Myosin drives autophagy in a pathway linking Atg1 to Atg9
The Atg1 kinase promotes autophagy, but its substrates have remained elusive. In this issue of the EMBO Journal, Tang et al identify a myosin light chain kinase as an Atg1 substrate, revealing a pathway that regulates Atg9 trafficking and autophagosome formation.
EMBO J 30 4, 636–651 (2011); published online December172010
Autophagy is a cellular process in which specialized autodegradative vesicles, the autophagosomes, are formed. Much progress has been made in understanding the molecular mechanism controlling autophagy, particularly the role of the Atg genes. In this issue, Tang et al identify a signalling pathway linking two main regulators, the Atg1 kinase—essential for the induction of the autophagosome—and the transmembrane protein Atg9, whose shuttling between the Golgi and the forming autophagosme provides a source of membrane for the new vesicle. This study provides the missing piece of the puzzle: Atg1 phosphorylates and activates a myosin light chain kinase, which in turn activates myosin to drive transport of Atg9.
During autophagy, the cell consumes its own contents in specialized double-membrane enclosed vesicles, the autophagosomes, and this is crucial for homeostasis and cellular stress responses. Recent studies have examined mechanisms controlling various steps of autophagy, including induction, nucleation, and expansion of the autophagosome, and trafficking and fusion to the lysosome to form the degradative autophagosome (Simonsen and Tooze, 2009). A group of Atg genes, originally isolated in yeast, control the early steps. At the signalling pathway's apex lies the Atg1 kinase (Ulk1/2 in mammals) and its associated proteins (Jung et al, 2010). The Atg1 complex is kept inactive by phosphorylation by the Target of Rapamycin (TOR; mTOR in mammals) kinase, a sensor of energy and nutrient availability that regulates cell growth and protein synthesis (Jung et al, 2010). Upon nutrient deprivation, TOR is silenced, enabling activation of Atg1/Ulk1 and consequently, autophagy. Two factors are needed downstream of Atg1/Ulk1 for the initiation of autophagosome formation: the Class III PI3 kinase Vps34 (Simonsen and Tooze, 2009), and trafficking of Atg9, a transmembrane protein, which is thought to provide a membrane source by shuttling from pre-existing membrane sites to the forming autophagosome (Webber and Tooze, 2010). Until recently, it was not known how Atg1/Ulk1 activates autophagy, since its only known substrates were members of its own complex. Now, Tang et al describe a new Atg1 substrate, discovered through a combined approach involving Drosophila and human cells (Tang et al, 2010). They identified a signalling pathway induced by Atg1/Ulk1 leading to myosin II activation, which they propose mediates trafficking of Atg9. This is the second mechanism to explain Atg1/Ulk1 activity, following a recently published paper in which Ulk1 was shown to phosphorylate AMBRA1, a Beclin-1-binding protein that is part of the Vps34 complex. AMBRA1 phosphorylation leads to its release from dynein light chain and the cytoskeleton, enabling Vps34/Beclin-1 complex formation and activation (Di Bartolomeo et al, 2010).
Tang et al show that Drosophila Atg1 and human Ulk1 both lead to increased phosphorylation of the myosin II regulatory light chain (Spaghetti-squash (Sqh) in Drosophila, MLC in human), which activates myosin. Moreover, phosphorylation of Sqh was necessary for autophagy in third instar wing imaginal disks. Atg1 was incapable of phosphorylating Sqh directly; instead the authors identify a new Drosophila MLCK-like kinase, Spaghetti-squash activator, or Sqa, which can phosphorylate Sqh in vitro and in vivo. Importantly, Atg1 binds and phosphorylates Sqa on the conserved Thr279, thereby activating it. Sqa and subsequent Sqh phosphorylation were required for Atg1-induced changes in actin organization and autophagy. Sqa phosphorylation on Thr279 was also shown to be necessary for starvation-induced myosin activation, and importantly, autophagosome formation, in larval fat body. The authors suggest that Sqa is a member of the DAP-kinase (DAPk) family, a group of death-associated kinases that phosphorylate MLC and induce autophagy (Bialik and Kimchi, 2006), and that ZIP kinase (ZIPk), specifically, is the mammalian Sqa homologue. In fact, phosphorylation of MLC required both Ulk1 and ZIPk in starved MCF7 cells. Moreover, inhibition of myosin II suppressed starvation-induced autophagy, as did depletion of ZIPk. Significantly, Sqa Thr279 corresponds to Thr265 on ZIPk, which is phosphorylated to activate ZIPk (Graves et al, 2005). The authors show that ZIPk interacts with Ulk1 but they do not assess direct transphosphorylation.
Using human cells, Tang et al identify a link between myosin contractility and autophagosome formation. Ulk1 was previously shown to be required for Atg9 redistribution from the trans-Golgi network to autophagosomes (Webber and Tooze, 2010). Now the authors show that both ZIPk and myosin are required for this translocation during starvation, and that Atg9 interacts with myosin in a ZIPk and Ulk1-dependent manner. Thus, by activating myosin II through ZIPk, Ulk1 regulates Atg9 trafficking to induce autophagy (Figure 1). Of note, the authors have not excluded other MLC kinases, particularly other DAPk family members, from participating in this pathway. In fact, ZIPk depletion only partially affected starvation-induced MLC phosphorylation and Atg9 trafficking, and other data were based on myosin depletion or inhibitors that could block the action of any of the MLC kinases.
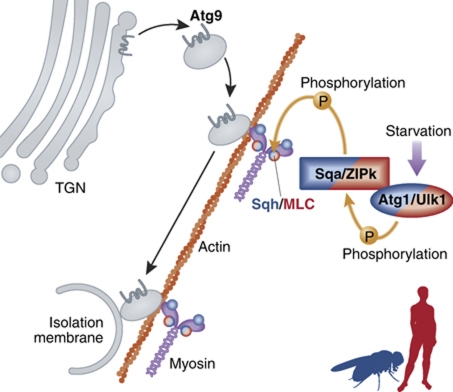
Figure 1.
Schematic of proposed Atg1/myosin/Atg9 signalling pathway. Upon activation by starvation, Atg1/Ulk1 phosphorylates and activates Sqa/ZIPk, which in turn phosphorylates Sqh/MLC to activate myosin II. Myosin drives trafficking of Atg9-containing membranes, which derive from fission of the trans-Golgi network (TGN), to the sites of autophagosome nucleation, the isolation membrane. Note that this model combines the data from Drosophila and human cells, and some details have not yet been confirmed in both organisms.
The human DAPk family includes DAPK1, DRP-1 (DAPK2), and ZIPk (DAPK3) and more distant relatives, DRAK1 and 2 (Bialik and Kimchi, 2006). Phylogenetic analysis indicates that DRP-1 and ZIPk are found only in vertebrates, but DAPK1 is a more ancient gene found also in some invertebrates. Curiously, Drosophila lacks a DAPK1 gene, although fly DRAK2 has been identified (Neubueser and Hipfner, 2010). Other arthropods have both DAPK1 and Sqa genes, the latter of which seems restricted to arthropods. Notably, the DAPk family is a distinct subgroup within the CaM kinase superfamily, while Sqa (CG1776) is found within the closely related MLCK family (Champagne et al, 2000). Tang et al classify Sqa as a homologue of ZIPk, since its protein domain organization resembles ZIPk more than the other kinases. However, Sqa and ZIPk are not orthologs, although, at least in Drosophila, Sqa fills the functional role of DAPk family members.
DAPK1 and ZIPk have been shown to phosphorylate MLC in vivo, leading to changes in cell morphology such as membrane blebbing (Bialik and Kimchi, 2006). DAPK1 has been previously shown to induce autophagy by various mechanisms independent of its cytoskeletal function, including direct phosphorylation of Beclin-1 (Zalckvar et al, 2009; Bialik and Kimchi, 2010). The data presented by Tang et al suggest that the autophagy-inducing activity of the DAPk family also derives from its ability to activate myosin. It will be interesting to determine in future studies whether additional trafficking or membrane-remodelling events that determine autophagosome formation are regulated by the Ulk1/DAPk family/myosin pathway.
Footnotes
The authors declare that they have no conflict of interest.
References
- Bialik S, Kimchi A (2006) The death-associated protein kinases: structure, function and beyond. Annu Rev Biochem 75: 189–210 [DOI] [PubMed] [Google Scholar]
- Bialik S, Kimchi A (2010) Lethal weapons: DAP-kinase, autophagy and cell death. Curr Opin Cell Biol 22: 199–205 [DOI] [PubMed] [Google Scholar]
- Champagne MB, Edwards KA, Erickson HP, Kiehart DP (2000) Drosophila stretchin-MLCK is a novel member of the Titin/Myosin light chain kinase family. J Mol Biol 300: 759–777 [DOI] [PubMed] [Google Scholar]
- Di Bartolomeo S, Corazzari M, Nazio F, Oliverio S, Lisi G, Antonioli M, Pagliarini V, Matteoni S, Fuoco C, Giunta L, D'Amelio M, Nardacci R, Romagnoli A, Piacentini M, Cecconi F, Fimia GM (2010) The dynamic interaction of AMBRA1 with the dynein motor complex regulates mammalian autophagy. J Cell Biol 191: 155–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves PR, Winkfield KM, Haystead TA (2005) Regulation of zipper-interacting protein kinase activity in vitro and in vivo by multisite phosphorylation. J Biol Chem 280: 9363–9374 [DOI] [PubMed] [Google Scholar]
- Jung CH, Ro S-H, Cao J, Otto NM, Kim D-H (2010) mTOR regulation of autophagy. FEBS Lett 584: 1287–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubueser D, Hipfner DR (2010) Overlapping roles of Drosophila Drak and Rok kinases in epithelial tissue morphogenesis. Mol Biol Cell 21: 2869–2879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A, Tooze SA (2009) Coordination of membrane events during autophagy by multiple class III PI3-kinase complexes. J Cell Biol 186: 773–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H-W, Wang Y-B, Wang S-L, Wu M-H, Lin S, Chen G-C (2011) Atg1-mediated myosin II activation regulates autophagosome formation during starvation-induced autophagy. EMBO J 30: 636–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber JL, Tooze SA (2010) New insights into the function of Atg9. FEBS Lett 584: 1319–1326 [DOI] [PubMed] [Google Scholar]
- Zalckvar E, Berissi H, Mizrachy L, Idelchuk Y, Koren I, Eisenstein M, Sabanay H, Pinkas-Kramarski R, Kimchi A (2009) DAP-kinase-mediated phosphorylation on the BH3 domain of beclin 1 promotes dissociation of beclin 1 from Bcl-XL and induction of autophagy. EMBO Rep 10: 285–292 [DOI] [PMC free article] [PubMed] [Google Scholar]