Abstract
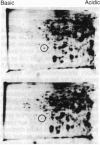
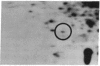
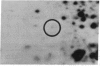
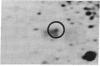
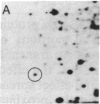
Our previous results indicated that protein synthesis was necessary for serotonin (5-hydroxytryptamine, 5-HT) to regulate the phase of the biological clock in the Aplysia eye. Also, we showed that 5-HT appeared to increase the synthesis of a 34-kDa protein with an isoelectric point of 7.2. Subsequent studies were carried out to quantitate the effect of 5-HT on the 34-kDa protein and to examine whether the 34-kDa protein was involved in the circadian timing system. The regional specificity of the effect of 5-HT on the 34-kDa protein was investigated. The proximal portion of the eye appeared to synthesize much more of the 34-kDa protein than the distal portion. Also, 5-HT had a much larger effect on the synthesis of the 34-kDa protein in the proximal portion than in the distal portion. The proximal location of synthesis and the 5-HT effect on the synthesis of the 34-kDa protein correlate with the proximal location of cells and processes that are necessary for the expression of the circadian rhythm. The relationship between the effect of 5-HT on the circadian rhythm and the effect of 5-HT on the 34-kDa protein was also examined. As 5-HT causes phase shifts in the rhythm by activating adenylate cyclase to increase cAMP, forskolin and 8-benzylthioadenosine 3',5'-cyclic monophosphate mimicked the effect of 5-HT on the 34-kDa protein. We also found that 5-HT significantly increased the synthesis of the 34-kDa protein at phases when 5-HT delays or advances the phase of the rhythm but did not increase the synthesis of the 34-kDa protein at a phase when 5-HT did not phase shift. This phase-dependent effect of 5-HT on the 34-kDa protein qualitatively accounts for the phase dependence of the effect of 5-HT on the circadian rhythm. These results, when considered together with our earlier data, suggest that the synthesis of the 34-kDa protein is directly involved in the phase shift produced by 5-HT. The 34-kDa protein is worthy of future investigation as a candidate for a component of the circadian oscillator.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Corrent G., Eskin A., Kay I. Entrainment of the circadian rhythm from the eye of Aplysia: role of serotonin. Am J Physiol. 1982 Mar;242(3):R326–R332. doi: 10.1152/ajpregu.1982.242.3.R326. [DOI] [PubMed] [Google Scholar]
- Corrent G., Eskin A. Transmitterlike action of serotonin in phase shifting a rhythm from the Aplysia eye. Am J Physiol. 1982 Mar;242(3):R333–R338. doi: 10.1152/ajpregu.1982.242.3.R333. [DOI] [PubMed] [Google Scholar]
- Corrent G., McAdoo D. J., Eskin A. Serotonin shifts the phase of the circadian rhythm from the Aplysia eye. Science. 1978 Dec 1;202(4371):977–979. doi: 10.1126/science.309655. [DOI] [PubMed] [Google Scholar]
- Eskin A., Corrent G., Lin C. Y., McAdoo D. J. Mechanism for shifting the phase of a circadian rhythm by serotonin: involvement of cAMP. Proc Natl Acad Sci U S A. 1982 Jan;79(2):660–664. doi: 10.1073/pnas.79.2.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskin A., Takahashi J. S. Adenylate cyclase activation shifts the phase of a circadian pacemaker. Science. 1983 Apr 1;220(4592):82–84. doi: 10.1126/science.6298939. [DOI] [PubMed] [Google Scholar]
- Eskin A., Yeung S. J., Klass M. R. Requirement for protein synthesis in the regulation of a circadian rhythm by serotonin. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7637–7641. doi: 10.1073/pnas.81.23.7637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman K. G., Strumwasser F. Regional specializations in the eye of Aplysia, a neuronal circadian oscillator. J Comp Neurol. 1984 Dec 20;230(4):593–613. doi: 10.1002/cne.902300408. [DOI] [PubMed] [Google Scholar]
- Jacklet J. W., Geronimo J. Circadian rhythm: population of interacting neurons. Science. 1971 Oct 15;174(4006):299–302. doi: 10.1126/science.174.4006.299. [DOI] [PubMed] [Google Scholar]
- Jacklet J. W. Neuronal circadian rhythm: phase shifting by a protein synthesis inhibitor. Science. 1977 Oct 7;198(4312):69–71. doi: 10.1126/science.897685. [DOI] [PubMed] [Google Scholar]
- Nadakavukaren J. J., Lickey M. E., Jordan W. P. Regulation of the circadian clock in the Aplysia eye: mimicry of neural action by serotonin. J Neurosci. 1986 Jan;6(1):14–21. doi: 10.1523/JNEUROSCI.06-01-00014.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Rothman B. S., Strumwasser F. Phase shifting the circadian rhythm of neuronal activity in the isolated Aplysia eye with puromycin and cycloheximide. Electrophysiological and biochemical studies. J Gen Physiol. 1976 Oct;68(4):359–384. doi: 10.1085/jgp.68.4.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamon K. B., Padgett W., Daly J. W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolum J. C., Strumwasser F. The differential effects of ionizing radiation on the circadian oscillator and other functions in the eye of Aplysia. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5542–5546. doi: 10.1073/pnas.77.9.5542. [DOI] [PMC free article] [PubMed] [Google Scholar]