Abstract
Plasmodium falciparum malaria is a major cause of morbidity and mortality in African children, and factors that determine the development of uncomplicated (UM) versus cerebral malaria (CM) are not fully understood. We studied the ex vivo responsiveness of microvascular endothelial cells to pro-inflammatory stimulation and compared the findings between CM and UM patients. In patients with fatal disease we compared the properties of vascular endothelial cells cultured from brain tissue to those cultured from subcutaneous tissue, and found them to be very similar. We then isolated, purified and cultured primary endothelial cells from aspirated subcutaneous tissue of patients with CM (ECCM) or UM (ECUM) and confirmed the identity of the cells before analysis. Upon TNF stimulation in vitro, ECCM displayed a significantly higher capacity to upregulate ICAM-1, VCAM-1 and CD61 and to produce IL-6 and MCP-1 but not RANTES compared with ECUM. The shedding of endothelial microparticles, a recently described parameter of severity in CM, and the cellular level of activated caspase-3 were both significantly greater in ECCM than in ECUM. These data suggest that inter-individual differences in the endothelial inflammatory response to TNF may be an additional factor influencing the clinical course of malaria.
Introduction
Cerebral malaria (CM) is an important life-threatening complication of Plasmodium falciparum infection. This neurological syndrome occurs in about 1% of infections but exhibits significant case fatality rates, contributing up to a million deaths annually among children in sub-Saharan Africa (Rowe et al., 2006). The pathophysiological mechanisms underlying CM are not fully understood and several hypotheses have been put forward, including mechanical obstruction of microvessels by P. falciparum-parasitized red blood cells (PRBC) and hyper-activation of host immune cells leading to the excessive release of pro-inflammatory cytokines (Berendt et al., 1994; Clark and Rockett, 1994; Grau and de Kossodo, 1994). These complementary theories may contribute to our understanding of pathogenesis, but they fail to explain why only a small percentage of malaria patients develop CM.
Sequestration of PRBC within brain microvasculature is a common feature of CM, and this phenomenon is mediated by several endothelial cell (EC) adhesion molecules including inter-cellular adhesion molecule (ICAM)-1, CD36, P-selectin and vascular cell adhesion molecule (VCAM)-1 (Ho and White, 1999). Most of these receptors are inducible in endothelium by pro-inflammatory cytokines such as TNF, a cytokine that has been postulated to play a crucial role in the pathogenesis of CM (Grau et al., 1989; Hunt and Grau, 2003). In P. falciparum-infected patients, the surface expression of vascular endothelial receptorshas been shown to be increased in several tissues at post-mortem, including the brain. A colocalization of PRBC and these receptors has also been observed, suggesting that endothelial activation plays a role in sequestration, and therefore potentially in pathogenesis (MacPherson et al., 1985; Turner et al., 1994). In addition to the upregulation of endothelial receptors mediating PRBC sequestration, TNF induces extensive alterations in microvascular EC including morphological reorganization (Pober and Cotran, 1990), release of membrane microparticles (MP) (Zwaal and Schroit, 1997), production of pro-inflammatory cytokines and apoptosis (Grell et al., 1999; Gnant et al., 2000; Kimura et al., 2003).
In an experimental model for CM, it has been reported that brain EC purified from CM-susceptible and CM-resistant mice exhibit different sensitivities to TNF. Brain EC isolated from susceptible mice had a greater capacity to produce interleukin (IL)-6 and to upregulate ICAM-1 and VCAM-1 in response to TNF than did brain EC from resistant mice (Lou et al., 1998). On this basis, we postulated that differences in the severity of human malarial disease might in part be due to a differential responsiveness of the host endothelium to systemic inflammation.
We studied the effect of TNF on cultured EC originating from Malawian children with different clinical syndromes. We compared upregulation of cytoadherence receptors, shedding of MP, release of chemokines and cytokines selected for their relevance in the pathophysiology of malaria and activation of pro-apoptotic factors in EC cultured from patients in the different clinical groups. Since the isolation, purification and culture of brain vascular EC is feasible only through post-mortem sampling, we used subcutaneous adipose tissue as a source of vascular EC from all patient groups. Wilairatana and colleagues described focal cytokine accumulations in the skin that were similar to those in the brain (Wilairatana et al., 2000). This was recently confirmed in humans, where the subcutaneous adipose tissue, but not dermal or epidermal skin layers, was a major site for sequestration (Seydel et al., 2006). Nakano and colleagues reported a correlation between PRBC sequestration in subcutaneous tissues and in brain microvasculature in a primate model of CM (Nakano et al., 1996).
In view of these data, and considering that purification and isolation of fat-derived EC has been extensively described and can be performed routinely in living patients (Kern et al., 1983), EC derived from human subcutaneous fat appeared to represent an original and relevant alternative model to test our hypothesis and compare endothelial reactivity to TNF in patients with uncomplicated malaria (UM) and CM.
Results
Success rates of brain and subcutaneous fat cell preparations
The success rate of skin cell preparation from tissue samples was of 73.3%, with six successful preparations out of eight UM patients and five out of eight CM patients respectively. In the case of brain cell preparations, we obtained six cell cultures out of the same eight patients (75% of success) but restricted our analyses to the five patients we successfully isolated, purified and cultured both brain and fat EC. The loss of approximately 25% of our samples in both patient categories was mainly due to either bacterial or fungal contamination, a risk considerably increased by the collection of tissue samples in a non-sterile environment (i.e. ward or mortuary). In one case of brain EC preparation, cells stopped growing after 2 days in culture with no known explanation.
Comparison of morphological and phenotypical characteristics between subcutaneous fat and brain-derived ECCM
Brain and subcutaneous fat-derived primary ECCM readily adhere to plastic substrate, form flat monolayers after plating in culture flasks, present a thin cobblestone-like morphology with central and prominent nuclei after 3 days in culture, and exhibit contact inhibition (Fig. 1). All of the primary ECCM constitutively expressed typical endothelial markers including von Willebrand factor (vWF) and platelet-endothelium cell adhesion molecule (PECAM)-1 (CD31), and showed an E-selectin (CD62E) inducible expression upon TNF stimulation, as shown in Fig. 1 and Table 1. Brain and subcutaneous fat EC constitutively expressed high levels of ICAM-1 (Fig. 1), αvβ3 integrin (CD51/CD61), CD146 and VCAM-1, and both ICAM-1 and VCAM-1 were significantly upregulated upon TNF stimulation (Table 1). However, brain-derived primary EC exhibited a peripheral pattern of positive staining for ZO-1, a constituent of endothelial tight junctions, as well as no CD36 expression, whereas subcutaneous fat EC did not express ZO-1 but were stained positively for CD36 (Fig. 1).
Fig. 1.

Immunofluorescence analysis of endothelial markers on subcutaneous fat and brain-derived ECCM monolayers. The figure shows evidence for the expression of several typical endothelial markers on submembrane or cell type surface, including vWF, CD31, ZO-1, ICAM-1, CD36 and CD62-E. Micrographs presented here are from patient PM91-derived cells in passage 3 and are representative of the results obtained with all the other CM patients. Magnification: × 100 for general morphology micrographs, × 400 for immunofluorescence micrographs.
Table 1.
Flow cytometric phenotyping of ECCM under resting and activated conditions
| Subcutaneous fat ECCM | Brain ECCM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Resting | TNF | Resting | TNF | |||||||
| CD | Antigen | Clone | MFI (a.u.) | % POS | MFI (a.u.) | % POS | MFI (a.u.) | % POS | MFI (a.u.) | % POS |
| IgG1 | 0.23 | 0.21 | 0.19 | 0.22 | ||||||
| CD31 | PECAM-1 | 5.6E | 30 | 89 | 34.7 | 78.9 | 25.9 | 78.8 | 27.3 | 81.4 |
| CD36 | GPIV | FA6-152 | 37.2 | 48.2 | 40.3 | 51 | 0.23 | 0.7 | 0.26 | 0.9 |
| CD40 | Bp50 | B-B20 | 0.42 | 2.8 | 0.46 | 6.1 | 0.24 | 3.5 | 0.6 | 6.3 |
| CD51 | αv | AMF7 | 20.2 | 100 | 19.7 | 100 | 24.3 | 100 | 25.8 | 100 |
| CD54 | ICAM-1 | 84H10 | 43.8 | 94.7 | 84.6 | 96 | 35 | 92.7 | 79.8 | 90.6 |
| CD61 | β3 | SZ21 | 2.1 | 100 | 34.1 | 100 | 3.7 | 100 | 31.6 | 100 |
| CD62E | E-selectin | CL2/6 | 0.26 | 2 | 0.32 | 2.7 | 0.4 | 1.3 | 0.22 | 0.7 |
| CD106 | VCAM-1 | 1G1 | 5.6 | 80 | 27.1 | 78.3 | 4.1 | 82.6 | 24.7 | 81.1 |
| CD146 | MCAM | OJ79c | 28.1 | 76 | 25.9 | 72.7 | 23 | 89.3 | 21.5 | 92.1 |
Subcutaneous fat- and brain-derived EC isolated from patients who died of CM were either left unstimulated or activated with TNF (10 ng ml−1) and stained by indirect immunolabelling after 12 h, except for CD62E, which was studied after 6 h. Results are representative of a series of three experiments performed in passage 2 and show both the mean fluorescence intensity (MFI) expressed in arbitrary units (a.u.) of the labelling with specific monoclonal antibodies and also the percentage of cells positively labelled with these antibodies in the whole population.
Variation of ICAM-1, VCAM-1 and CD61 upregulation in subcutaneous fat ECCM and ECUM in the presence of a pro-inflammatory stimulus
Under resting conditions, the basal expression of ICAM-1, VCAM-1 and CD61 did not show any significant difference between ECCM and ECUM in cell-based ELISA assays using subcutaneous fat ECs. In contrast, upon overnight stimulation with increasing doses of TNF, cells of both patient categories exhibited a dose-dependent upregulation of the three surface molecules. When the dose of TNF reached a range between 1 and 10 ng ml−1, a significantly higher upregulation of ICAM-1 (P < 0.05 and P < 0.001, respectively, Fig. 2A) and CD61 (P < 0.05 and P < 0.01, respectively, Fig. 2C) was observed in ECCM compared with ECUM. In the case of VCAM-1, a significant difference was only observed at a TNF concentration of 10 ng ml−1 (P < 0.05, Fig. 2B). All these results were confirmed by flow cytometry at the highest TNF concentration (10 ng ml−1, Fig. 2D–F). Brain-derived ECCM were also used as a comparator and when stimulated with the most effective dose of TNF no differences were observed in terms of ICAM-1, VCAM-1 and CD61 upregulation, when compared with subcutaneous fat-derived ECCM (data not shown).
Fig. 2.

Comparison of TNF-induced adhesion molecule upregulation between ECUM and ECCM of subcutaneous fat origin. Cells were seeded in a 96-well plate and stimulated by increasing concentrations of TNF. ECUM and ECCM were then fixed and the expression of ICAM-1, VCAM-1 and CD61 was measured by cell-based ELISA (A, B and C respectively). Results are expressed as mean ± SD of two experiments (Dunn's test, *P < 0.05, **P < 0.01 and ***P < 0.001). As a control, each experiment was confirmed by flow cytometry (D–F). The results of one representative reading obtained with the greatest concentration of TNF are shown here.
Shedding of endothelial membrane MP
ECCM and ECUM were exposed to two concentrations of TNF, for which significant effects have been observed in previous assays (1 and 10 ng ml−1 respectively), to compare MP formation. Both cell types produced significantly higher MP numbers than in resting conditions and a dose-dependent increase of MP production was observed (Fig. 3). In both stimulation conditions the number of MP released by 1000 EC was significantly higher in ECCM than in ECUM (P < 0.01), ECCM releasing up to 5420 MP per 1000 EC when stimulated with 10 ng ml−1 TNF (Fig. 3).
Fig. 3.
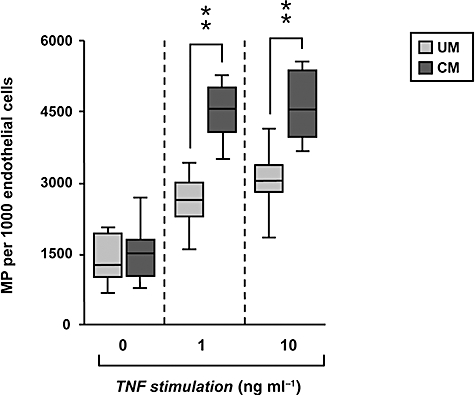
Quantification of TNF-induced endothelial MP release by subcutaneous fat-derived ECUM and ECCM. Cells were cultured and left resting or stimulated with TNF for 6 h before analysis. MP production was quantified by flow cytometry for each condition. Results (two determinations in three experiments) are expressed in numbers of MP labelled with annexin V-FITC, extracted from culture supernatants of 1000 EC (Dunn's test, **P < 0.01).
MCP-1, RANTES and IL-6 release by ECCM and ECUM upon TNF stimulation
To determine whether the release of MCP-1, RANTES and IL-6 by ECUM and ECCM varied upon inflammatory stimulus, we performed a series of ELISA in the presence of increasing concentrations of TNF, ranging from 0.01 to 10 ng ml−1. The release of both MCP-1 and IL-6 was induced by TNF in ECUM and ECCM and this effect was dose-dependent. Significantly higher levels of MCP-1 and IL-6 were produced by ECUM than by ECCM when TNF concentrations reached 1 and 10 ng ml−1 respectively (Fig. 4). Conversely, there was no significant effect of TNF on release of RANTES by either cell types, nor was there a clear tendency or statistically significant difference in its production between ECCM and ECUM (Fig. 4).
Fig. 4.

Comparison of TNF-induced production of MCP-1, RANTES, IL-6 and cleaved Caspase-3 between ECUM and ECCM. Subcutaneous fat-derived EC from both patient categories were stimulated with different doses of TNF. In the case of MCP-1, RANTES and IL-6, supernatants were collected after 12 h of incubation and concentrations were measured by ELISA. For activated Caspase-3, supernatants and cell extracts were pooled before analysis by ELISA. Results are expressed as mean ± SD of three determinations in two experiments (Dunn's test, *P < 0.05 and ***P < 0.001).
Activation of caspase-3 in TNF-stimulated ECCM and ECUM
The level of activated caspase-3 in ECCM than in ECUM was clearly modulated by the amount of cytokine added to the medium. Caspase-3 activation was significantly higher in ECCM than in ECUM at TNF concentrations of 0.1 ng ml−1 and above (P < 0.05).
Discussion
A central component of CM pathophysiology appears to be the activation of microvascular EC, resulting both from the cytoadherence of infected erythrocytes to EC and from the pro-inflammatory effects of locally released cytokines (Wassmer et al., 2003). The consequences of this endothelial inflammation are numerous and include: (i) the upregulation of endothelial receptors, enhancing PRBC sequestration, (ii) an increased shedding of endothelial MP, (iii) further release of cytokines and chemokines, and (iv) the trigger of a TNF-dependent pro-apoptotic pathway (as reviewed in Combes et al., 2006). Since brain microvascular EC are profoundly affected in these ways by TNF, variation in the responsiveness of EC to TNF in different individuals could be a factor affecting the severity of disease in patients with malaria.
Several studies indicate that microvascular EC differ from large vascular EC in morphology or function (Grau and Lou, 1993), and EC derived from different organs also differ in response to some cytokines (Belloni et al., 1992). In addition, there are considerable variations in both adhesion molecule expression and the functional properties of EC depending on their position within the vascular bed of a particular tissue (Ley et al., 1992). To examine the possibility that our findings with cultured subcutaneous fat-derived EC might not reflect the properties of intracerebral vascular EC, we took the opportunity to examine EC from both brain and subcutaneous fat in patients dying of CM who came to autopsy. Both EC types exhibited similar endothelial features. Cells of both origins were shown to express major pro-inflammatory cytokine-induced endothelial adhesion molecules such as VCAM-1 and ICAM-1 (Springer, 1990), which were upregulated to a similar degree upon activation by TNF (Table 1). Two discrepancies confirming previously published data were observed between subcutaneous fat- and brain-derived ECCM: the former expressed CD36 and not ZO-1 whereas the latter expressed ZO-1 but not CD36. ZO-1 plays a crucial role in the permeability of the blood–brain barrier (Staddon et al., 1995), and its presence has been widely acknowledged as a hallmark of adjacent brain EC (Collins et al., 2006). In contrast, the expression of CD36, a non-inducible receptor for PRBC, has been reported to be low and irregular in cerebral microvasculature in several studies (Turner et al., 1994), as opposed to microvascular beds from subcutaneous and visceral adipose tissue (Bonen et al., 2006). However, these two differences were irrelevant to our study since variation in EC monolayer permeability was not one of our read-out parameters and CD36 expression is not regulated by TNF. Our results show that EC cultured from subcutaneous fat closely resemble cerebral vascular EC in several properties relevant to the pathogenesis of malaria, which suggests they may be useful in an ex vivo model.
Making use of this model, we have shown that subcutaneous fat-derived ECCM and ECUM display significantly different ex vivo responsiveness to TNF. We demonstrated that, when compared with ECUM responses, ECCM express significantly higher levels of ICAM-1, VCAM-1 and CD61, produce more endothelial MP, MCP-1 and IL-6 and are more prone to undergo apoptosis upon stimulation with TNF.
While sequestration of PRBC in the brain has been widely described in malaria, its association with disease severity and especially with CM is still debated. Specific binding of PRBC to ICAM-1 has been reported to be higher in CM patients compared with patients with uncomplicated disease (Newbold et al., 1997). Our results are in line with the former, and indicate that ECCM upregulate much higher levels of ICAM-1 on their surface than ECUM, providing an increased number of PRBC binding sites. This inter-individual difference in ICAM-1 upregulation upon TNF stimulation has also been described in human umbilical vein EC (HUVEC). In a recently published study, Beck and colleagues isolated 30 lines of HUVEC and upon stimulation with TNF, some EC, i.e. type I responders, displayed a low ICAM-1 upregulation while type II responders exhibited a significantly higher level of the receptor (Beck et al., 2006). However, we cannot exclude the possibility of a long-lasting effect of malaria parasite-derived glycosylphosphatidylinositol, which has been reported to alter cell-surface expression of various adhesion molecules on HUVEC (Schofield et al., 1996). Such stimulation could well alter the progeny of cells derived from patients with severe malaria despite their time in culture.
In addition to ICAM-1, VCAM-1 and CD61 upregulation was also shown to be more pronounced in ECCM compared with ECUM. Since VCAM-1 acts as a receptor for PRBC (Ockenhouse et al., 1992), this phenomenon might also contribute to the amplification of sequestration in CM patients. The upregulation of CD61, which is involved in the binding of activated platelets to EC, may account for the phenomenon of platelet accumulation in cerebral microvessels, a histological feature of fatal CM (for review see Moxon et al., 2009). However, no commensurate upregulation of CD51, its pairing beta integrin chain to form the αvβ3 receptor, was measured and the significance of this finding will require further investigation.
Inter-individual differences in the endothelial inflammatory response to TNF in our study also included the production of membrane MP. A recent study in Malawian children demonstrated significantly higher levels of circulating endothelial MP during the acute phase of CM compared with levels observed in parasitaemic controls. Levels of endothelial MP correlated positively with plasma levels of TNF and returned to normal following recovery (Combes et al., 2004). In the present study, in vitro cultures of ECCM released significantly higher numbers of MP when exposed to TNF than did cultures of ECUM. We do not know whether this increased release plays a role in the cascade of events leading to CM, but recent analyses in a mouse model provided evidence for both pro-inflammatory and pro-coagulant effects of MP (Combes et al., 2005), suggesting that MP could potentially enhance the severity of intracerebral pathology.
In addition to monocytes, the vascular endothelium is a major source from which circulating cytokines and chemokines can be derived, especially in inflammatory conditions. This is particularly true for IL-6, MCP-1 and RANTES (Krishnaswamy et al., 1999). Using the ex vivo model we showed that ECUM release less MCP-1 and IL-6 under TNF stimulation than ECCM, while the release of RANTES is similar in both groups. MCP-1 is a CC chemokine produced by macrophages and EC in response to diverse stimuli, including TNF (Murao et al., 1999), attracting both monocytes and lymphocytes (Carr et al., 1994). Mononuclear leucocytes have been identified among the sequestered cells in brain venules in fatal paediatric CM (Grau et al., 2003), and monocytes expressing the appropriate ligand can bind to ICAM-1. Local MCP-1 release coupled to upregulation of ICAM-1 might together be responsible for both the recruitment and the binding of monocytes on cerebral microvasculature (Beekhuizen et al., 1990).
Like MCP-1, RANTES is a chemokine that can be released by EC under a pro-inflammatory stimulus, but there was no difference in RANTES production in cells from both patient categories. These results suggest a selective triggering by TNF of the expression and secretion of MCP-1 but not of RANTES, similar to the one reported in studies of human herpesvirus 8-infected HUVEC cultures (Caselli et al., 2007). Alternatively, there may be a post-transcriptional inhibition of RANTES release. Our findings are in keeping with the reported observation that low plasma levels of RANTES are associated with mortality in children with CM (John et al., 2006).
The higher level of IL-6 by ECCM than ECUM observed in this study is in line with data showing that elevated plasma levels of IL-6 are associated with severe malaria (Larkin et al., 2009). IL-6 together with TNF can increase cerebral EC permeability (Duchini et al., 1996), which could contribute to the cerebral oedema observed in CM (Patnaik et al., 1994). IL-6 might also aggravate PRBC and leucocyte cytoadherence by upregulating ICAM-1 and VCAM-1 on the EC surface (Watson et al., 1996). IL-6 has been reported to inhibit the ability of plasma enzyme ADAMTS13 to cleave full-length VWF (Bernardo et al., 2004), possibly leading to procoagulant events characteristically seen in severe malaria, including the adhesion and clumping of platelets to circulating or endothelial surface-anchored ultra large VWF multimers (Bridges et al., 2010).
The induction of endothelial apoptosis during CM has been widely described in both in vitro (Wassmer et al., 2006) and murine models of the pathology (Wiese et al., 2006). Its occurrence in vivo during human CM, however, remains debated. In the present study we observed a significantly higher level of caspase-3 cleavage in ECCM compared with ECUM, which is consistent with previous results. However, since circulating levels of the cytokine rarely exceed 1 pg ml−1 during human malaria infection, the induction of apoptosis is likely to involve a massive local release of TNF in areas of intense sequestration. This is in line with previous reports of focal accumulation of TNF in the brain of patients who died of CM (Udomsangpetch et al., 1997). Whether these findings accurately reflect the completion of the whole pro-apoptotic cascade in vivo is not known.
Taken together, our findings demonstrate a difference in responsiveness to TNF between EC derived from CM patients and those derived from UM patients. One explanation for this difference might be that EC responsiveness in vitro is affected by the severity of disease in the patient from whom the EC were collected. This explanation is unconvincing, because the EC with which we worked had been cultured through several cell cycles during a period of up to 3 weeks. Any influence of conditions prevailing in vivo is unlikely to have persisted throughout this period. An alternative explanation is that there is an inherent diversity among individuals in the responsiveness of their vascular EC to pro-inflammatory stimuli. This might result from (i) a variation in TNF ‘intake’ by the cells, (ii) a long-term effect due to specific P. falciparum exposure, (iii) a genetic inter-individual variation in reactivity to inflammation or (iv) a combination of all the factors. The differences we observed between ECCM and ECUM would then suggest that greater antecedent EC responsiveness predisposed some individuals to the development of more severe disease. Inherent diversity of individuals in the responsiveness of their EC to TNF stimulation has recently been reported by Beck and colleagues in a study of cultured lines of HUVEC (Beck et al., 2006) and in the fever effect on PRBC cytoadherence in individual HUVEC isolated from Kenyan mothers (A.G. Craig, unpubl. data). In addition, a recent study in Ugandan children and Thai adults revealed that TNF levels do not clearly discriminate between UM and CM patients (Lovegrove et al., 2009), suggesting that a differential responsiveness to this cytokine rather than a differential production might account for the clinical outcome of malaria infection.
Experimental procedures
Patients
We studied cases of UM and CM admitted to the paediatric research ward of the Queen Elizabeth Central Hospital, Blantyre, Malawi between January 2007 and August 2009. All patients with UM (n = 6; four males and two females; mean age 4.7 years, mean parasitaemia 53 349 parasites µl−1, parasitaemia range 151 500) had P. falciparum parasitaemia and a packed cell volume above 25% and were fully conscious (Blantyre coma score 5/5). UM patients' relatives gave fully informed consent for a subcutaneous fat tissue sample to be taken. This was performed by needle aspiration using a hollow bore ‘menghini’ needle on the upper external thigh after application of local anaesthetic cream for 2 h to temporarily numb the surface of the skin (EMLA Cream 5%, AstraZeneca). Patients with CM (n = 7; five males and two females; mean age 3.3 years, mean parasitaemia 43 141 parasites µl−1, parasitaemia range 90 724) were admitted to the hospital in coma (Blantyre coma score 2/5 or less), had P. falciparum parasitaemia with no other clinically evident cause of unconsciousness and were found to have malarial retinopathy (for review, see Beare et al., 2006). They were treated with quinine, as described elsewhere (Taylor et al., 2004). Two patients who recovered had subcutaneous fat tissue sample collected in the ward as described above. Five patients died of CM and once consent was granted from parents or guardians (Taylor et al., 2004), a full autopsy was conducted at the mortuary of the Queen Elizabeth Central Hospital, during which brain and subcutaneous fat tissue samples were collected (approximately 5 mm3). The autopsy or the subsequent histological studies confirmed the diagnosis for these five CM patients. This study was conducted according to the principles expressed in the Declaration of Helsinki and was approved by the ethical review committees of the College of Medicine, University of Malawi, the Liverpool School of Tropical Medicine and Michigan State University. The parents, relatives or guardians of all patients provided written informed consent for the collection of samples and subsequent analysis.
Isolation, purification and culture of EC derived from patients
Subcutaneous fat tissue-derived EC were obtained from all patients (fatal CM at autopsy and UM in the ward, Fig. 5) while brain EC were only obtained in fatal CM cases (Fig. 6). For clarity, EC derived from CM patients will be referred to as ECCM and EC derived from UM patients as ECUM. Cells were isolated following a protocol adapted from Hutley et al. (2001) as described below.
Fig. 5.
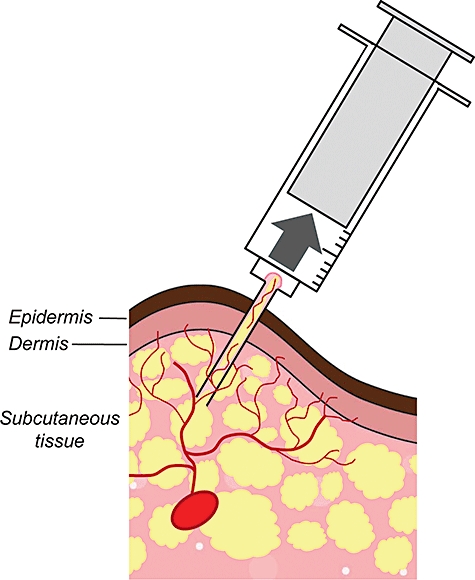
Schematic representation of needle aspiration biopsy. The diagram shows the technique used to isolate subcutaneous fat-derived fat EC by inserting a hollow bore ‘menghini’ needle in the upper external thigh of patients with malaria (UM and CM) after application of a local anaesthetic for 2 h. A strong manual aspiration force represented by the arrow allowed the sampling of approximately 5 mm3 of adipose tissue, from which EC were isolated, selected and cultured before analysis.
Fig. 6.

Method for sampling and isolating ECCM from brain and subcutaneous fat tissue. ECCM were collected at autopsy and isolated from both subcutaneous fat and brain tissue from patients who died of CM (n = 7). Brain ECCM were only used for a phenotypical comparison with subcutaneous fat-derived ECCM in the first part of our study to assess the relevance of the latter as an accurate model of cerebral endothelium.
Isolation
Briefly, biopsies were transported to the laboratory in PBS and transferred to gentleMACS™ Dissociator C-tubes (Miltenyi Biotec). Both brain and subcutaneous tissues were treated as follows: (i) samples were finely minced and incubated for 15 min at 37°C in digest solution (in mM: 25 Hepes, 5 glucose, 120 sodium chloride, 50 potassium chloride and 1 calcium chloride) containing 3 mg ml−1 type II collagenase and 1.5% bovine serum albumin (BSA). The ratio of digest solution to tissue was 4:1. C-tubes were then connected to the gentleMACS™ Dissociator and spun for 60 s before being incubated for 15 additional minutes at 37°C. The operation was repeated three times. The resultant digest material was filtered through a 250 µm mesh (Sefar), and adipocytes and free oil were separated from the stromovascular components by centrifugation at 250 g for 5 min at 4°C. (ii) The stromovascular pellet was resuspended, washed and centrifuged in DPBS containing 10% BSA (500 g, 5 min, 4°C). This was repeated and followed by a final wash in DPBS alone. (iii) The resulting pellet was incubated in 0.25% trypsin containing 1 mM EDTA (Invitrogen) for 15 min at room temperature with gentle automated agitation. Trypsin was neutralized by addition of Hanks' balanced salt solution (HBSS) containing 5% fetal bovine serum (FBS; Sigma). (iv) Large fragments of connective tissue were then removed by filtration through 100 µm mesh (Sefar). (v) The filtrate was centrifuged (500 g, 5 min, 4°C), and the pellet was resuspended and plated into 1% gelatine-coated 25 cm2 culture flasks (Corning) in DMEM-F12 medium (Invitrogen) supplemented with EGM-2 MV SingleQuots (Lonza), 150 µg ml−1 EC growth supplement (ECGS, Sigma) and 30 ng ml−1β-EC growth factor (β-ECGF). This mixed cell population was cultured for 3–5 days at 37°C in 5% CO2.
Selection of microvascular endothelial cells (MVEC) with anti-CD31 Dynabeads
After a culture period of 7–10 days, the cells were incubated with Trypsin-EDTA for 4–5 min at 37°C, followed by neutralization of trypsin with HBSS containing 5% FBS and centrifugation. The pelleted cells were resuspended in 1 ml of PBS containing 0.1% FBS and incubated with 50 µl of anti-CD31-coated Dynabeads under gentle agitation (20 min, 4°C). The cell/bead suspension was brought to a total volume of 10 ml with 0.1% FBS in PBS, and EC were selected using a magnetic particle concentrator for 3 min at room temperature. With the tube still in the magnet, non-selected cells were discarded and EC were then washed with a further 10 ml of PBS containing 0.1% FBS and reselected using the magnetic particle concentrator (3 min). This wash/selection procedure was repeated five times. Selected cells (EC) were plated on to 1% gelatin-coated culture flask in EC growth medium as described above.
Cell culture
Cells were maintained at 37°C in an atmosphere of 5% CO2. The medium was changed every 2–3 days. As EC numbers increased, the concentration of β-ECGF in the growth medium was decreased from 30 to 10 ng ml−1. Brain and subcutaneous fat tissue-derived EC were used in experimental work between passages 2 and 4.
Characterization and phenotypic comparison of subcutaneous fat and brain-derived ECCM
In order to assess the relevance of subcutaneous fat-derived EC as a representative model for cerebral endothelium, cells obtained from brain and adipose tissue at autopsy were characterized and compared as described below.
Morphology
Cultures were examined by inverted phase-contrast microscopy for the characteristic cobblestone-like morphology of EC.
Immunofluorescence
Confluent EC were rinsed with PBS, fixed with 1% w/v of paraformaldehyde (PFA) for 15 min on ice, washed with PBS and incubated with FITC-coupled mouse anti-human CD31, CD54, CD36 (PECAM-1, clone 5.6E; ICAM-1, clone 84H10 and clone FA6.152, Beckman Coulter Immunotech), mouse anti-human VWF (clone F6/86, DAKO Cytomation) revealed by a secondary goat anti-mouse Alexa488® -coupled mAb (Molecular Probes) or FITC-coupled anti-ZO-1 (clone ZO-1-1A12, Zymed Laboratory). A non-specific isotype-matched mouse IgG1 (Beckman-Coulter Immunotech) was used for all controls. The expression of E-selectin (CD62E) was also investigated by use of a monoclonal antibody (clone CL2/6, Serotec) revealed by a secondary goat anti-mouse Alexa594®-coupled mAb (Molecular Probes). In this case, the immunofluorescence analysis was performed with cells pre-treated for 6 h with TNF (10 ng ml−1, Sigma).
Flow cytometry
Confluent monolayers of EC were stimulated, or not, with TNF (overnight or 6 h, 10 ng ml−1) before analysis. EC were then harvested by a short trypsin-EDTA treatment, washed, and labelled by indirect labelling using mouse anti-human CD106, CD54, CD51, CD61, CD31, CD36 (Beckman-Coulter Immunotech), CD40 (Diaclone), CD62E and CD146 (Serotec) mAbs as first step (as detailed in Table 1) and then with secondary goat anti-mouse Alexa488® -coupled mAb (Molecular Probes, Eugene, USA). A non-specific isotype-matched mouse IgG1 (Beckman-Coulter Immunotech) was used for all controls. Cells were then resuspended in PBS before flow cytometry analysis on a FACScalibur (Becton Dickinson). Alexa488® signal was detected in the FL1 channel (488 nm laser and 530/30 band-pass filter). The area corresponding to EC was defined, and mean fluorescence intensities (MFI) of the positive cell populations were measured for each antigen.
Determination of adhesion molecules expression by TNF-activated ECUM and ECCM using cell-based ELISA and flow cytometry
Endothelial cells were seeded on 1% gelatin pre-coated flat-bottom 96-well plates (104 cells well−1) and grown to confluence. Cells were then washed with DMEM-F12 medium and stimulated overnight separately with increasing concentrations of TNF (0, 0.01, 0.1, 1 and 10 ng ml−1). After stimulation, cells were washed a second time with DMEM-F12, fixed with −20°C methanol for 15 min at room temperature and incubated with PBS containing 5% FCS and 0.05% Tween (Merck) to block non-specific binding. Cells were then incubated, respectively, with monoclonal antibodies to human ICAM-1, VCAM-1, CD61 (Beckman-Coulter Immunotech) and CD62-E (Serotec), all at 10 µg ml−1 for 40 min at room temperature. For ELISA, the plate was then washed three times in PBS/Tween 20 and incubated for 45 min under mild shaking with 1 mg ml−1 goat anti-mouse IgG alkaline phosphatase conjugate (Promega). Cells were then washed twice with HBSS containing 5% BSA and once with 2.5 M diethanolamine (pH 9.5). Finally, the substrate solution was added, consisting of 0.58 mg ml−1 Attophos fluorescent substrate (Promega) and 2.4 mg ml−1 endogenous phosphatase activity blocking agent levamisole hydrochloride (Sigma), diluted in diethanolamine buffer. After 5 min, fluorescence was measured at an excitation wavelength 450 nm and an emission wavelength 580 nm in an automated ELISA reader. For flow cytometry analysis, cells were grown to confluence in 12-well plates and stimulated overnight with 10 ng ml−1 TNF. EC were then harvested by a short trypsin-EDTA treatment, washed, and labelled with monoclonal antibodies to human anti-ICAM-1, VCAM-1, CD61 (Beckman-Coulter Immunotech) and CD62-E (Serotec) as described above.
Analysis of MP production by ECUM and ECCM by flow cytometry
Confluent ECUM and ECCM were left unstimulated or activated by TNF (1 and 10 ng ml−1) for 6 h before analysis. Culture supernatants were then collected and centrifuged at 1500 g for 15 min to discard EC and debris. Endothelial MP were labelled using annexin V-FITC and resuspended in binding buffer (Beckman-Coulter Immunotech) as previously described (Combes et al., 2004). MP present in supernatants were then quantified by flow cytometry.
Quantification of MCP-1, RANTES and IL-6 release and caspase-3 activation levels by ELISA
Confluent cells were washed with DMEM-F12 medium and stimulated separately overnight with fresh culture medium containing increasing concentrations of TNF (0, 0.01, 0.1, 1 and 10 ng ml−1). For MCP-1, RANTES and IL-6, cell supernatants were harvested, centrifuged to remove cell debris and stored at −80°C. After thawing, samples were analysed for the presence of MCP-1, RANTES and IL-6 using specific quantitative enzyme-linked immunosorbent assays (ELISA) kits (R&D System Quantikine for MCP-1 and RANTES; Becton Dickinson OptEIA for IL-6). For active Caspase-3, cells were washed in PBS and cell extracts were prepared according to the manufacturer's instruction before being analysed immediately by ELISA (R&D System Quantikine). Each sample was tested in triplicate.
Statistics
Statistical analyses were performed with STATA 8 ©. Data were analysed by the Kruskall–Wallis and Dunn's pairwise tests. Results are expressed as means ± standard deviations. A value of P < 0.05 was considered significant.
Acknowledgments
We would like to thank the clinicians and the nurses of the Paediatric Research Ward, Queen Elizabeth Central Hospital, Blantyre, Malawi for providing patient care. For conducting the autopsies, we thank mortuary attendants D. Kotokwa and W. Namanya, and pathologists Dr S. Kamiza, Dr C. Dzamalala and Dr D. Milner. We are grateful to the patients' families for the privilege of conducting the present study. This work was funded by grants from the Wellcome Trust, UK (to S.C.W., Grant #080948/Z/06/Z) and from the National Institutes of Health, USA (to T.E.T., Grant AI R01-34969).
References
- Beare NA, Taylor TE, Harding SP, Lewallen S, Molyneux ME. Malarial retinopathy: a newly established diagnostic sign in severe malaria. Am J Trop Med Hyg. 2006;75:790–797. [PMC free article] [PubMed] [Google Scholar]
- Beck GC, Rafat N, Brinkkoetter P, Hanusch C, Schulte J, Haak M, et al. Heterogeneity in lipopolysaccharide responsiveness of endothelial cells identified by gene expression profiling: role of transcription factors. Clin Exp Immunol. 2006;143:523–533. doi: 10.1111/j.1365-2249.2006.03005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekhuizen H, Corsel-van Tilburg AJ, van Furth R. Characterization of monocyte adherence to human macrovascular and microvascular endothelial cells. J Immunol. 1990;145:510–518. [PubMed] [Google Scholar]
- Belloni PN, Carney DH, Nicolson GL. Organ-derived microvessel endothelial cells exhibit differential responsiveness to thrombin and other growth factors. Microvasc Res. 1992;43:20–45. doi: 10.1016/0026-2862(92)90004-9. [DOI] [PubMed] [Google Scholar]
- Berendt AR, Tumer GD, Newbold CI. Cerebral malaria: the sequestration hypothesis. Parasitol Today. 1994;10:412–414. doi: 10.1016/0169-4758(94)90238-0. [DOI] [PubMed] [Google Scholar]
- Bernardo A, Ball C, Nolasco L, Moake JF, Dong JF. Effects of inflammatory cytokines on the release and cleavage of the endothelial cell-derived ultralarge von Willebrand factor multimers under flow. Blood. 2004;104:100–106. doi: 10.1182/blood-2004-01-0107. [DOI] [PubMed] [Google Scholar]
- Bonen A, Tandon NN, Glatz JF, Luiken JJ, Heigenhauser GJ. The fatty acid transporter FAT/CD36 is upregulated in subcutaneous and visceral adipose tissues in human obesity and type 2 diabetes. Int J Obes (Lond) 2006;30:877–883. doi: 10.1038/sj.ijo.0803212. [DOI] [PubMed] [Google Scholar]
- Bridges DJ, Bunn J, van Mourik JA, Grau G, Preston RJ, Molyneux M, et al. Rapid activation of endothelial cells enables P. falciparum adhesion to platelet decorated von Willebrand factor strings. Blood. 2010;115:1472–1474. doi: 10.1182/blood-2009-07-235150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci USA. 1994;91:3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli E, Fiorentini S, Amici C, Di Luca D, Caruso A, Santoro MG. Human herpesvirus 8 acute infection of endothelial cells induces monocyte chemoattractant protein 1-dependent capillary-like structure formation: role of the IKK/NF-kappaB pathway. Blood. 2007;109:2718–2726. doi: 10.1182/blood-2006-03-012500. [DOI] [PubMed] [Google Scholar]
- Clark IA, Rockett KA. The cytokine theory of human cerebral malaria. Parasitol Today. 1994;10:410–412. doi: 10.1016/0169-4758(94)90237-2. [DOI] [PubMed] [Google Scholar]
- Collins NT, Cummins PM, Colgan OC, Ferguson G, Birney YA, Murphy RP, et al. Cyclic strain-mediated regulation of vascular endothelial occludin and ZO-1: influence on intercellular tight junction assembly and function. Arterioscler Thromb Vasc Biol. 2006;26:62–68. doi: 10.1161/01.ATV.0000194097.92824.b3. [DOI] [PubMed] [Google Scholar]
- Combes V, Taylor TE, Juhan-Vague I, Mege JL, Mwenechanya J, Tembo M, et al. Circulating endothelial microparticles in Malawian children with severe falciparum malaria complicated with coma. JAMA. 2004;291:2542–2544. doi: 10.1001/jama.291.21.2542-b. [DOI] [PubMed] [Google Scholar]
- Combes V, Coltel N, Alibert M, van Eck M, Raymond C, Juhan-Vague I, et al. ABCA1 gene deletion protects against cerebral malaria: potential pathogenic role of microparticles in neuropathology. Am J Pathol. 2005;166:295–302. doi: 10.1016/S0002-9440(10)62253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combes V, Coltel N, Faille D, Wassmer SC, Grau GE. Cerebral malaria: role of microparticles and platelets in alterations of the blood–brain barrier. Int J Parasitol. 2006;36:541–546. doi: 10.1016/j.ijpara.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Duchini A, Govindarajan S, Santucci M, Zampi G, Hofman FM. Effects of tumor necrosis factor-alpha and interleukin-6 on fluid-phase permeability and ammonia diffusion in CNS-derived endothelial cells. J Investig Med. 1996;44:474–482. [PubMed] [Google Scholar]
- Gnant MF, Turner EM, Alexander HR., Jr Effects of hyperthermia and tumour necrosis factor on inflammatory cytokine secretion and procoagulant activity in endothelial cells. Cytokine. 2000;12:339–347. doi: 10.1006/cyto.1999.0568. [DOI] [PubMed] [Google Scholar]
- Grau GE, de Kossodo S. Cerebral malaria: mediators, mechanical obstruction or more? Parasitol Today. 1994;10:408–409. doi: 10.1016/0169-4758(94)90236-4. [DOI] [PubMed] [Google Scholar]
- Grau GE, Lou J. TNF in vascular pathology: the importance of platelet–endothelium interactions. Res Immunol. 1993;144:355–363. doi: 10.1016/s0923-2494(93)80080-i. [DOI] [PubMed] [Google Scholar]
- Grau GE, Taylor TE, Molyneux ME, Wirima JJ, Vassalli P, Hommel M, Lambert PH. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med. 1989;320:1586–1591. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- Grau GE, Mackenzie CD, Carr RA, Redard M, Pizzolato G, Allasia C, et al. Platelet accumulation in brain microvessels in fatal pediatric cerebral malaria. J Infect Dis. 2003;187:461–466. doi: 10.1086/367960. [DOI] [PubMed] [Google Scholar]
- Grell M, Zimmermann G, Gottfried E, Chen CM, Grunwald U, Huang DC, et al. Induction of cell death by tumour necrosis factor (TNF) receptor 2, CD40 and CD30: a role for TNF-R1 activation by endogenous membrane-anchored TNF. EMBO J. 1999;18:3034–3043. doi: 10.1093/emboj/18.11.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M, White NJ. Molecular mechanisms of cytoadherence in malaria. Am J Physiol. 1999;276:C1231–C1242. doi: 10.1152/ajpcell.1999.276.6.C1231. [DOI] [PubMed] [Google Scholar]
- Hunt NH, Grau GE. Cytokines: accelerators and brakes in the pathogenesis of cerebral malaria. Trends Immunol. 2003;24:491–499. doi: 10.1016/s1471-4906(03)00229-1. [DOI] [PubMed] [Google Scholar]
- Hutley LJ, Herington AC, Shurety W, Cheung C, Vesey DA, Cameron DP, Prins JB. Human adipose tissue endothelial cells promote preadipocyte proliferation. Am J Physiol Endocrinol Metab. 2001;281:E1037–E1044. doi: 10.1152/ajpendo.2001.281.5.E1037. [DOI] [PubMed] [Google Scholar]
- John CC, Opika-Opoka R, Byarugaba J, Idro R, Boivin MJ. Low levels of RANTES are associated with mortality in children with cerebral malaria. J Infect Dis. 2006;194:837–845. doi: 10.1086/506623. [DOI] [PubMed] [Google Scholar]
- Kern PA, Knedler A, Eckel RH. Isolation and culture of microvascular endothelium from human adipose tissue. J Clin Invest. 1983;71:1822–1829. doi: 10.1172/JCI110937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H, Gules I, Meguro T, Zhang JH. Cytotoxicity of cytokines in cerebral microvascular endothelial cell. Brain Res. 2003;990:148–156. doi: 10.1016/s0006-8993(03)03450-4. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy G, Kelley J, Yerra L, Smith JK, Chi DS. Human endothelium as a source of multifunctional cytokines: molecular regulation and possible role in human disease. J Interferon Cytokine Res. 1999;19:91–104. doi: 10.1089/107999099314234. [DOI] [PubMed] [Google Scholar]
- Larkin D, de Laat B, Jenkins PV, Bunn J, Craig AG, Terraube V, et al. Severe Plasmodium falciparum malaria is associated with circulating ultra-large von Willebrand multimers and ADAMTS13 inhibition. PLoS Pathog. 2009;5:e1000349. doi: 10.1371/journal.ppat.1000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K, Gaehtgens P, Spanel-Borowski K. Differential adhesion of granulocytes to five distinct phenotypes of cultured microvascular endothelial cells. Microvasc Res. 1992;43:119–133. doi: 10.1016/0026-2862(92)90011-d. [DOI] [PubMed] [Google Scholar]
- Lou J, Gasche Y, Zheng L, Critico B, Monso-Hinard C, Juillard P, et al. Differential reactivity of brain microvascular endothelial cells to TNF reflects the genetic susceptibility to cerebral malaria. Eur J Immunol. 1998;28:3989–4000. doi: 10.1002/(SICI)1521-4141(199812)28:12<3989::AID-IMMU3989>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Lovegrove FE, Tangpukdee N, Opoka RO, Lafferty EI, Rajwans N, Hawkes M, et al. Serum angiopoietin-1 and -2 levels discriminate cerebral malaria from uncomplicated malaria and predict clinical outcome in African children. PLoS ONE. 2009;4:e4912. doi: 10.1371/journal.pone.0004912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson GG, Warrell MJ, White NJ, Looareesuwan S, Warrell DA. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985;119:385–401. [PMC free article] [PubMed] [Google Scholar]
- Moxon CA, Heyderman RS, Wassmer SC. Dysregulation of coagulation in cerebral malaria. Mol Biochem Parasitol. 2009;166:99–108. doi: 10.1016/j.molbiopara.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murao K, Imachi H, Momoi A, Sayo Y, Hosokawa H, Sato M, et al. Thiazolidinedione inhibits the production of monocyte chemoattractant protein-1 in cytokine-treated human vascular endothelial cells. FEBS Lett. 1999;454:27–30. doi: 10.1016/s0014-5793(99)00765-6. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Fujioka H, Luc KD, Rabbege JR, Todd GD, Collins WE, Aikawa M. A correlation of the sequestration rate of Plasmodium coatneyi-infected erythrocytes in cerebral and subcutaneous tissues of a rhesus monkey. Am J Trop Med Hyg. 1996;55:311–314. doi: 10.4269/ajtmh.1996.55.311. [DOI] [PubMed] [Google Scholar]
- Newbold C, Warn P, Black G, Berendt A, Craig A, Snow B, et al. Receptor-specific adhesion and clinical disease in Plasmodium falciparum. Am J Trop Med Hyg. 1997;57:389–398. doi: 10.4269/ajtmh.1997.57.389. [DOI] [PubMed] [Google Scholar]
- Ockenhouse CF, Tegoshi T, Maeno Y, Benjamin C, Ho M, Kan KE, et al. Human vascular endothelial cell adhesion receptors for Plasmodium falciparum-infected erythrocytes: roles for endothelial leukocyte adhesion molecule 1 and vascular cell adhesion molecule 1. J Exp Med. 1992;176:1183–1189. doi: 10.1084/jem.176.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patnaik JK, Das BS, Mishra SK, Mohanty S, Satpathy SK, Mohanty D. Vascular clogging, mononuclear cell margination, and enhanced vascular permeability in the pathogenesis of human cerebral malaria. Am J Trop Med Hyg. 1994;51:642–647. [PubMed] [Google Scholar]
- Pober JS, Cotran RS. Cytokines and endothelial cell biology. Physiol Rev. 1990;70:427–451. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- Rowe AK, Rowe SY, Snow RW, Korenromp EL, Schellenberg JR, Stein C, et al. The burden of malaria mortality among African children in the year 2000. Int J Epidemiol. 2006;35:691–704. doi: 10.1093/ije/dyl027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield L, Novakovic S, Gerold P, Schwarz RT, McConville MJ, Tachado SD. Glycosylphosphatidylinositol toxin of Plasmodium up-regulates intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and E-selectin expression in vascular endothelial cells and increases leukocyte and parasite cytoadherence via tyrosine kinase-dependent signal transduction. J Immunol. 1996;156:1886–1896. [PubMed] [Google Scholar]
- Seydel KB, Milner DA, Jr, Kamiza SB, Molyneux ME, Taylor TE. The distribution and intensity of parasite sequestration in comatose Malawian children. J Infect Dis. 2006;194:208–205. doi: 10.1086/505078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Staddon JM, Herrenknecht K, Smales C, Rubin LL. Evidence that tyrosine phosphorylation may increase tight junction permeability. J Cell Sci. 1995;108(Part 2):609–619. doi: 10.1242/jcs.108.2.609. [DOI] [PubMed] [Google Scholar]
- Taylor TE, Fu WJ, Carr RA, Whitten RO, Mueller JS, Fosiko NG, et al. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med. 2004;10:143–145. doi: 10.1038/nm986. [DOI] [PubMed] [Google Scholar]
- Turner GD, Morrison H, Jones M, Davis TM, Looareesuwan S, Buley ID, et al. An immunohistochemical study of the pathology of fatal malaria. Evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule-1 in cerebral sequestration. Am J Pathol. 1994;145:1057–1069. [PMC free article] [PubMed] [Google Scholar]
- Udomsangpetch R, Chivapat S, Viriyavejakul P, Riganti M, Wilairatana P, Pongponratin E, Looareesuwan S. Involvement of cytokines in the histopathology of cerebral malaria. Am J Trop Med Hyg. 1997;57:501–506. doi: 10.4269/ajtmh.1997.57.501. [DOI] [PubMed] [Google Scholar]
- Wassmer SC, Combes V, Grau GE. Pathophysiology of cerebral malaria: role of host cells in the modulation of cytoadhesion. Ann N Y Acad Sci. 2003;992:30–38. doi: 10.1111/j.1749-6632.2003.tb03135.x. [DOI] [PubMed] [Google Scholar]
- Wassmer SC, Combes V, Candal FJ, Juhan-Vague I, Grau GE. Platelets potentiate brain endothelial alterations induced by Plasmodium falciparum. Infect Immun. 2006;74:645–653. doi: 10.1128/IAI.74.1.645-653.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C, Whittaker S, Smith N, Vora AJ, Dumonde DC, Brown KA. IL-6 acts on endothelial cells to preferentially increase their adherence for lymphocytes. Clin Exp Immunol. 1996;105:112–119. doi: 10.1046/j.1365-2249.1996.d01-717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese L, Kurtzhals JA, Penkowa M. Neuronal apoptosis, metallothionein expression and proinflammatory responses during cerebral malaria in mice. Exp Neurol. 2006;200:216–226. doi: 10.1016/j.expneurol.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Wilairatana P, Riganti M, Puchadapirom P, Punpoowong B, Vannaphan S, Udomsangpetch R, et al. Prognostic significance of skin and subcutaneous fat sequestration of parasites in severe falciparum malaria. Southeast Asian J Trop Med Public Health. 2000;31:203–212. [PubMed] [Google Scholar]
- Zwaal RF, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89:1121–1132. [PubMed] [Google Scholar]


