Abstract
Clusterin is a secretory glycoprotein, which is highly up-regulated in a variety of normal and injury tissues undergoing apoptosis including infarct region of the myocardium. Here, we report that clusterin protects H9c2 cardiomyocytes from H2O2-induced apoptosis by triggering the activation of Akt and GSK-3β. Treatment with H2O2 induces apoptosis of H9c2 cells by promoting caspase cleavage and cytochrome c release from mitochondria. However, co-treatment with clusterin reverses the induction of apoptotic signaling by H2O2, thereby recovers cell viability. The protective effect of clusterin on H2O2-induced apoptosis is impaired by PI3K inhibitor LY294002, which effectively suppresses clusterin-induced activation of Akt and GSK-3β. In addition, the protective effect of clusterin is independednt on its receptor megalin, because inhibition of megalin has no effect on clusturin-mediated Akt/GSK-3β phosphoylation and H9c2 cell viability. Collectively, these results suggest that clusterin has a role protecting cardiomyocytes from oxidative stress and the Akt/GSK-3β signaling mediates anti-apoptotic effect of clusterin.
Keywords: apoptosis; clusterin; glycogen synthase kinase 3β; myocytes, cardiac; oxidative stress; proto-oncogene proteins c-akt
Introduction
The reactive oxygen species (ROS) and free radicals are widely believed to be important in pathological events causing apoptotic cell death. In particular, they play central roles in cardiac pathophysiology when the heart is subjected to significant oxidative stress governed by ischemia and reperfusion (Maclellan and Schneider, 1997; Saraste et al., 1997; Lee et al., 2009). Clusterin, also known as apolipoprotein J (apo J) or testosterone-repressed prostate message-2 (TRPM-2), was originally isolated from the rat testis (Blaschuk et al., 1983). Clusterin is a hetero-dimeric glycoprotein secreted by a number of cell types (Jones and Jomary, 2002). Clusterin is an extracellular chaperone protein, which has a cytoprotective effect in response to diverse stresses (Poon et al., 2000; Whang et al., 2005). Previous studies suggest that clusterin plays important roles in cell adhesion, spermatogenesis, tumor metastasis, and lipid transportation (Wilson and Easterbrook-Smith, 2000; Jones and Jomary, 2002; Lau et al., 2006). In addition to chaperone activity, clusterin may have an antiapoptotic function. With regard to the role of clusterin in apoptotic cell death, multiple lines of evidence have demonstrated its cytoprotective effects in several tumors, epithelial cells, endothelial cells and so on (Kim et al., 2007, 2010; Sallman et al., 2007). Moreover, clusterin protects cells from heat shock and TNF-α, suggesting that clusterin could be a survival factor related to the apoptotic pathway (Sensibar et al., 1995; Miyake et al., 2000; Trougakos and Gonos, 2006). The anti-apoptotic effect of clusterin was also reported in the cardiac system based on its increased expression in the injured heart (Swertfeger et al., 1996; Ishikawa et al., 1998; McLaughlin et al., 2000; Miyata et al., 2001). It is previously reported that clusterin protects cardiomyocytes from ischemia-induced cell death (Krijnen et al., 2005) or ethanol-induced cardiac injury (Li et al., 2007). Despite the potential cardioprotective effect of clusterin, little is known about the action of clusterin against the individual oxidative stress, and the mechanism by which clusterin represents cardioprotection needs to be elucidated in detail.
In the present study, therefore, we investigated the protective effect of clusterin on the survival of H9c2 cardiomyocytes against H2O2 stimulus as well as the underlying signaling pathway. We found that clusterin exerts cardioprotection against H2O2-induced oxidative injury and the activation of Akt/GSK-3β signaling pathway plays an important role in mediating the anti-apoptotic effect of clusterin.
Results
Effect of single treatment of H2O2 or clusterin on H9c2 cells viability
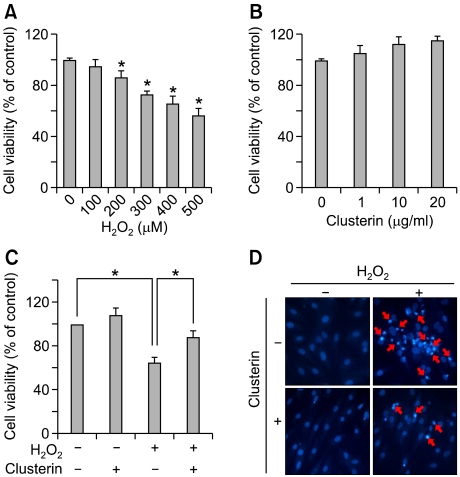
We first determined the dose at which cytotoxicity develops in a period of 24 h upon H2O2 exposure in H9c2 cells through the MTS assay. Cells were treated with increasing doses of H2O2 for 24 h. As shown in Figure 1A, H2O2 impaired cell viability in a concentration-dependent manner over the tested concentration range (100-500 µM). A maximum reduction was 56 ± 2% of the control group and it was observed at 500 µM of H2O2. We chose the level of H2O2 400 µM for use in our subsequent experiments based on this result. In order to evaluate whether clusterin is cytotoxic to H9c2 cells, we determined the viability of cells treated with clusterin (1-20 µg) for 24 h using the MTS assay. Cell viability was not significantly affected by treatment with any tested clusterin concentrations (Figure 1B).
Figure 1.
Clusterin attenuates H2O2-induced apoptotic cell death. (A) H9c2 cells were incubated with indicated concentrations of H2O2 (100-500 µM) for 24 h, then cell viability was measured by MTS assay. (B) After treatment of clusterin (1-20 µg/ml) for 24 h, cell viability was determined by MTS assay. (C) H9c2 cells were treated with 10 µg/ml clusterin in the presence or absence of 400 µM H2O2 for 24 h. Cell viability was measured by MTS assay. (D) Cell apoptosis was evaluated by DAPI staining. Each value represents the mean ± SD of three independent experiments (*P < 0.05).
Effects of clusterin on H2O2-induced apoptosis in H9c2 cells
In order to determine the effects of clusterin on H2O2-induced cell death, the H9c2 cells were pretreated with 10 µg clusterin for 2 h, after which co-incubated with 400 µM of H2O2 for additional 24 h. As shown in Figure 1C, co-treatment of clusterin relieved reduced viability of H2O2-stimulated cells. To clarify whether clusterin protects H9c2 cells from apoptosis, we compared apoptotic nuclei and DNA fragmentation in H2O2-treated cells with or without clusterin. As shown in Figure 1D, most nuclei in either control or clusterin alone group displayed uniform blue chromatin with organized structure. In contrast, intense DAPI stained nuclei that indicate cells undergoing apoptosis were frequently observed in H2O2-stimulated cells compared to control cells. However, co-treatment with clusterin decreased the rate of apoptotic nuclei induced by H2O2. These data propose that clusterin has a protective role in H2O2-induced apoptotic cell death of H9c2 cells.
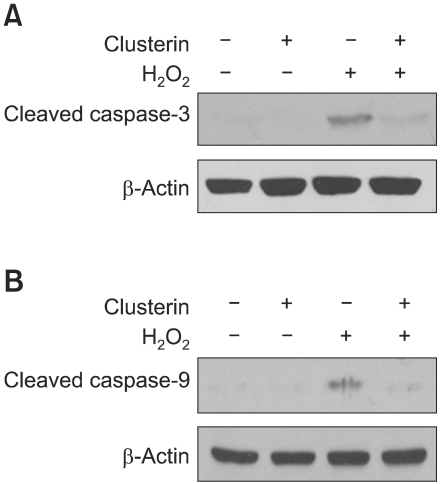
Caspases are key mediators of cell death and caspase-3 is an executioner for the death program in many cells in response to oxidant, such as H2O2. Thus we examined the effect of clusterin on H2O2-stimulated caspase-3 and caspae-9 activation. As shown in Figure 2, H2O2 treatment was associated with significant increase in caspase-9 and caspase-3 cleavage as compared to control. Clusterin pretreatment significantly decreased the level of both cleaved caspase-9 and -3 in H2O2-treated H9c2 cells, indicating that clusterin attenuated H2O2-induced apoptosis occurring through caspase activation.
Figure 2.
Clusterin inhibits H2O2-induced caspase-3 and caspase-9 activation in H9c2 cells. After 2 h pre-incubation with 10 µg/ml clusterin, H9c2 cells were treated with 400 µM H2O2 for 24 h. The activity of caspase-9 (A) caspase-3 (B) was assessed by western blot analysis.
Effect of clusterin on mitochondria-mediated apoptosis in H9c2 cells
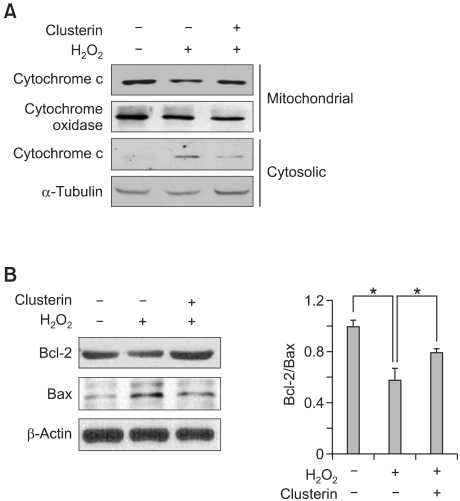
Changes in the induction of Bcl-2 family proteins are closely related to an imbalance in the mitochondrial homeostasis. In particular, the release of cytochrome c into the cytosol is an important step for the induction of apoptosis in a number of different cell types, which is tightly regulated by the equilibrium between the anti-apoptotic Bcl-2 and pro-apoptotic Bad and Bax. As shown in Figure 3A, cytochrome c mainly located in mitochondria and could not be found in cytosol of myocytes in control group. H2O2 treatment reduced the level of cytochrome c in mitochondria but increased it in cytosol, indicating its translocation from mitochondria to cytosol. However, the level of cytosolic cytochrome c was lower in clusterin co-treatment group compared to H2O2 single treatment indicating that clusterin might inhibit translocation of cytochrome c. Next, we examined the level of Bcl-2 and Bax protein. As shown in Figure 3B, H2O2 treatment resulted in decreased Bcl-2/Bax ratio compared to control, but clusterin treatment recovered the ratio of Bcl-2/Bax proteins.
Figure 3.
Clusterin reduces cytochrome c release and the ratio of Bcl-2/Bax. After 2 h pre-incubation with 10 µg/ml clusterin, H9c2 cells were incubated in the presence of 400 µM H2O2 for 12 h. (A) Cytochrome c release from mitochondria to cytosol was measured by western blot analysis. (B) Bcl-2 and Bax expression were assessed by western blot analysis. Each value represents the mean ± SD of three independent experiments (*P < 0.05).
Effects of clusterin on Akt and GSK-3β phosphorylation
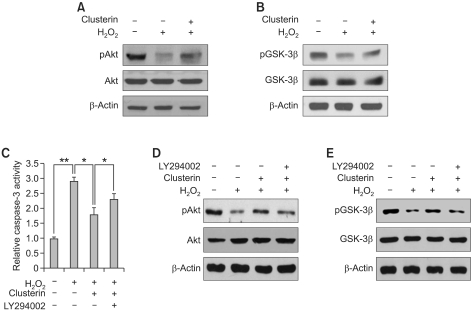
To explore the potential signaling pathways contributing to the anti-apoptotic function of clusterin, we examined Akt and GSK-3β activation. As shown in Figure 4, H2O2 treatment decreased both Akt (Figure 4A) and GSK-3β phosphorylation (Figure 4B) compared to control, but the decreased phosphorylation of Akt and GSK-3β were recovered by clusterin treatment. To determine whether the Akt/GSK-3β signaling pathway is involved in the pro-survival function of clusterin, H9c2 cells were pre-incubated with PI3K inhibitor LY294002 and thereafter treated with H2O2 in the absence or presence of clusterin for 24 h. LY294002 reversed the protective effects of clusterin on H2O2-induced apoptosis as determined by the caspase-3 activity (Figure 4C). The efficacy of inhibitor was confirmed through the reduction of phosphorylation of Akt (Figure 4D) and GSK-3β (Figure 4E). Therefore, these data imply that the apoptotic effect of clusterin on oxidative stress-induced apoptosis of cardiomyocytes is mediated at least in part through Akt/GSK-3β signaling.
Figure 4.
Cardioprotective effect of clusterin is mediated by the activation of Akt/GSK-3β signaling. (A, B) After 2 h pre-incubation with 10 µg/ml clusterin, H9c2 cells were treated with 400 µM H2O2 for 12 h. Western blot analysis was performed to examine phosphorylation of Akt and GSK-3β. (C) H9c2 cells were pre-incubated with 10 µM LY294002 and treated with H2O2 alone or in combination with clusterin. Then, caspase-3 activity was measured. (D, E) Phosphorylation of Akt and GSK-3β was examined in LY294002 pre-treated cells by western blot analysis. Each value represents the mean ± SD of three independent experiments (*P < 0.05, **P < 0.01).
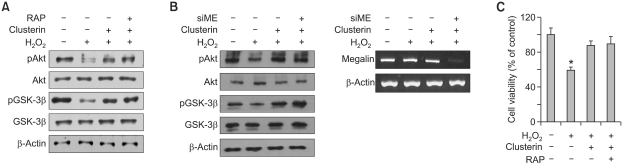
Independency of megalin on the protective effect of clusterin
Megalin is described as a receptor of clusterin (Zlokovic et al., 1996). Clustein is bound and taken up by megalin (Kounnas et al., 1995). Even though clusterin certainly has a survival effect in various cell types, there are some controversial results about whether the survival effect of clusterin is dependent or independent on its receptor megalin (Girton et al., 2002; Ammar and Closset, 2008; Van Dijk et al., 2010). We examined whether or not megalin is involved in the survival effect of clusterin in H9c2 cells using either RAP, an inhibitor of megalin (Van Dijk et al., 2010), or megalin siRNA. H9c2 cells were treated with clusterin with and without RAP, and the phosphoryations of Akt and GSK-3β were analyzed. Decreased phosphorylations of Akt and GSK-3β by H2O2 were recovered by clusterin treatment but co-treatment of RAP did not alter the effect of clusterin (Figure 5A). In addition, knock-down of megalin using siRNA did not affect Akt and GSK-3β phosphorylation (Figure 5B). Furthermore, there was no change in cell viability after treatment of RAP with clusterin as determined by the MTS assay (Figure 5C). Therefore, these results suggest that clusterin does not exert its protective effect through megalin in H9c2 cells.
Figure 5.
Cardioprotective effect of clusterin is megalin independent. (A) After 2 h pre-incubation with 10 µg/ml clusterin and/or 0.5 µM RAP, H9c2 cells were treated with 400 µM H2O2 for 12 h. Western blot analysis was performed to examine phosphorylation of Akt and GSK-3β. (B) H9c2 cells were transfected with either negative control siRNA (20 nM) or megalin siRNA (20 nM). After 24 h, the transfectants were exposed to 400 uM H2O2 for 12 h. RT-PCR analysis was carried out using specific primers for megalin and β-actin to show the efficient knock-down of megalin (right) and western blot analysis was performed to examine phosphorylation of Akt and GSK-3β (left). (C) After 2h pre-incubation with 10 µg/ml clusterin and/or 0.5 µM RAP, H9c2 cells were treated with 400 µM H2O2 for 24 h. Cell viability was measured by MTS assay. Each value represents the mean ± SD of three independent experiments (*P < 0.05).
Discussion
During ischemia and reperfusion, cell death of cardiomyocytes causes direct loss of heart function. Therefore, massive amount of studies has focused on ways to protect cardiomyocytes from cytotoxic stimuli such as oxidative stress. Due to its importance in heart function, cardiomyocyte seems to have mechanisms protecting itself against cytotoxic stimuli (Lamendola et al., 2009). One of the self-protective mechanisms of cardiomyocyte could be an induction of cytoprotective protein, clusterin. Despite some controversy about the exact location of clusterin up-regulation, it seems to be clear that clusterin expression increases in the myocardium of the infarcted heart (Vakeva et al., 1993; Silkensen et al., 1998). In particular, recent report showed that the factors released from conditioned medium of mouse embryonic stem cell, including clusterin, cystain-c, osteoponin, and TIMP-1, inhibited H2O2-induced apoptosis in H9c2 cells (Singla and McDonald, 2007). Consistently with these results, our study presents new direct evidence showing the protective effect of clusterin on H2O2-induced apoptosis in H9c2 cells. Clusterin was previously reported to decrease intracellular ROS level in human corneal endotherila cells and human retinal pigment epithelial cells (Shin et al., 2009; Kim et al., 2010). Compatible with these previous studies, we also observed that clusterin treatment in H9c2 cells strongly inhibited H2O2-enhanced intracellular ROS production (Supplemental Data Figure S1). Similar to the pattern of intracellular ROS production, H2O2-induced cell death was also significantly inhibited by clusterin treatment (Figures 1C and D) and accordingly clusterin significantly attenuates H2O2-induced cytochrome c release and apoptosis in H9c2 cells. These data imply that clusterin may protect H9c2 cells from oxidative stress through inhibition of ROS production.
However, clusterin represented cytoprotection in H9c2 cells at much higher concentration compared to in retinal pigment epithelium cells against the same H2O2 concentration (Kim et al., 2010). In the previous study, we observed anti-apoptotic effect of clusterin in retinal pigment epithelium at 1 µg/ml concentration (Kim et al., 2010) whereas it showed anti-apoptotic effect in H9c2 cells at more than 10 µg/ml concentration (Figure 1). The possible explanation of this difference is that H9c2 cell might express clusterin higher than retinal pigment epithelial cell upon oxidative stress. In accordance with this, Krijnen et al. (2005) showed that clusterin expression increased by ischemic challenge in H9c2 cells. Thus, our previous and current data may inform that clusterin administration should be differently adapted in different tissues. In addition to the functional study of clusterin, we also showed the mechanisms whereby it exerts anti-apoptotic effect in H9c2 cells.
We observed that clusterin regulates the expression of Bcl-2 family members such as Bcl-2 and Bax, which is important to control cytochrome c release (Pollack et al., 2002; Susnow et al., 2009). Co-treatment of clusterin with H2O2 increased anti-apoptotic protein Bcl-2 and decreased pro-apoptotic protein Bax compared to H2O2-treated H9c2 cells. Accordingly, H2O2-induced cytochrome c release and caspase activation were also reduced by co-treatment of clusterin.
Besides Bcl-2 family, GSK-3β is also recognized as a critical factor mediating cytochrome c release from mitochondria to cytosol (Park et al., 2006). Akt directly phosphorylates GSK-3β at Ser9 which negatively regulates its kinase activity. Thereafter, phosphorylated GSK-3β inhibits the opening of mitochondrial permeability transition pore (mPTP) (Juhaszova et al., 2009). In this study, clusterin recovered the decrease in Akt and GSK-3β phosphorylation induced by H2O2 (Figures 4A and B). Activation of Akt/GSK-3β seems to be responsible for anti-apoptotic effect of clusterin because LY294002, the inhibitor of PI3K which is an upstream activator of Akt, could abolish cytoprotection by clusterin (Figure 4C). Indeed, accumulating evidence supports that Akt might protect injured heart through normalizing mitochondrial regulation (Miyamoto et al., 2009) and GSK-3β is recognized as a potential therapeutic target for cardiac protection (Miura and Miki, 2009). In particular, the previous report suggested that phosphorylated GSK-3β provides cardioprotection against myocardial I/R injury (Gross et al., 2004). In our results, the treatment of LY294002, however, did not completely recovered caspase-3 activity compared to H2O2-treated cells (Figure 4C), suggesting that additional pathway(s) could be involved in anti-apoptotic effect of clusterin.
Similar to our results, Ammar and Closset (2008) previously reported that clusterin protects TNF-alpha-induced apoptosis through activation of PI 3-kinase/Akt pathway in prostatic cells. In this study, they also showed that the activation of PI 3-kinase/Akt pathway by clusterin is dependent on the expression and phosphorylation of megalin, the receptor of clusterin. However, in the present study, clusterin-induced Akt phosphorylation seems to be meglin independent even though we observed similar activation of Akt pathway by clusterin in H9c2 cells. To examine whether or not megalin is involved in the survival effect of clusterin in H9c2 cells, we used either RAP, an inhibitor of megalin, or megalin siRNA. Expression of megalin was not changed by clusterin treatment in H9c2 cells and the inhibition of megalin using RAP and siRNA has no effect on clusterin-induced Akt/GSK-3β phosphorylation (Figure 5), suggesting that clusterin does not exert its protective effect through megalin in H9c2. These differences could be explained by the different system used. While Ammar and Closset used prostatic cells, we used H9c2 cardiomyocytes. Therefore, we could say that clusterin shows similar cytoprotective effect mediating PI 3-kinase/Akt pathway in both prostatic cells and H9c2 cardiomyocytes but it may utilize different upstream mediator; it involves megalin in prostatic cells but not in H9c2 cardiomyocytes. In accordance with our results, Van Dijk et al. (2010) previously reported that endogeneous clusterin does not co-localize with megalin in H9c2 cells and its protective effect on cardiomyocytes after acute myocardial infarction in vivo and ischemia in vitro is independent on its receptor, megalin.
In summary, our study reveals that clusterin protects H9c2 cardiomyocytes against oxidative stress by inhibiting mitochondria-mediated apoptosis and that the activation of the Akt/GSK-3β signaling pathway is involved in the cardioprotective effect of clusterin. Therefore, clusterin could be applicable to therapeutics for heart injury after ischemia and reperfusion.
Methods
Materials
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), and other tissue culture reagents were purchased from Invitrogen (Carlsbad, CA). DAPI (4,6-diamidino-2-phenylindole) was obtained from Sigma (St. Louis, MO). Receptor-associated protein (RAP) was obtained from Progen (Heidelberg, Germany). Rabbit polyclonal anti-Bcl-2, and anti-Bax antbodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal anti-Akt, anti-p-Aktser473, anti-caspase-3, anti-caspase-9, anti-GSK-3β, and anti-p-GSK-3βser9 antibodies were purchased from Cell Signaling (Danvers, MA). All other reagents, including H2O2 and LY294002, were purchased from Sigma.
Cell culture
H9c2 cardiomyocytes were obtained from American Type Culture Collection (Rockville, MD) and cultured in DMEM medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 µg/mL streptomycin.
Purification of clusterin
Clusterin was purified from human serum as previously described (Shin et al., 2006). Briefly, human serum in 0.5 mM phenylmethylsulfonyl fluoride (PMSF) was stirred with 12% polyethylene glycol (PEG, Sigma) overnight at 4℃. After centrifugation, the supernatant was reprecipitated with 23% PEG. This precipitate was subjected to DEAE-sepharose and heparin sepharose column chromatography (GE Healthcare Life Science, Buckinghamshire, England). The serum clusterin was finally purified by affinity chromatography using cyanogen bromide-activated sepharose covalently conjugated with monoclonal clusterin antibody. The eluted protein was dialyzed and lyophilized prior to being stored at -70℃.
Cell viability
Cell viability was determined colorimetrically using MTS assay. H9c2 cells were cultured in 48-well plates and treated with H2O2 and/or clusterin. After 24 h, MTS was added to each well according to the manufacturer's instruction (Promega, Madison, WI). After 2 h incubation at 37℃, the cell viability was determined by measuring the absorbance at 490 nm using microplate spectrophotometer (Molecular Device, Sunnyvale, CA). Three independent experiments were performed for each experimental condition.
DAPI staining
Apoptosis of H9c2 cardiomyocytes were determined by DAPI staining, which allows determination and quantification of cells with fragmented and condensed chromatin. After washing with phosphate-buffered saline (PBS), H9c2 cells were fixed for 10 min with a 4% paraformaldehyde at room temperature. Then, the cells were washed with PBS and stained with DAPI (5 µg/ml in PBS) at room temperature for 5 min. Cell nuclei analysis was conducted with the TCS NT fluorescence imaging system comprising of inverted microscope (Leica, Germany).
Caspase-3 activity assay
Caspase-3 activity was measured by a cleavage of fluorogenic substrate according to the manufacturer's recommendation (BD Bioscience, San Jose, CA). H9c2 cells were pre-incubated with LY294002 (10 µM) for 30 min, then incubated with 400 µM H2O2 or in combination with 10 µg/ml clusterin for 24 h. Following treatment, cell lysate and caspase-3 substrate (Ac-DEVD-AMC) were added to the reaction buffer and incubated for 1 h at 37℃. Cleaved substrate by caspase-3 was determined using microplate spectrofluorometer (Molecular Device, Sunnyvale, CA) with a 380 nm excitation filter and a 460 nm emission filter. Assays were performed in three independent experiments.
Western blot analysis
Cells were lysed in a whole cell extract buffer containing 10 mM HEPES (pH 7.9), 1 mM EDTA, 5% Glycerol, 1 mM DTT and 400 mM NaCl. Protein concentration was determined with the use of a BCA protein assay kit following the manufacturer's instruction (Thermo Scientific, Rockford, IL). Protein samples of whole cell lysate were mixed with an equal volume of 5 × SDS sample buffer, boiled for 5 min, and then separated by 8-15% SDS-PAGE gels. After electrophoresis, proteins were transferred to nitrocellulose membrane. The membranes were blocked in 5% non-fat dry milk for 1 h, rinsed, and incubated with specific antibodies against clusterin, caspase-3, caspase-9, Akt and p-Akt in PBS-T (PBS containing 0.1% Tween-20) overnight at 4℃. Primary antibody was removed by washing the membranes 3 times in PBS-T, and incubated for 1 h with horseradish peroxidase-conjugated secondary antibody. Following 3 times of washing in PBS-T, immuno-positive bands were visualized using chemiluminescent reagent (Amersham, Piscataway, NJ) and exposed to X-ray film (Agfa, Germany).
Cell fractionation
Cultured cells were washed with PBS and then Dounce-homogenized (40 strokes) in homogenization buffer (20 mM HEPES, pH 7.4, 10 mM KCl, 1.5 mM MgCl2, 1 mM EGTA, 0.1 mM EDTA, 1 mM dithiothreitol, 250 mM sucrose and 0.1 mM PMSF) and centrifuged twice at 4℃ for 5 min at 1,000 g. The supernatants were transferred to new tubes and the pellets were discarded to remove nuclei and unbroken cells. The supernatant were re-centrifuged at 4℃ for 15 min at 14,000 g. The collected pellets was washed twice by homogenization buffer and resuspended with lysis buffer for mitochondria fraction, and the supernatants were used for cytosolic fraction. Protein concentration was determined using the BCA assay. Proteins were analyzed by SDS-PAGE as described above.
siRNA transfection
siRNA targeting rat megalin was chemically synthesized and purified in the 2-deprotected and desalted form (Bioneer, Daejeon, Korea). The sequences of megalin siRNA pair were 5'-CAGUGAUGAGCUUCCUACA-3' and 5'-UGUAGGAAGCUCAUCACUG-3'. Nonspecific siRNA (Cat. no: SN-1002, Bioneer, Daejeon, Korea) was used as a control for comparison. Transfection of siRNA was performed using lipofectamine and plus (Invitrogen, Rockville, MD), according to the manufacturer's instructions. Briefly, H9c2 cells were seeded at 2 × 105 cells in a 6-well plate. After 24 h, cells were transfected at the final concentration of 20 nM siRNA duplexes. The efficacy of knockdown was assessed RT-PCR using a megalin specific primer.
Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis
Total RNA from cells was isolated using TRI Reagent® (Molecular Research Center, Cincinnati, OH), according to the manufacturer's instructions. First-stranded cDNA was synthesized with 5 µg of total RNA and oligo(dT)18 by M-MLV reverse transcriptase (Invitrogen, Rockville, MD). Equal amounts of cDNA were subsequently amplified by PCR using PCR reaction kit (Bioneer, Daejeon, Korea) and specific primer for megalin (5'-GGTGTGTGACGAGGAT-3' and 5'-AGTTGCAATTGCGCTCATCG-3') and β-actin (5'-ATTGCCGATAGTGATGACCT-3' and 5'-CGTGAAAAGATGACCCAGAT-3'). PCR was performed with an initial denaturation step followed by denaturation, annealing, and extension. PCR products were separated on 1% agarose gels and visualized using SYBR® Safe DNA gel stain (Invitrogen, Rockville, MD) under UV transillumination.
Statistical analysis
Data were expressed as mean ± SD. Differences were analyzed for significance by Student's t-test. The results were considered significant at P < 0.05.
Supplemental data
Supplemental Data include a Figure and can be found with this article online at http://e-emm.or.kr/article/article_files/SP-43-1-07.pdf.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Education, Science & Technology (MEST) through the Creative Research Initiative Program (Grant R16-2004-001010010, 2009) and the grant No. R31-2008-000-10103-0 from the WCU project of the MEST and the NRF.
Abbreviations
- Apo J
apolipoprotein J
- ROS
reactive oxygen species
- TRPM-2
testosterone-repressed prostate message-2
Supplemental data
Supplemental Data
References
- 1.Ammar H, Closset JL. Clusterin activates survival through the phosphatidylinositol 3-kinase/Akt pathway. J Biol Chem. 2008;283:12851–12861. doi: 10.1074/jbc.M800403200. [DOI] [PubMed] [Google Scholar]
- 2.Blaschuk O, Burdzy K, Fritz IB. Purification and characterization of a cell-aggregating factor (clusterin), the major glycoprotein in ram rete testis fluid. J Biol Chem. 1983;258:7714–7720. [PubMed] [Google Scholar]
- 3.Girton RA, Sundin DP, Rosenberg ME. Clusterin protects renal tubular epithelial cells from gentamicin-mediated cytotoxicity. Am J Physiol Renal Physiol. 2002;282:F703–F709. doi: 10.1152/ajprenal.00060.2001. [DOI] [PubMed] [Google Scholar]
- 4.Gross ER, Hsu AK, Gross GJ. Opioid-induced cardioprotection occurs via glycogen synthase kinase beta inhibition during reperfusion in intact rat hearts. Circ Res. 2004;94:960–966. doi: 10.1161/01.RES.0000122392.33172.09. [DOI] [PubMed] [Google Scholar]
- 5.Ishikawa Y, Akasaka Y, Ishii T, Komiyama K, Masuda S, Asuwa N, Choi-Miura NH, Tomita M. Distribution and synthesis of apolipoprotein J in the atherosclerotic aorta. Arterioscler Thromb Vasc Biol. 1998;18:665–672. doi: 10.1161/01.atv.18.4.665. [DOI] [PubMed] [Google Scholar]
- 6.Jones SE, Jomary C. Clusterin. Int J Biochem Cell Biol. 2002;34:427–431. doi: 10.1016/s1357-2725(01)00155-8. [DOI] [PubMed] [Google Scholar]
- 7.Juhaszova M, Zorov DB, Yaniv Y, Nuss HB, Wang S, Sollott SJ. Role of glycogen synthase kinase-3beta in cardioprotection. Circ Res. 2009;104:1240–1252. doi: 10.1161/CIRCRESAHA.109.197996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JH, Yu YS, Kim JH, Kim KW, Min BH. The role of clusterin in in vitro ischemia of human retinal endothelial cells. Curr Eye Res. 2007;32:693–698. doi: 10.1080/02713680701487871. [DOI] [PubMed] [Google Scholar]
- 9.Kim JH, Kim JH, Jun HO, Yu YS, Min BH, Park KH, Kim KW. Protective effect of clusterin from oxidative stress-induced apoptosis in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2010;51:561–566. doi: 10.1167/iovs.09-3774. [DOI] [PubMed] [Google Scholar]
- 10.Kounnas MZ, Loukinova EB, Stefansson S, Harmony JA, Brewer BH, Strickland DK, Argraves WS. Identification of glycoprotein 330 as an endocytic receptor for apolipoprotein J/clusterin. J Biol Chem. 1995;270:13070–13075. doi: 10.1074/jbc.270.22.13070. [DOI] [PubMed] [Google Scholar]
- 11.Krijnen PA, Cillessen SA, Manoe R, Muller A, Visser CA, Meijer CJ, Musters RJ, Hack CE, Aarden LA, Niessen HW. Clusterin: a protective mediator for ischemic cardiomyocytes? Am J Physiol Heart Circ Physiol. 2005;289:H2193–H2202. doi: 10.1152/ajpheart.00355.2005. [DOI] [PubMed] [Google Scholar]
- 12.Lamendola P, Di Monaco A, Barone L, Pisanello C, Lanza GA, Crea F. Mechanisms of myocardial cell protection from ischemia/reperfusion injury and potential clinical implications. G Ital Cardiol (Rome) 2009;10:28–36. [PubMed] [Google Scholar]
- 13.Lau SH, Sham JS, Xie D, Tzang CH, Tang D, Ma N, Hu L, Wang Y, Wen JM, Xiao G, Zhang WM, Lau GK, Yang M, Guan XY. Clusterin plays an important role in hepatocellular carcinoma metastasis. Oncogene. 2006;25:1242–1250. doi: 10.1038/sj.onc.1209141. [DOI] [PubMed] [Google Scholar]
- 14.Lee MJ, Kwak YK, You KR, Lee BH, Kim DG. Involvement of GADD153 and cardiac ankyrin repeat protein in cardiac ischemia-reperfusion injury. Exp Mol Med. 2009;41:243–252. doi: 10.3858/emm.2009.41.4.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Sagar MB, Wassler M, Shelat H, Geng YJ. Apolipoprotein-J prevention of fetal cardiac myoblast apoptosis induced by ethanol. Biochem Biophys Res Commun. 2007;357:157–161. doi: 10.1016/j.bbrc.2007.03.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maclellan WR, Schneider MD. Death by design. Programmed cell death in cardiovascular biology and disease. Circ Res. 1997;81:137–144. doi: 10.1161/01.res.81.2.137. [DOI] [PubMed] [Google Scholar]
- 17.McLaughlin L, Zhu G, Mistry M, Ley-Ebert C, Stuart WD, Florio CJ, Groen PA, Witt SA, Kimball TR, Witte DP, Harmony JA, Aronow BJ. Apolipoprotein J/clusterin limits the severity of murine autoimmune myocarditis. J Clin Invest. 2000;106:1105–1113. doi: 10.1172/JCI9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miura T, Miki T. GSK-3beta, a therapeutic target for cardiomyocyte protection. Circ J. 2009;73:1184–1192. doi: 10.1253/circj.cj-09-0284. [DOI] [PubMed] [Google Scholar]
- 19.Miyake H, Nelson C, Rennie PS, Gleave ME. Testosterone-repressed prostate message-2 is an antiapoptotic gene involved in progression to androgen independence in prostate cancer. Cancer Res. 2000;60:170–176. [PubMed] [Google Scholar]
- 20.Miyamoto S, Murphy AN, Brown JH. Akt mediated mitochondrial protection in the heart: metabolic and survival pathways to the rescue. J Bioenerg Biomembr. 2009;41:169–180. doi: 10.1007/s10863-009-9205-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyata M, Biro S, Kaieda H, Eto H, Orihara K, Kihara T, Obata H, Matsushita N, Matsuyama T, Tei C. Apolipoprotein J/clusterin is induced in vascular smooth muscle cells after vascular injury. Circulation. 2001;104:1407–1412. doi: 10.1161/hc3701.095583. [DOI] [PubMed] [Google Scholar]
- 22.Park SS, Zhao H, Mueller RA, Xu Z. Bradykinin prevents reperfusion injury by targeting mitochondrial permeability transition pore through glycogen synthase kinase 3beta. J Mol Cell Cardiol. 2006;40:708–716. doi: 10.1016/j.yjmcc.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 23.Pollack M, Phaneuf S, Leeuwenburgh C. The role of apoptosis in the normal aging brain, skeletal muscles, and heart. Ann N Y Acad Sci. 2002;959:93–107. doi: 10.1111/j.1749-6632.2002.tb02086.x. [DOI] [PubMed] [Google Scholar]
- 24.Poon S, Easterbrook-Smith SB, Rybchyn MS, Carver JA, Wilson MR. Clusterin is an ATP independent chaperone with very broad substrate specificity that stabilizes stressed proteins in a folding competent state. Biochemistry. 2000;39:15953–15960. doi: 10.1021/bi002189x. [DOI] [PubMed] [Google Scholar]
- 25.Sallman DA, Chen X, Zhong B, Gilvary DL, Zhou J, Wei S, Djeu JY. Clusterin mediates TRAIL resistance in prostate tumor cells. Mol Cancer Ther. 2007;6:2938–2947. doi: 10.1158/1535-7163.MCT-07-0345. [DOI] [PubMed] [Google Scholar]
- 26.Saraste A, Pulkki K, Kallajoki M, Henriksen K, Parvinen M, Volpio-Pulkki LM. Apoptosis in human acute myocardial infarction. Circulation. 1997;95:320–323. doi: 10.1161/01.cir.95.2.320. [DOI] [PubMed] [Google Scholar]
- 27.Sensibar JA, Sutkowski DM, Raffo A, Buttyan R, Griswold MD, Sylvester SR, Kozlowski JM, Lee C. Prevention of cell death induced by tumor necrosis factor alpha in LNCaP cells by overexpression of sulfated glycoprotein-2 (clusterin) Cancer Res. 1995;55:2431–2437. [PubMed] [Google Scholar]
- 28.Shin YJ, Kang SW, Jeong SY, Shim YJ, Kim YH, Kim BM, Kee SH, Park JJ, Park IS, Min BH. Clusterin enhances proliferation of primary astrocytes through extracellular signal-regulated kinase activation. Neuroreport. 2006;17:1871–1875. doi: 10.1097/WNR.0b013e328010ac99. [DOI] [PubMed] [Google Scholar]
- 29.Shin YJ, Kim JH, Seo JM, Lee SM, Hyon JY, Yu YS, Wee WR. Protective effect of clusterin on oxidative stress-induced cell death of human corneal endothelial cells. Mol Vis. 2009;15:2789–2795. [PMC free article] [PubMed] [Google Scholar]
- 30.Silkensen JR, Hirsch AT, Lunzer MM, Chmielewski D, Manivel JC, Muellerleile MR, Rosenberg ME. Temporal induction of clusterin in the peri-infarct zone after experimental myocardial infarction in the rat. J Lab Clin Med. 1998;131:28–35. doi: 10.1016/s0022-2143(98)90074-9. [DOI] [PubMed] [Google Scholar]
- 31.Singla DK, McDonald DE. Factors released from embryonic stem cells inhibit apoptosis of H9c2 cells. Am J Physiol Heart Circ Physiol. 2007;293:H1590–H1595. doi: 10.1152/ajpheart.00431.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Susnow N, Zeng L, Margineantu D, Hockenbery DM. Bcl-2 family proteins as regulator of oxidative stress. Semin Cancer Biol. 2009;19:42–49. doi: 10.1016/j.semcancer.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swertfeger DK, Witte DP, Stuart WD, Rockman HA, Harmony JA. Apolipoprotein J/clusterin induction in myocarditis: A localized response gene to myocardial injury. Am J Pathol. 1996;148:1971–1983. [PMC free article] [PubMed] [Google Scholar]
- 34.Trougakos IP, Gonos ES. Regulation of clusterin/apolipoprotein J, a functional homologue to the small heat shock proteins, by oxidative stress in ageing and age-related diseases. Free Radic Res. 2006;40:1324–1334. doi: 10.1080/10715760600902310. [DOI] [PubMed] [Google Scholar]
- 35.Vakeva A, Laurila P, Meri S. Co-deposition of clusterin with the complement membrane attack complex in myocardial infarction. Immunology. 1993;80:177–182. [PMC free article] [PubMed] [Google Scholar]
- 36.Van Dijk A, Vermond RA, Krijnen PA, Juffermans LJ, Hahn NE, Makker SP, Aarden LA, Hack E, Spreeuwenberg M, van Rossum BC, Meischl C, Paulus WJ, Van Milligen FJ, Niessen HW. Intravenous clusterin administration reduces myocardial infarct size in rats. Eur J Clin Invest. 2010;40:893–902. doi: 10.1111/j.1365-2362.2010.02345.x. [DOI] [PubMed] [Google Scholar]
- 37.Whang WK, Park HS, Ham I, Oh M, Namkoong H, Kim HK, Hwang DW, Hur SY, Kim TE, Park YG, Kim JR, Kim JW. Natural compounds,fraxin and chemicals structurally related to fraxin protect cells from oxidative stress. Exp Mol Med. 2005;37:436–446. doi: 10.1038/emm.2005.54. [DOI] [PubMed] [Google Scholar]
- 38.Wilson MR, Easterbrook-Smith SB. Clusterin is a secreted mammalian chaperone. Trends Biochem Sci. 2000;25:95–98. doi: 10.1016/s0968-0004(99)01534-0. [DOI] [PubMed] [Google Scholar]
- 39.Zlokovic BV, Martel CL, Matsubara E, McComb JG, Zheng G, McCluskey RT, Frangione B, Ghiso J. Glycoprotein 330/megalin: probable role in receptor-mediated transport of apolipoprotein J alone and in a complex with Alzheimer disease amyloid beta at the blood-brain and blood-cerebrospinal fluid barriers. Proc Natl Acad Sci USA. 1996;93:4229–4234. doi: 10.1073/pnas.93.9.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data