Abstract
The aim of this work is to explore the efficacy , safety, and patients' satisfaction of laparoscopic uterosacral nerve ablation (LUNA) in relief of pain in women with chronic pelvic pain in whom diagnostic laparoscopy reveals either no pathology or mild endometriosis (AFS score ≤5). The study was a prospective, single-blind, randomized trial with 12 months follow-up. It was conducted at the endoscopy unit of the Gynecology Department of El Minia University Hospital, Egypt. One hundred ninety Egyptian women consented to participate in the study. These eligible patients were randomized using computer-generated tables and were divided into two equal groups, including the control group (diagnostic laparoscopy with no pelvic denervation) and the study group (diagnostic laparoscopy plus LUNA). Diagnostic laparoscopy with or without laparoscopic uterosacral nerve ablation was done. There were no statistically significant difference between both groups regarding the efficacy and the overall success rate (between group I and group II, it was 77.64%, 76.47%, and 74.11% versus 79.06%, 75.58%, and 73.25% at 3, 6, and 12 months, respectively) and the cumulative patients' satisfaction rate (it was 74.11%, 74.11%, and 71.76% versus 75.58%, 75.58%, and 72.09% at 3, 6, and 12 months between group I and group II, respectively; P ≤ 0.05). There was no statistically significant difference between both groups as regards the effectiveness of LUNA in the treatment of primary (spasmodic) and secondary (congestive) dysmenorrhea (P ≤ 0.05), while there was a statistically significant difference between both groups in the treatment of dyspareunia (P ≥ 0.05). LUNA can be a last alternative option in well-selected patients for control of chronic pelvic pain without endometriosis; however, its effectiveness may not extend to other indications. Also, preliminary experience in the treatment of primary deep dyspareunia presents a promising perspective on the management of deep dyspareunia, especially if it will involve a team of social, psychological, and gynecological specialists.
Keywords: Chronic pelvic pain, LUNA, Dysmenorrhea, Laparoscopy, Dyspareunia
Background
Chronic pelvic pain (CPP) can be defined as intermittent or constant pain in the lower abdomen or pelvis of at least 6 months’ duration, not occurring exclusively with menstruation or intercourse, and not associated with pregnancy; it is a symptom, not a diagnosis and dysmenorrhea, deep dyspareunia, and intermenstrual pain constitute its main symptom complex [1]. CPP is one of the commonest symptomatology in gynecological outpatient clinics. It accounts for 10% of office visits to gynecologists, general clinics, and about a quarter of outpatient consultations in general gynecological practice and for 40–60% of all diagnostic laparoscopies [2]. In our department, it varies from 40–46% of all diagnostic laparoscopy annually. CPP has a profound impact on a woman’s personal health and quality of life, including an economic impact through loss of working hours [1].
Laparoscopy is a valuable tool in the evaluation of undiagnosed CPP, as it can establish a definite diagnosis and modify the treatment without resorting to exploratory laparotomy [3]. In the absence of pathology, there is no established treatment, therefore, treatment is sometimes unsuccessful, and hysterectomy often becomes the final resort. Therefore, a conservative surgery, if shown to be effective, would represent a major improvement in its management [1]. Recent developments in minimal access surgery using laparoscopy make ablation of the nerve plexuses and ganglions in the uterosacral ligaments (laparoscopic uterosacral nerve ablation (LUNA)) a practicable treatment option [4].
Some recent reports of randomized controlled studies have clarified some roles of LUNA in the control of pelvic pain [5, 6] ,but systematic review and recent Cochrane reviews [7, 8] have shown that the currently available research evidence on LUNA is inconclusive, and therefore, further research is required among patients in different communities with different demographic and clinical characteristics to generate effectiveness evidence in the form of a high-quality, randomized, controlled trial to assess the principal hypothesis that, in women with chronic pelvic pain in whom diagnostic laparoscopy reveals either no pathology or mild endometriosis (American Fertility Society (AFS) score ≤5), LUNA alleviates pain and improves life quality [4].
Aim of the work
The primary aim of this work is to explore the efficacy and the safety of LUNA in the relief of pain in women with CPP in whom diagnostic laparoscopy reveals either no pathology or mild endometriosis (AFS score ≤5). The secondary aim was to evaluate the patients' satisfaction from the procedure during 3, 6, and 12 months follow-up, and to test the hypothesis that response to LUNA differs according to type of the pain by two secondary analyses: (1) women with primary or secondary dysmenorrhea, and (2) women with deep dyspareunia.
Patients and methods
This prospective, randomized, controlled trial was conducted at the endoscopy unit of the Gynecology Department at El Minia University Hospital, Egypt between the period of June 2004 to December 2008 with single-blind assessment of outcomes in eligible consenting patients randomized at diagnostic laparoscopy to LUNA (study group) or to no pelvic denervation (control group).
Eligibility
All new patients (280 women) who presented to the Gynecology outpatient clinic with pelvic pain (cyclical or noncyclical) and/or dyspareunia and who required diagnostic laparoscopy for evaluation of these conditions were invited to participate. When they consented to participate, they were registered prior to randomization (registered patients). Only 190 patients were eligible to randomization into one of the study groups according to strict inclusions criteria, and the study protocol was in concordance with the protocol guidelines of the LUNA Trial Collaboration [3] (Fig. 1).
Inclusion criteria
Fig. 1.
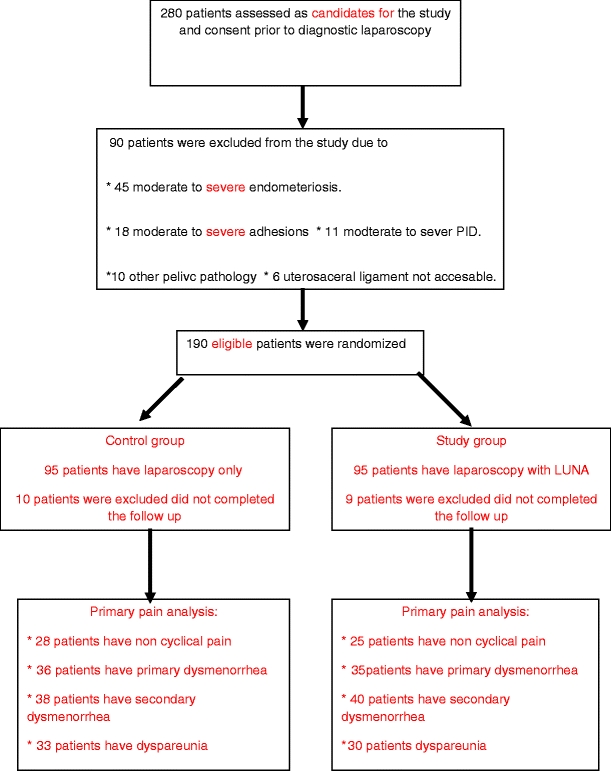
Flow chart of the study protocol
Patients with pelvic pain of longer than 6 months in duration, that was located within the true pelvis or between and below the anterior iliac crests, associated with functional disability, did not respond to medical treatment, and planned diagnostic laparoscopy revealed no pelvic pathology were considered as eligible patients for the study.
-
2.
Exclusion criteria
Patients with previous LUNA, moderate and severe endometriosis (AFS score >5), previous surgery for endometriosis or for pelvic inflammatory disease, previous hysterectomy, and adnexal pathology were excluded from the study.
Eligible patients were thoroughly counseled, and informed consent was taken from all the patients. Complete history was taken, including the patients' present symptoms, past medical history, medication used, known allergies, and previous similar attack(s), and method(s) of treatment. Detailed, general, abdominal, and pelvic examinations were performed, including a vaginal examination using sterile speculum. Cervical cytology, pelvic and vaginal ultrasound was done for all patients. At this point, the women were randomly assigned to diagnostic laparoscopy to start therapy according to protocol that has been approved by the medical ethical committee of the department.
Randomization
Consenting patients (280 patients) were subjected to diagnostic laparoscopy first, and in the same sitting, consenting eligible patients (190 patients) were randomized using computer-generated tables into two equal groups, including the control group (diagnostic laparoscopy with no pelvic denervation) and the study group (diagnostic laparoscopy plus uterosacral nerve ablation).
The patients were allocated to groups using a chance procedure, blocking, and stratification. Stratified block randomization was employed to ensure that there was nearly equal numbers of patients in the two groups within the prognostic subgroups, even if the study ends prematurely so that a chance imbalance in the stratification variable does not have an effect on the outcome.
Blinding
In this study, patients were kept blind to their treatment allocation until the follow-up in the trial was completed to avoid the placebo effect that may occur if they know they have received the active treatment. Unfortunately, single-blind approach was used, as it was difficult to keep the surgeon blind to the procedure.
Interventions
Routine preparation was made for a diagnostic laparoscopy with the patient under general anesthesia. Following pneumoperitoneum, a laparoscope was used to visualize the pelvis. Before embarking on operative laparoscopy, an anatomical pelvic assessment was performed to identify pelvic structures and pathology. At this stage, patients with pathology outlined in the exclusion criteria were excluded. It is expected that around 30% to 50% of women will be unsuitable for LUNA at operation and were “registered only” cases. Eligible patients were randomized into one of the study groups: first group, the control group, laparoscopy without pelvic denervation, and the second group, the study group, laparoscopy with uterosacral nerve ablation.
The posterior leaf of the broad ligament was carefully inspected to identify the course of the ureters, which, on rare occasions, could be particularly close to the uterosacral ligaments. Care was also taken to note thin-walled pelvic veins, which often lie lateral to the uterosacral ligaments. If accidentally punctured, they may cause troublesome bleeding, requiring further endoscopic endocoagulation. The uterosacral ligaments were identified by manipulation of the uterus in the right and left lateral planes. Clear identification of the uterosacral ligaments was a prerequisite for treatment, and ablation of the ligament was carried out using 5-mm bipolar electrodiathermy using bipolar Meyerland forceps (the main unit of the diathermy is adjusted at 30 W, and energy is applied for 5 s in order to deliver a dose power of coagulation 150 J to every uterosacral ligament), and then complete transaction of the uterosacral ligaments was done using a 5-mm curved scissors supplied with the ability to use monopolar electrodiathermy if needed. The ablation was started as close to the posterior aspect of the cervix as possible and continued for a minimum of 1 cm posterolaterally on either side. The aim of the procedure was to destroy the sensory nerve fibers and the secondary ganglia as they leave the uterus and come to lie within the uterosacral ligaments [3]. Patients with moderate pelvic adhesion obliterating the douglas pouch or making access to either uterosacral ligament difficult were excluded from the study.
Following surgery, the surgeon filled the operation details on a post-surgery form, including the laparoscopic finding, technique, the operative time, intraoperative blood loss, and intraoperative and postoperative complications.
Follow-up protocol and statistical analysis
All patients were followed up after 3, 6, and 12 months after the ablation. Follow-up visits included: history taking, clinical examination, and ultrasound (abdominal and vaginal) examination. The effectiveness of the procedure was estimated using a 10-cm visual analog scale (VAS), anchored at one end as no pain at all, and at the other as the worst imaginable pain. Also, the VAS ratings were obtained at 3, 6, and 12 months for each of the types of pain: noncyclical pain (pain at any other time other than during periods or during intercourse), primary (spasmodic) dysmenorrhea, secondary (congestive) dysmenorrhea, and dyspareunia (pain during intercourse). The success rate was defined as the percentage of women who reported no, minimal, or tolerable pain during the period of follow-up and without hysterectomy or repeated LUNA, and this PAS was calculated as percentage to simplify the results (*excellent = 10–9, **good = 6–8 PAS , ***tolerable = 3–5, ****minimal = 0–2 PAS). The patients’ satisfaction was estimated by asking the patients a direct question (did the procedure improve your health status?, regarding the need for additional treatments, resource usage, days off work, and complications of surgery), and the patients’ answers determine the degree of satisfaction (if it is excellent [4], good [3], average [2], or no improvement [1]). Also, the option if the pain was "worse than before" was evaluated and was only restricted to complications of the procedure that worsen the patients' condition. All patients were followed up for long-term complication and the need for additional treatment (medical or surgical), which was considered as treatment failure. Statistical analysis was performed on an IBM personal computer using SPSS statistical package for windows (SOSS, Inc, USA). Results were expressed as mean ± SD for quantitative characteristics, and number and percentage for qualitative characteristics. Among different groups, statistical comparison was made using chi-square X2 test or Fisher exact test for qualitative characteristics and one-way ANOVA for numerical results among different groups. P values of ≤0.05 were considered as the level of significance.
Findings
There was no statistically significant difference between both groups regarding demographic and clinical characteristics of patients (Tables 1 and 2). The efficacy was comparable between group I and group II at 3, 6, and 12 months of follow-up (P ≤ 0.05). Also, the overall success rate between group I and group II was comparable to each other, as it was 77.64%, 76.47%, and 74.11% versus 79.06%, 75.58%, and 73.25% at 3, 6, and 12 months, respectively(P ≤ 0.05; Table 3). Patients' satisfaction rate did not vary significantly between group I and group II at 3, 6, and 12 months follow-up (P ≤ 0.05). The cumulative satisfaction rate was 74.11%, 74.11%, and 71.76% versus 75.58%, 75.58%, and 72.09% at 3, 6, and 12 months between group I and group II, respectively (P ≤ 0.05; Table 4). In evaluating the effectiveness of LUNA (when compared to a control or no treatment) in the treatment of primary (spasmodic) dysmenorrhea and secondary (congestive) dysmenorrhea, there was no statistically significant difference between both groups, (P ≤ 0.05); on the other hand, as regarding the effectiveness of LUNA in the treatment of dyspareunia, there was a statistically significant difference between both groups, (P ≥ 0.05; Fig. 2). There were no statistically significant difference between group I and group II regarding the intraoperative and postoperative complications (P ≤ 0.05; Table 2).
Table 1.
Clinical characteristics of patients in both study groups
| Group I (control) N = 85 | Group II (study) N = 86 | P value | ||
|---|---|---|---|---|
| Age (years) | Range | 25–45 | 24–43 | NS |
| (Mean ± SD) | 31.90 ± 2.44 | 30.25 ± 2.55 | ||
| Weight (kg) | Range | 48–75 | 50–72 | NS |
| (Mean ± SD) | 66–11 ± 5.31 | 67.18 ± 4.34 | ||
| Height (cm) | Range | 153–170 | 152–173 | NS |
| (Mean ± SD) | 163 ± 1.17 | 161 ± 2.10 | ||
| BMI (kg/m2) | Range | 24.40–29.55 | 23.66–28.41 | NS |
| (Mean ± SD) | 24.80 ± 1.46 | 25.23 ± 1.25 | ||
| Parity (N) | Range | 1–8 | 1–7 | NS |
| (Mean ± SD) | 4.78 ± 1.65 | 5.43 ± 1.61 | ||
| Clinical presentation(s), N (%) | ||||
| Acyclic lower abdominal pain | 28 (32.9%) | 25 (29%) | NS | |
| Congestive dysmenorrhea | 38 (44.7%) | 40 (46.5%) | NS | |
| Spasmodic dysmenorrhea | 36 (42.3%) | 35 (40.6%) | NS | |
| Deep dyspareunia | 33 (38.8%) | 30 (34.8%) | NS | |
| Pervaginal findings, N (%) | ||||
| Pelvic tenderness | 18/85 (21.1%) | 19/86 (22.9%) | NS | |
| Localized to one fornix | 6/18 (33.3%) | 7/19 (36.8%) | NS | |
| Bilateral | 3/18 (16.6%) | 3/19 (15.7%) | NS | |
| Diffuse | 9/18 (50%) | 12/19 (63.3%) | NS | |
| Fornical fullness | 14/85 (16.4%) | 12/86 (13.9%) | NS | |
| Unilateral | 5/14 (35.7%) | 5/12 (41.6%) | NS | |
| Bilateral | 9/14 (64.2%) | 7/12 (85.3%) | NS | |
| Cul-de-sac nodularity | 3/85 (3.5%) | 2/86 (2.3) | NS | |
| Fixed retroverted uterus | 1/85 (1.1%) | 1/86 (1.1%) | NS | |
| No significant finding | 16/85 (18.8%) | 18/86 (20.9%) | NS | |
NS not significant
Table 2.
Operative and postoperative data for both study groups
| Group I (N = 85) | Group II (N = 86) | P value | |||
|---|---|---|---|---|---|
| Operative time | Range (min) | 25–35 | 30–48 | S | |
| (Mean ± SD) | 27.50 ± 4.51 | 36.33 ± 7.68 | |||
| Intraoperative complications (N %) | |||||
| Bleeding | 4/85 (4.70%) | 5/86 (5.8%) | NS | ||
| Visceral injuries | 0 | 0 | NS | ||
| Vascular injuries | 0 | 0 | NS | ||
| Conversion to open surgery | 0 | 0 | NS | ||
| Discharge time | Range (days) | 1–2 | 1–2 | NS | |
| (Mean ± SD) | 1.33 ± 0.22 | 1.33 ± 0.22 | |||
| aPostoperative complications, N (%) | |||||
| Fever | 5/85 (5.88%) | 6/86 (6.9%) | NS | ||
| Postoperative bleeding | 0 | 0 | NS | ||
| Constipation | 0 | 3 (3.48%) | NS | ||
| Urinary urgency | 0 | 4 (4.65%) | NS | ||
| Uterine prolapse | 0 | 2 (2.32%) | NS | ||
| Painless labor | 0 | 0 | NS | ||
| Time to return to normal lifestyle | Range (days) | 4–10 | 5–10 | NS | |
| (Mean ± SD) | 6.78 ± 1.55 | 6.34 ± 1.22 | |||
aIncluding immediate and remote complications during the period of follow-up
S significant, NS not significant
Table 3.
The summation of success rate according to the mean PAS after treatment at 3, 6, 12 month follow-up in both study groups
| Group I (N = 85) | Group II (N = 86 ) | P value | |||||
|---|---|---|---|---|---|---|---|
| 3MS | 6MS | 12MS | 3MS | 6MS | 12MS | ||
| aNo pain, N (%) | 20 (23.52%) | 18 (21.17%) | 18 (21.17%) | 21 (24.41%) | 19 (22.09%) | 18 (20.93%) | NS |
| bMinimal pain, N (%) | 19 (22.35%) | 19 (22.35%) | 17 (20%) | 20 (23.25%) | 19 (22.09%) | 18 (20.93%) | NS |
| cTolerable pain, N (%) | 27 (31.76%) | 28 (32.94%) | 28 (32.94%) | 27 (31.39%) | 28 (32.55%) | 27 (31.39%) | NS |
| dSevere pain, N (%) | 19 (22.35%) | 20 (23.52%) | 22 (25.88%) | 18 (20.93%) | 21 (24.41%) | 22 (25.58%) | NS |
| Overall success rate | (66/85) (77.64%) | (65/85) (76.47%) | (63/85) 74.11% | (68/86) 79.06% | (65/86) 75.58 % | (63/86) 73.25% | NS |
NS not significant
aexcellent = 10–9
bgood = 6–8 PAS
ctolerable = 3–5
dminimal = 0–2 PAS
Table 4.
Patients' satisfaction rate of the treatment during 3, 6, and 12 months follow-up in both study groups
| Group I (N = 85) | Group II (N = 86) | P value | |||||
|---|---|---|---|---|---|---|---|
| 3MS | 6MS | 12MS | 3MS | 6MS | 12MS | ||
| Excellent | 19/85 (22.35%) | 17/85 (20%) | 17/85 (20%) | 20/86 (23.25%) | 19/86 (22.09%) | 19/86 (22.09%) | NS |
| Good | 26/85 (30.58 %) | 24/85 (28.23%) | 22/85 (25.88%) | 25/86 (29.06%) | 23/86 (26.74 %) | 22/86 (23.25 %) | NS |
| Moderate | 18/85 (21.17%) | 22/85 (25.88%) | 22/85 (25.88%) | 20/86 (23.86 %) | 23/86 (26.74%) | 21/86 (24.41%) | NS |
| No improvement | 22/85 (25.88%) | 22/85 (25.88%) | 24/85 (28.23%) | 21/86 (24.41%) | 21/86 (24.41%) | 24/86 (27.90%) | NS |
| aCumulative satisfaction rate | 63/85 (74.11%) | 63/85 (74.11%) | 61/85 (71.76%) | 65/86 (75.58%) | 65/86 (75.58%) | 62/86 (72.09%) | NS |
aCumulative satisfaction rate = Excellent + Good + Moderate.
Worse than before = restricted only to patients with surgery complications, and it was 0%
Fig. 2.
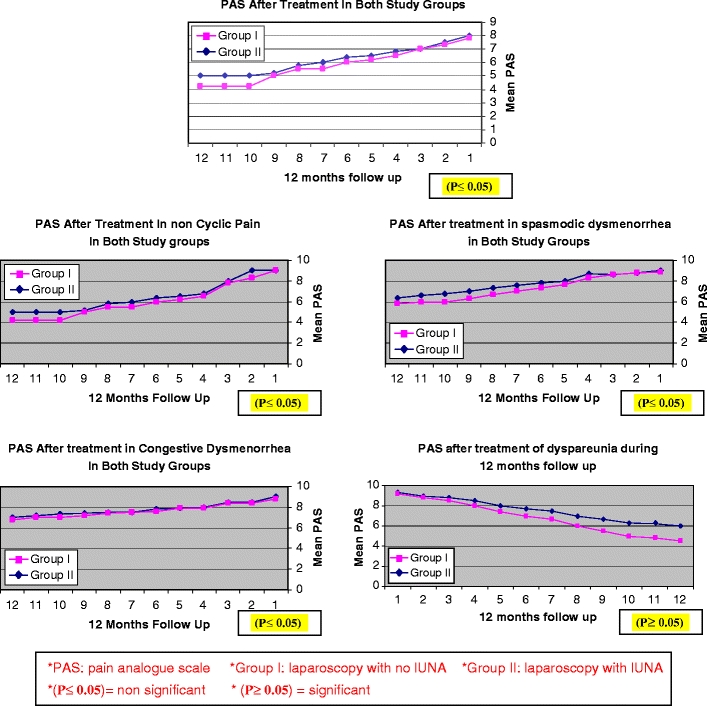
Effect of LUNA at 12 months follow-up in both study groups
Discussion
Definitive diagnosis of CPP is usually by laparoscopy or laparotomy. Treatment for CPP depends on the underlying cause. In some patients, a cause cannot be identified, so conservative treatments will be tried. If these medical treatments fail, conservative surgical treatment options which include vaginal uterosacral ligament resection, uterine nerve ablation, and presacral neurectomy may be beneficial [1–4].
In the present study, There was no statistically significant difference as regards the efficacy and the overall success rate between group I and group II at 3, 6, and 12 months of follow-up (P ≤ 0.05). This is in agreement with the results of other studies, in which LUNA was used to treat patients with CPP in whom laparoscopy revealed no pathology as the systematic review (Cochrane) and meta-analysis including six randomized, controlled trials (RCTs) [9], reported that there were no significant differences overall in pain relief between women treated with LUNA and controls (women treated with diagnostic laparoscopy or conservative surgery alone) as pain relief up to 6 months (five studies, N = 258): odds ratio (OR) 1.15, 95% confidence interval (CI) 0.66 to 1.99, pain relief up to 12 months (four studies, N = 285): OR 1.20, 95% CI 0.72 to 1.99 and pain relief up to 36 months (one study, N = 116): OR 0.84, 95% CI 0.39 to 1.80). The review states that the effect of treatment may overlap with the placebo effect of laparoscopy, reducing differences in short-term efficacy between groups, and the lack of power to detect a clinically important difference was an issue of concern in the trials with null results, and they stated that lack of sustained long-term benefit could be due to regrowth of nerves or pain signals being transferred via alternative routes and consequently, because of this last speculation, in the present study, uterosacral nerve cauterization using bipolar electrosurgical current and then nerve transaction was used rather than any procedure alone. In a recent randomized, controlled trial [10], 80 women were studied with CPP treated with LUNA or vaginal uterosacral nerve resection with a follow-up till 12 months, there were no significant differences between the two study groups with regard to pain relief. The same results were obtained from recent meta-analysis [11] used by collecting individual patient data from the existing trials to provide a comprehensive assessment of the effectiveness of LUNA that will be generalizable in various clinical contexts. Also, in the most recent multicentric, randomized, controlled trial [12] including 487 women with CPP lasting longer than 6 months without or with minimal endometriosis, adhesions, or pelvic inflammatory disease, recruited to the study by consultant gynecological surgeons from 18 UK hospitals, with follow-up conducted by questionnaires mailed at 3 and 6 months and at 1, 2, 3, and 5 years, it concluded that after a median follow-up of 69 months, there were no significant differences reported on the visual analog pain scales for the worst pain between the LUNA group and the no LUNA group for quality of life.
The cumulative satisfaction rate was 74.11%, 74.11%, and 71.76% versus 75.58%, 75.58%, and 72.09% at 3, 6, 12 months between group I and group II, respectively (P ≤ 0.05). This results go hand in hand with the overall satisfaction rate in the meta-analysis study [9] and other studies [13, 14], as it stated that there is no significant difference at the satisfaction rate, or the need of additional treatment between the study and the control group among six RCTs they evaluated.
As regarding the effectiveness of LUNA (when compared to a control or no treatment) in treatment of primary (spasmodic) dysmenorrhea and secondary (congestive) dysmenorrhea, there was no statistically significant difference between both groups, (P ≤ 0.05); on the other hand, as regarding the effectiveness of LUNA in the treatment of dyspareunia, there was a statistically significant difference between both groups, (P ≥ 0.05). The previous results were in agreement with that of the report of Proctor et al. [9], as in his systematic review of six RCTs, he reported that, for women with primary dysmenorrhea, the OR for pain relief at 6 and 12 months was 0.67 (95% CI 0.17 to 2.61) and 0.10 (95% CI 0.03 to 0.03), respectively, in favor of LUNA. For women with secondary dysmenorrhea, the OR for pain relief at 6 and 12 months was 1.03 (95% CI 0.52 to 2.02) and 0.77 (95% CI 0.43 to 1.39), respectively. In Cochrane Database of Systematic Reviews [15], regarding the treatment of primary dysmenorrhea, there was some evidence of the effectiveness of LUNA when compared to a control or no treatment.
In the present study, endometriosis with an AFS score of >5 was excluded from the study, as in the majority of women who had endometriosis that was treated with conservative laparoscopic procedure alone or with conservative medical therapy, as well as with LUNA, it will be difficult to attribute their response to the LUNA alone or to the adjuvant therapy of endometriosis, and this is in agreement with the conclusion of different studies as that of Guyer et al. [13] and Davis et al. [14]. Also, in a randomized trial of 180 patients with symptomatic endometriosis, the addition of LUNA to conservative laparoscopic surgery for endometriosis did not reduce the medium- or long-term frequency and severity of recurrent dysmenorrheal [6]. Another randomized study of 67 patients with CPP and laparoscopic evidence of endometriosis found no significant difference in the pain outcome [5].
In the present study, the effectiveness of the LUNA in relief or improvement of deep dyspareunia was taken with great interest and discussion because to my knowledge, there has been few similar reports specifically on the treatment of primary deep dyspareunia by the LUNA procedure, as Jung et al. [16] found that of his 12-month follow-up study of LUNA for treating primary deep dyspareunia, the satisfactory rates at 3 and 12 months were 66.7% and 50%, respectively, while on the other hand, Vercellini et al. [6] found no significant advantage on sexual satisfaction by the LUNA procedure. In a multicentric, randomized, controlled trial (JAMA trial) [12], after a median follow-up of 69 months, there were no significant differences reported on the visual analog pain scales for the worst pain (mean difference between the LUNA group and the no LUNA group, −0.04 cm (95% CI −0.33 to 0.25 cm; P = 0 .80), noncyclical pain (−0.11 cm, 95% CI, −0.50 to 0.29 cm]; P = 0.60), dysmenorrhea (−0.09 cm, 95% CI, −0.49 to 0.30 cm; P = 0.60), or dyspareunia (0.18 cm, 95% CI, −0.22 to 0.62 cm; P = 0.40), and they concluded that in women with CPP, LUNA did not result in improvements in pain, dysmenorrhea, dyspareunia, or quality of life compared with laparoscopy without pelvic denervation. However, in our opinion, deep dyspareunia is very complicated in its pathogenesis, which includes both physical and psychiatric/psychologic aspects; hence, a verified system comprising of clarified definitions and criteria in the assessment of satisfaction or improvement is mandatory, and will be very difficult to be practiced in our community and with our patients with the lack of group evaluators, including social personnel who are qualified for appraising a sexologic and psychiatric/psychologic questionnaire to give more informed insight, and so these results need to be evaluated carefully in a large, randomized trial before it will be generalized.
In the present study, there was no statistically significant difference between group I and group II regarding intraoperative and postoperative complications (P ≤ 0.05); only urge incontinence was significantly observed among patients with LUNA. All of these complications were minimal and treated conservatively with no additional measures. This was in agreement with that of Yuan [1] who stated that the adverse events of LUNA were less common. Also, a meta-analysis [9] found that few complications were reported, while two case reports described a total of five women with uterine prolapse after having LUNA; three women were young nulliparous, and the other two women had a history of vaginal childbirth [14, 16]. In Cochrane Database of Systematic Reviews [15], adverse events were significantly more common for presacral neurectomy; however, the majority had complications such as constipation, which may spontaneously improve.
The National Institute for Health and Clinical Excellence [17] examined LUNA for CPP and will publish guidance on its safety and efficacy to the NHS in England, Wales, Scotland, and Northern Ireland. This overview is based on one Cochrane systematic review and meta-analysis [9–14]. They concluded that there was some evidence for the effectiveness of LUNA when compared with controls or no treatment in women with primary dysmenorrhea, and there were no significant differences in short-term pain relief between LUNA and LPSN. On the other hand, The European Society for Human Reproduction and Embryology published a guideline for the diagnosis and treatment of endometriosis in 2005 [18]. This guideline states that “ablation of endometriotic lesions reduces endometriosis-associated pain and the smallest effect is seen in patients with minimal disease; there is no evidence that also performing LUNA is necessary”. A guideline published by the Royal College of Obstetricians and Gynecologists states that “There is no evidence that laparoscopic uterine nerve ablation is necessary when ablating endometriotic lesions and laparoscopic uterine nerve ablation by itself has no effect on dysmenorrhea associated with endometriosis” [19]. So, both uncontrolled or controlled randomized studies and the present study had proven that LUNA showed no statistically significant difference in complete relief of CPP, but it has a role in substantial reduction in CPP level in well-selected patients, and this may be due the complicated anatomical and physiological picture of CPP as anatomically, at least five pathways transmit signals from noxious stimuli in the pelvis, and these nerve trunks vary in location and can intersect, with the potential for neuronal crosstalk so LUNA may obliterate some of the nerve fibers, but others are interwoven with the pelvic arteries and ureters [12]. This explanation that LUNA has no role in reducing CPP was an objective one, not subjective, and if we generalized this opinion, at the same step, it will be of no value to ligate the uterine arteries or the internal iliac arteries to stop severe postpartum hemorrhage, as the uterus has many other blood sources, but the philosophy of the LUNA is that it has a short-term effect in complete or incomplete relief of CPP that will give the patients the chance to return to their normal lifestyle, and then accommodation and less pain habituation will take place making their PAS fall to its minimal level, especially when the patients know that their diagnostic laparoscopy was free, and these results, if obtained from the LUNA, will be considered as a relative success event, not an absolute one. So, after this controversial explanations, LUNA, in well-selected patients, can be added as an adjuvant last treatment in patients with CPP when other options of treatment failed, and laparoscopy was recommended for diagnosis, but it is not recommended as an indication for LUNA in patients with CPP.
Conclusion
In conclusion, LUNA can be a last alternative option in well-selected patients for control of CPP without endometriosis; however, its effectiveness may not extend to other indications. Also, preliminary experience in the treatment of primary deep dyspareunia presents a promising perspective on the management of deep dyspareunia, especially if it will involve a team of social, psychological, and gynecological specialists.
Acknowledgments
To all my colleges in the endoscopy unit , El-Minia university hospital, and all of my patients for their cooperation. Also with great appreciation to my colleges in the statistical section of the public health department about their cooperation in performing these results.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Yuan Ch-Ch. Laparoscopic uterosacral nerve ablation and chronic pelvic pain. J Chin Med Assoc. 2006;69(3):101–103. doi: 10.1016/S1726-4901(09)70185-6. [DOI] [PubMed] [Google Scholar]
- 2.Hebbar S, Chawla C. Role of laparoscopy in evaluation of chronic pelvic pain. J Min Access Surg. 2005;1(issue 3):116–120. doi: 10.4103/0972-9941.18995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The LUNA Trial Collaboration: a randomized controlled trial to assess the efficacy of laparoscopic uterosacral nerve ablation (LUNA) in the treatment of chronic pelvic pain: the trial protocol. BMC Womens Health. 2003 3:6. Published online 2003 December 8. doi:10.1186/1472-6874-3-6 [DOI] [PMC free article] [PubMed]
- 4.Hammoud A, Gago LA, Diamond MP. Adhesions in patients with chronic pelvic pain: a role for adhesiolysis? Fertil Steril. 2004;82:1483–1491. doi: 10.1016/j.fertnstert.2004.07.948. [DOI] [PubMed] [Google Scholar]
- 5.Johnson NP, Farquhar CM, Crossley S, Yu Y, Van Peperstraten AM, Sprecher M, Suckling J. A double-blind randomized controlled trial of laparoscopic uterine nerve ablation for women with chronic pelvic pain. BJOG. 2004;111:950–959. doi: 10.1111/j.1471-0528.2004.00233.x. [DOI] [PubMed] [Google Scholar]
- 6.Vercellini P, Aimi G, Busacca M, Apolone G, Uglietti A, Crosignani PG. Laparoscopic uterosacral ligament resection for dysmenorrhea associated with endometriosis: results of a randomized, controlled trial. Fertil Steril. 2003;80:310–319. doi: 10.1016/S0015-0282(03)00613-7. [DOI] [PubMed] [Google Scholar]
- 7.Proctor ML, Latthe PM, Farquhar CM (2005) Surgical interruption of pelvic nerve pathways for primary and secondary dysmenorrhea. Cochrane Database of Systematic Reviews Issue 4: CD001896 [DOI] [PMC free article] [PubMed]
- 8.Stones, RW, Mountfield, J (2000) Interventions for treating chronic pelvic pain in women. [update of Cochrane Database Syst Rev. ;(2): CD000387 ; 10796713.]. [Review] [15 refs]. Cochrane Database Syst Rev. p. CD000387 [DOI] [PubMed]
- 9.Wilson ML, Farquhar CM, Sinclair OJ, Johnson NP (2000) Surgical interruption of pelvic nerve pathways for primary and secondary dysmenorrhea. [Review] [6 refs]. Cochrane Database Syst Rev.: (2000) CD001896 [DOI] [PubMed]
- 10.Palomba S, Russo T, Falbo A. Laparoscopic uterine nerve ablation versus vaginal uterosacral ligament resection in postmenopausal women with intractable midline chronic pelvic pain: a randomized study. Eur J Obstet Gynaecol. 2006;129:84–91. doi: 10.1016/j.ejogrb.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Xiong T, Daniels J, Middleton L, Champaneria R, Khan KS, Gray R, Johnson N, Lichten EM, Sutton C, Jones KD, Chen FP, Vercellini P, Aimi G, Lui WM (2007) International LUNA IPD Meta-analysis Collaborative Group: meta-analysis using individual patient data from randomised trials to assess the effectiveness of laparoscopic uterosacral nerve ablation in the treatment of chronic pelvic pain: a proposed protocol. BJOG. Dec;114(12):1580, e1-7 [DOI] [PubMed]
- 12.Daniels J, Gray R, Hills RK, Latthe P, Buckley L, Gupta J, Selman T, Adey E, Xiong T, Champaneria R, Lilford R, Khan KS, LUNA Trial Collaboration Laparoscopic uterosacral nerve ablation for alleviating chronic pelvic pain: a randomized controlled trial. JAMA. 2009;302(9):955–961. doi: 10.1001/jama.2009.1268. [DOI] [PubMed] [Google Scholar]
- 13.Guyer C, Moors A, Louden K. An audit of conservative surgery for endometriosis in a district general hospital 1995–1998. J Obstet Gynaecol. 2000;20(5):514–516. doi: 10.1080/014436100434721. [DOI] [PubMed] [Google Scholar]
- 14.Davis GD. Uterine prolapse after laparoscopic uterosacral transection in nulliparous airborne trainees. J Reprod Med. 1996;41:279–282. [PubMed] [Google Scholar]
- 15.Proctor ML, Latthe PM, Farquhar CM, Khan KS, and Johnson NP: Surgical interruption of pelvic nerve pathways for primary and secondary dysmenorrhea Cochrane Database of Systematic Reviews 2007 Issue 3 Copyright © 2007 The Cochrane Collaboration. Published by Wiley [DOI] [PMC free article] [PubMed]
- 16.Juang CM, Yen MS, Horng HC, Cheng CY, Yu HC, Chang CM. Treatment of primary deep dyspareunia with laproscopic uterosacral nerve ablation procedure: a pilot study. J Chin Med Assoc. 2006;69:110–114. doi: 10.1016/S1726-4901(09)70187-X. [DOI] [PubMed] [Google Scholar]
- 17.National institute for health and clinical excellence: interventional procedure overview of laparoscopic uterine nerve ablation (LUNA) for chronic pelvic pain. February 2007. Available from www.nice.org.uk/ip376overview
- 18.Kennedy S, Bergqvist A, Chapron C. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20(10):2698–2704. doi: 10.1093/humrep/dei135. [DOI] [PubMed] [Google Scholar]
- 19.Royal College of Obstetricians and Gynaecologists (2006) The investigation and management of endometriosis. Green-top guideline no. 24. London


