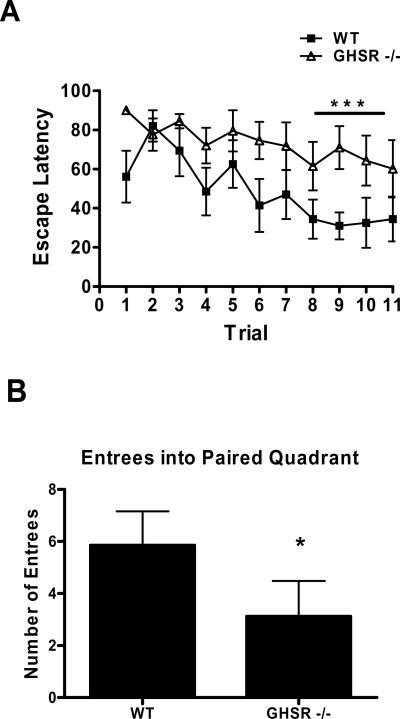
We then addressed hippocampal dependent learning in GHSR −/− mice. Three days of training, in which each mouse was allowed to independently navigate to a submerged platform within the MWM, induced significant decreases in escape latencies in wild type mice (p<0.01); however this effect was absent in GHSR −/− mice (Figure 3A). After training GHSR −/− mice were unable to efficiently locate the position where the platform had been as evidenced by a reduced number of entrees into the paired quadrant (Fig 3B), suggesting that GHSR −/− mice display deficiencies in the expression of spatial learning.
Figure 3.
A) Acquisition of spatial learning across eleven trials of training and B) expression of spatial learning on test day only represented as total number of entrees into paired quadrant in wild type and GHSR −/− mice *=p<0.01.
3.4 Food Anticipatory Behavior
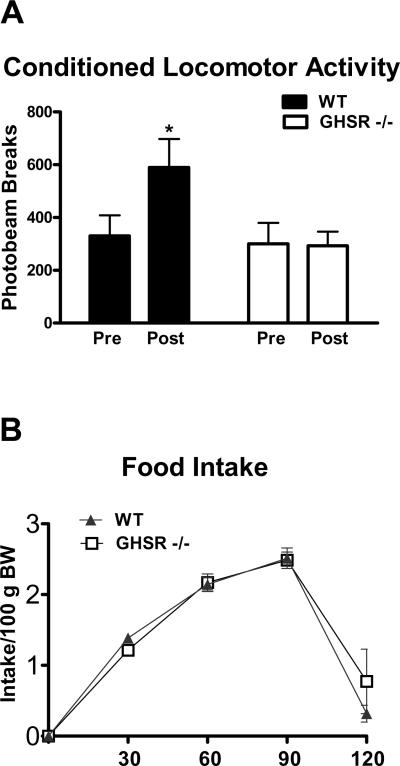
The meal feeding protocol employed here significantly increased anticipatory locomotor activity in wild type mice (p<0.01); however this effect was absent in GHSR −/− mice (Figure 4A). After training wild type and GHSR −/− mice consumed equal amounts of food. Specifically both groups consumed their daily ration of chow in the first two hours of the four hour feeding period (Fig 4B), suggesting that each group was capable of adapting intake levels to cope with the restricted feeding schedule.
Figure 4.
A) Food anticipatory activity and B) feeding responses in wild type and GHSR −/− mice after 14 days of meal feeding *=p<0.01.
4.Discussion
The goal of the current experiments was to test the hypothesis that ghrelin receptor signaling is necessary for the processing of emotionally relevant stimuli, hippocampal-dependent learning and food anticipation. Here, we report that mice lacking functional ghrelin receptors (GHSR −/−) display impaired hippocampal function and are unable to acquire anticipatory locomotor activity associated with restricted feeding. Importantly, the ability to anticipate meals prepares an organism for the consumption, absorption and metabolism of a caloric load (Woods 1991). This concept assumes that peripheral hormones which convey information relating to energy balance can access central circuitry responsible for eliciting feeding. Here, we assume that gastric ghrelin accessing central circuitry is responsible for the behavioral effects observed. However, future studies are required to rule out any contribution from the central ghrelin system. Meal onset is controlled by many factors including, pre-meal surges in metabolic hormones (Drazen et al., 2006), time of day, and learned associations with stimuli that predict meal delivery (Petrovich and Gallaher 2007, Sclafani et al., 1997, Woods 2009). In the context of learning, meal anticipation alone can drive food consumption independent of metabolic need, and this is exemplified by the ability of learned environmental cues to stimulate feeding in sated animals (Weingarten 1983, for review see Holland and Petrovich 2005). Moreover, environmental cues that signal the availability of palatable foods activate brain reward circuitry (Schroeder et al., 2001, Schlitz et al., 2007), an effect that presumably occurs through learning. Furthermore, the propensity of cues to induce feeding in periods of satiation suggests that learning may also be a significant contributing factor to the current rise in obesity in humans (Zheng and Berthoud 2007). Taken together, the ability to predict food availability can be modified by learning and yields both adaptive and maladaptive effects on food intake.
The hippocampus is one brain structure implicated in the regulation of learning and memory which also mediates feeding. For example, humans with hippocampal damage will initiate feeding only minutes after completing a meal (Hebben et al., 1985, Rozin et al., 1998). In addition, food sated animals with hippocampal lesions show increased appetitive responding relative to controls (Davidson and Jarrard 1993), suggesting that intact hippocampal function is necessary for the inhibitory control of food intake. Ghrelin receptors are present within the hippocampus, raising the possibility that ghrelin receptor signaling within hippocamapal neurons may mediate hippocampal function. This possibility was examined by investigating spatial learning in GHSR −/− mice. Here, we report that mice lacking a functional ghrelin receptor were unable to acquire normal spatial learning. Moreover, when tested after training, GHSR −/− mice displayed decreased retention of the spatial learning task. This finding is in agreement with previous studies which report that ghrelin binds to hippocampal neurons where it promotes long term potentiation, dendritic spine formation and enhanced spatial memory performance using a spontaneous alternation task (Diano et al., 2006). Our observations support these finding and in addition, suggest that signaling through the ghrelin receptor is necessary for hippocampal mediated behaviors.
It has been suggested that the hippocampus and amygdala represent two functionally distinct neuronal substrates in regards to their ability to modify learning and memory systems (Ito et al., 2005, Phillips and Le Doux 1992, Jeffery et al., 2004, LeDoux et al., 1990, Parkinson et al., 2000). The amygdala is hypothesized to integrate emotional experience with memory to gain control of future behaviors (Ito et al., 2005). Restricted feeding induces neuronal activation within the stress-associated brain regions and leads to an increased release of stress hormones at the predicted time of meal (Poulin and Timofeeva 2008) suggesting that reducing food availability can be an emotionally salient event. Ghrelin positive neurons exist within the hypothalamus, and these neurons project to the amygdala (Cowley et al., 2003). When administered directly into the amygdala, ghrelin modulates anxiolytic behavior (Carlini et al., 2003) suggesting that this hypothalamic-amygdalar projection is functionally relevant. Collectively, these observations led us to examine the possibility that ghrelin receptor signaling may regulate amygdala-dependent function. The GHSR −/− mice used here displayed normal step though latencies in the passive avoidance paradigm, suggesting that deletion of the ghrelin receptor had no effect on amygdalar-dependent learning. It is of interest to note here that both intracranial (Diano et al., 2006) and intra-amygdalar (Carlini et al., 2003) ghrelin administration augments step down passive avoidance latencies. One interpretation of these data is that ghrelin acts upon a yet to be identified receptor within the amygdala, thus deletion of this specific GHSR was without affect. It is also possible that in these studies, ghrelin spread to adjacent brain regions or in the case on third ventricular injections, acted synaptically through hypothalamic circuits which express GHSR and project to the amygdala (Cowely et al., 2003) to exert its effects of anxiety-like behavior. In either case, our results suggest that signaling through the GHSR does not alter amygdalar-dependent learning.
Food anticipatory activity (FAA) is one way to measure habituated feeding responses. When food availability is restricted to a few hours a day, rodents develop food anticipatory activity patterns in which increases in activity are detected within 1–3 hours prior to meal delivery. This behavior is thought to recapitulate foraging strategies that might be employed in the wild to procure a meal (Stephan et al., 2001). Functional anatomical studies suggest that FAA is controlled by a distributed set of nuclei comprised of the hippocampus, periventricular thalamic nucleus and the dorsomedial nucleus of the hypothalamus (Poulin and Timofeeva 2008). Ghrelin receptors are present within this septohippocampal-thalamo-hypothalamic circuitry hypothesized to mediate FAA (Guan et al., 1997) suggesting that ghrelin is capable of signaling within these regions. In the present study, wild type and GHSR −/− mice habitutated to the meal feeding regimen as evidenced by their ability to become calorically replete within a four hour timeframe. Additionally, anticipatory increases in locomotor activity were antecedent to the habituated feeding response. However, this behavioral anticipatory response was absent in GHSR −/− mice despite the fact that these mice initiated feeding normally thus suggesting that intact ghrelin signaling is necessary for FAA but not meal initiation. In terms of meal initiation, the only endogenous neural peptide which reliably initiates feeding is neuropeptide Y (NPY). Rodents are nocturnal animals and thus typically consume their largest meal at the onset of the dark period. Functional deletion of the NPY gene attenuates meal initiation during this period indicating that endogenous NPY signaling is required for meal onset under normal circumstances (Sindelar et al., 2005). Both ghrelin and centrally produced orexin activate NPY containing neurons in the arcuate nucleus (Wang et al., 2002, van den Top et al., 2004) thus it is possible that in the absence of functional ghrelin receptor orexin may be capable of activating NPY neurons and thus initiating the feeding response.
The finding that ghrelin signaling modulates meal anticipation is consistent with a recently published study which reported that GHSR-KO mice displayed attenuated FAA (Blum et al., 2009). In that study, GHSR-KO mice were capable of eliciting food anticipatory responding, but not to the same degree observed in wild type control mice. One difference between these studies is that the GHSR −/− mice and GHSR-KO mice were generated in different laboratories and were backcrossed to somewhat different strains to maintain the colony. Moreover, the GHSR −/− mice used here did not display FAA, that is, the locomotor pattern after fourteen days of meal feeding was not significantly different from activity levels prior to meal feeding, whereas in the former study the FAA activity levels were merely attenuated. It is possible that these differences are due to the strain differences present in the two different mouse models, or the method of monitoring FAA. However, it is clear that both studies confirm that ghrelin receptor signaling modulates FAA. Consistent with this notion is the observation that central ghrelin administration increases locomotor activity in ad libitum fed rodents (Jerlhag et al., 2006). Thus it is possible that apart from its effects on food consumption, pre-prandial rises in plasma ghrelin facilitate meal seeking behavior.
It is important to mention here that each of these findings could be source specific as we used a single genetic mouse model to examine each function. Thus, future studies are needed to confirm each of these findings in isolation as well as in combination with different methodologies to alter ghrelin receptor function.
In summary, the studies presented here suggest that ghrelin receptor signaling is required for hippocampal but not amygdalar dependent learning. In addition, we report that ghrelin receptor signaling mediates the increases in locomotor activity that precede meal delivery, but not habituated feeding under a restricted access regimen. It has been suggested that the initiation of feeding is controlled by indicators that reliably signal the presence of a meal (Woods 2009) which can be nutrient signals or environmental stimuli as well as stimuli associated with food itself (Holland and Gallagher 2004). Thus, cues which are learned and associated with the delivery of a particular meal represent an important aspect of feeding behavior, especially when the availability of food is limited. Collectively, these results suggest that ghrelin receptor signaling is necessary for adaptations in the anticipatory responses that accompany restricted feeding; perhaps through its ability to regulate learning.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ader R, Weinjen WAJM, Moleman P. Retention of passive avoidance response as a function of the intensity and duration of electric shock. Psychosom Sci. 1972;26:126–128. [Google Scholar]
- Abizaid A, Liu ZW, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116(12):3229–39. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20(1):1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- Blum ID, Patterson Z, et al. Reduced anticipatory locomotor responses to scheduled meals in ghrelin receptor deficient mice. Neuroscience. 2009;164(2):351–9. doi: 10.1016/j.neuroscience.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlini VP, Monzon ME, et al. Ghrelin increases anxiety-like behavior and memory retention in rats. Biochem Biophys Res Commun. 2002;299(5):739–43. doi: 10.1016/s0006-291x(02)02740-7. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37(4):649–61. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Purnell JQ, et al. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50(8):1714–9. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Jarrard LE. A role for hippocampus in the utilization of hunger signals. Behav Neural Biol. 1993;59(2):167–71. doi: 10.1016/0163-1047(93)90925-8. [DOI] [PubMed] [Google Scholar]
- Diano S, Farr SA, et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9(3):381–8. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- Drazen DL, Vahl TP, et al. Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology. 2006;147(1):23–30. doi: 10.1210/en.2005-0973. [DOI] [PubMed] [Google Scholar]
- Guan XM, Yu H, et al. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res. 1997;48(1):23–9. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- Hebben N, Corkin S, et al. Diminished ability to interpret and report internal states after bilateral medial temporal resection: case H.M. Behav Neurosci. 1985;99(6):1031–9. doi: 10.1037//0735-7044.99.6.1031. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala-frontal interactions and reward expectancy. Curr Opin Neurobiol. 2004;14(2):148–55. doi: 10.1016/j.conb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Holland PC, Petrovich GD. A neural systems analysis of the potentiation of feeding by conditioned stimuli. Physiol Behav. 2005;86(5):747–61. doi: 10.1016/j.physbeh.2005.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst B, Cygankiewicz A, et al. High constitutive signaling of the ghrelin receptor--identification of a potent inverse agonist. Mol Endocrinol. 2003;17(11):2201–10. doi: 10.1210/me.2003-0069. [DOI] [PubMed] [Google Scholar]
- Holst B, Schwartz TW. Constitutive ghrelin receptor activity as a signaling set-point in appetite regulation. Trends Pharmacol Sci. 2004;25(3):113–7. doi: 10.1016/j.tips.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Ito R, Everitt BJ, et al. The hippocampus and appetitive Pavlovian conditioning: effects of excitotoxic hippocampal lesions on conditioned locomotor activity and autoshaping. Hippocampus. 2005;15(6):713–21. doi: 10.1002/hipo.20094. [DOI] [PubMed] [Google Scholar]
- Jeffery KJ, Anderson MI, Hayman R, Chakraborty S. A proposed architecture for the neural representation of spatial context. Neurosci Biobehav Rev. 2004;(28):201–18. doi: 10.1016/j.neubiorev.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Dickson SL, Andersson M, Svensson L, Engel JA. Ghrelin stimulates locomotor activity and accumbal dopamine-overflow via central cholinergic systems in mice: implications for its involvement in brain reward. Addic Biol. 2006;1:45–54. doi: 10.1111/j.1369-1600.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Cicchetti P, et al. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990;10(4):1062–9. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda Y, Tanaka T, et al. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem Biophys Res Commun. 2000;276(3):905–8. doi: 10.1006/bbrc.2000.3568. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Angeles-Castellanos M, et al. Entrainment by a palatable meal induces food-anticipatory activity and c-Fos expression in reward-related areas of the brain. Neuroscience. 2005;133(1):293–303. doi: 10.1016/j.neuroscience.2005.01.064. [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409(6817):194–8. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Robbins TW, Everitt BJ. Dissociable roles for the central and basolateral amygdala in appetitive emotional learning. Eur J Neurosci. 2000;(12):405–13. doi: 10.1046/j.1460-9568.2000.00960.x. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Gallagher M. Control of food consumption by learned cues: a forebrain-hypothalamic network. Physiol Behav. 2007;91(4):397–403. doi: 10.1016/j.physbeh.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Holland PC, et al. Amygdalar and prefrontal pathways to the lateral hypothalamus are activated by a learned cue that stimulates eating. J Neurosci. 2005;25(36):8295–302. doi: 10.1523/JNEUROSCI.2480-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Ross CA, et al. Learned contextual cue potentiates eating in rats. Physiol Behav. 2007;90(2–3):362–7. doi: 10.1016/j.physbeh.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106(2):274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Poulin AM, Timofeeva E. The dynamics of neuronal activation during food anticipation and feeding in the brain of food-entrained rats. Brain Res. 2008;1227:128–41. doi: 10.1016/j.brainres.2008.06.039. [DOI] [PubMed] [Google Scholar]
- Schiltz CA, Bremer QZ, Landry CF, Kelley AE. Food-associated cues alter forebrain functional connectivity as assessed with immediate early gene and proenkephalin expression. BMC Biology. 2007;5:16. doi: 10.1186/1741-7007-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder BE, Binzak JM, et al. A common profile of prefrontal cortical activation following exposure to nicotine- or chocolate-associated contextual cues. Neuroscience. 2001;105(3):535–45. doi: 10.1016/s0306-4522(01)00221-4. [DOI] [PubMed] [Google Scholar]
- Sclafani A. Learned controls of ingestive behaviour. Appetite. 1997;29(2):153–8. doi: 10.1006/appe.1997.0120. [DOI] [PubMed] [Google Scholar]
- Sindelar DK, Palmiter RD, Woods SC, Schwartz MW. Attenuated feeding responses to circadian and palatability cues in mice lacking neuropeptide Y. Peptides. 2005;26:2597–2602. doi: 10.1016/j.peptides.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Stephan FK. The other circadian system: food as a zeitgeber. J Biol Rythms. 2001;17:284–292. doi: 10.1177/074873040201700402. [DOI] [PubMed] [Google Scholar]
- Sugino T, Hasegawa Y, et al. A transient ghrelin surge occurs just before feeding in a scheduled meal-fed sheep. Biochem Biophys Res Commun. 2002;295(2):255–60. doi: 10.1016/s0006-291x(02)00654-x. [DOI] [PubMed] [Google Scholar]
- Tolle V, Bassant MH, et al. Ultradian rhythmicity of ghrelin secretion in relation with GH, feeding behavior, and sleep-wake patterns in rats. Endocrinology. 2002;143(4):1353–61. doi: 10.1210/endo.143.4.8712. [DOI] [PubMed] [Google Scholar]
- Tschop M, Smiley DL, et al. Ghrelin induces adiposity in rodents. Nature. 2000;407(6806):908–13. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- Tschop M, Wawarta R, et al. Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest. 2001;24(6):RC19–21. doi: 10.1007/BF03351037. [DOI] [PubMed] [Google Scholar]
- Van den Top M, Lee K, Whyment A, Blanks A, Spanswick D. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nature Neurosci. 2004;7:493–494. doi: 10.1038/nn1226. [DOI] [PubMed] [Google Scholar]
- Wang L, Saint-Pierre D, Tache Y. Peripheral ghrelin selectively increases c-Fos expression in neuropeptide Y-synthesizing neurons in mouse hypothalamic arcuate nucleus. Neuroscience letters. 2002;31:47–51. doi: 10.1016/s0304-3940(02)00241-0. [DOI] [PubMed] [Google Scholar]
- Weingarten HP. Conditioned cues elicit feeding in sated rats: a role for learning in meal initiation. Science. 1983;220(4595):431–3. doi: 10.1126/science.6836286. [DOI] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36(2):229–40. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- Woods SC. The control of food intake: behavioral versus molecular perspectives. Cell Metab. 2009;9(6):489–98. doi: 10.1016/j.cmet.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SC. The eating paradox: how we tolerate food. Psychol Rev. 1991;98(4):488–505. doi: 10.1037/0033-295x.98.4.488. [DOI] [PubMed] [Google Scholar]
- Zheng H, Berthoud HR. Eating for pleasure or calories. Curr Opin Pharmacol. 2007;7(6):607–12. doi: 10.1016/j.coph.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, et al. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27(41):11075–82. doi: 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman JM, Nakano Y, et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest. 2005;115(12):3564–72. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]