Abstract
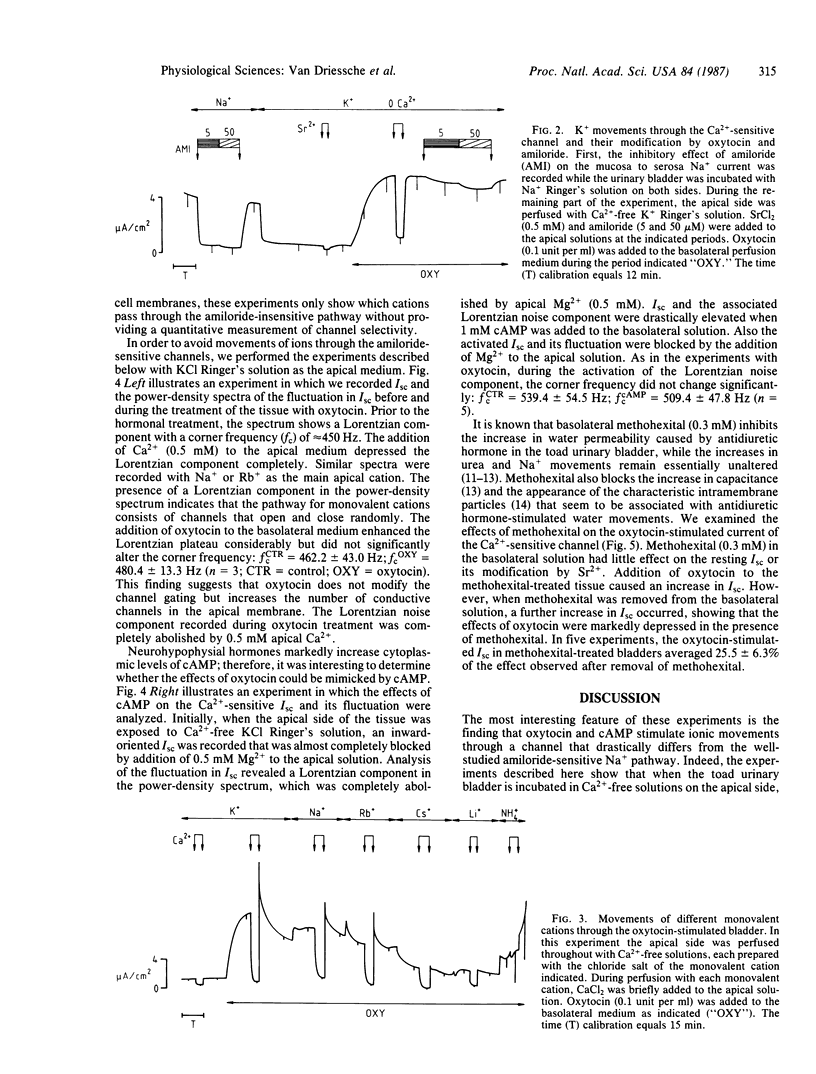
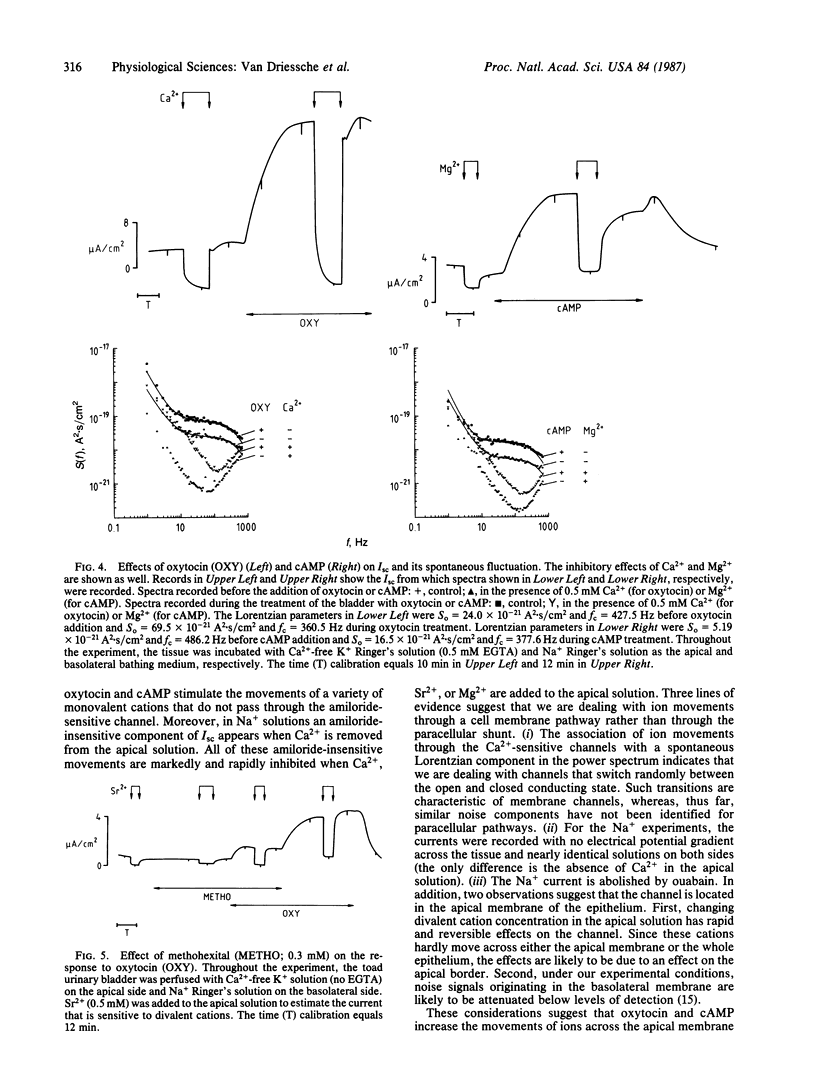
The effects of oxytocin and cAMP on ion transport were investigated in toad urinary bladders incubated with Ca2+-free solutions on the apical side. Under these conditions both oxytocin and cAMP markedly stimulated the movements of Na+, K+, Rb+, Cs+, Li+, and NH4+ through a pathway that is insensitive to amiloride. The amiloride-insensitive currents were inhibited by the addition of Ca2+, Sr2+, or Mg2+ to the apical solution. The movement of the monovalent cations was associated with a spontaneous Lorentzian component in the power spectrum of the fluctuation in short-circuit current. The plateau of the Lorentzian component was enhanced by oxytocin and cAMP and was depressed by divalent cations. Methohexital inhibited the stimulation of monovalent cation movements caused by oxytocin. These findings suggest that oxytocin and cAMP activate at least two kinds of ionic channels in the apical membrane of toad urinary bladder: the well-known amiloride-sensitive channel and an amiloride-insensitive channel that allows the movement of several monovalent cations and is blocked by Ca2+ and other divalent cations.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., McCleskey E. W., Palade P. T. A non-selective cation conductance in frog muscle membrane blocked by micromolar external calcium ions. J Physiol. 1984 Aug;353:565–583. doi: 10.1113/jphysiol.1984.sp015351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Kunze D. L., Yatani A. The agonist effect of dihydropyridines on Ca channels. Nature. 1984 Oct 11;311(5986):570–572. doi: 10.1038/311570a0. [DOI] [PubMed] [Google Scholar]
- De Sousa R. C., Grosso A. Osmotic water flow across the abdominal skin of the toad bufo marinus: effect of vasopressin and isoprenaline. J Physiol. 1982 Aug;329:281–296. doi: 10.1113/jphysiol.1982.sp014303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erne P., Bürgisser E., Bühler F. R., Dubach B., Kühnis H., Meier M., Rogg H. Enhancement of calcium influx in human platelets by CGP 28392, a novel dihydropyridine. Biochem Biophys Res Commun. 1984 Feb 14;118(3):842–847. doi: 10.1016/0006-291x(84)91471-2. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hess P., Tsien R. W. Mechanism of ion permeation through calcium channels. 1984 May 31-Jun 6Nature. 309(5967):453–456. doi: 10.1038/309453a0. [DOI] [PubMed] [Google Scholar]
- Kachadorian W. A., Levine S. D., Wade J. B., Di Scala V. A., Hays R. M. Relationship of aggregated intramembranous particles to water permeability in vasopressin-treated toad urinary bladder. J Clin Invest. 1977 Mar;59(3):576–581. doi: 10.1172/JCI108673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. High selectivity of calcium channels in single dialysed heart cells of the guinea-pig. J Physiol. 1984 Sep;354:253–272. doi: 10.1113/jphysiol.1984.sp015374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S. D., Levine R. D., Worthington R. E., Hays R. M. Selective inhibition of osmotic water flow by general anesthetics to toad urinary bladder. J Clin Invest. 1976 Oct;58(4):980–988. doi: 10.1172/JCI108552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macknight A. D., DiBona D. R., Leaf A. Sodium transport across toad urinary bladder: a model "tight" epithelium. Physiol Rev. 1980 Jul;60(3):615–715. doi: 10.1152/physrev.1980.60.3.615. [DOI] [PubMed] [Google Scholar]
- McCleskey E. W., Almers W. The Ca channel in skeletal muscle is a large pore. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7149–7153. doi: 10.1073/pnas.82.20.7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H., Scholz H. The regulation of the calcium conductance of cardiac muscle by adrenaline. J Physiol. 1977 Jan;264(1):49–62. doi: 10.1113/jphysiol.1977.sp011657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoen H. F., Erlij D. Basolateral membrane responses to transport modifiers in the frog skin epithelium. Pflugers Arch. 1985;405 (Suppl 1):S33–S38. doi: 10.1007/BF00581777. [DOI] [PubMed] [Google Scholar]
- Sperelakis N., Schneider J. A. A metabolic control mechanism for calcium ion influx that may protect the ventricular myocardial cell. Am J Cardiol. 1976 Jun;37(7):1079–1085. doi: 10.1016/0002-9149(76)90428-8. [DOI] [PubMed] [Google Scholar]
- Stetson D. L., Lewis S. A., Alles W., Wade J. B. Evaluation by capacitance measurements of antidiuretic hormone induced membrane area changes in toad bladder. Biochim Biophys Acta. 1982 Jul 28;689(2):267–274. doi: 10.1016/0005-2736(82)90259-0. [DOI] [PubMed] [Google Scholar]
- Van Driessche W., Erlij D. Noise analysis of inward and outward Na+ currents across the apical border of ouabain-treated frog skin. Pflugers Arch. 1983 Aug;398(3):179–188. doi: 10.1007/BF00657149. [DOI] [PubMed] [Google Scholar]
- Van Driessche W., Lindemann B. Low-noise amplification of voltage and current fluctuations arising in epithelia. Rev Sci Instrum. 1978 Jan;49(1):53–57. [PubMed] [Google Scholar]
- Van Driessche W., Zeiske W. Ba2+-induced conductance fluctuations of spontaneously fluctuating K+ channels in the apical membrane of frog skin (Rana temporaria). J Membr Biol. 1980 Aug 21;56(1):31–42. doi: 10.1007/BF01869349. [DOI] [PubMed] [Google Scholar]
- Van Driessche W., Zeiske W. Ca2+-sensitive, spontaneously fluctuating, cation channels in the apical membrane of the adult frog skin epithelium. Pflugers Arch. 1985 Oct;405(3):250–259. doi: 10.1007/BF00582569. [DOI] [PubMed] [Google Scholar]
- Van Driessche W., Zeiske W. Ionic channels in epithelial cell membranes. Physiol Rev. 1985 Oct;65(4):833–903. doi: 10.1152/physrev.1985.65.4.833. [DOI] [PubMed] [Google Scholar]



