Abstract
Biliverdin reductase A (BLVRA), an enzyme that converts biliverdin to bilirubin, has recently emerged as a key regulator of the cellular redox cycle. However, the role of BLVRA in the aging process remains unclear. To study the role of BLVRA in the aging process, we compared the stress responses of young and senescent human diploid fibroblasts (HDFs) to the reactive oxygen species (ROS) inducer, hydrogen peroxide (H2O2). H2O2 markedly induced BLVRA activity in young HDFs, but not in senescent HDFs. Additionally, depletion of BLVRA reduced the H2O2-dependent induction of heme oxygenase-1 (HO-1) in young HDFs, but not in senescent cells, suggesting an aging-dependent differential modulation of responses to oxidative stress. The role of BLVRA in the regulation of cellular senescence was confirmed when lentiviral RNAitransfected stable primary HDFs with reduced BLVRA expression showed upregulation of the CDK inhibitor family members p16, p53, and p21, followed by cell cycle arrest in G0-G1 phase with high expression of senescence-associated β-galactosidase. Taken together, these data support the notion that BLVRA contributes significantly to modulation of the aging process by adjusting the cellular oxidative status.
Keywords: biliverdin reductase, cell aging, fibroblasts, heme oxygenase-1, reactive oxygen species
Introduction
Reactive oxygen species (ROS) are involved in the pathogenesis of various human diseases and in the aging process. ROS-related oxidative stress is the result of an imbalance between the generation and scavenging of ROS. Thus, restoration of the balance would be necessary for modulation of the aging process. A sub-lethal concentration of prooxidants induces premature cellular senescence (Chen and Ames, 1994; Chen et al., 1998), and ROS levels are higher in old cells than in younger cells (Hagen et al., 1997; Lee et al., 1999). Therefore, cells treated with antioxidants or grown under low oxygen conditions may maintain a youthful state for a longer time (Chen et al., 1995; Yuan et al., 1995).
Biliverdin reductase A (BLVRA) is an evolutionarily conserved enzyme that converts biliverdin to bilirubin (Beale and Cornejo, 1984; Schluchter and Glazer, 1997; Maines, 2005). Recent studies have shown that BLVRA has ROS-scavenging abilities through its production of bilirubin, a potent physiological antioxidant (Baranano et al., 2002; Florczyk et al., 2008; Wu et al., 2008; Sedlak et al., 2009). As little as 10 nM bilirubin can protect cells against almost 10,000-fold higher concentrations of H2O2 (Baranano et al., 2002). The high efficacy of bilirubin as an antioxidant is attributable to renewing cycle in which bilirubin is oxidized to biliverdin, which is recycled back to bilirubin by BLVRA (Baranano et al., 2002; Sedlak and Snyder, 2004). Biliverdin is a product of heme cleavage by heme oxygenase (HO). Two heme oxygenase isoforms have been reported, a constitutive isoform (HO-2) and an inducible antioxidant enzyme (HO-1). Recently, Tudor et al. (2008) have shown that BLVRA is involved in the regulation of HO-1 expression. This efficient antioxidative system consisting of cyclic cellular redox control and induction of HO-1 by BLVRA may fortify the capacity of bilirubin as one of the most effective physiological defense molecules against cellular oxidative stress.
Despite the reported efficient capacity of the BLVRA-associated system for protection against oxidative stress, its role in the adjustment of the aging process has not been thoroughly studied. In the present study, we examined the role of BLVRA in the cellular response to oxidative stress and in the maintenance of a youthful cellular state, using human diploid fibroblast cells (HDF).
Results
Aging dependent differences in oxidative stress-induced BLVRA expression
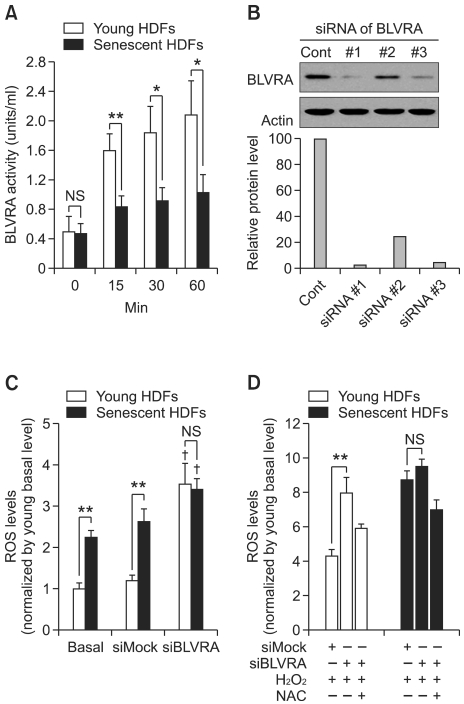
In order to test the relationship of BLVRA with cellular senescence, we first compared BLVRA activity in young and senescent HDF cells in response to H2O2 treatment. In senescent cells, BLVRA activity was not significantly changed by H2O2 in contrast to the significant increased activity in young cells (Figure 1A). For the role of BLVRA in ROS generation, the intracellular levels of ROS was monitored after knockdown of BLVRA by specific siRNA with use of the redox-sensitive fluorophore 2',7'-dichlorofluorescein diacetate (DCF). As shown in Figure 1C, ROS levels increased more than three-fold on day 4 after simple knockdown of BLVRA. In addition, the role of BLVRA against exogenous oxidative stress was tested after exposure of the cells to H2O2. Depletion of BLVRA led to the increased sensitivity to oxidative stress in young cells (Figure 1D). In contrast, the senescent cells did not show any significant changes toward oxidative stress by BLVRA depletion. These data suggest strongly that BLVRA would be involved in reduction of cellular oxidative stress, especially in the young cells.
Figure 1.
BVRA depletion markedly increases the ROS generation in young HDF cells. (A) Young and senescent HDF cells were treated with 1 mM H2O2 for the indicated periods. After lysis, the cell extract was prepared, and the reductase activity was measured at pH 8.7. The activity analysis was repeated using three separate preparations of HDF *P ≤ 0.05 and **P ≤ 0.01 when compared with young HDFs. (B) Young HDFs were transfected with three different siRNAs of BLVRA and control siRNA using Oligofectamine. After 72 h, cells were harvested. Protein expression of BLVRA were analyzed by Western blotting using anti-BLVRA antibody (upper panel). All proteins were normalized to the densitometric signal of the actinin loading control (lower panel). (C) After transfection with BLVRA siRNA or a control siRNA for 72 h, cells were stained with dichlorofluorescein diacetate (DCF-DA), fixed and immediately analyzed by FACS. The data are mean ± SEM of three independent experiments. A double asterisk (**) denotes P < 0.01 in Student's t-test. (†) denotes P < 0.01 compared with non-transfected cells (ANOVA, Dunnett was used as post-test). (D) Effect of BLVRA deficiency on intracellular ROS level in young and senescent cells undergoing oxidative stress. After transfection with BLVRA siRNA or a control siRNA for 72 h, cells were treated with 1 mM H2O2 with/without NAC (20 mM), then stained with dichlorofluorescein diacetate (DCF-DA), fixed and immediately analyzed by FACS. The data are mean ± SEM of three independent experiments. A double asterisk (**) denotes P < 0.01 in Student's t-test. DCF: dichlorofluorescein.
Aging dependent differences in induction of HO-1 by depletion of BLVRA
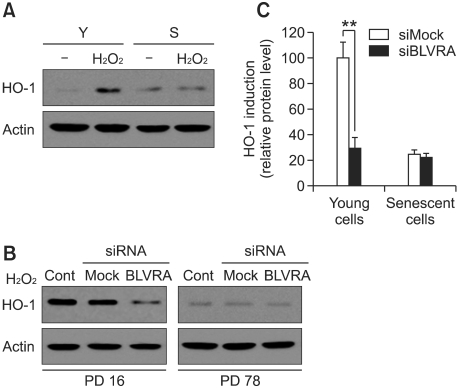
HO-1 is one of the critical proteins responsible for regulating cellular stress. The induction of HO-1 is reported to be regulated by BLVRA (Tudor et al., 2008). Western blot analysis indicated that H2O2 dependent induction of HO-1 was much higher in young cells than in old cells (Figure 2A). Moreover, it is confirmed that this H2O2 dependent induction of HO-1 was effectively reduced by BLVRA depletion in young cells. However, this effect was not evident in senescent cells (Figure 2B).
Figure 2.
Failure of H2O2-induced HO-1 induction in senescent HDFs. (A) Young and s enescent HDF cells were treated with 500 uM H2O2 for 4 h. After lysis, the cell extract was prepared, and the level of HO-1 were determined by Western blotting using anti-HO-1antibody. (B) Young and Senescent HDFs were transfected with siRNAs of BLVRA and control siRNA using Oligofectamine. After 72 h, HDF cells were treated with 500 uM H2O2 for 4 h. After lysis, the cell extract was prepared and protein expression of BLVRA were analyzed by Western blotting using anti-BLVRA antibody (C) Quantitative graph of HO-1. The results shown are representative of three independent experiments; the histograms represent average and error bars represent standard deviations of the means. A double asterisk (**) denotes P < 0.01 in Student's t-test.
Increased sensitivity to oxidative stress by depletion of BLVRA
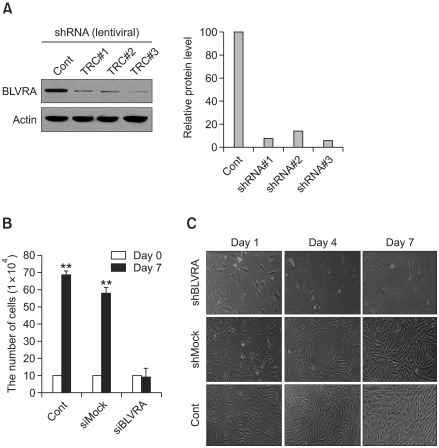
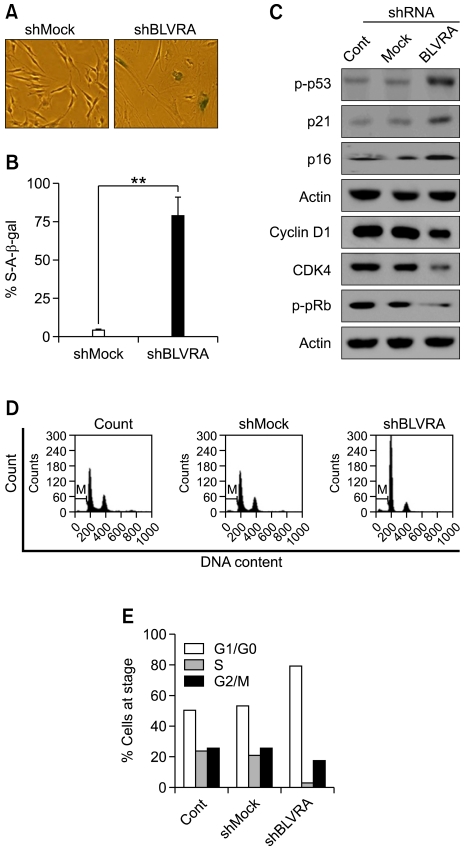
In order to assess the role of endogenous BLVRA in cellular protection against physiological levels of oxidative stress, we monitored the differences in proliferation rates of HDF cells for 7 days after transfection with shRNA against BLVRA or mock shRNA. Efficacy of this shRNA was sufficient to suppress BLVRA expression (Figure 3A). Depletion of BLVRA could reduce the cell number by > 60% in young HDFs even without additive exogenous oxidative stress. In contrast, mock shRNA did not significantly affect cell proliferation (Figure 3B). In addition, morphological analysis indicated that HDF cells became enlarged and flattened after BLVRA shRNA treatment (Figure 3C). Moreover, knockdown of BLVRA led to induce the expression of the senescence marker SA-β-gal (Figures 4A and 4B). These data strongly implicate that knockdown of BLVRA would induce the premature senescence in HDF cells and that the enzyme might be one of the major cellular protective systems against oxidative stress in the physiological condition.
Figure 3.
Effects of BLVRA depletion on cell proliferation and cell death. (A) Three individual clones from shRNA target set (sigma NM_002467) were co-transfected with a lentivirus packaging plasmid into HDF cells. 24 h post-infection, 2.5 mg/ml of puromycin was added to select for infected cells. 72 h post-selection, cells were harvested. BLVRA protein level was detected by western blot analysis (left panel). All proteins were normalized to the densitometric signal of the actinin loading control (right panel). Actin was used as an loading control. (B) Exponentially growing HDF cells were transfected with shBLVRA along with shMock. (C) Morphologies of senescent HDFs after BLVRA downregulation were determined by light microscopy.
Figure 4.
hBVRA knockdown induces premature senescence in HDF cells. (A) hBVRA knockdown causes expression of SA-β-gal. shRNAs of BLVRA were transfected into HDF cells. Seven days post-transfection, cells were fixed and incubated with X-gal at pH 6.0 overnight and senescence-like phenotype was imaged with microscopy. (B) Percentage of cells that are positive senescence-associated β-galactosidase activity in three independent HDF populations from shMock or shBLVRA-transfected cells. (C) The effects of BLVRA knockdown on cell cycle regulators in HDF cells. Western blot analysis of whole-cell lysates from HDF cells collected at 7 days post-shBVRA transfection. On the top of gels, mock indicates lysate from control shRNA-transfected cells. p16ink4a, p21waf1, and p-p53, cyclin D1, p16/INK4a, p21/Cip1, phospho-pRb (Ser 795) on the right side of gels indicate the proteins detected with corresponding antibodies. Loading of equal amounts of protein was confirmed by probing for β-actin. (D, E). A representative diagram of cell cycle histogram showed HDF cell arrest in G0-G1 when cells were subjected to BLVRA shRNA knockdown (and mock shRNA) for 3 days prior to cell cycle analysis with flow cytometry.
Cell cycle arrest by depletion of BLVRA
In addition to senescence-associated β-galactosidase expression, we assessed the expression of p16ink4a, p21waf1, and p53 by Western blot analysis, which are highly up-regulated in various forms of senescent cells (Kastan et al., 1991; Johnson et al., 1994; Alcorta et al., 1996; Hara et al., 1996). Transfection of the cells with shRNA-BLVRA induced the expression levels of p53, 16, and p21 significantly (Figure 4C), indicating the arrest of the cell cycles. In order to understand the mode of cell cycle arrest by BLVRA knockdown, we carried out the flow cytometry. Seven days after transfection, HDF cells were grown to 70% confluence and cell cycle analysis was performed by propidium iodide uptake. As shown in Figures 4D and 4E, BLVRA knockdown cells were arrested in the G0-G1 phase of the cell cycle to approximately 78% of the cells whereas 53% of random shRNA-treated cells were arrested in the G0-G1 phase of the cell cycle. At the same time, approximately 24% and 26% of control cells progressed into the S and G2-M phases, respectively. In contrast, only 4% and 20% of BLVRA knockdown cells progressed into the S and G2-M phases, respectively. Furthermore, Western blot analysis demonstrated that protein levels of cyclin D1 and CDK4 were markedly reduced 3 days post-transfection with shRNA-BLVRA, compared with the negligible changes in mock shRNA-transfected cells (Figure 4C). We also observed a dramatic decrease in Rb phosphorylation (Ser 795) in cell lysates from 7 days post-transfection with BLVRA shRNA (Figure 4C). Down-regulation of cyclin-D1 and CDK4, induced by depletion of BLVRA, confirmed the G0-G1 cell cycle arrest (Figure 4C).
Discussion
BLVRA was initially known as an enzyme that converts biliverdin to bilirubin. Since then, numerous studies have shown that BLVRA has extra functions in cell signaling, gene control, metabolism, cell growth, and apoptosis (Salim et al., 2001; Kravets et al., 2004; Lerner-Marmarosh et al., 2005), and BLVRA may play a role in the pathogenesis of diseases such as diabetes or cancer (Lerner-Marmarosh et al., 2005; Florczyk et al., 2008; Wu et al., 2008). Moreover, because BLVRA is a leucine zipper-like DNA binding protein, it may serve as a transcription factor for activator protein 1 (AP-1)-regulated genes such as HO-1 in the nucleus (Kravets et al., 2004; Tudor et al., 2008). Nevertheless, the primary physiological function of BLVRA is the production of bilirubin, a major natural and potent antioxidant. In recent studies, BLVRA administration effectively ameliorated oxidative stress-mediated pathological states, and when cellular BLVRA activity was suppressed by small interfering RNA (siRNA), ROS levels and cell death markedly increased (Baranano et al., 2002; Sedlak et al., 2009). We also observed that BLVRA knockdown significantly increased ROS levels in young HDFs (Figure 1C).
The efficient antioxidant capacity of this system is potentiated by the redox cyclic nature of bilirubin to biliverdin in addition to HO-1 induction by BLVRA (Baranano et al., 2002; Sedlak and Snyder, 2004). BLVRA has been reported to function in the transcriptional activation of HO-1 after oxidative stress (Maines et al., 2001; Ahmad et al., 2002; Wang and de Montellano, 2003; Kravets et al., 2004). HO-1 is an inducible oxidative stress-responsive gene that is induced by various stimuli, including heme, H2O2, metals, cytokines, and growth factors, and is associated with various pathological disorders such as hypertension, atherosclerosis, and hypoxic injury (Guyton et al., 1996; Otterbein and Choi, 2000; Dennery, 2004; Aggeli et al., 2006) One major role of HO-1 is to protect cells from oxidative stress via the degradation of prooxidant heme to free iron, carbon monoxide (CO), and biliverdin (Maines, 1997). Numerous studies have indicated the close relationship between ROS and aging (Martin et al., 1996; Sohal and Weindruch, 1996; Beckman and Ames, 1998). In this regard, BLVRA may also contribute to the induction of cellular senescence, although a direct role of BLVRA in cellular senescence has not been thoroughly studied.
To compare the oxidative stress responses of young and senescent fibroblasts, we examined the effect of H2O2 on HO-1 induction and on BLVRA activity. Treatment with H2O2 markedly increased BLVRA activity in young fibroblasts, but not in senescent cells. Moreover, BLVRA depletion decreased HO-1 induction in H2O2-treated young cells, in contrast to the negligible induction of HO-1 in senescent cells. Interestingly, we could not observe any significant qualitative change in the BLVRA protein expression level in young and senescent cells (data not shown), whereas HO-1 induction after oxidative stress differed significantly between young and senescent cells (Figure 2A). These differences between young and old cells may be related to a difference in the endogenous oxidative stress level, that is, a high stress level in senescent cells and a low level in young cells, which would result in different oxidative stress-related signaling such as the Nrf-1 pathway (Lee et al., 2002). Alternatively, these differences between young and old cells may be explained by aging-dependent differences in the efficiency of oxidative stress-related signal trafficking into the nucleus. Nuclear localization of BLVRA is important for its signal transduction and induction of HO-1 (Maines et al., 2001), and we recently demonstrated that the expression levels of genes involved in nucleocytoplasmic trafficking were markedly decreased in senescent HDFs (Kim et al., 2010). These data suggest that nuclear trafficking of BLVRA and oxidative signaling may be impaired in senescent HDFs. Further studies are needed to better understand the nuclear translocation of BLVRA and its contribution to HO-1 induction in young and senescent cells.
In the present study, we explored the molecular role of BLVRA in cellular senescence. Specifically, we investigated the effects of BLVRA knockdown by short hairpin RNA (shRNA) on cell viability and cell cycle progression in human diploid fibroblasts. After BLVRA depletion, the HDFs were arrested for a prolonged period. Most BLVRA shRNA-transfected HDFs developed premature senescence, with decreases in cyclin D1 and phosphorylated pRb concomitantly with increases in p16/INK4a and senescence-associated β-galactosidase. To confirm the role of high ROS in the induction of premature senescence in HDFs, we treated cells with N-acetyl cysteine (NAC), a potent ROS scavenger. Transfection of cells with the BLVRA gene reduced ROS levels by about 35% (Figure 1D), and ROS scavenging by NAC effectively suppressed cellular senescence induced by BLVRA knockdown (data not shown). The simple depletion of BLVRA reduced the viability of the cells, suggesting that BLVRA is most likely an essential component for cell survival under physiological oxidative stress. In contrast to its knockdown, BLVA overexpression rescued the young cell-like morphological changes in senescent fibroblasts to some extent, but without restoration of mitogenic potential (data not shown), indicating that BLVRA overexpression alone is not sufficient to prevent senescence-related cell cycle arrest.
The Hayflick limit describes the phenomenon of normal mitotic cells undergoing a finite number of cell divisions and then entering replicative senescence, after which they cannot divide further and become unresponsive to mitogenic stimuli (Hayflick, 1965). In addition to replicative senescence, cells can also exhibit stress-induced or premature cellular senescence upon exposure to oxidants, DNA-damaging agents, histone deacetylase inhibitors, or overexpression of certain oncogenes (Chen et al., 1995; Serrano et al., 1997; Robles and Adami, 1998; Zhu et al., 1998; Choi and Kim, 2004). Compared with early-passage cells, senescent HDFs contain higher levels of oxidative DNA lesions (Chen et al., 1995), presenting the possibility that oxidative damage may be responsible for triggering the activation of cell cycle checkpoints in senescent cells. Senescent cells contain an elevated p21 level (Noda et al., 1994), hypo-phosphorylated pRb (Stein et al., 1990), and reduced E2F activity (Dimri et al., 1996), which are responsible for arrest in G0 phase of the cell cycle. Senescence in culture is thought to reflect the in vivo aging process and might have evolved as a tumor suppression mechanism (Cristofalo and Pignolo, 1993; Campisi, 1996). Senescent cells also express high levels of p16, which inhibits the inactivating phosphorylation of pRb (Alcorta et al., 1996; Hara et al., 1996; Ohtani et al., 2001). Thus, cellular senescence may, to some extent, provide an important barrier to genomic instability and tumorigenesis (Campisi and d'Adda di Fagagna, 2007; Schmitt, 2007). In this regard, the significance of the mechanism by which BLVRA activity is elevated in tumor tissues (Maines et al., 1999) and the relationship between serum bilirubin levels and cancer mortality (McCarty, 2007) remain to be elucidated.
Taken together, these results suggest that BLVRA may be among the most effective physiological ROS scavenging systems and may play an important role in regulating cellular senescence. Additionally, BLVRA may provide a new target for developing a strategy to control aging and cancer.
Methods
Cell culture and SA β-galactosidase staining
Human diploid fibroblasts (HDFs) were isolated from the foreskin of a 4-year-old boy and cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin in a 5% CO2 incubator. Cells were subcultured serially at a ratio of 1:4. We defined young cells as those resulting from < 22 population doublings, and old cells were from > 68 population doublings. Cellular senescence of all of the old cells was confirmed by their delayed population doubling times and by a senescence-associated β-galactosidase activity assay as described by Dimri et al. (1995). After being grown in a semi-confluent state, senescence-associated β-galactosidase, pH 6.0, activity was examined. Cells were washed with phosphate-buffered saline and fixed with 2% paraformaldehyde containing 0.2% glutaraldehyde in phosphate-buffered saline for 5 min at room temperature. After washing with phosphate-buffered saline, cells were incubated with β-galactosidase reagent (1 mg/ml 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal), 40 mm citric acid/sodium phosphate buffer, pH 6.0, 5 mm potassium ferrocyanide/potassium ferricyanide, 150 mm NaCl, 2 mm MgCl2) at 37℃. HeLa and A498 cells were purchased from the Korean Cell Line Bank (KCLB, Seoul, Korea). Cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% heat-inactivated FBS at 37 5% CO2 and 95% O2 in a humidified cell incubator, and the medium was changed every 2 to 3 days. Cells were subcultured serially at a ratio of 1:3.
Enzyme assays
Biliverdin Reductase Assay - The enzyme activity was determine by measuring the rate of bilirubin formation. BLVRA activity was measured in HDF cells plated in 60-mm plastic dishes and pooled samples of cells using a colorimetric reaction to measure the formation of bilirubin, as described by manufacturer (Sigma, St. Louis, MO). Cells were lysed in 150 µl of extraction buffer, and the assay was performed using 150 µg of total protein. Bilirubin levels were measured at 450 nm at 37℃ 18 min after start of the reaction. BVR activity was expressed as units/ml with 1 unit of biliverdin reductase converting 1 nanomole of biliverdin to bilirubin in an NADPH-dependent fashion at pH 8.5 at 37℃.
Cell-cycle analysis
Cells were seeded in 100 mm dish at 3 × 105 cells per dish. After treatment with sh-BLVRA, cells were harvested using trypsinization and combined with floating cells collected from the medium. Cells were then washed twice with PBS, and fixed with ice cold 70% ethanol. Cells were pelleted by centrifugation and the 70% ethanol was discarded. Cells were then stained with 50 µg/ml propidium iodide (PI) plus RNase A for 20 min, analyzed for DNA content on a FACScan flow cytometer (Becton Dickinson FACSorter) using CELLQuest software.
Western-blot analysis
hBLVRA, p16, p21, p53, CDK1, Cyclin D1 and p-pRb band were determined by Western blotting. Briefly, cells were lysed with lysis buffer [50 mM Tris pH74, 150 mM NaCl, 1 mM EDTA pH8.0, 1 mM protease inhibitor (Roche), 1 mM PMSF, 1 mM NaF, 1 mM sodium orthovanadate], and protein contents were determined using Bradford reagent. Equal amounts of protein (40 µg) were then separated by SDS-PAGE and transferred to nitrocellulose membranes (Schleicher & Schuell Bioscience Inc). After blocking with TBS containing Tween-20 in the presence of 2.5% nonfat dry milk, the membranes were incubated with the primary antibodies at 4℃ for 16 h. Secondary antibodies were added for 1 h at RT. The antibody-antigen complexes were detected using the ECL detection system (Pierce).
RNA interference and transfection
The Lentiviral Transduction Particles are perchused from sigma co. (NM_000712: The base vector pLKO.1-puro was used. TRCN0000046388 sequence: CCGGGCAGAAGAAATCCAGAAATATCTCGAGATATTTCTGGATTTCTTCTGCTTTTTG, TRCN0000046390 Sequence: CCGGGCGAAAGGAAGATCAGTATATCTCGAGATATACTGATCTTCCTTTCGCTTTTTG, TRCN0000046389 Sequence: CCGGCCATTTCAAGTCTGGGTCCTTCTCGAGAAGGACCCAGACTTGAAATGGTTTTTG) For puromycin selection, cells in 6 cell plate added 0.5-2 µg/ml of puromycin to selected wells and examine viability every 2 day for 10-14 days. Then replace the media containing puromycin every 3 days. RNA interference expression vector or control vector were transfected into HDF cells using Lipofectamine™ 2000 (Invitrogen, CA) according to the manufacturers' instructions. Briefly, the complexes of diluted RNA interference expression vector or control vector and Lipofectamine™ 2000 were added to wells containing 90% confluence cells and Opti-MEM medium (Invitrogen, CA). 6 h later, medium was changed to DMEM with 10% FBS.
The specific siRNA for BLVRA was purchased from BIONEER Co. RNA interference (RNAi) of the BVRA transcript was performed as described (Elbashir et al., 2001). Briefly, senescent HDFs (5 × 104) were plated in a 100 mm dish, transfected with 0.5 nmole of siRNA and Oligofectamine™ reagent in serum-free medium and incubated for 4 h at 37℃ in a CO2 incubator. Following incubation, the cells were supplied with growth medium containing 10% fetal bovine seerum.
Measurement of intracellular ROS level
The cellular levels of ROS were determined using dichlorodihydrofluorescein diacetate (DCF-DA) (Sigma-Aldrich Co., Ltd.). siRNA treated cells were stained with 50 µM of DCF-DA for 30 min and then harvested. The fluorescent intensities were quantified using an cytometer (Becton Dickinson FACSorter). To examine the effect of N-acetylcystein (NAC) (Sigma-Aldrich Co., Ltd.), cell were treated with 20 mM of NAC for 2 days.
3-(4,5-dimethylthiazal-z-yl)-2,5-diphenylterazolium (MTT) assays
Cells were plated in 24-well tissue culture plates and allowed to attach overnight. MTT was added to each well to a final concentration of 200 µg/ml, and cells were incubated for 4 h. After removing the medium completely, the formazan product was solubilized with dimethlysulfoxide. Optical densities (OD) were measured at 490 nm. Each experiment was performed three times. Error bars represent standard deviations of the means.
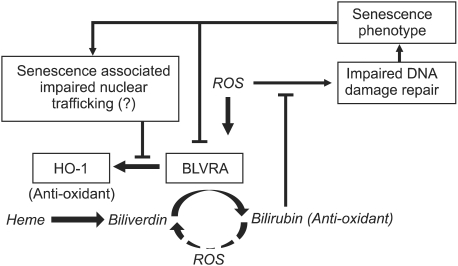
Figure 5.
Feedback loop of the physiological antioxidative defense system. ROS, reactive oxygen species; HO-1, heme oxygenase-1; BLVRA, biliverdin reductase-1.
Acknowledgements
This work was supported by grants from the National Research Foundation (NRF) through the Ageing and Apoptosis Research Center at Seoul National University (RII-2002-097-08001-0) and Dual Aging and Cancer Project.
Abbreviations
- HDF
human diploid fibroblast
- SA-β-gal
senescence associated β-galactosidase
- BLVRA
biliverdin reductase A
- HO-1
heme oxygenase-1
- ROS
reactive oxygen species
- DCF
dichlorofluorescein diacetate
References
- 1.Aggeli IK, Gaitanaki C, Beis I. Involvement of JNKs and p38-MAPK/MSK1 pathways in H2O2-induced upregulation of heme oxygenase-1 mRNA in H9c2 cells. Cell Signal. 2006;18:1801–1812. doi: 10.1016/j.cellsig.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad Z, Salim M, Maines MD. Human biliverdin reductase is a leucine zipper-like DNA-binding protein and functions in transcriptional activation of heme oxygenase-1 by oxidative stress. J Biol Chem. 2002;277:9226–9232. doi: 10.1074/jbc.M108239200. [DOI] [PubMed] [Google Scholar]
- 3.Alcorta DA, Xiong Y, Phelps D, Hannon G, Beach D, Barrett JC. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc Natl Acad Sci USA. 1996;93:13742–13747. doi: 10.1073/pnas.93.24.13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baranano DE, Rao M, Ferris CD, Snyder SH. Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci USA. 2002;99:16093–16098. doi: 10.1073/pnas.252626999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beale SI, Cornejo J. Enzymatic heme oxygenase activity in soluble extracts of the unicellular red alga, Cyanidium caldarium. Arch Biochem Biophys. 1984;235:371–384. doi: 10.1016/0003-9861(84)90210-8. [DOI] [PubMed] [Google Scholar]
- 6.Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 7.Campisi J. Replicative senescence: an old lives' tale? Cell. 1996;84:497–500. doi: 10.1016/s0092-8674(00)81023-5. [DOI] [PubMed] [Google Scholar]
- 8.Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 9.Chen Q, Ames BN. Senescence-like growth arrest induced by hydrogen peroxide in human diploid fibroblast F65 cells. Proc Natl Acad Sci USA. 1994;91:4130–4134. doi: 10.1073/pnas.91.10.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Q, Fischer A, Reagan JD, Yan LJ, Ames BN. Oxidative DNA damage and senescence of human diploid fibroblast cells. Proc Natl Acad Sci USA. 1995;92:4337–4341. doi: 10.1073/pnas.92.10.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen QM, Bartholomew JC, Campisi J, Acosta M, Reagan JD, Ames BN. Molecular analysis of H2O2-induced senescent-like growth arrest in normal human fibroblasts: p53 and Rb control G1 arrest but not cell replication. Biochem J. 1998;332:43–50. doi: 10.1042/bj3320043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi BH, Kim JS. Age-related decline in expression of calnexin. Exp Mol Med. 2004;36:499–503. doi: 10.1038/emm.2004.63. [DOI] [PubMed] [Google Scholar]
- 13.Cristofalo VJ, Pignolo RJ. Replicative senescence of human fibroblast-like cells in culture. Physiol Rev. 1993;73:617–638. doi: 10.1152/physrev.1993.73.3.617. [DOI] [PubMed] [Google Scholar]
- 14.Dennery PA. Introduction to serial review on heme oxygenase in human disease. Free Radic Biol Med. 2004;37:1095–1096. doi: 10.1016/j.freeradbiomed.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dimri GP, Nakanishi M, Desprez PY, Smith JR, Campisi J. Inhibition of E2F activity by the cyclin-dependent protein kinase inhibitor p21 in cells expressing or lacking a functional retinoblastoma protein. Mol Cell Biol. 1996;16:2987–2997. doi: 10.1128/mcb.16.6.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 18.Florczyk UM, Jozkowicz A, Dulak J. Biliverdin reductase: new features of an old enzyme and its potential therapeutic significance. Pharmacol Rep. 2008;60:38–48. [PMC free article] [PubMed] [Google Scholar]
- 19.Guyton KZ, Spitz DR, Holbrook NJ. Expression of stress response genes GADD153, c-jun, and heme oxygenase-1 in H2O2- and O2-resistant fibroblasts. Free Radic Biol Med. 1996;20:735–741. doi: 10.1016/0891-5849(95)02151-5. [DOI] [PubMed] [Google Scholar]
- 20.Hagen TM, Yowe DL, Bartholomew JC, Wehr CM, Do KL, Park JY, Ames BN. Mitochondrial decay in hepatocytes from old rats: membrane potential declines, heterogeneity and oxidants increase. Proc Natl Acad Sci USA. 1997;94:3064–3069. doi: 10.1073/pnas.94.7.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hara E, Smith R, Parry D, Tahara H, Stone S, Peters G. Regulation of p16CDKN2 expression and its implications for cell immortalization and senescence. Mol Cell Biol. 1996;16:859–867. doi: 10.1128/mcb.16.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 23.Huang TJ, Trakshel GM, Maines MD. Detection of 10 variants of biliverdin reductase in rat liver by two-dimensional gel electrophoresis. J Biol Chem. 1989;264:7844–7849. [PubMed] [Google Scholar]
- 24.Huang TJ, Maines MD. Bromobenzene-mediated alteration in activity and electrophoretic pattern of biliverdin reductase variants in rat kidney. Mol Pharmacol. 1990;37:25–29. [PubMed] [Google Scholar]
- 25.Johnson M, Dimitrov D, Vojta PJ, Barrett JC, Noda A, Pereira-Smith OM, Smith JR. Evidence for a p53-independent pathway for upregulation of SDI1/CIP1/WAF1/p21 RNA in human cells. Mol Carcinog. 1994;11:59–64. doi: 10.1002/mc.2940110202. [DOI] [PubMed] [Google Scholar]
- 26.Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991;51:6304–6311. [PubMed] [Google Scholar]
- 27.Kim SY, Ryu SJ, Ahn HJ, Choi HR, Kang HT, Park SC. Senescence-related functional nuclear barrier by down-regulation of nucleo-cytoplasmic trafficking gene expression. Biochem Biophys Res Commun. 2010;391:28–32. doi: 10.1016/j.bbrc.2009.10.154. [DOI] [PubMed] [Google Scholar]
- 28.Kravets A, Hu Z, Miralem T, Torno MD, Maines MD. Biliverdin reductase, a novel regulator for induction of activating transcription factor-2 and heme oxygenase-1. J Biol Chem. 2004;279:19916–19923. doi: 10.1074/jbc.M314251200. [DOI] [PubMed] [Google Scholar]
- 29.Lee AC, Fenster BE, Ito H, Takeda K, Bae NS, Hirai T, Yu ZX, Ferrans VJ, Howard BH, Finkel T. Ras proteins induce senescence by altering the intracellular levels of reactive oxygen species. J Biol Chem. 1999;274:7936–7940. doi: 10.1074/jbc.274.12.7936. [DOI] [PubMed] [Google Scholar]
- 30.Lee HC, Yin PH, Chi CW, Wei YH. Increase in mitochondrial mass in human fibroblasts under oxidative stress and during replicative cell senescence. J Biomed Sci. 2002;9:517–526. doi: 10.1007/BF02254978. [DOI] [PubMed] [Google Scholar]
- 31.Lerner-Marmarosh N, Shen J, Torno MD, Kravets A, Hu Z, Maines MD. Human biliverdin reductase: a member of the insulin receptor substrate family with serine/threonine/tyrosine kinase activity. Proc Natl Acad Sci USA. 2005;102:7109–7114. doi: 10.1073/pnas.0502173102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 33.Maines MD, Mayer RD, Erturk E, Huang TJ, Disantagnese A. The oxidoreductase, biliverdin reductase, is induced in human renal carcinoma--pH and cofactor-specific increase in activity. J Urol. 1999;162:1467–1472. [PubMed] [Google Scholar]
- 34.Maines MD, Ewing JF, Huang TJ, Panahian N. Nuclear localization of biliverdin reductase in the rat kidney: response to nephrotoxins that induce heme oxygenase-1. J Pharmacol Exp Ther. 2001;296:1091–1097. [PubMed] [Google Scholar]
- 35.Maines MD. New insights into biliverdin reductase functions: linking heme metabolism to cell signaling. Physiology (Bethesda) 2005;20:382–389. doi: 10.1152/physiol.00029.2005. [DOI] [PubMed] [Google Scholar]
- 36.Martin GM, Austad SN, Johnson TE. Genetic analysis of ageing: role of oxidative damage and environmental stresses. Nat Genet. 1996;13:25–34. doi: 10.1038/ng0596-25. [DOI] [PubMed] [Google Scholar]
- 37.McCarty MF. "Iatrogenic Gilbert syndrome"--a strategy for reducing vascular and cancer risk by increasing plasma unconjugated bilirubin. Med Hypotheses. 2007;69:974–994. doi: 10.1016/j.mehy.2006.12.069. [DOI] [PubMed] [Google Scholar]
- 38.Noda A, Ning Y, Venable SF, Pereira-Smith OM, Smith JR. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res. 1994;211:90–98. doi: 10.1006/excr.1994.1063. [DOI] [PubMed] [Google Scholar]
- 39.Ohtani N, Zebedee Z, Huot TJ, Stinson JA, Sugimoto M, Ohashi Y, Sharrocks AD, Peters G, Hara E. Opposing effects of Ets and Id proteins on p16INK4a expression during cellular senescence. Nature. 2001;409:1067–1070. doi: 10.1038/35059131. [DOI] [PubMed] [Google Scholar]
- 40.Otterbein LE, Choi AM. Heme oxygenase: colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1029–L1037. doi: 10.1152/ajplung.2000.279.6.L1029. [DOI] [PubMed] [Google Scholar]
- 41.Robles SJ, Adami GR. Agents that cause DNA double strand breaks lead to p16INK4a enrichment and the premature senescence of normal fibroblasts. Oncogene. 1998;16:1113–1123. doi: 10.1038/sj.onc.1201862. [DOI] [PubMed] [Google Scholar]
- 42.Salim M, Brown-Kipphut BA, Maines MD. Human biliverdin reductase is autophosphorylated, and phosphorylation is required for bilirubin formation. J Biol Chem. 2001;276:10929–10934. doi: 10.1074/jbc.M010753200. [DOI] [PubMed] [Google Scholar]
- 43.Schluchter WM, Glazer AN. Characterization of cyanobacterial biliverdin reductase. Conversion of biliverdin to bilirubin is important for normal phycobiliprotein biosynthesis. J Biol Chem. 1997;272:13562–13569. doi: 10.1074/jbc.272.21.13562. [DOI] [PubMed] [Google Scholar]
- 44.Schmitt CA. Cellular senescence and cancer treatment. Biochim Biophys Acta. 2007;1775:5–20. doi: 10.1016/j.bbcan.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 45.Sedlak TW, Snyder SH. Bilirubin benefits: cellular protection by a biliverdin reductase antioxidant cycle. Pediatrics. 2004;113:1776–1782. doi: 10.1542/peds.113.6.1776. [DOI] [PubMed] [Google Scholar]
- 46.Sedlak TW, Saleh M, Higginson DS, Paul BD, Juluri KR, Snyder SH. Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proc Natl Acad Sci USA. 2009;106:5171–5176. doi: 10.1073/pnas.0813132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 48.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stein GH, Beeson M, Gordon L. Failure to phosphorylate the retinoblastoma gene product in senescent human fibroblasts. Science. 1990;249:666–669. doi: 10.1126/science.2166342. [DOI] [PubMed] [Google Scholar]
- 50.Tudor C, Lerner-Marmarosh N, Engelborghs Y, Gibbs PE, Maines MD. Biliverdin reductase is a transporter of haem into the nucleus and is essential for regulation of HO-1 gene expression by haematin. Biochem J. 2008;413:405–416. doi: 10.1042/BJ20080018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J, de Montellano PR. The binding sites on human heme oxygenase-1 for cytochrome p450 reductase and biliverdin reductase. J Biol Chem. 2003;278:20069–20076. doi: 10.1074/jbc.M300989200. [DOI] [PubMed] [Google Scholar]
- 52.Wu B, Liu X, Shen J. Old biliverdin reductase: links to insulin resistance and may be a novel therapeutic target. Med Hypotheses. 2008;71:73–76. doi: 10.1016/j.mehy.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 53.Yuan H, Kaneko T, Matsuo M. Relevance of oxidative stress to the limited replicative capacity of cultured human diploid cells: the limit of cumulative population doublings increases under low concentrations of oxygen and decreases in response to aminotriazole. Mech Ageing Dev. 1995;81:159–168. doi: 10.1016/0047-6374(95)01584-m. [DOI] [PubMed] [Google Scholar]
- 54.Zhu J, Woods D, McMahon M, Bishop JM. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 1998;12:2997–3007. doi: 10.1101/gad.12.19.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]