Abstract
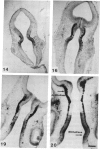
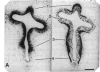
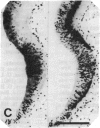
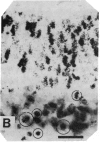
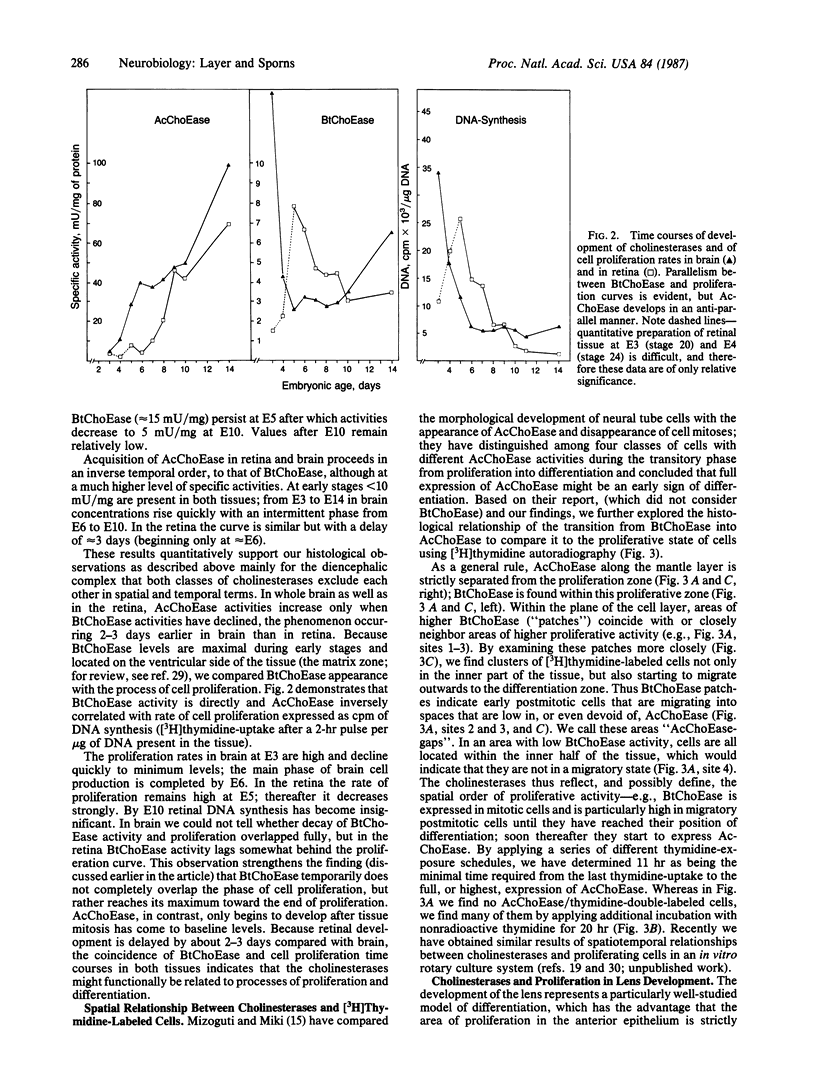
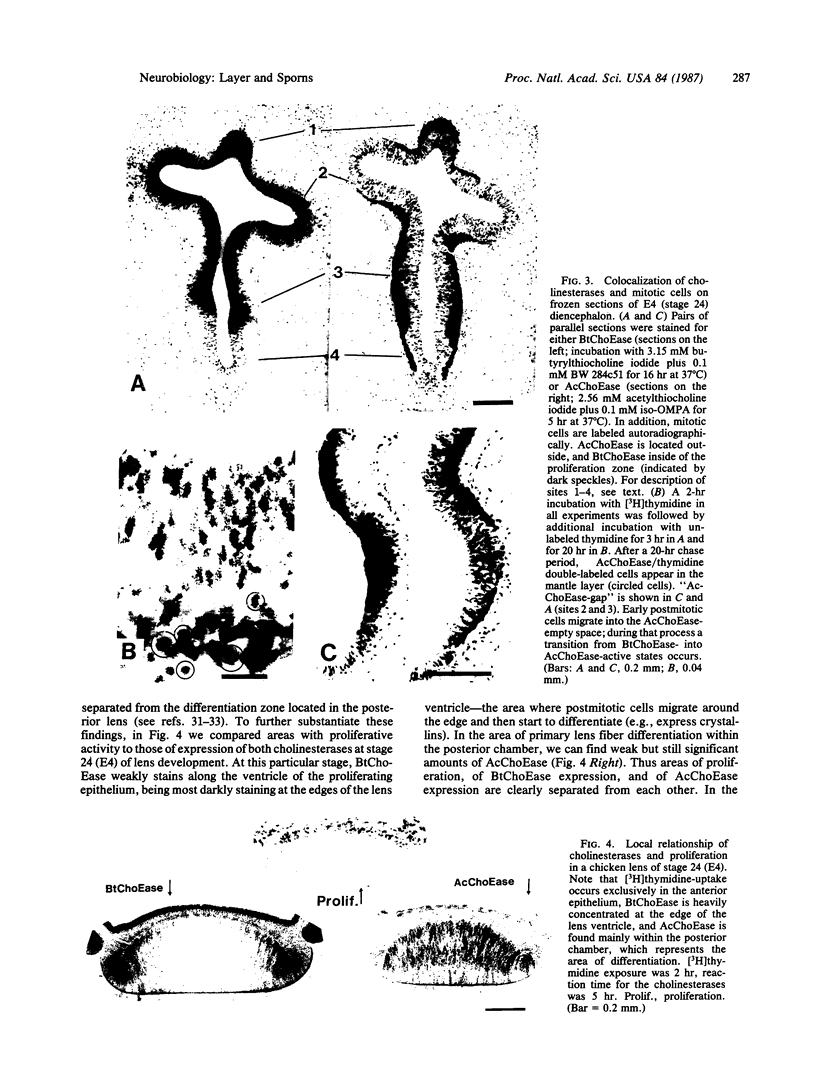
Close relationships between acetylcholinesterase (AcChoEase; acetylcholine acetylhydrolase, true cholinesterase, EC, 3.1.1.7) and butyrylcholinesterase (BtChoEase, acylcholine acylhydrolase, pseudocholinesterase, EC, 3.1.1.8) with cell proliferation were observed in the early chicken brain. These include the following: BtChoEase is transiently accumulating in patchy fashion on the ventricular side of the neuroepithelium shortly before AcChoEase appears in cell bodies along the opposing mantle layer. The amount of BtChoEase in retina and brain is greatest in the early phase (E3-E5, or incubation periods of 3-5 days); in retina it decreases about 2 days later than in brain. However, AcChoEase expression increases with time, in inverse order to that of BtChoEase. In both tissues decrease of cell proliferation is closely followed by decrease in BtChoEase. A double-labeling technique of cholinesterase staining together with [3H]thymidine autoradiography reveals proliferation zones that are diffusely stained by BtChoEase but not by AcChoEase. Patches intensely stained for BtChoEase accompany clusters of cells in final stages of mitosis on their way to the differentiation zone, where they begin expressing AcChoEase. By applying different thymidine pulses, we identify an 11-hr lag from the last thymidine-uptake to full AcChoEase expression. (iv) These findings are confirmed by studying lens development, where areas of proliferation and differentiation are well separated. The spatiotemporal pattern of the transition of neuroblasts from a proliferating into a differentiating state correlates with the expression of BtChoEase just before and during mitosis and that of AcChoEase about 11 hr after mitosis. Thus cholinesterases could be involved in the regulation of this transition.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bottenstein J. E., Sato G. H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979 Jan;76(1):514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubb I. W., Ranieri E., White G. H., Hodgson A. J. The enkephalins are amongst the peptides hydrolyzed by purified acetylcholinesterase. Neuroscience. 1983 Dec;10(4):1369–1377. doi: 10.1016/0306-4522(83)90118-5. [DOI] [PubMed] [Google Scholar]
- Coyle J. T., Price D. L., DeLong M. R. Alzheimer's disease: a disorder of cortical cholinergic innervation. Science. 1983 Mar 11;219(4589):1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- Drews U. Cholinesterase in embryonic development. Prog Histochem Cytochem. 1975;7(3):1–52. [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Falugi C., Raineri M. Acetylcholinesterase (AChE) and pseudocholinesterase (BuChE) activity distribution pattern in early developing chick limbs. J Embryol Exp Morphol. 1985 Apr;86:89–108. [PubMed] [Google Scholar]
- Fitzpatrick-McElligott S., Stent G. S. Appearance and localization of acetylcholinesterase in embryos of the leech Helobdella triserialis. J Neurosci. 1981 Aug;1(8):901–907. doi: 10.1523/JNEUROSCI.01-08-00901.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel A. M., Hickey T. L. Chemospecificity of ontogenetic units in the striatum: demonstration by combining [3H]thymidine neuronography and histochemical staining. Proc Natl Acad Sci U S A. 1982 Jan;79(1):198–202. doi: 10.1073/pnas.79.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel A. M., Ragsdale C. W., Jr Pseudocholinesterase staining in the primary visual pathway of the macaque monkey. Nature. 1982 Sep 30;299(5882):439–442. doi: 10.1038/299439a0. [DOI] [PubMed] [Google Scholar]
- Illing R. B., Graybiel A. M. Convergence of afferents from frontal cortex and substantia nigra onto acetylcholinesterase-rich patches of the cat's superior colliculus. Neuroscience. 1985 Feb;14(2):455–482. doi: 10.1016/0306-4522(85)90303-3. [DOI] [PubMed] [Google Scholar]
- KARNOVSKY M. J., ROOTS L. A "DIRECT-COLORING" THIOCHOLINE METHOD FOR CHOLINESTERASES. J Histochem Cytochem. 1964 Mar;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- Koelle W. A., Koelle G. B., Smyrl E. G. Effects of persistent selective suppression of ganglionic butyrylcholinesterase on steady state and regenerating levels of acetylcholinesterase: implications regarding function of butyrylcholinesterase and regulation of protein synthesis. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2936–2938. doi: 10.1073/pnas.73.8.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Layer P. G. Comparative localization of acetylcholinesterase and pseudocholinesterase during morphogenesis of the chicken brain. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6413–6417. doi: 10.1073/pnas.80.20.6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchbanks R. M. Biochemistry of Alzheimer's dementia. J Neurochem. 1982 Jul;39(1):9–15. doi: 10.1111/j.1471-4159.1982.tb04695.x. [DOI] [PubMed] [Google Scholar]
- Massoulié J., Bon S. The molecular forms of cholinesterase and acetylcholinesterase in vertebrates. Annu Rev Neurosci. 1982;5:57–106. doi: 10.1146/annurev.ne.05.030182.000421. [DOI] [PubMed] [Google Scholar]
- Pannese E., Luciano L., Iurato S., Reale E. Cholinesterase activity in spinal ganglia neuroblasts: a histochemical study at the electron microscope. J Ultrastruct Res. 1971 Jul;36(1):46–67. doi: 10.1016/s0022-5320(71)80088-6. [DOI] [PubMed] [Google Scholar]
- Ramakrishna T. V., Balasubramanian N. Spectrophotometric determination of nitrite. Z Gesamte Hyg. 1984 Aug;30(8):467–468. [PubMed] [Google Scholar]
- Richards G. M. Modifications of the diphenylamine reaction giving increased sensitivity and simplicity in the estimation of DNA. Anal Biochem. 1974 Feb;57(2):369–376. doi: 10.1016/0003-2697(74)90091-8. [DOI] [PubMed] [Google Scholar]
- Smith A. D., Wald N. J., Cuckle H. S., Stirrat G. M., Bobrow M., Lagercrantz H. Amniotic-fluid acetylcholinesterase as a possible diagnostic test for neural-tube defects in early pregnancy. Lancet. 1979 Mar 31;1(8118):685–688. doi: 10.1016/s0140-6736(79)91144-9. [DOI] [PubMed] [Google Scholar]
- Soreq H., Zevin-Sonkin D., Avni A., Hall L. M., Spierer P. A human acetylcholinesterase gene identified by homology to the Ace region of Drosophila. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1827–1831. doi: 10.1073/pnas.82.6.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennyson V. M., Brzin M. The appearance of acetylcholinesterase in the dorsal root neuroblast of the rabbit embryo. A study by electron microscope cytochemistry and microgasometric analysis with the magnetic diver. J Cell Biol. 1970 Jul;46(1):64–80. doi: 10.1083/jcb.46.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer G., Layer P. G. An in vitro model of proliferation and differentiation of the chick retina: coaggregates of retinal and pigment epithelial cells. J Neurosci. 1986 Jul;6(7):1885–1896. doi: 10.1523/JNEUROSCI.06-07-01885.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer G., Layer P. G., Gierer A. Reaggregation of embryonic chick retina cells: pigment epithelial cells induce a high order of stratification. Neurosci Lett. 1984 Jul 27;48(2):191–196. doi: 10.1016/0304-3940(84)90018-1. [DOI] [PubMed] [Google Scholar]