Abstract
This descriptive study used stakeholder input to prioritize evidence-based strategies for improving depression care and to select incentives for mental health clinicians to adopt those strategies, and to conduct a feasibility test of an incentive-based program in a managed behavioral healthcare organization (MBHO). In two rounds of interviews and a stakeholder meeting, MBHO administrators and clinicians selected increasing combination treatment (antidepressant plus psychotherapy) rates as the program goal; and paying a bonus for case reviews, clinician feedback, and clinician education as incentives. We assessed program feasibility with case review and clinician surveys from a large independent practice association that contracts with the MBHO. Findings suggest that providing incentives for mental health clinicians is feasible and the incentive program did increase awareness. However, adoption may be challenging because of administrative barriers and limited clinical data available to MBHOs.
Keywords: Provider behavior change, incentives, mental health services, depression, quality improvement
Introduction
Despite the tremendous progress made toward improving the quality of care for depression (Dietrich et al., 2004; Katon et al., 1996; Katon et al., 2006; Katon et al., 1995; Korsen, Scott, Dietrich, & Oxman, 2003; Meredith, Jackson-Triche, Rubenstein, Camp, & Wells, 2000; Meredith et al., 2006; Rost, Fortney, Fischer, & Smith, 2002; Rubenstein et al., 2006; Schoenbaum et al., 2001; Sherbourne et al., 2001; Unützer et al., 2002; K. Wells et al., 2004; K. B. Wells et al., 2000), a wide gap remains between ideal care and usual practice (Institute of Medicine, 2001). Evidence-based treatments for depression may include antidepressant medication, psychotherapy (cognitive behavioral therapy or interpersonal therapy), and in some cases (i.e., when either form of therapy alone is insufficient) the combination of both medication and psychotherapy (Klerman, Weissman, Markowitz, et al., 1994; Jindal & Thase, 2005; Frank, Novick, & Kupfer, 2006; Keller, McCullough, Klein et al., 2000, US Department of Health and Human Services, 1999). In particular, combination treatment has been found helpful for moderate and severe depression.
One option for increasing the rate of effective mental health care is to provide incentives for clinicians to practice evidence-based care. However, most of the research on clinician incentives is based in primary care settings and relatively little is known about how to do so for mental health clinicians in managed behavioral healthcare organizations (MBHOs). A potential approach receiving increasing interest among managed care organizations, purchasers, and researchers is to use clinician financial incentives (Bailit Health Purchasing and Sixth Man Consulting, March 2002).
The “Quality Chasm” report (Institute of Medicine, 2001), suggested that current payment systems include many barriers inhibiting high-quality care and recommends that organizations build stronger quality improvement (QI) incentives into these systems. The effectiveness of incentives may depend on a number of factors, including whether the incentive is tied to the performance of individual clinicians vs. groups of clinicians; the amount of the incentive; and the interaction between performance-based payment systems and non-financial interventions (Balilit Health Purchasing and Sixth Man Consulting, March 2002).
Financial incentives may influence clinician performance in one of two ways. Clinicians may either be paid a higher price for providing a particular service or paid a bonus for meeting a specified target. Non-financial clinician incentives may also influence performance improvement (Balilit Health Purchasing and Sixth Man Consulting, March 2002) by linking good performance with non-monetary reward. Many of these approaches are based on social psychological models that predict behavior change through social influence from and comparison with peers, behavioral capacity (knowledge and skills), expectations, and how these factors interact with the practice environment (Ajzen & Fishbein, 1980; Bandura, 1986). For example, performance profiling may be a valuable QI tool if good performance data are available and it is presented to clinicians in a format that makes it easy for clinicians to compare their performance with their peers. Publicizing performance and practical interventions that alleviate clinician burden, such as eliminating administrative requirements or providing technical assistance for QI, may also motivate clinicians to provide more evidence-based care. Clinician education, while generally not sufficient alone for producing enduring behavior change (Davis, Thomson, Oxman, & Haynes, 1995), may reinforce and motivate good performance when paired with financial incentives.
Another critical factor for determining the success of interventions is whether they can be implemented in real world settings. Practical challenges include the potential for clinician resistance to performance monitoring and the complexity of implementing system-level changes to accommodate an incentive program. If QI programs fail because of clinician resistance, then designing programs that are informed by the clinicians who provide mental health services is likely to increase implementation success, particularly in conjunction with incentives. All of these questions about the use of stakeholder input and incentives are only recently being explored in the context of depression care in the managed behavioral health sector.
Only recently have programs emerged to align incentives in the treatment of depression. Most of these programs targeted primary care clinicians, for example the eight demonstration programs funded under the Robert Wood Johnson Foundation program Depression in Primary Care: Linking Clinical and System Strategies (Barry & Frank, 2006; Frank, Huskamp, & Pincus, 2003; Pincus, Pechura, Keyser, Bachman, & Houtsinger, 2006). These programs used a variety of approaches to change the economic and organizational environment to improve care for depression, including developing new payment and billing mechanisms as well as restructuring delivery systems to better target care (Feldman, Ong, Lee, & Perez-Stable, 2006; Grazier & Klinkman, 2006; Labby, Spofford, Robison, & Ralston, 2006; Thomas, Waxmonsky, McGinnis, & Barry, 2006). A recent study identified 24 programs that use pay-for-performance approaches in behavioral health (Bremer, Scholle, Keyser, Houtsinger, & Pincus, 2008). Of the 13 programs that targeted behavioral health specialists, nine were sponsored by behavioral health plans and only two focused specifically on depression as a target condition. The majority of the programs used group- or practice-level incentives, but a few offered incentives at the individual clinician level. The most common types of behaviors incentivized included achieving performance targets (i.e., 20% meeting the National Committee for Quality Assurance's Health Plan Employer Data and Information Set/HEDIS measures for antidepressant medication management). The types of incentives varied widely but only one program used a non-financial incentive (recognition of exemplary performance). None of these studies looked at incentivizing mental health clinicians to improve depression care.
Our study sought to identify a promising incentive-based program with potential to improve depression care by mental health specialty clinicians in a large MBHO and test the feasibility of implementation. In particular, we considered both financial and non-financial incentives and also direct involvement of key MBHO stakeholders in selecting and prioritizing incentives and evidence-based clinical behaviors to target in the intervention. We define evidence-based behaviors (e.g., practices) as preferential use of therapeutic interventions for which systematic empirical research has provided evidence of statistically significant effectiveness as treatments with a reasonable chance that the behavioral change will improve quality of care for depression. Our study involved three stages. First, we interviewed, benefits consultants to represent the purchaser perspective, and administrators and clinicians from the MBHO in two rounds – first asking them to identify the target of the QI program and second asking them to select incentives. Next, we convened a meeting of stakeholders in which they prioritized the program target and incentives. Lastly, we used case review and clinician surveys to test the feasibility of implementing such a program successfully within a MBHO.
Methods
Study Approach
We drew upon principles from community-based participatory research (CBPR) (Hohman & Shear, 2002; Lasker & Weiss, 2003; Minkler & Wallerstein, 2003). Our goal was to work collaboratively with the purchaser, MBHO, and clinician stakeholders in all stages of the research so that their values and perspectives were incorporated into the final QI program. We then tested the feasibility of implementing the program in a clinician group that contracts with the MHBO.
Community Partners
The study community partner was OptumHealth Behavioral Solutions (OHBS, formerly United Behavioral Health), the largest MBHO in the country. OHBS serves 2,531 large and small employers from different industries, health plans, and public sector entities with over 42 million members nationwide. Service products include employee assistance programs, behavioral health, and disability services. OHBS's clinical network has over 81,000 clinicians from multiple specialties and over 5,000 facilities with locations in every state. The study (described below) was implemented in a large regional Independent Practice Association (IPA) that contracts with OHBS. This IPA provides comprehensive behavioral health care through a network of 3,000 contracted clinicians serving a current membership of 1.4 million residents.
Study Design
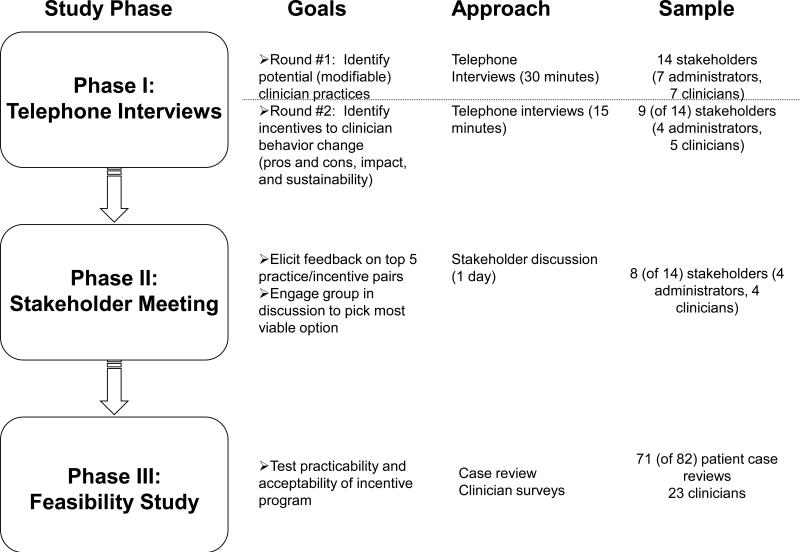
. The study had three phases as depicted in Figure 1. Phase I involved two rounds of semi-structured telephone interviews with stakeholders to elicit feedback about aspects of depression care needing improvement and types of incentives to pair with different behavior changes. Phase II, consisted of a stakeholder meeting to present the findings from the interviews and ask the stakeholders to identify the most promising clinician behaviors and the best incentive methods to pair with each behavior. Phase III involved working closely with the contracting IPA to implement a small feasibility study.
Figure 1.
Overview of Study Methods
Telephone Interviews
We identified 29 stakeholders to target for interviews. This was a purposeful sample to select as much variation as possible within each key targeted group (administrators, benefit consultant representatives, and specialty clinicians of all types (psychiatrists, psychologists, and masters-level therapists)). Stakeholders were recruited with the assistance of OHBS team members (FA and RB). Participants were recruited with an initial contact letter co-signed by researchers from all partnered institutions (UCLA, OHBS, and RAND) with follow-up phone contact two weeks later to confirm participation and schedule the interviews. The initial round of 30-minute semi-structured interviews (conducted from December 2004 through March 2005) used an open-ended format to identify professional characteristics, evidence-based clinician behaviors to improve depression care, accountability for care, and the advantages and disadvantages of the various strategies identified by the respondents (up to 5). Separate versions were tailored for the different stakeholder types. The second round of interviews (conducted in May and June 2005) involved 15-minute calls focused on the stakeholder's experience with clinician-based incentives, suggestions for which incentives might best be paired with each of the top five behaviors identified in the first round, and opinions about implementation barriers associated with different behavior-incentive approaches.
Stakeholder Meeting
In November 2005, we invited a subset of the interview participants to a daylong stakeholder meeting. Prior to the meeting, we distributed a “pre-work” packet that included a 1-page survey, to be returned in advance of the meeting. The survey asked participants to rank the top five behaviors and select one financial and one non-financial incentive for each behavior. Participants were paid a $500 honorarium and meals and travel were covered by the project funds.
After the research team made brief presentations to review the study and explain the meeting goals, the summary of pre-work survey results provided a launching point for the 5-hour working session (1 hour devoted to each of the five behavior-incentive pairs previously identified by the stakeholders). We then used formal group process methods (Fink, Kosecoff, Chassin, & Brook, 1984; National Institutes of Health, 1983) based on modified Delphi techniques (Dalkey, 1967, 1969; Helmer, 1966) to achieve consensus among stakeholders to select an evidence-based strategy for improving depression care that is feasible from both organizational and individual clinician perspectives. The stakeholders selected combination therapy with bonus payment plus feedback and education as the top-rated behavior-incentive pair (described in more detail below).
Feasibility Study
OHBS used behavioral health claims to identify patients with moderate to severe depression diagnoses and no record of receiving combination treatment. Those cases were forwarded to the IPA manager who contacted clinicians to request a case review and provide clinical information not available from claims (e.g., whether the patient had declined combination treatment previously or was in maintenance therapy, and a clinical assessment of whether combination treatment would be appropriate for the patient currently). Clinicians received a $10 bonus payment for submitting each case review form to the IPA. For patients identified as potentially benefiting from combination treatment (patients with moderate to severe depression diagnoses who did not appear to be receiving combination treatment), the patient's clinician was asked to encourage them to seek additional treatment. The IPA mailed patients information about combination treatment for depression and assisted with referrals and scheduling. We administered a post-program survey to clinicians via “Survey Monkey” on the Internet.
Results
Participant Characteristics
Of the 29 stakeholders identified for the first round of interviews, 14 could be reached and were available within the project time frame. Participants in the first round included four OHBS administrative staff known to 2 of the authors (RB & FA), 1 administrator, 2 quality/clinical education specialists, and 1 psychiatry medical director) , seven independent practice providers from the OHBS clinical panel unknown to the authors (1 psychiatrist, 2 psychologists, and 4 masters-level therapists) and 3 outside benefits consultants representing the employer perspective with no affiliation to OHBS. Participants represented 10 different states and eight were women. Nine of these individuals (4 administrators and 5 clinicians) also participated in the second interview round. Of the 14 who participated in the first interview, eight also participated in the panel meeting (3 administrators, 2 benefits consultants and 4 clinicians).
Telephone Interviews
The research team coded the clinical behaviors that were identified by participants in the first round of interviews into 10 categories, ranked them in terms of importance for improving depression care, and assigned priority by summing the rankings to identify the top five: 1) combination therapy (getting patients who need it into counseling and on an antidepressant medication), 2) patient education, 3) improving the quality of antidepressant medication management, 4) improving capacity for rapid access to care, and 5) clinician education.
In the second interview round, participants identified incentive strategies that could motivate clinicians to improve depression care. Consistent with incentive strategies outlined in the literature, this study identified two types of financial incentives (Table 1): paying a higher price for a service and paying a bonus if a target is met (Balilit Health Purchasing and Sixth Man Consulting, March 2002; Institute of Medicine, 2001). Three types of non-financial incentives were identified: social influence methods (Ajzen & Fishbein, 1980; Bandura, 1986), technical assistance, and reducing administrative requirements.
Table 1.
Classification of Incentives (Round #2 Interviews)
| Financial Incentives (e.g., Money) | Non-Financial Incentives (e.g., Resources and Opportunity) |
|---|---|
| 1. Pay a Higher Price for a Service | 1. Use Social Influence Methods (e.g., feedback, recognition, peer pressure) |
| A. Create new billing code | |
| B. Increase amount of reimbursement | A. Recognize through extra credential/autonomy |
| C. Pay more to increase access to psychiatrists | B. Provide feedback on patient outcomes (to clinicians) or on clinician outcomes (to patients) |
| 2. Pay a Bonus if a Target is Met | |
| A. Pay a bonus for incorporating patient education into treatment | 2. Provide Technical Assistance |
| A. CEU-based education/training | |
| B. Provide readily available information (free) through plan | |
| C. Access to preferred physicians | |
| 3. Reduce Administrative Requirements | |
| A. Remove authorization requirement | |
| B. Increase amount of approved sessions |
Stakeholder Meeting
Data from the pre-panel survey confirmed priority rankings and paired the incentives with each clinical behavior. Table 2 shows, for each of the clinical behaviors ranked in the top five, all incentives mentioned by at least one participant and the behaviors respondents linked to those interventions. For combination therapy, there were two dominant financial incentives (paying more to increase psychiatry access and bonus for patient education), each mentioned by three individuals. Five panel participants mentioned one dominant non-financial incentive--to provide feedback on patient/clinician outcomes.
Table 2.
Top 5 Behaviors and Selected Incentives*
| Type of Incentive | CT | AT | RA | PE | CE |
|---|---|---|---|---|---|
| Financial | |||||
| 1A: Create new billing code | 1 | 0 | 1 | 1 | 1 |
| 1B: Increase amount of reimbursement | 2 | 3 | 3 | 0 | 2 |
| 1C: Pay more to increase access to psychiatrists | 3 | 3 | 4 | 1 | 1 |
| 2A: Bonus for incorporating patient education into treatment | 3 | 3 | 1 | 7 | 4 |
| Non-Financial | |||||
| 1A: Provide feedback on patient or clinician outcomes | 5 | 4 | 1 | 1 | 1 |
| 1B: Recognize through extra credential/autonomy | 2 | 0 | 1 | 2 | 1 |
| 2A: CEU-based education/training | 0 | 0 | 0 | 1 | 5 |
| 2B: Provide readily available information (free) through plan | 0 | 1 | 0 | 4 | 2 |
| 2C: Access to preferred physicians | 1 | 4 | 4 | 0 | 0 |
| 3A: Remove authorization requirement | 1 | 0 | 3 | 1 | 0 |
| 3B: Increase amount of approved sessions | 0 | 0 | 0 | 0 | 0 |
Behaviors (columns) are ordered by rank (highest on the left): CT=Combination Therapy, AT=Antidepressant Treatment, RA=Rapid Access to Care, PE=Patient Education, CE=Clinician Education. Entries are the number of pre-panel survey respondents selecting each type of incentive. The highest ranked incentives for each of the behaviors are shown in italics.
At the end of the stakeholder meeting, the panel participants completed a post-discussion ranking form allowing them to reprioritize the five behavior-incentive pairs. Rankings (tallied by the research team and fed back to the stakeholders) ranged from a low of 16 (highest priority) to a high of 26 (lowest priority). The top two choices were antidepressant treatment and combination therapy. Based on these rankings , stakeholders decided to design a program to address both behavior-incentive pairs since antidepressant treatment is a component of combination therapy. The objective was to incentivize clinicians to consider combination therapy for their patients with moderate to severe depression who might benefit from the addition of either medications or psychotherapy and thereby increase the percentage of patients in combination treatment if after case review, the clinician recommended either adding an antidepressant medication if being seen solely by a mental health specialist or adding psychotherapy if being seen only by a psychiatrist. The financial incentives selected were paying clinicians a bonus for reviewing each patient case to identify current case status (not completely available from administrative claims data) and to determine whether patients might benefit from combination therapy. Non-financial incentives selected were providing feedback to the clinician about each patient case and educating therapists about combination treatment.
Feasibility Study
Eighty-nine patients were identified as candidates for combination treatment from behavioral health administrative claims for psychotherapy and/or medication management data during a 5-week period (May 19 through June 27, 2008). Sixty-four (72%) of these patients were women. The initial IPA administrative case review determined that seven patients were already in combination treatment with a psychotherapist and psychiatrist but the claims data had not yet been processed. Clinical case review forms were requested from the 23 clinicians (12 psychiatrists and 11 psychotherapists) treating the remaining 82 patients. Of the case review forms returned (n=71), 42 were candidates for combination therapy (36 did not have therapists and 6 did not have psychiatrists). This number is essentially 71 minus the 29 patients determined to already be on combination therapy. Of the 36 needing medication, 30 refused, it was not indicated for 2, and 4 received therapy referrals. Of the 6 needing therapy, 3 refused, and it was not indicated for 3. Due to budget constraints, we were limited to paying $10 per case review. Twenty (87%) responded and seventy-one (87%) of the forms were returned (59 or 87% from psychiatrists and 12 or 86% from therapists).
In summary, of these 71 case reviews, 29 (41%) patients were already receiving combination treatment through private pay or other health benefits. Among patients with monotherapy 33 (78%) declined the addition of either medications or psychotherapy for combination treatment , for five patients combination treatment was contraindicated, while four were referred for psychotherapy.
Half of the 20 clinicians (5 psychiatrists and 5 psychotherapists) who participated in the incentive program completed a survey (e.g., response rate was 50%). These clinicians treat patients that are predominantly covered by managed care and with private health insurance (Table 3). Most clinicians (60%) indicated that they liked the financial incentives for case review, but they also reported that the incentives would not have an impact on their delivery of care. A few clinicians noted that they should be held accountable for patient care but that financial incentives were not an appropriate method for ensuring accountability. Most (80%) reported that they would participate in another incentive program like this one.
Table 3.
Results of Clinician Survey (N=20)
| Practice Characteristics | N | % |
|---|---|---|
| Percent of patients not covered by managed care | N/A | 17 |
| Percent of patients with public health coverage | N/A | 5 |
| Number of managed care panels | 8 | N/A |
| Clinician Attitudes and Preferences | N | % |
| N and Percent of clinicians who agreed that: | ||
| Using incentives to improve care for depression is acceptable | 4 | 40 |
| Clinicians should be held accountable for care | 6 | 60 |
| Like receiving a bonus payment for each case review | 6 | 60 |
| Bonus motivated obtaining a patient referral for more treatment | 1 | 10 |
| Would prefer some other incentive | 1 | 10 |
| $10 payment per patient was sufficient | 3 | 30 |
| Would participate in another incentive program like this one | 8 | 80 |
Discussion
Our study is important because it contributes information about improving the care process through incentivizing clinician behavior change. Use of clinician incentives is a challenging topic and is understudied with specialty mental health clinicians. This article synthesized the literature in this area and provides lessons learned through the feasibility study. A major strength of the study was our partnership with the largest MBHO in the country. In addition, we incorporated a collaborative approach to engage the MBHO and provider community in the process of selecting key facets of a QI program, including the target and the incentives. The unique methods of staged interviews and consensus discussion served to identify a QI approach that had a high chance of success in terms of receptivity, ease of use, and maintenance over time. By designing and implementing a program that considers the opinions and attitudes of practicing clinicians, the program may have been more acceptable to those clinicians targeted for the behavior change. Additionally, by eliciting input from administrators and quality managers within the organization, changes can be better aligned with existing institutional structures and systems, making implementation more feasible. This is consistent with strategies for success identified in the pay for performance literature (HealthCare Benchmarks and Quality Improvement, 2004). Our study also contributed additional information about the use of both financial (bonus payment) and non-financial incentives (education and feedback) to individual clinicians to encourage improvement in care for depression. Previous work has primarily centered on financial incentives (Bremer et al., 2008).
Several key factors were considered to guide us in eliciting evidence-based behaviors to target and incentives for change. As a starting point, we targeted evidence-based behaviors that are likely to be adopted and that were expected to improve quality of care for depression. Behaviors and incentives were also identified based on how well they fit within the organizational culture and capability. For example, access to psychiatrists is limited within managed care because availability of psychiatrists is limited in clinical networks. Therefore, an intervention focused on increasing utilization of psychiatrist services would have been constrained by the number of available psychiatrists.
Stakeholders in this study may have selected and prioritized particular behaviors and incentives because QI initiatives that target HEDIS already focus on other issues related to quality of care (i.e. visits and refills) at the population level, yet none focus on evidence-based psychotherapy alone or on coordinating care for combination treatment at an individual level. Additionally, if the incentive pairing was successful, then it could also be developed into a HEDIS measure, particularly since accreditation is an important concern of MBHOs. Also, because payers are asking for greater integration of medical care with behavioral health care services, an incentive program such as this pilot could increase referrals between both systems of care.
These challenges may be insurmountable at the present time. Incentive-based approaches that pay for increasing referrals could raise legal concerns about kickbacks. In fact, an early proposal to provide monetary incentives for clinicians to refer patients to combination treatment was ruled out, due to concerns that direct clinician-to-clinician referrals sometimes are viewed as a conflict of interest among providers. Another challenge is having adequate data sources for screening. Even if medical and pharmacy data are accessible, it is still difficult to track use of care for those paying out of pocket. Out-of-plan utilization has become more common with the shortage of psychiatrists nationwide who are leaving MBHO panels and charging directly for services. Additionally, fragmented medical care often leads to fragmented, and therefore incomplete, data sources.
Our study did not produce much increase in combination therapy. This may be because the magnitude of the problem is not as great as we had thought, since many patients were actually receiving combination therapy or were on maintenance treatment schedules. Although at some point clinicians had discussed combination treatment with their patients, the majority of those eligible for combination treatment refused to add medication management or psychotherapy, at the time of the study. This study demonstrated that behavioral claims alone do not provide a full explanation of provider practice patterns as patients can receive care from providers not reimbursed through their behavioral health benefit or can simply decline any additional treatment; both of which are not captured in behavioral health claims. Future QI efforts would benefit by incorporating additional data sources like pharmacy and/or medical claims, as well as clinical information reflecting the process of care and patient treatment preferences.
The data from the feasibility study are limited for several reasons. The sample was small and captures only a brief window of time. Additionally, the study focused on administrative feasibility from the clinician perspective without attempting to engage directly patients suffering from depression. In addition, the incentive structures established are unlikely to work uniformly across individual clinicians and across settings as there is substantial evidence from Social Cognitive Theory and Goal Setting Theory (Bandura, 1986; Locke and Latham, 1990), suggesting that intrinsic and extrinsic motivation vary depending on both intra-personal and inter-personal factors. The large network that participated in this study has inherently different characteristics (practice structure, volume, etc.) relative to the solo practitioners and small practices making up the largest proportion of clinicians contracting with MBHOs. Thus, while the findings may generalize to other large group practices, they may not generalize to solo or small group practices. Further, even within this IPA, the sample may have been biased toward psychiatrists who responded at a higher rate relative to the general population. Psychiatrists account for only 18% of the IPA network but comprised 52% of incentive program participants. The validity of an expert survey is also uncertain given the potential to select clinical behaviors that are best suited to the personal treatment styles of stakeholders who participated. Another limitation of this study is that patient refusal was assessed via clinician self-report which could have been subject to socially desirable responding. Although the use of administrative claims data to identify cases in need of quality of care improvement allows for an efficient method of assessing care for large patient populations, claims have limited clinical information for an adequate assessment of quality of care because the diagnosis does not always accurately reflect the condition being treated, improvement in the course of treatment, or the patients’ satisfaction or preferences for treatment. In addition, since depression care can be provided in various settings (i.e. primary care and specialty care) the use of only behavioral claims and not medical/pharmacy claims also limited our ability to adequately assess the actual depression care received. Another limitation of using claims data is that they typically require a 90-day lag for processing by insurance companies which is challenging for real-time interventions. Finally, it is unclear from this study whether the psychotherapy being provided actually conforms to evidence-based practice. The scope of the present study was confined to understanding the process of implementing an incentive-based QI program but future work is sorely needed to better understand this issue of psychotherapy content. Accordingly, additional work is needed to understand the cost of implementing an incentive-based depression treatment program in a MBHO as we did not collect data on costs for this feasibility study.
Our study suggests that the level and/or availability of combination treatment within this IPA was good, although this only became apparent once patient cases were reviewed beyond what could be ascertained through behavioral claims data. It is uncertain whether the modest financial incentive would be sufficient to change clinician behavior to improve care but it did motivate clinicians to take a closer look at their patients’ treatment plan; a first step in addressing any potential gaps in care. Such an approach may subtly shape clinician behavior by simply increasing clinician awareness; and combined with non-financial incentives such as patient education, may hold promise with additional study.
Implications for Behavioral Health
Evidence-based strategies, such as collaborative care strategies for engaging behavior and practice change, are available for improving depression care. However, few studies have simultaneously addressed individual- and system-level fit of these interventions prior to implementation. Lessons from a span of literatures were incorporated in an effort to implement an intervention to improve depression care that is both acceptable to clinicians and that fits well within the organizational environment and culture. In the process of identifying and implementing a relatively small incentive program, numerous practical issues were encountered that are likely to arise in other incentive programs. Identifying and measuring specific desired changes in clinician behavior is difficult. In fact, administrative data can give a very incomplete picture of the prevalence of a clinician's practice pattern and of the reasons for its use or non-use. Funds to provide incentives or to administer programs are limited. Direct payments to improve quality of care are viewed with mixed feelings by clinicians and may require legal review. Implementing payment mechanisms and behavior monitoring systems may involve complex and costly administrative and IT systems changes. Changes in operational procedures and corporate reorganizations can place difficult competing demands upon both systems and technical staff required to implement incentive programs. As a result of these factors, incorporating incentive programs in the MBHO environment, while feasible, is likely to be challenging because of resource and administrative barriers and lack of adequate clinical data. Nevertheless, we were able to demonstrate that when an incentive was offered, providers took the time to review the treatment plan for those cases. Thus, the intervention did raise awareness which is the first step toward changing behavior and ultimately improved quality of care.
Acknowledgements
The study team thanks Michael Schoenbaum, Ph.D. for his early contributions to conceptualizing this work and Gery Ryan, Ph.D. for his guidance on the qualitative research processes used. The authors also wish to acknowledge Mindy Morefield, M.A. and Yuting Wong, B.A. for their able research assistance and Jony Weiss, M.P.H. for her panel facilitation skills. Participation by members of the OHBS community in all phases of the research study vastly improved the promise of QI for depression and are greatly appreciated for their contribution. The authors also thank Richard Rodriquez at OHBS and Randy Davis, PhD at College Health IPA for their guidance with decisions about implementation and Florence (Toni) Christopher for assistance with manuscript preparation.
This research was supported by a grant to Dr. Wells from NIMH (grant # P30MH068639). Earlier versions of this article were presented at the annual meeting of AcademyHealth, Boston, Massachusetts, June, 2005 and the Academy of Psychosomatic Medicine, Tucson, Arizona, November, 2006.
Contributor Information
Lisa S. Meredith, RAND Corporation 1776 Main Street Santa Monica, CA 90407-2138 Phone: (310) 393-0411 ext. 7365 FAX: (310) 850-8152 lisa_meredith@rand.org.
Robert B. Branstrom, Optum Health Behavioral Solutions 425 Market St. 14th floor San Francisco CA 94105 Phone: (415) 547-5530 Fax: (415) 547-5512 robert_b_branstrom@uhc.com.
Ruth Fikes, VP of Product Management and Compliance College Health IPA 17100 Pioneer Blvd., Suite 420 Cerritos, CA 90701 Phone: (800) 779-3825 Fax: (877) 349-1135 rfikes@chipa.com.
Francisca Azocar, Research and Evaluation Optum Health Behavioral Solutions 425 Market St. 14th floor San Francisco CA 94105 Phone: (415) 547-6148 Fax: (415) 547-5512 francisca_azocar@uhc.com.
Susan L. Ettner, David Geffen School of Medicine at UCLA Division of General Internal Medicine and Health Services Research 911 Broxton Plaza, Room 106 Box 951736 Los Angeles, CA 90095-1736 Phone: (310) 794-2289 Fax: (310) 794-0732 settner@mednet.ucla.edu.
References
- Ajzen I, Fishbein M. Understanding attitudes and predicting social behavior. Prentice-Hall; Englewood Cliffs, NJ: 1980. [Google Scholar]
- Bailit Health Purchasing and Sixth Man Consulting The Growing Case for Using Physician Incentives to Improve Health Care Quality, the National Health Care Purchasing Institute, December 2001. Mar, 2002.
- Bandura A. Social foundations of thought and action: A social cognitive theory. Prentice-Hall; Englewood Cliffs, NJ: 1986. [Google Scholar]
- Barry CL, Frank RG. Commentary: an economic perspective on implementing evidence-based depression care. Administration and Policy in Mental Health. 2006;33(1):21–25. doi: 10.1007/s10488-005-4234-2. [DOI] [PubMed] [Google Scholar]
- Bremer RW, Scholle SH, Keyser D, Houtsinger JV, Pincus HA. Pay for performance in behavioral health. Psychiatric Services. 2008;59(12):1419–1429. doi: 10.1176/ps.2008.59.12.1419. [DOI] [PubMed] [Google Scholar]
- Dalkey ND. Delphi. RAND Corporation; Santa Monica, CA: 1967. [Google Scholar]
- Dalkey ND. The Delphi method: An experimental study of group opinion. RAND Corporation; Santa Monica, CA: 1969. [Google Scholar]
- Davis DA, Thomson MA, Oxman A, D., Haynes RB. Changing physician performance: a systematic review of the effect of continuing medical education strategies. Journal of the American Medical Association. 1995;274:700–705. doi: 10.1001/jama.274.9.700. [DOI] [PubMed] [Google Scholar]
- Dietrich AJ, Oxman TE, Williams JW, Jr., Kroenke K, Schulberg HC, Bruce M, et al. Going to scale: re-engineering systems for primary care treatment of depression. Annals of Family Medicine. 2004;2(4):301–304. doi: 10.1370/afm.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman MD, Ong MK, Lee DL, Perez-Stable EJ. Realigning economic incentives for depression care at UCSF. Administration and Policy in Mental Health. 2006;33(1):34–38. doi: 10.1007/s10488-005-4233-3. [DOI] [PubMed] [Google Scholar]
- Fink A, Kosecoff J, Chassin M, Brook RH. Consensus methods: characteristics and guidelines for use. American Journal of Public Health. 1984;74(9):979–983. doi: 10.2105/ajph.74.9.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank RG, Huskamp HA, Pincus HA. Aligning incentives in the treatment of depression in primary care with evidence-based practice. Psychiatric Services. 2003;54(5):682–687. doi: 10.1176/appi.ps.54.5.682. [DOI] [PubMed] [Google Scholar]
- Frank E, Novick D, Kupfer DJ. Antidepressants and psychotherapy: A clinical research review. Focus. 2006;7:263–272. doi: 10.31887/DCNS.2005.7.3/efrank. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grazier KL, Klinkman MS. The economics of integrated depression care: the University of Michigan study. Administration and Policy in Mental Health. 2006;33(1):16–20. doi: 10.1007/s10488-005-4231-5. [DOI] [PubMed] [Google Scholar]
- HealthCare Benchmarks and Quality Improvement [December 15, 2008];Leapfrog offers web-based compendium of incentives [Electronic Version] 2004 http://findarticles.com/p/articles/mi_m0NUZ/is_9_11/ai_n6179865. from http://findarticles.com/p/articles/mi_m0NUZ/is_9_11/ai_n6179865.
- Helmer D. Social technology. Basic Books; New York, NY: 1966. [Google Scholar]
- Hohman AA, Shear KM. Community-based Intervention Research: Coping with the “Noise” of Real Life in Study Design. American Journal of Psychiatry. 2002;159:201–207. doi: 10.1176/appi.ajp.159.2.201. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine . Crossing the Quality Chasm: A New Health System for the 21st Century. National Academy Press; Washington DC: 2001. [PubMed] [Google Scholar]
- Jindal RD, Thase ME. Integrating Psychotherapy and Pharmacotherapy to Improve Outcomes Among Patients With Mood Disorders. Focus. 2005;3:114–121. doi: 10.1176/appi.ps.54.11.1484. [DOI] [PubMed] [Google Scholar]
- Katon W, Robinson P, Von Korff M, Lin E, Bush T, Ludman E, et al. A multifaceted intervention to improve treatment of depression in primary care. Archives of General Psychiatry. 1996;53(10):924–932. doi: 10.1001/archpsyc.1996.01830100072009. [DOI] [PubMed] [Google Scholar]
- Katon W, Unützer J, Fan MY, Williams JW, Jr., Schoenbaum M, Lin EH, et al. Cost-effectiveness and net benefit of enhanced treatment of depression for older adults with diabetes and depression. Diabetes Care. 2006;29(2):265–270. doi: 10.2337/diacare.29.02.06.dc05-1572. [DOI] [PubMed] [Google Scholar]
- Katon W, Von Korff M, Lin E, Walker E, Simon GE, Bush T, et al. Collaborative management to achieve treatment guidelines. Impact on depression in primary care. Journal of the American Medical Association. 1995;273(13):1026–1031. [PubMed] [Google Scholar]
- Keller MB, McCullough JP, Klein DN, Arnow B, Dunner Dl, Gelenberg, et al. A comparison of nefazodone, the cognitive behavioral analysis system of psychotherapy, and their combination for the treatment of chronic depression. The New England Journal of Medicine. 2000;342:1462–1470. doi: 10.1056/NEJM200005183422001. [DOI] [PubMed] [Google Scholar]
- Klerman GL, Weissman MM, Markowitz J, et al. Medication and psychotherapy. In: Bergin AE, Garfield SL, editors. Handbook of Psychotherapy and Behaivor Change. Raven; New York: 1994. [Google Scholar]
- Korsen N, Scott P, Dietrich AJ, Oxman T. Implementing an office system to improve primary care management of depression. Psychiatric Quarterly. 2003;74(1):45–60. doi: 10.1023/a:1021193606120. [DOI] [PubMed] [Google Scholar]
- Labby D, Spofford M, Robison J, Ralston R. The economics of depression in primary care: de-fragmentation in the Oregon Medicaid market. Administration and Policy in Mental Health. 2006;33(1):39–42. doi: 10.1007/s10488-005-4232-4. [DOI] [PubMed] [Google Scholar]
- Lasker RD, Weiss ES. Creating partnership synergy: The critical role of community stakeholders. Journal of Health Services Administration. 2003;26:119–139. [PubMed] [Google Scholar]
- Locke EA, Latham GP. A Theory of Goal Setting and Performance. Prentice-Hall; Englewood Cliffs, NJ: 1990. [Google Scholar]
- Meredith LS, Jackson-Triche MD, N., Rubenstein LV, Camp P, Wells KB. Quality improvement for depression enhances long-term treatment knowledge for primary care clinicians. Journal of General Internal Medicine. 2000;15(12):868–877. doi: 10.1046/j.1525-1497.2000.91149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith LS, Mendel P, Pearson M, Wu SY, Joyce G, Straus JB, et al. Implementation and maintenance of quality improvement for treating depression in primary care. Psychiatric Services. 2006;57(1):48–55. doi: 10.1176/appi.ps.57.1.48. [DOI] [PubMed] [Google Scholar]
- Minkler M, Wallerstein N. Community-based participatory research for health. Jossey-Bass; San Francisco, CA: 2003. [Google Scholar]
- National Institutes of Health . Consensus Development Program Staff: Assessing the NIH Consensus Development Program: Study Design. RAND Corporation; Santa Monica, CA: 1983. [Google Scholar]
- Pincus HA, Pechura C, Keyser D, Bachman J, Houtsinger JK. Depression in primary care: learning lessons in a national quality improvement program. Administration and Policy in Mental Health. 2006;33(1):2–15. doi: 10.1007/s10488-005-4227-1. [DOI] [PubMed] [Google Scholar]
- Rost K, Fortney J, Fischer E, Smith J. Use, quality, and outcomes of care for mental health: the rural perspective. Medical Care Research and Review. 2002;59(3):231–265. doi: 10.1177/1077558702059003001. discussion 266-271. [DOI] [PubMed] [Google Scholar]
- Rubenstein LV, Meredith LS, Parker LE, Gordon NP, Hickey SC, Oken C, et al. Impacts of evidence-based quality improvement on depression in primary care: a randomized experiment. Journal of General Internal Medicine. 2006;21(10):1027–1035. doi: 10.1111/j.1525-1497.2006.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum M, Unützer J, Sherbourne CD, Duan N, Rubenstein LV, Miranda J, et al. Cost-effectiveness of practiced-initiated quality improvement for depression: Results of a randomized controlled trial. Journal of the American Medical Association. 2001;286(11):1325–1330. doi: 10.1001/jama.286.11.1325. [DOI] [PubMed] [Google Scholar]
- Sherbourne CD, Wells KB, Duan N, Miranda J, Unutzer J, Jaycox L, et al. Long-term effectiveness of disseminating quality improvement for depression in primary care. Archives of General Psychiatry. 2001;58(7):696–703. doi: 10.1001/archpsyc.58.7.696. [DOI] [PubMed] [Google Scholar]
- Thomas MR, Waxmonsky JA, McGinnis GF, Barry CL. Realigning clinical and economic incentives to support depression management within a medicaid population: the Colorado access experience. Administration and Policy in Mental Health. 2006;33(1):26–33. doi: 10.1007/s10488-005-4229-z. [DOI] [PubMed] [Google Scholar]
- Unützer J, Katon W, Callahan CM, Williams JW, Jr., Hunkeler E, Harpole L, et al. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. Journal of the American Medical Association. 2002;288(22):2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services . Mental Health: A Report of the Surgeon General. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Mental Health Services, National Institues of Health, National Institutes of Mental Health; Rockville, MD: 1999. Ref Type: Report. [Google Scholar]
- Wells K, Sherbourne C, Schoenbaum M, Ettner S, Duan N, Miranda J, et al. Five-year impact of quality improvement for depression: results of a group-level randomized controlled trial. Archives of General Psychiatry. 2004;61(4):378–386. doi: 10.1001/archpsyc.61.4.378. [DOI] [PubMed] [Google Scholar]
- Wells KB, Sherbourne C, Schoenbaum M, Duan N, Meredith L, Unutzer J, et al. Impact of disseminating quality improvement programs for depression in managed primary care: a randomized controlled trial. Journal of the American Medical Association. 2000;283(2):212–220. doi: 10.1001/jama.283.2.212. [DOI] [PubMed] [Google Scholar]