Abstract
Importance of the field
Photoacoustic imaging is an imaging modality that derives image contrast from the optical absorption coefficient of the tissue being imaged. The imaging technique is able to differentiate between healthy and diseased tissue with either deeper penetration or higher resolution than other functional imaging modalities currently available. From a clinical standpoint, photoacoustic imaging has demonstrated safety and effectiveness in diagnosing diseased tissue regions using either endogenous tissue contrast or exogenous contrast agents. Furthermore, the potential of photoacoustic imaging has been demonstrated in various therapeutic interventions ranging from drug delivery and release to image-guided therapy and monitoring.
Areas covered in this review
This article reviews the current state of photoacoustic imaging in biomedicine from a technological perspective, highlights various biomedical and clinical applications of photoacoustic imaging, and gives insights on future directions.
What the reader will gain
Readers will learn about the various applications of photoacoustic imaging, as well as the various contrast agents that can be used to assist photoacoustic imaging. This review will highlight both pre-clinical and clinical uses for photoacoustic imaging, as well as discuss some of the challenges that must be addressed to move photoacoustic imaging into the clinical realm.
Take home message
Photoacoustic imaging offers unique advantages over existing imaging modalities. The imaging field is broad with many exciting applications for detecting and diagnosing diseased tissue or processes. Photoacoustics is also used in therapeutic applications to identify and characterize the pathology and then to monitor the treatment. Although the technology is still in its infancy, much work has been done in the pre-clinical arena, and photoacoustic imaging is fast approaching the clinical setting.
Keywords: Atherosclerosis, cancer, contrast agents, drug delivery, molecular imaging, nanoparticles, optoacoustics, photoacoustics, therapy, ultrasound
1. Introduction
1.1. Basic Fundamentals
Biomedical photoacoustic (also known as optoacoustic) imaging is an imaging modality that derives its contrast from the optical absorption properties of tissue and other objects being imaged [1, 2]. Imaging is performed through the detection of photoacoustic signals generated from the energy absorption events caused by of pulsed laser illumination. Since physiological and pathological changes often alter tissue composition and its associated optical absorption, the magnitude of the received photoacoustic signal can reveal different characteristics of living tissue. Many imaging applications have taken advantage of the strong potential of photoacoustic imaging from detection of cancer [3] to diagnosis of vulnerable atherosclerotic plaques [4, 5]. Since image contrast is obtained from optical absorption properties, imaging can be performed using endogenous or exogenous contrast in tissue. The primary endogenous optical absorber in tissue in the near infrared spectrum is hemoglobin. The absorption coefficient of hemoglobin is several orders of magnitude greater than the absorption of surrounding tissues [6]. In vivo photoacoustic imaging of blood vasculature [7] has been used to monitor tumor angiogenesis, vasa vasorum in atheroscelerotic plaques, blood oxygenation [8], functional brain mapping [9], and also skin melanomas [10]. At wavelengths above 1100 nm, optical absorption of other tissue components such as lipid may dominate, which is helpful in imaging other tissue types [5, 11]. To further improve photoacoustic contrast, various exogenous contrast agents have been introduced to target specific regions or pathologies. These contrast agents include the use of dyes [7, 12], nanoparticles [13, 14] or other absorbers [15] targeted to various receptors on the surface of diseased cells.
Photoacoustic imaging operates through the use of a pulsed energy irradiated into tissue. Energy absorbed by tissue is converted into heat, whereupon rapid thermal expansion occurs and causes emission of an acoustic pressure wave proportional to the fluence of the irradiated energy, Φ, the absorption coefficient of the medium being irradiated, μa, and the Grüneisen coefficient of tissue, Γ. This relationship is described in equation 1, where μa is dependent on the wavelength of light, and fluence is dependent on the wavelength of light and the depth to which the photons propagate. The resulting acoustic transients are wideband and can be detected by an ultrasonic transducer. Reconstruction of the resultant image is performed by inversely calculating the photoacoustic signal source both temporally and spatially. Two-dimensional images or 3D tomographic images can be constructed depending on the scan parameters used in data acquisition.
| (Eq. 1) |
Multi-wavelength, or spectroscopic, photoacoustic imaging can be used to reconstruct the local optical absorption spectrum in the imaged regions of interest [5]. The optical absorption coefficient can be backcalculated at corresponding wavelengths by normalizing the acquired photoacoustic signals to their fluence, the Grüneisen coefficient of the environment, and also accounting for wavelength-dependent scattering [3]. Examining the unique absorption spectra from the corresponding photoacoustic image can determine the composition of lipid-filled plaques or cancerous neoplasia growth. The ability to image at different wavelengths and fluences, as well as perform different signal processing methods on the photoacoustic signal, provide the necessary framework for developing new clinical methods to accurately image in vivo tissue in real-time.
In this paper, we review the current state of photoacoustic imaging in terms of technology and implementation, biomedical applications, and future directions.
1.2. Components/Systems
Systems for photoacoustic imaging require two primary components: a pulsed energy source and an acoustic detector. Usually, a pulsed nanosecond laser is used as the energy source. The laser pulse is delivered to the tissue through a combination of optical fibers and/or mirrors. Acoustic detectors can be single element or array-based ultrasound transducers of various center frequencies. An ultrasound receiver is required to receive photoacoustic signals, and a microprocessor is used to handle data acquisition, image reconstruction and display. With these basic components in place, a photoacoustic imaging system can take on many different configurations.
The simplest photoacoustic imaging setup uses a focused single-element ultrasound transducer. Typically seen in photoacoustic microscopy (PAM) systems, this configuration is able to detect photoacoustic signals from a single A-line (i.e., the axial line along the ultrasound beam of the transducer). Imaging requires the transducer to be mechanically scanned if a 2-D imaging plane is desired. This type of imaging requires minimal image reconstruction, as the amplitude at every position along the A-line is mapped to a corresponding image pixel. Photoacoustic microscopy systems use transducers with high center frequencies and have the advantage of high spatial resolution at relatively high imaging depth compared to optical imaging modalities. Additional scanning geometries can be used to generate photoacoustic tomographic (PAT) images, which rely on reconstruction methods to form images [16]. To improve image acquisition speed and reduce the need for mechanical scanning, ultrasound array transducers can be used for real-time photoacoustic imaging to be performed [17]. Image reconstruction, called beamforming, is required to process signals captured by array elements. Beamforming methods have been developed to improve spatial resolution and increase image contrast [17]. Real-time or near real-time photoacoustic images can be obtained as long as the pulse repetition rate of the laser source is fast enough (i.e., 30 pulses per second). Many array-based systems have been used for in vivo photoacoustic imaging [18, 19].
In addition to photoacoustic imaging from the surface of the skin, several applications of photoacoustic imaging are based on endoscopic probes. Endoscopic imaging requires the use of a catheter-based probe for both light delivery and acoustic detection similar to endoscopic ultrasound or optical coherence tomography [20]. A combined catheter probe has proven to be technically challenging, with several groups trying different techniques to implement a light delivery system, ultrasound transducer and a mechanical scanning system in one probe [16, 21, 22].
1.3. Hybrid Imaging Modalities
Several other imaging modalities have been proposed to complement the absorption-based contrast modality of photoacoustic imaging. The most common hybrid imaging technology combined with photoacoustic imaging is ultrasound. This combination of ultrasound and photoacoustic imaging is based on the complementary nature of these imaging modalities [23, 24]. Ultrasound has been in widespread clinical practice for many years. It is a relatively inexpensive, easy to use, real-time imaging technology. Its non-invasiveness has allowed it to be extensively used in many diagnostic settings such as obstetrics, mammography, cardiology, and also for needle guidance in tissue biopsy. Ultrasound is very successful at imaging tissue structure and morphology. However, ultrasound is limited by its ability to identify all abnormalities in tissue. Acoustic contrast between healthy and diseased tissue can be very limited, hindering effective diagnosis. Photoacoustic imaging is able to complement and enhance ultrasound imaging by providing physiological and functional assessment of tissue based on changes in optical absorption [25]. The shared use of an ultrasound transducer for signal detection increases the ease of integration and decreases the cost for combined imaging. Since ultrasound is already readily available in clinical settings, all the necessary prerequisites for combined imaging are in place, allowing for ease of integration into the diagnostic procedure.
Magneto-photoacoustic imaging is a technique based on the combination of magneto-motive ultrasound (MMUS) and photoacoustic imaging. MMUS is capable of detecting the presence of hybrid magnetic particles [26]. In situations where photoacoustic signal is used to detect the aggregation of nanoparticles [27], the absence of photoacoustic signal can indicate either the lack of particle aggregation or the complete absence of particles. Magneto-photoacoustic imaging, using hybrid magnetic plasmonic nanoparticles, can confirm the presence or absence of these particles and photoacoustic imaging can further identify the state of the nanoparticles [28, 29].
Photoacoustic imaging has also been combined with optical coherence tomography (OCT) to provide complementary contrast for imaging tissue [30, 31]. The combination of photoacoustic and OCT provides comprehensive anatomical and functional information of biological tissues. The capabilities of this technique was demonstrated by imaging the anatomy and microvasculature of a mouse ear in vivo [30]. Furthermore, functional imaging could be measured together [31]: photoacoustics can be used to measure oxygen saturation [32], while OCT can be used to measure blood flow as slow as 10 μm/s [33].
1.4. Comparisons with Other Imaging Modalities
One of the primary benefits of photoacoustic imaging is its ability to image functional information due to the strong wavelength-dependent optical absorption oxygenated and deoxygenated blood, or contrast agents. Many functional imaging modalities, such as positron emission tomography (PET) and single photon emission computed tomography (SPECT), rely on the use of nuclear radioisotopes for gamma-ray detection but have poor spatial resolution. Optical imaging techniques can be used to obtain functional information, but suffer from poor spatial resolution at depths beyond 0.5 mm due to the high optical scattering properties of tissue. Acoustic scattering in tissue, however, is several orders of magnitude weaker than optical scattering, and therefore, photoacoustic imaging overcomes the depth limitation of optical techniques by detecting acoustic phonons instead of ballistic photons. Photoacoustics can visualize light-tissue interaction with high spatial resolution at depths of several tens of millimeters, depending on the laser wavelength and transducer frequency used. Generally, spatial resolution of photoacoustic imaging beyond 0.5 mm is determined by the ultrasound transducer. However, within the penetration depth of ballistic photons, incorporating fine optical focusing results in optical-resolution photoacoustic microscopy (OR-PAM) [34]. In OR-PAM imaging, lateral resolution is increased, while axial resolution is still determined by the transducer bandwidth.
1.5. Broad Applications of Photoacoustic Imaging
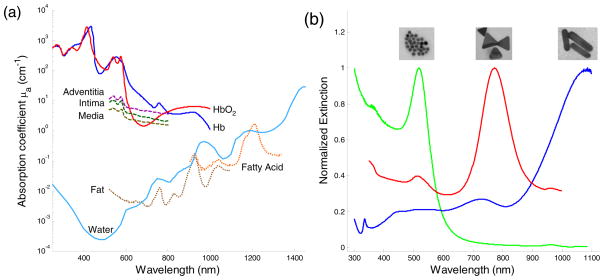
Photoacoustic imaging is ideal for imaging in areas where differences in optical absorption exist (Fig. 1a). These contrast differences can occur naturally, such as in atherosclerotic plaques or angiogenesis in tumor. In atherosclerosis, the presence of a large lipid pool or a thin fibrous cap is a strong indicator of vulnerable plaques and can be investigated with multi-wavelength intravascular photoacoustic (IVPA) imaging [5, 11, 25]. Plaque formation is usually accompanied by the development of a micro-network of blood vessels that serve to feed the plaque. This angiogenic growth can serve as an indicator of plaque growth and vulnerability. Tumor growth is also dependent on angiogenesis which can be an early indicator of various cancers, such as breast cancer [35].
Figure 1.
(a) Optical absorption spectrum of different tissue types which could be used as endogenous contrast agents in vivo. (b) Exogenous contrast agents such as gold nanospheres (16 nm diameter), silver nanotriangles (200 nm on edge), and gold nanorods (10 nm by 40 nm) and their corresponding extinction spectrum. Tunable peaks show that nanoparticles can be used as contrast agents in various applications.
Contrast can also come from exogenous sources (Fig. 1b). For example, contrast agent labeled antibodies can be injected to specifically target regions of interest for photoacoustic imaging. Photoacoustics has also been proposed as a detection method for guiding metal needles or detecting foreign objects embedded in vivo [36].
2. Diagnostic Imaging
2.1. Endogenous Contrast Agents
The intrinsic optical absorption property of tissue constituents can be used to identify tissue components. Because the amplitude of the photoacoustic response is proportional to the optical absorption coefficient, photoacoustic imaging can differentiate tissue types based on the endogenous contrast in tissue. Figure 1a shows the optical absorption spectrum of common tissue constituents [6, 37, 38]. Blood (oxygenated and de-oxygenated hemoglobin) dominates in the visible to near infrared wavelength range. The absorption of water is very low compared to blood in the same wavelength range, but starts to increase and becomes dominant in the infrared wavelengths.
Some types of tissue, such as blood and melanoma cells, can be imaged at a single wavelength in the visible range because of their high optical absorption contrast compared to surrounding tissues [39]. However, to differentiate tissue types with lower optical absorption, multi-wavelength imaging is necessary. Spectroscopic photoacoustic imaging has been widely investigated to detect the oxygen saturation in blood vessels. Changes of oxygen saturation in the brain, skin or tumors usually indicate changes in their physiological condition [2, 40, 41]. For example, the irregular vasculature in a tumor region causes insufficient blood perfusion and leads to hypoxemia that can be detected by spectroscopic photoacoustic imaging [41].
Fatty tissue, as another example, can also be differentiated from other tissue types based on the optical absorption peak of fatty acid around 1210 nm. At wavelengths higher than 1000 nm, the optical absorption from blood is low and overall background photoacoustic signal from native tissue is more homogeneous [42]. The relatively sharp absorption peak of fatty acid at 1210 nm makes detection within a small wavelength range possible, reducing the effect of wavelength-dependent optical property changes on the spectroscopic analysis (such as optical scattering). Moreover, photoacoustic imaging may detect fatty tissue with higher sensitivity in vivo because fatty tissues have higher Grüneisen coefficients than water [43].
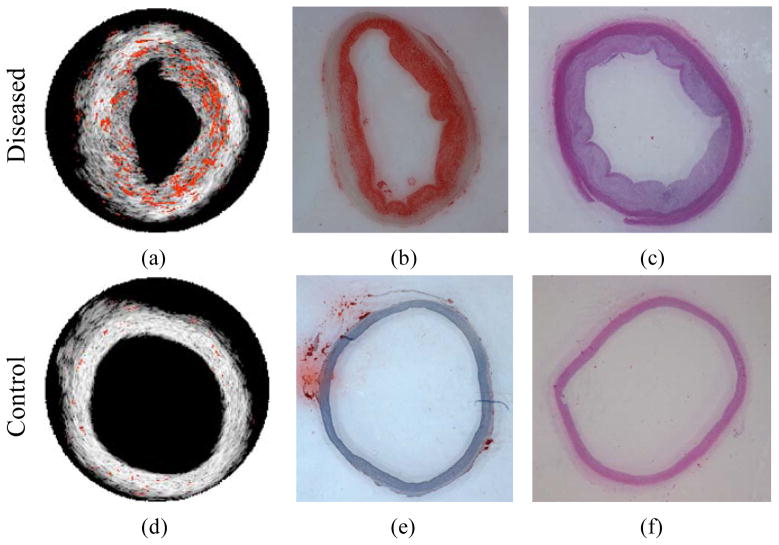
Spectroscopic photoacoustic detection of fatty tissue is of interest in atherosclerotic plaque detection because lipid is one of the most important pathological relevant constituents in atherosclerosis [11]. Plaques with a large lipid pool and thin fibrous cap are typically classified as rupture-prone plaques [44]. In Figure 2a, lipid in an atherosclerotic plaque is imaged using an intravascular photoacoustic (IVPA) imaging system operating from 1200–1230 nm [11]. The identified lipid regions were color-coded in red and overlaid onto the intravascular ultrasound (IVUS) image to illustrate the morphology of the vessel wall. Lipid is primarily located in the thickened intimal layer of the aorta. H&E and Oil Red O stains for tissue morphology and lipid (Figs. 2b and 2c) confirmed the finding from spectroscopic IVPA imaging. In contrast, no lipid was detected in the normal, healthy vessel (Figure 2d–f).
Figure 2.
Lipid regions (orange color) were demonstrated on top of the IVUS images for diseased (a) and control (d) rabbit aorta. (b, e) Oil Red O stain for lipid and (c, f) H&E stain closed to the imaged cross-section of diseased and control rabbit aortas. Reproduced with permission from Wang B, Su JL, Amirian J, et al. Detection of lipid in atherosclerotic vessels using ultrasound-guided spectroscopic intravascular photoacoustic imaging. Opt Express 2010 Mar 1;18(5):4889–97. [11].
Spectroscopic photoacoustic imaging can detect tissues with intrinsically different optical absorption contrast. However, data on the optical absorption properties of certain tissue constituents are limited. In the visible to NIR wavelength range, blood inside of the tissues greatly affects the measurements of their optical properties. For example, the optical absorption spectra of the vessel intima, media and adventitia resemble that of blood (Figure 1a). It is challenging to differentiate tissue from blood in this wavelength range. As a result, exogenous contrast is usually required for additional contrast in the NIR region.
The main challenge in spectroscopic photoacoustic imaging is that the optical properties of tissue are unknown, which greatly affects laser fluence compensation for quantitative measurements [45, 46]. Without a priori knowledge of the tissue properties through which the light passes, imaging in a small wavelength range can reduce errors in the reconstruction process [46]. However, inverse algorithms have been developed to estimate both the ultrasonic measurement and the photoacoustic image reconstruction [47–49].
2.2. Exogenous Contrast Agents
Exogenous contrast agents can produce photoacoustic contrast with several fold higher magnitude than intrinsic endogenous contrast alone. Plasmonic noble metal nanoparticles, primarily made from gold [27, 50–52] and silver [53, 54], are the most frequently used exogenous contrast agents. The superior optical absorption properties of plamonic nanoparticles are due to their ability to undergo local surface plasmon resonance. When an electromagnetic wave interacts with a plasmonic nanoparticle, which has a wavelength and size of similar magnitude, the free surface electrons oscillate with the polarity of the inciting wave. The energy from the electromagnetic wave is absorbed, causing oscillation of the electrons, and is released as heat, resulting in high attenuation (absorption) of the incident electromagnetic wave. Multiple absorption peaks at different wavelengths arise from the different orientations in which the nanoparticle could interact with the inciting wave. Plasmonic nanoparticles are highly optically absorbing, and their optical absorption can be increased by shape/composition modifications, due to surface area and volume effects [55]. The synthesis of these nanoparticles can be easily tuned to produce a variety of different shapes, including nanospheres [27, 50], nanoshells [51, 56], nanorods [52, 57], and nanocages [54, 58]. Additionally, the peak absorption wavelength may be varied by changing synthesis conditions to control the size and shape of the nanoparticle as shown in Figure 1b.
Other categories of exogenous contrast agents exist, such as organic dyes [59, 60]. As with plasmonic nanoparticles, organic dyes, such as indocyanine green [59, 60] and Evan’s blue [61], have distinct absorption properties. However, organic dyes are prone to photobleaching, which limits their ability to produce sustained photoacoustic transients. Fluorescent proteins also allow for photoacoustic imaging of cellular phenomenon in vivo [62].
With respect to contrast agent toxicity and immunogenicity, research on gold nanoparticles has advanced to the in vivo clinical trial stage [63]. Previous studies have examined the in vitro cytotoxicity of nanoparticles [64] many of which can be used as contrast agents for photoacoustic imaging. As with most biomedical nanoparticle applications, the size, shape, and surface properties affect the biodistribution and efficacy of nanoparticle contrast agents in vivo [65, 66], and each parameter should be designed for optimal uptake, distribution and retention in the targeted tissue or organ to be imaged.
With respect to the optical properties, a strong optical absorption in the near infrared often allows for deep imaging within biological tissues; however, many contrast agents used in photoacoustic imaging possess strong visible optical absorption spectra. A contrast agent with tunable optical absorption properties is useful for multiplex applications, where multiple contrast agents may be targeted to differing tissue or cell types.
The strong optical absorption of a contrast agent can result in a large temperature increase of the particle and surrounding tissue when illuminated with a laser of sufficient power and appropriate pulse duration. In diagnostic photoacoustic imaging the increase in bulk tissue temperature does not exceed a small fraction of a degree. However, the use of a continuous wave laser can produce a large thermal heating effect due to energy absorption by nanoparticles and could cause cell injury—an effect that can be used for photothermal therapy [67, 68]. Unique to photoacoustic imaging or photothermal therapies, the thermal stability of nanoparticles should also be considered. Gold nanorods, for example, may change shape and become more spherical, thereby shifting their peak absorption wavelength as they absorb laser fluence and heat [69]. Silica-coatings have been found to enhance thermal stability of gold nanorods (Fig. 3a).
Figure 3.
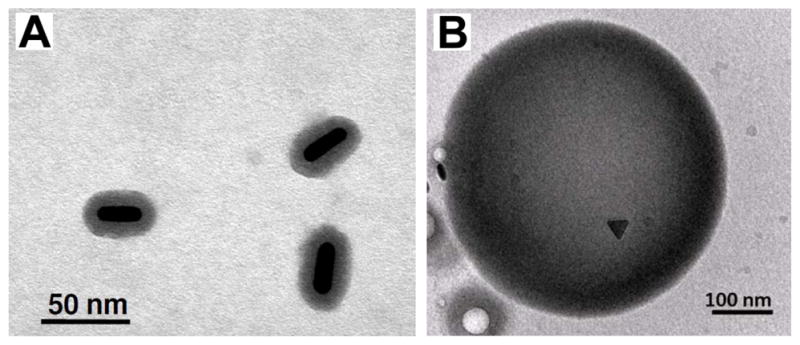
(a) Silica-coated gold nanorods with enhanced thermal stability. Reproduced with permission from[69]. (b) cTEM image of a dual ultrasound and photoacoustic contrast agent. Perfluorocarbon droplets loaded with silver nanotriangles. Reproduced with permission from Wilson K, Homan K, Emelianov S. Synthesis of a dual contrast agent for ultrasound and photoacoustic imaging. In: Samuel A, Ramesh R, editors. Reporters, Markers, Dyes, Nanoparticles, and Molecular Probes for Biomedical Applications II; 2010; San Francisco, CA, USA; 2010. p. 75760M [53]. (b) Silica-coated gold nanorods with enhanced thermal stability. Reproduced with permission from Chen YS, Frey W, Kim S, et al. Enhanced thermal stability of silica-coated gold nanorods for photoacoustic imaging and image-guided therapy. Opt Express 2010 Apr 26;18(9):8867–78. [69].
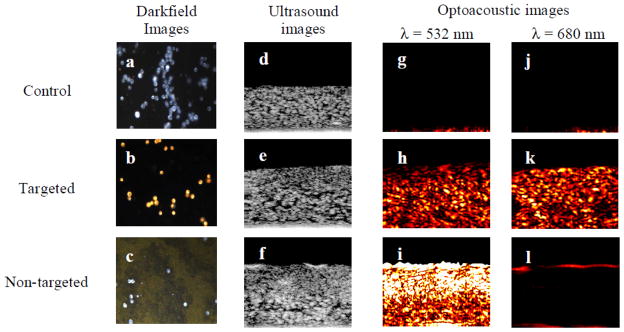
Applications of exogeneous contrast agents for photoacoustic imaging take advantage of the unique targeting capabilities of nanoparticles. Diseases, such as cancer [3, 70] and atherosclerosis [71], may be imaged more effectively using a photoacoustic contrast agent. Imaging of specific tumor cell types using targeted nanoparticles [3, 70], and of the metastasis of tumor cells [72] have been demonstrated. Additionally, physiology, such as detailed microvasculature [59], may be imaged easily with a contrast agent injected into the bloodstream. Most promisingly, exogeneous contrast agents may provide molecular imaging capabilities in vivo at depths not possible with other imaging modalities. This possibility has led to the demonstration of exogenous contrast agents combined with photoacoustic imaging to analyze gene expression [73], enzyme concentrations [74], the differential uptake of targeted nanoparticles in tumor cells [3, 27], and the pharmacokinetics of drug delivery [75]. Gold nanoparticles have been used to target epithelial growth factor receptor (EGFR), a factor that is over-expressed in epithelial cancer cells [27]. The clustering of targeted gold nanoparticles interacting with cancer cells causes plasmon resonance coupling to occur between nanoparticles, resulting in a red-shift in the absorbance spectra of the nanoparticles (Fig. 4). This spectral shift is detectable using spectroscopic photoacoustic imaging, and can be used to differentiate cancer cells from surrounding benign cells [27].
Figure 4.
Darkfield, ultrasound and photoacoustic images (λ = 532 nm and 680 nm) of control, targeted and non-targeted tissue phantoms. The darkfield images measure 440 μm by 340 μm field of view. The ultrasound and optoacoustic images measure 2 mm by 1.67 mm. The targeted EGFR cells show high photoacoustic signal due to the plasmon resonance coupling resulting from clustering gold nanoparticles. Reproduced from Mallidi S, Larson T, Aaron J, et al. Molecular specific optoacoustic imaging with plasmonic nanoparticles. Opt Express 2007 May 28;15(11):6583–8 with permission of the Optical Society of America. [27]
Exogenous contrast agents for photoacoustic imaging and therapy have been combined with therapeutic functionality. These multimodal contrast agents have been demonstrated for photoacoustic imaging combined with MRI with gadolinium doped, gold speckled silica nanoparticles [76], as well as with ferromagnetic cobalt and gold particles [77]. Multimodal contrast agents for photoacoustic and ultrasound imaging have been created using a combination of perfluorocarbon droplets and plasmonic nanoparticles [53] (Fig. 3b), and polymer spheres loaded with organic dye [78].
3. Image-Guided Therapy
Photoacoustic imaging has also been proposed as a tool for guiding therapy in vivo in addition to diagnosing pathologies. Changes over time can be investigated in vivo to track the effectiveness of therapy.
3.1. Thermal Imaging
Photoacoustic imaging can be applied to noninvasively measure a temperature map by tracking the temperature-induced changes in photoacoustic signal amplitude [79]. This photoacoustic-based thermal imaging can be used for photothermal and high-intensity focused ultrasound (HIFU) therapies where accurate and real-time measurements of temperature distributions are necessary to maximize therapy outcomes and minimize normal tissue damage [14].
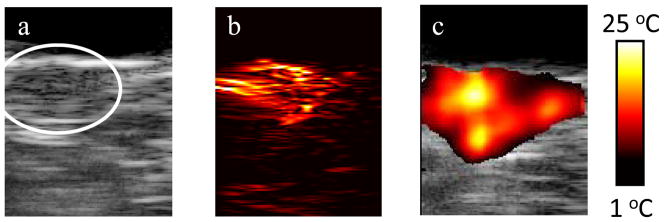
The combination of ultrasound, photoacoustic, and thermal imaging has a great synergistic effect to plan, guide and monitor the outcome of photothermal therapies [3, 14]. Combined ultrasound and photoacoustic imaging can first be used prior to therapy to identify the size and location of the tumor, and confirm the uptake of optical contrast agents, such as nanoparticles in the tumor. Photoacoustic-based thermal imaging can then be performed during therapy to monitor temperature. This concept has been demonstrated in vivo using a mouse model of cancer [80]. Gold nanorods were directly injected into the subcutaneous tumor in a nude mouse prior to performing photothermal therapy. In vivo ultrasound and photoacoustic imaging performed after the injection showed both tumor location (Fig. 5a) and the presence of nanoparticles (Fig. 5b) [81]. Photoacoustic-based thermal imaging showed significant temperature increases within the tumor which can cause tumor necrosis (Fig. 5c).
Figure 5.
(a) Ultrasound, (b) photoacoustic and (c) thermal image of a subcutaneous tumor in nude mouse. Reprinted with permission from Mallidi S, Wang B, Mehrmohammadi M, et al. Ultrasound-based imaging of nanoparticles: from molecular and cellular imaging to therapy guidance. Proceedings of the 2009 IEEE Ultrasonics Symposium 2009:27–36 (2009) [81].
Aside from this, noninvasive photoacoustic temperature measurements can be used for ophthalmology applications such as photocoagulation, photo dynamic therapy, selective retina treatment (SRT), and transpupillary thermotherapy (TTT). For example, photoacoustic-based temperature measurements were successfully performed for SRT [82] and TTT [83].
3.2. Drug Delivery/Release
Research using photoacoustic imaging to guide photothermal therapy or trigger drug release is still in its infancy. Initial strategies proposed to combine drug delivery with photoacoustic imaging involve using photoacoustic contrast agents as multiplexed drug carriers.
Gold nanorods are photoacoustic contrast agents that can be easily multiplexed by conjugating drugs, targeting moieties, or other molecules of interest to their surface [84]. Drugs can be chemically modified with a linker that allows for facile attachment to the gold surface. The linker can have a thiol group that readily attaches to the gold surface via thiolate bonds, or other photocleavable linkers. For example, gold nanorods conjugated to Etanercept (an rheumatic drug) were intra-articularly injected into ex vivo rat tail joints, and photoacoustic tomography (PAT) was used to visualize the distribution of the conjugates. The sensitivity of photoacoustic imaging to detect the gold nanorods was determined to be 10 pM in joint connective tissue [85].
Other photoacoustic contrast agents with potential as drug carriers are hollow gold structures. Both hollow gold nanocages and nanoshells absorb NIR light and have the capacity to house large payloads of drugs in their interior while leaving their exterior available for surface functionalization with targeting moieties [86, 87] (Fig. 6a, b). It was shown that 63% by weight doxorubicin (a breast and ovarian chemotherapeutic agent) could be loaded by weight in hollow gold nanospheres [88]. NIR light was used to trigger release of the drug by using the light for heat conversion at the nanosphere surface. After release, the doxorubicin exhibited cytotoxic effects on MDA-MB-231 breast cancer cells. Others demonstrated that the gold hollow nanospheres could double as photothermal agents, demonstrating an enhanced killing effect in vitro when both drugs and photothermal therapy were used in combination. These hollow gold structures have been used to enhance contrast in photoacoustic imaging [89].
Figure 6.
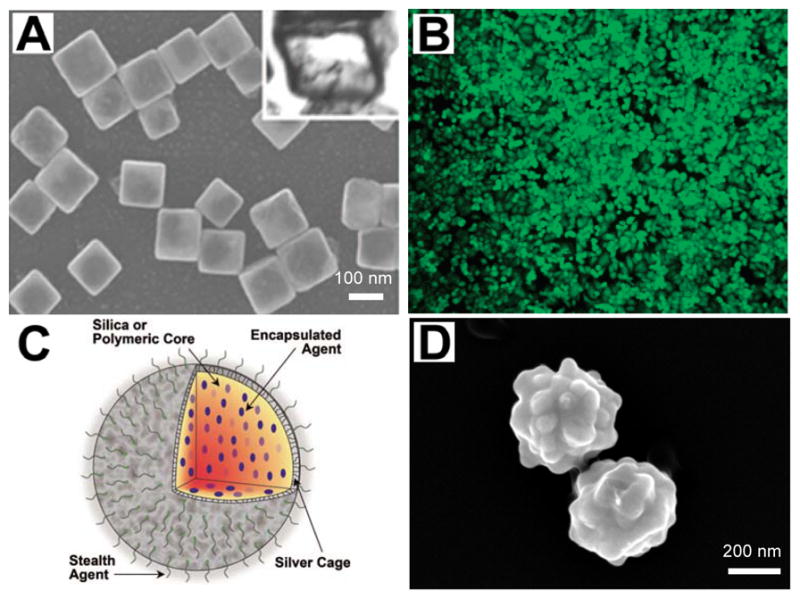
Examples of several multiplexed nanoparticles used as an imaging contrast agent, as a drug delivery vehicle, and/or for image-guided therapy. (a)_SEM image of hollow nanocages used to contain therapy products. Reproduced with permission from Skrabalak SE, Chen J, Sun Y, et al. Gold nanocages: synthesis, properties, and applications. Acc Chem Res 2008 Dec;41(12):1587–95 [86] (b) Gold nanoshells for use in photothermal applications. Reproduced with permission from Loo C, Lowery A, Halas N, et al. Immunotargeted Nanoshells for Integrated Cancer Imaging and Therapy. Nano Letters 2005;5(4):709–11. [87]. (c) Diagram demonstrating a multifunctional nanosystem platform utilizing a silver cage with a silica core. Reproduced with permission from Homan K, Shah J, Gomez S, et al. Silver nanosystems for photoacoustic imaging and image-guided therapy. J Biomed Opt 2010 Mar-Apr;15(2):021316 [92]. (d) Silver coated PLGA as a carrier for imaging contrast agents. Image courtesy of K.A. Homan. All figures used with permission.
Though using noble metal structures with drugs conjugated directly to their surface is a commonly used example of a multiplexed drug carrier, new hybrid carriers are also being explored. Traditionally, nano-forms of medicine involved encapsulation of drugs in polymers or liposomal complexes for delivery. Now hybrid polymer-silver or polymer-gold structures are being explored for this purpose where the drugs are housed in the polymer and the metal is used to heat the structure and trigger drug release [90–92] (Fig. 6c, d). In one approach, hollow gold nanoshells were either encapsulated in the liposome with drugs or tethered to the lipid bilayer via a linker, and it was shown that NIR light could be used to disrupt the stability of the lipid membrane and cause the inner contents to be released [93]. If the ratio of the noble metal content to the drugs and polymer or lipid system is known, photoacoustic imaging can be used to monitor drug release processes using these hybrid systems.
The combination of photoacoustics with nanocarriers is the most common way researchers have approached multiplexed strategies of imaging and drug therapy, but photoacoustics can also be used without nanocarriers to monitor drug diffusion. Several examples of this approach come from dermatology and optometry. In one study, photoacoustic spectroscopy was used to monitor nitroglycerin concentration transdermally in an in vitro model [94]. Others used photoacoustics to monitor the absorption kinetics of different types of sunscreen applied to skin samples. Their results showed that light absorption by the sunscreen plus skin system stabilized between 25 and 45 minutes after sunscreen application [95]. Photoacoustics was also used to monitor diffusion of dyes in ocular tissue-bearing phantoms, showing the potential of photoacoustics to be sensitive to specific analytes in the eye while using laser pulses below the threshold for retinal damage [96].
3.3. Imaging of Coronary Artery Stents
Photoacoustic imaging has been used to intravascularly image arterial stents deployed in vessels containing severe stenosis. These stenotic vessels can be due to atherosclerotic plaques, which can also be detected with photoacoustic imaging [4]. While stenting procedures are largely successful, they bring about several issues including restenosis, hyperplasia and stent drift, all of which require additional follow-up visits to track changes over time. These changes require imaging that can visualize stent longitudinal location and cross-sectional apposition as it relates to the lumen wall. There exist several imaging modalities that can qualitatively assess the location of the stent in vivo. However, issues with metallic susceptibility make it difficult to accurately visualize individual stent struts in relation to the lumen wall. Imaging modalities such as OCT are able to visualize stent struts but are unable to penetrate greater than 1–2 mm through the lumen wall.
Coronary stents have been shown to be well-visualized with IVPA imaging combined with IVUS [97]. Using a high frequency transducer, images showed good depth penetration and sufficient resolution (on the order of tens of micrometers) (Fig. 7). Three-dimensional tomography can be obtained by performing pullback imaging within the vessel. Imaging in 3D is important since recent studies have shown that stent positioning can drift over time, leading to the need to detect stent shape and location with respect to the site of atherosclerosis while also determining the progression of plaque vulnerability [98]. This makes the combined IVUS/IVPA imaging a natural and feasible method in the diagnosis and treatment of atherosclerosis. This initial study showed that IVUS/IVPA is a promising modality to image stents in vivo.
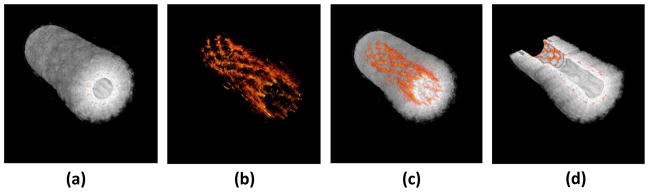
Figure 7.
3D-reconstruction of a stent embedded in a PVA phantom. Individual cross sections can show the position of the stent within the vessel. (a) Ultrasound 3D reconstruction of the phantom showing the structure of the vessel. (b) Photoacoustic reconstruction of the stent structure which can be used to assess the condition of the stent. (c) Photoacoustic image of the stent overlaid with the ultrasound image of the vessel can show the position of both. (d) Cutaway image of the reconstruction, allowing for accurate assessment of the stent within the vessel. Reproduced with permission from Su JL, Wang B, Emelianov SY. Photoacoustic imaging of coronary artery stents. Opt Express 2009 Oct 26;17(22):19894–901 [97].
Though photoacoustic imaging is well-suited for imaging stents, it is also effective for imaging other clinical metal implants [36]. Many of these devices, such as needles, staples, and brachytherapy seeds, are manufactured out of stainless steel which has strong biocompatibility, durability and high resistance to degradability in the body. Photoacoustic imaging of metal implants in vivo is highly advantageous due to the high optical absorption coefficient of stainless steel relative to that of background tissue [6, 99]. The differences in optical absorption at physiological temperatures between metal and tissue are upwards of two to three orders of magnitude in the near infrared region.
4. Conclusions
Due to the ability to measure differences in optical absorption, photoacoustic imaging is well suited to measure functional information in vivo. The added benefit of photoacoustic imaging is that it can penetrate to deep tissue structures with better resolution than other functional imaging modalities. Furthermore, the functional measurements that photoacoustic imaging can provide have increased interest in combining photoacoustic with other complementary modalities. This has led to an explosion in hybrid imaging technologies that have included photoacoustic imaging for diagnosing specific pathologies.
Since photoacoustic imaging obtains its contrast from differences in optical absorption, there are a wide variety of endogenous and exogenous contrast agents available. Generally speaking, endogenous contrast agents are less absorbing than exogenous ones, but the ease of imaging naturally occurring contrast in vivo is an attractive proposition for both clinicians and patients. Endogenous contrast agents include, but are not limited to, hemoglobin found in blood, and lipid in atherosclerotic plaques. Though these endogenous contrast agents may differ only slightly in their absorption coefficient compared with healthy background tissue at a given wavelength, the optical spectra of these endogenous agents are unique and can be differentiated through the use of multi-wavelength photoacoustic imaging. Multi-wavelength photoacoustic imaging allows for differentiation of several tissue constituents in the same image. However, from a hardware perspective, this requires a tunable wavelength light source which can increase the hardware costs.
The use of exogenous contrast agents can help simplify the imaging hardware, since generally these contrast agents are highly absorbing and can be tuned to a specific wavelength. Various contrast agents can be targeted to specific tissue with surface modifiers. The benefit to targeted contrast agents is a higher signal-to-noise ratio and cellular/molecular photoacoustic imaging. As with any exogenous contrast agent, safety and biocompatibility are very important and must be dealt with. Many of the dyes in use today, such as indocyanine green or methylene blue, are already FDA-approved contrast agents suitable for photoacoustic imaging.
Several photoacoustic image-guided therapy applications were discussed. Photoacoustic imaging features the ability to image foreign objects with high optical absorbing properties. Temperature-based imaging is also capable due to the temperature dependence on the photoacoustic signal. The exogenous contrast agents can also be multiplexed to function as drug delivery carriers which are triggered remotely.
5. Expert Opinion
Photoacoustic imaging is a relatively new but rapidly developing field which has enjoyed tremendous growth in recent years. The ability to image differences in optical absorption in tissue makes it an effective strategy for differentiating between healthy and diseased tissue. Current imaging modalities for diagnosing diseased tissue suffer from lack of penetration depth, appropriate resolution or sufficient contrast in several of the applications mentioned in this paper. Studies have shown the effectiveness of photoacoustic imaging for detecting relevant functional features of tissue. Furthermore, real-time photoacoustic imaging can be performed in vivo. Real time imaging is critical in many diagnostic and therapeutic applications of photoacoustic imaging in clinical environments.
From a clinical standpoint, a combined ultrasound and photoacoustic imaging system can be easily implemented due to the presence of a shared detector and associated electronics. Furthermore, such a system will be readily accepted by clinicians familiar with ultrasound imaging. However, the addition of a tunable pulsed laser source with a large spectral range would add significant cost to ultrasound systems. A more cost-effective option would be to use a single-wavelength laser with repetition rates sufficient for imaging in real-time.
Photoacoustic imaging can reliably detect regions with high optical absorption which means diseased tissue can be imaged with high contrast in the presence of surrounding healthy tissue. When combined with ultrasound, the photoacoustic image can be visualized in the context of the ultrasound image. The ultrasound image can provide depth and position information of the optical absorber when overlaid with the photoacoustic image. Clinically speaking, the high resolution of photoacoustic imaging can allow accurate localization of diseased tissue such as cancerous tumors. Another application is the imaging and tracking of metal objects in vivo. Photoacoustic images of metal objects co-registered with ultrasound background image can be a useful tool to monitor and localize implanted metal that may otherwise be lost due to lack of contrast with present imaging modalities. The potential is there to identify other foreign objects in vivo by using spectroscopic imaging to differentiate between non-tissue materials. Secondly, quantitative information from photoacoustic signals must take into account light attenuation from depth-dependence and optical properties. The photoacoustic information alone cannot measure this light attenuation. Work has been done utilizing other methods to inversely calculate or to estimate the light attenuation of specific tissue or other absorbers. Monte Carlo simulations offer rough calculations for fluence estimations but do not take into account minute changes in optical properties, or fluence attenuations due to other absorbers. Since these parameters can change based on wavelength, multi-wavelength imaging across a wide optical spectra can introduce large errors in the calculated absorption spectrum. For simple geometries and tissue structures, ultrasound may assist by providing additional information about the background. However, this could only provide a very limited estimation of optical properties, and could only be applied for small, uniform tissue volumes where the optical properties would not change much. Techniques for quantifying chromophore concentrations may truly require application-specific methods that take into account prior knowledge of particular imaging scenarios to simplify the calculations.
Design of combined imaging probes contains challenges that still need to be met. The transducer’s size is a major factor in endoscopic applications where size constraints exist. Intravascular imaging catheters are heavily limited by size which affects light delivery through an optical fiber to the vessel lumen. Small fibers can burn out if used to deliver high fluence. Though ANSI standards exist for laser safety levels, light attenuation means that deeply penetrating landmarks require high fluences to image these landmarks with sufficient contrast. Larger external use transducers may not have size constraints, but must still be developed to appropriately deliver light into tissue at the imaging plane. These concerns must be addressed in the near future for photoacoustic imaging to be used effectively.
The combination of ultrasound-guided photoacoustic imaging with molecularly targeted contrast agents can be used to non-invasively study the development and treatment of cancer and other pathologies in preclinical settings. Indeed, this imaging system, capable of simultaneous anatomical, functional, cellular and molecular visualization of cancer in small animals, will have a significant impact on many aspects of cancer research. However, significant challenges with the interface between nanotechnology (targeted contrast agents) and biology cannot be ignored. In most studies to date only 5 to 15% of a systemically injected dose of nanoparticles actually accumulates in a tumor or other pathological region of interest in mice. The majority of the dose accumulates in other filtering organs such as the spleen and liver. Sufficient renal clearance of nanoparticles has only been demonstrated for particles below 5.5 nm [100]. Thus, the plasmonic nanoparticles discussed extensively as contrast agents here will have limited renal clearance and the majority of the dose will accumulate in the liver, spleen, and kidneys. Even though only a limited amount of the injected dose accumulates in the pathological region of interest, that concentration has been more than sufficient to provide high imaging contrast or therapy of the pathology as required. The long term effect of accumulated nanoparticles in other areas of the body is a subject of intense investigation by researchers. Most metal nanoparticles formulations are proven non-toxic in in vitro testing, but long term in vivo studies are still pending. In fact, the two biggest challenges with nanoparticles are delivery in vivo and toxicity. Research on improving the delivery in vivo spans from: (1) making smaller and/or biodegradable nanoformulations, (2) using ultrasound to enhance nanoparticle extravasation through blood vessels or uptake in cells, and (3) using magnet fields to guide nanoparticle accumulation in specific regions. Toxicity is also being investigated. Overall, while nanotechnology has its challenges in vivo, researchers are finding ways to meet these challenges since nanoparticle contrast agents can provide unprecedented information about functional, cellular, and molecular changes non-invasively.
Indeed, research in the photoacoustic field is expanding towards imaging targeted contrast agents that are multi-functional—capable of functional imaging along with delivering therapy. Multiplexed contrast agents have evolved from the need to treat the diseased tissue after detection and diagnosis has been made. Therefore, the future of photoacoustic imaging includes targeted nanoparticles that can be triggered to deliver drugs remotely, as needed. There is also a need to use nanoparticles that can absorb light in order to deliver photothermal therapy to specific tissue regions. In the coming years, we expect image-guided drug delivery using a combination of photoacoustics with multiplexed nanoparticles to be a rapidly expanding research area. Future challenges involve creating bioconjugated nanoparticles that can be specifically targeted and aggregated, but cleared after imaging and therapy is complete.
Article Highlights.
Photoacoustic imaging is an imaging modality capable of mapping the optical absorption coefficient of tissue. It is implemented by pairing a pulsed light source with an acoustic detector. Various types of lasers and ultrasound receivers can be integrated into a photoacoustic system depending on the imaging application.
Though well-suited for combined imaging with several other imaging modalities, photoacoustic imaging is complementary in nature and synergetic with ultrasound; therefore, the two imaging technologies are often combined.
The photoacoustic imaging modality can be used for diagnostic imaging by utilizing endogenous contrast between various tissues in vivo. Furthermore, the endogenous tissue contrast can be used to uniquely differentiate tissue composition through multi-wavelength, or spectroscopic, imaging.
Exogenous contrast agents such as free or encapsulated dyes and plasmonic nanoparticles, characterized by their high absorption coefficients, can be employed in photoacoustic imaging. The properties of these contrast agents will affect the biodistribution and efficacy of nanoparticle contrast agents in vivo. Each parameter should be designed for optimal uptake, distribution and retention of the contrast agent in the targeted tissue or organ to be imaged.
Photoacoustic imaging is also well-suited for visualizing foreign objects in vivo such as stents, needles, and brachytherapy seeds, due to the high absorption coefficient of the metal or composite materials. This ability makes photoacoustic imaging an excellent tool to guide diagnostic procedures or to monitor therapeutic interventions where metal implants are used.
Photoacoustic signal changes with temperature. Therefore, photoacoustic imaging can be used in thermal medicine. For example, the use of combined ultrasound and photoacoustic imaging is demonstrated in photothermal therapy to plan therapeutic procedures, to monitor the effectiveness of the treatment plan and to assess the outcome of the therapy.
Photoacoustic imaging can also be used to monitor drug delivery and release. Initial strategies combining drug delivery with photoacoustic imaging involve using hybrid particles as dual photoacoustic imaging contrast agents and drug carriers.
Footnotes
Declaration of Interest
The authors were supported by an NIH grant (# HL096981).
References
- 1.Kruger RA. Photoacoustic ultrasound. Med Phys. 1994 Jan;21(1):127–31. doi: 10.1118/1.597367. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Pang Y, Ku G, et al. Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain. Nat Biotechnol. 2003 Jul;21(7):803–6. doi: 10.1038/nbt839. [DOI] [PubMed] [Google Scholar]
- 3.Mallidi S, Larson T, Tam J, et al. Multiwavelength Photoacoustic Imaging and Plasmon Resonance Coupling of Gold Nanoparticles for Selective Detection of Cancer. Nano Lett. 2009 Aug;9(8):2825–31. doi: 10.1021/nl802929u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sethuraman S, Amirian JH, Litovsky SH, et al. Ex vivo Characterization of Atherosclerosis using Intravascular Photoacoustic Imaging. Opt Express. 2007 Dec 10;15(25):16657–66. doi: 10.1364/oe.15.016657. [DOI] [PubMed] [Google Scholar]
- 5.Sethuraman S, Amirian JH, Litovsky SH, et al. Spectroscopic intravascular photoacoustic imaging to differentiate atherosclerotic plaques. Opt Express. 2008 Mar 3;16(5):3362–7. doi: 10.1364/oe.16.003362. [DOI] [PubMed] [Google Scholar]
- 6.Prahl SA. Optical properties spectra compiled by Scott Prahl. 2001 [cited July 1, 2010]; Available from: http://omlc.ogi.edu/spectra/
- 7.Hu S, Wang LV. Photoacoustic imaging and characterization of the microvasculature. Journal of Biomedical Optics. 2010;15(1):011101–15. doi: 10.1117/1.3281673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laufer JG, Elwell CE, Delpy DT, et al. Spatially resolved blood oxygenation measurements using time-resolved photoacoustic spectroscopy. Adv Exp Med Biol. 2006;578:155–60. doi: 10.1007/0-387-29540-2_25. [DOI] [PubMed] [Google Scholar]
- 9.Stein EW, Maslov K, Wang LV. Noninvasive, in vivo imaging of blood-oxygenation dynamics within the mouse brain using photoacoustic microscopy. J Biomed Opt. 2009 Mar-Apr;14(2):020502. doi: 10.1117/1.3095799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oh JT, Li ML, Zhang HF, et al. Three-dimensional imaging of skin melanoma in vivo by dual-wavelength photoacoustic microscopy. J Biomed Opt. 2006 May-Jun;11(3):34032. doi: 10.1117/1.2210907. [DOI] [PubMed] [Google Scholar]
- 11.Wang B, Su JL, Amirian J, et al. Detection of lipid in atherosclerotic vessels using ultrasound-guided spectroscopic intravascular photoacoustic imaging. Opt Express. 2010 Mar 1;18(5):4889–97. doi: 10.1364/OE.18.004889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDonald MA, Jankovic L, Shahzad K, et al. Acoustic fingerprints of dye-labeled protein submicrosphere photoacoustic contrast agents. J Biomed Opt. 2009 May-Jun;14(3):034032. doi: 10.1117/1.3155519. [DOI] [PubMed] [Google Scholar]
- 13.Li PC, Wei CW, Liao CK, et al. Photoacoustic imaging of multiple targets using gold nanorods. IEEE Trans Ultrason Ferroelectr Freq Control. 2007 Aug;54(8):1642–7. doi: 10.1109/tuffc.2007.435. [DOI] [PubMed] [Google Scholar]
- 14.Shah J, Park S, Aglyamov S, et al. Photoacoustic imaging and temperature measurement for photothermal cancer therapy. J Biomed Opt. 2008 May-Jun;13(3):034024. doi: 10.1117/1.294036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shashkov EV, Everts M, Galanzha EI, et al. Quantum dots as multimodal photoacoustic and photothermal contrast agents. Nano Lett. 2008 Nov;8(11):3953–8. doi: 10.1021/nl802442x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang LV. Prospects of photoacoustic tomography. Med Phys. 2008 Dec;35(12):5758–67. doi: 10.1118/1.3013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park S, Karpiouk AB, Aglyamov SR, et al. Adaptive beamforming for photoacoustic imaging. Opt Lett. 2008 Jun 15;33(12):1291–3. doi: 10.1364/ol.33.001291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kharine A, Manohar S, Seeton R, et al. Poly(vinyl alcohol) gels for use as tissue phantoms in photoacoustic mammography. Phys Med Biol. 2003 Feb 7;48(3):357–70. doi: 10.1088/0031-9155/48/3/306. [DOI] [PubMed] [Google Scholar]
- 19.Song L, Kim C, Maslov K, et al. High-speed dynamic 3D photoacoustic imaging of sentinel lymph node in a murine model using an ultrasound array. Med Phys. 2009 Aug;36(8):3724–9. doi: 10.1118/1.3168598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang JM, Maslov K, Yang HC, et al. Photoacoustic endoscopy. Opt Lett. 2009 May 15;34(10):1591–3. doi: 10.1364/ol.34.001591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsieh Bao-Yu, Chen Sung-Liang, Ling Tao, et al. Design and fabrication of an integrated intravascular ultrasound/photoacoustic scan head. In: Oraevsky AA, Wang LV, editors. Photons Plus Ultrasound: Imaging and Sensing. San Francisco, CA: 2010. [Google Scholar]
- 22.Karpiouk AB, Wang B, Emelianov SY. Development of a catheter for combined intravascular ultrasound and photoacoustic imaging. Rev Sci Instrum. 2010 Jan;81(1):014901. doi: 10.1063/1.3274197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emelianov SY, Aglyamov SR, Karpiouk AB, et al. Synergy and applications of combined ultrasound, elasticity, and photoacoustic imaging. IEEE Ultrasonics Symposium; 2006; Vancouver, British Columbia, Canada. 2006. pp. 405–15. [Google Scholar]
- 24.Niederhauser JJ, Jaeger M, Lemor R, et al. Combined ultrasound and optoacoustic system for real-time high-contrast vascular imaging in vivo. IEEE Trans Med Imaging. 2005 Apr;24(4):436–40. doi: 10.1109/tmi.2004.843199. [DOI] [PubMed] [Google Scholar]
- 25.Sethuraman S, Aglyamov SR, Amirian JH, et al. Intravascular photoacoustic imaging using an IVUS imaging catheter. IEEE Trans Ultrason Ferroelectr Freq Control. 2007 May;54(5):978–86. doi: 10.1109/tuffc.2007.343. [DOI] [PubMed] [Google Scholar]
- 26.Mehrmohammadi M, Oh J, Aglyamov SR, et al. Pulsed magneto-acoustic imaging. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:4771–4. doi: 10.1109/IEMBS.2009.5334214. [DOI] [PubMed] [Google Scholar]
- 27.Mallidi S, Larson T, Aaron J, et al. Molecular specific optoacoustic imaging with plasmonic nanoparticles. Opt Express. 2007 May 28;15(11):6583–8. doi: 10.1364/oe.15.006583. [DOI] [PubMed] [Google Scholar]
- 28.Jia C, Huang S-W, Jin Y, et al. Integration of Photoacoustic, Ultrasound and Magnetomotive System. In: Oraevsky AA, Wang LV, editors. Photons Plus Ultrasound: Imaging and Sensing. San Francisco, CA, USA: 2010. [Google Scholar]
- 29.Qu M, Kim S, Mehrmohammadi M, et al. Combined photoacoustic and magneto-motive ultrasound imaging. In: Oraevsky AA, Wang LV, editors. Photons Plus Ultrasound: Imaging and Sensing. San Francisco, CA, USA: 2010. p. 756433. [Google Scholar]
- 30.Jiao S, Xie Z, Zhang HF, et al. Simultaneous multimodal imaging with integrated photoacoustic microscopy and optical coherence tomography. Opt Lett. 2009 Oct 1;34(19):2961–3. doi: 10.1364/OL.34.002961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li L, Maslov K, Ku G, et al. Three-dimensional combined photoacoustic and optical coherence microscopy for in vivo microcirculation studies. Opt Express. 2009 Sep 14;17(19):16450–5. doi: 10.1364/OE.17.016450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang HF, Maslow, Sivaramakrishnan M, et al. Imaging of hemoglobin oxygen saturation variations in single vessels in vivo using photoacoustic microscopy. Appl Phys Lett. 2007;90(5):053901. [Google Scholar]
- 33.Zhao Y, Chen Z, Saxer C, et al. Phase-resolved optical coherence tomography and optical Doppler tomography for imaging blood flow in human skin with fast scanning speed and high velocity sensitivity. Opt Lett. 2000 Jan 15;25(2):114–6. doi: 10.1364/ol.25.000114. [DOI] [PubMed] [Google Scholar]
- 34.Maslov K, Zhang HF, Hu S, et al. Optical-resolution photoacoustic microscopy for in vivo imaging of single capillaries. Opt Lett. 2008 May 1;33(9):929–31. doi: 10.1364/ol.33.000929. [DOI] [PubMed] [Google Scholar]
- 35.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995 Jan;1(1):27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 36.Su JL, Karpiouk AB, Wang B, et al. Photoacoustic imaging of clinical metal needles in tissue. J Biomed Opt. 2010 Mar-Apr;15(2):021309. doi: 10.1117/1.3368686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsai CL, Chen JC, Wang WJ. Near-infrared absorption property of biological soft tissue constituents. Journal of Medical and Biological Engineering. 2001;21(1):7–14. [Google Scholar]
- 38.Anderson RR, Farinelli W, Laubach H, et al. Selective photothermolysis of lipid-rich tissues: a free electron laser study. Lasers Surg Med. 2006;38(10):913–9. doi: 10.1002/lsm.20393. [DOI] [PubMed] [Google Scholar]
- 39.Viator JA, Au G, Paltauf G, et al. Clinical testing of a photoacoustic probe for port wine stain depth determination. Lasers Surg Med. 2002;30(2):141–8. doi: 10.1002/lsm.10015. [DOI] [PubMed] [Google Scholar]
- 40.Zhang HF, Maslov K, Stoica G, et al. Functional photoacoustic microscopy for high- resolution and noninvasive in vivo imaging. Nature Biotechnology. 2006;24:848–51. doi: 10.1038/nbt1220. [DOI] [PubMed] [Google Scholar]
- 41.Meng-Lin L, Jung-Taek O, Xueyi X, et al. Simultaneous Molecular and Hypoxia Imaging of Brain Tumors In Vivo Using Spectroscopic Photoacoustic Tomography. Proceedings of the IEEE. 2008;96(3):481–89. [Google Scholar]
- 42.Homan K, Kim S, Chen Y-S, et al. Prospects of molecular photoacoustic imaging at 1064 nm wavelength. Opt Lett. 2010;35(15):2663–65. doi: 10.1364/OL.35.002663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oraevsky AA, Karabutov AA. Optoacoustic Tomography. CRC Press; 2003. [DOI] [PubMed] [Google Scholar]
- 44.Virmani R, Burke AP, Farb A, et al. Pathology of the unstable plaque. Progress in Cardiovascular Diseases. 2002;44(5):349–56. doi: 10.1053/pcad.2002.122475. [DOI] [PubMed] [Google Scholar]
- 45.Laufer J, Delpy D, Elwell C, et al. Quantitative spatially resolved measurement of tissue chromophore concentrations. Physics in Medicine and Biology. 2007;52:141–68. doi: 10.1088/0031-9155/52/1/010. [DOI] [PubMed] [Google Scholar]
- 46.Maslov K, et al. Effects of wavelength-dependent fluence attenuation on the noninvasive photoacoustic imaging of hemoglobin oxygen saturation in subcutaneous vasculature in vivo. Inverse Problems. 2007;23(6):S113. [Google Scholar]
- 47.Xu M, Wang LV. Photoacoustic imaging in biomedicine. Review of Scientific Instruments. 2006;77(4):041101–22. [Google Scholar]
- 48.Cox BT, Arridge SR, Beard PC. Estimating chromophore distributions from multiwavelength photoacoustic images. J Opt Soc Am A Opt Image Sci Vis. 2009 Feb;26(2):443–55. doi: 10.1364/josaa.26.000443. [DOI] [PubMed] [Google Scholar]
- 49.Modgil D, Anastasio MA, Riviere PJL. Image reconstruction in photoacoustic tomography with variable speed of sound using a higher-order geometrical acoustics approximation. Journal of Biomedical Optics. 2010;15(2):021308. doi: 10.1117/1.3333550. [DOI] [PubMed] [Google Scholar]
- 50.Oraevsky AA, Karabutov AA, Savateeva EV. Hybrid and Novel Imaging and New Optical Instrumentation for Biomedical Applications (SPIE); 2001. Munich, Germany: 2001. Enhancement of optoacoustic tissue contrast with absorbing nanoparticles; pp. 60–69. [Google Scholar]
- 51.Wang YW, Xie XY, Wang XD, et al. Photoacoustic tomography of a nanoshell contrast agent in the in vivo rat brain. Nano Letters. 2004 Sep;4(9):1689–92. [Google Scholar]
- 52.Eghtedari M, Oraevsky A, Copland JA, et al. High sensitivity of in vivo detection of gold nanorods using a laser optoacoustic imaging system. Nano Letters. 2007 Jul;7(7):1914–18. doi: 10.1021/nl070557d. [DOI] [PubMed] [Google Scholar]
- 53.Wilson K, Homan K, Emelianov S. Synthesis of a dual contrast agent for ultrasound and photoacoustic imaging. In: Samuel A, Ramesh R, editors. Reporters, Markers, Dyes, Nanoparticles, and Molecular Probes for Biomedical Applications II; 2010. San Francisco, CA, USA: 2010. p. 75760M. [Google Scholar]
- 54.Homan K, Shah J, Gomez S, et al. Photons Plus Ultrasound: Imaging and Sensing 2009. San Jose, CA, USA: SPIE; 2009. Combined ultrasound and photoacoustic imaging of pancreatic cancer using nanocage contrast agents; pp. 71771M–6. [Google Scholar]
- 55.Burda C, Chen X, Narayanan R, et al. Chemistry and properties of nanocrystals of different shapes. Chem Rev. 2005 Apr;105(4):1025–102. doi: 10.1021/cr030063a. [DOI] [PubMed] [Google Scholar]
- 56.Lu W, Huang Q, Ku G, et al. Photoacoustic imaging of living mouse brain vasculature using hollow gold nanospheres. Biomaterials. 2010;31(9):2617–26. doi: 10.1016/j.biomaterials.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li PC, Wei CW, Liao CK, et al. Multiple targeting in photoacoustic imaging using bioconjugated gold nanorods - art. no. 60860M. In: Oraevsky AA, Wang LV, editors. Photons Plus Ultrasound: Imaging and Sensing 2006. Bellingham: Spie-Int Soc Optical Engineering; 2006. pp. M860–M60. [Google Scholar]
- 58.Yang XM, Skrabalak SE, Li ZY, et al. Photoacoustic tomography of a rat cerebral cortex in vivo with au nanocages as an optical contrast agent. Nano Letters. 2007 Dec;7(12):3798–802. doi: 10.1021/nl072349r. [DOI] [PubMed] [Google Scholar]
- 59.Wang XD, Ku G, Wegiel MA, et al. Noninvasive photoacoustic angiography of animal brains in vivo with near-infrared light and an optical contrast agent. Opt Lett. 2004 Apr;29(7):730–32. doi: 10.1364/ol.29.000730. [DOI] [PubMed] [Google Scholar]
- 60.Ku G, Wang LHV. Deeply penetrating photoacoustic tomography in biological tissues enhanced with an optical contrast agent. Opt Lett. 2005 Mar;30(5):507–09. doi: 10.1364/ol.30.000507. [DOI] [PubMed] [Google Scholar]
- 61.Yao J, Maslov K, Hu S, et al. Evans blue dye-enhanced capillary-resolution photoacoustic microscopy in vivo. J Biomed Opt. 2009;14(5):054049. doi: 10.1117/1.3251044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Razansky D, Distel M, Vinegoni C, et al. Multispectral opto-acoustic tomography of deep-seated fluorescent proteins in vivo. Nat Photon. 2009;3(7):412–17. [Google Scholar]
- 63.Goel R, Shah N, Visaria R, et al. Biodistribution of TNF-alpha-coated gold nanoparticles in an in vivo model system. Nanomedicine. 2009;4(4):401–10. doi: 10.2217/nnm.09.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lewinski N, Colvin V, Drezek R. Small. 1. Vol. 4. 2008. Cytotoxicity of nanoparticles. [DOI] [PubMed] [Google Scholar]
- 65.Owens DE, III, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. International Journal of Pharmaceutics. 2006;307(1):93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 66.Phillips MA, Gran ML, Peppas NA. Targeted nanodelivery of drugs and diagnostics. Nano Today. 2010;5(2):143–59. doi: 10.1016/j.nantod.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pitsillides CM, Joe EK, Wei X, et al. Selective Cell Targeting with Light-Absorbing Microparticles and Nanoparticles. Biophysical Journal. 2003;84(6):4023–32. doi: 10.1016/S0006-3495(03)75128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gobin AM, Lee MH, Halas NJ, et al. Near-infrared resonant nanoshells for combined optical imaging and photothermal cancer therapy. Nano Lett. 2007 Jul;7(7):1929–34. doi: 10.1021/nl070610y. [DOI] [PubMed] [Google Scholar]
- 69.Chen YS, Frey W, Kim S, et al. Enhanced thermal stability of silica-coated gold nanorods for photoacoustic imaging and image-guided therapy. Opt Express. 2010 Apr 26;18(9):8867–78. doi: 10.1364/OE.18.008867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Copland JA, Eghtedari M, Popov VL, et al. Bioconjugated gold nanoparticles as a molecular based contrast agent: implications for imaging of deep tumors using optoacoustic tomography. Molecular Imaging & Biology. 2004;6(5):341–49. doi: 10.1016/j.mibio.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 71.Wang B, Yantsen E, Larson T, et al. Plasmonic Intravascular Photoacoustic Imaging for Detection of Macrophages in Atherosclerotic Plaques. Nano Letters. 2009 Jun;9(6):2212–17. doi: 10.1021/nl801852e. [DOI] [PubMed] [Google Scholar]
- 72.Galanzha EI, Kokoska MS, Shashkov EV, et al. In vivo fiber-based multicolor photoacoustic detection and photothermal purging of metastasis in sentinel lymph nodes targeted by nanoparticles. J Biophotonics. 2009 Sep;2(8–9):528–39. doi: 10.1002/jbio.200910046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li L, Zemp RJ, Lungu G, et al. Imaging of gene expression in vivo with photoacoustic tomography - art. no. 608608. In: Oraevsky AA, Wang LV, editors. Photons Plus Ultrasound: Imaging and Sensing 2006. Bellingham: Spie-Int Soc Optical Engineering; 2006. pp. 8608–08. [Google Scholar]
- 74.La Riviere PJ, Green A, Norris JR. Development of a protease-sensitive molecular imaging agent for optoacoustic tomography. In: Oraevsky AA, Wang LV, editors. Photons Plus Ultrasound: Imaging and Sensing; 2007. San Jose, CA, USA: 2007. pp. K4370–K70. [Google Scholar]
- 75.Chamberland DL, Agarwal A, Kotov N, et al. Photoacoustic tomography of joints aided by an Etanercept-conjugated gold nanoparticle contrast agentóan ex vivo preliminary rat study. Nanotechnology. 2008;19(9):095101. doi: 10.1088/0957-4484/19/9/095101. [DOI] [PubMed] [Google Scholar]
- 76.Sharma P, Brown SC, Bengtsson N, et al. Gold-Speckled Multimodal Nanoparticles for Noninvasive Bioimaging. Chemistry of Materials. 2008;20(19):6087–94. doi: 10.1021/cm801020s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bouchard L-S, Anwar MS, Liu GL, et al. Picomolar sensitivity MRI and photoacoustic imaging of cobalt nanoparticles. Proceedings of the National Academy of Sciences. 2009 March 17;106(11):4085–89. doi: 10.1073/pnas.0813019106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim C, Qin R, Xu JS, et al. Multifunctional microbubbles and nanobubbles for photoacoustic and ultrasound imaging. J Biomed Opt. 2010;15(1):010510–3. doi: 10.1117/1.3302808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Larina IV, Larin KV, Esenaliev RO. Real-time optoacoustic monitoring of temperature in tissues. J Phys D: Appl Phys. 2005;38:2633–39. [Google Scholar]
- 80.Shah J, Karpiouk AB, Emelianov SY. Real-Time photoacoustic and ultrasound imaging to monitor photothermal therapy in mice. Abstract and presentation at the 2009 SPIE Photonics West Symposium: Photons Plus Ultrasound: Imaging and Sensing. 2009 [Google Scholar]
- 81.Mallidi S, Wang B, Mehrmohammadi M, et al. Ultrasound-based imaging of nanoparticles: from molecular and cellular imaging to therapy guidance. Proceedings of the 2009 IEEE Ultrasonics Symposium; 2009. pp. 27–36. [Google Scholar]
- 82.Schule G, Huttmann G, Framme C, et al. Noninvasive optoacoustic temperature determination at the fundus of the eye during laser irradiation. J Biomed Opt. 2004 Jan-Feb;9(1):173–9. doi: 10.1117/1.1627338. [DOI] [PubMed] [Google Scholar]
- 83.Kandulla J, Elsner H, Birngruber R, et al. Noninvasive optoacoustic online retinal temperature determination during continuous-wave laser irradiation. J Biomed Opt. 2006 Jul-Aug;11(4):041111. doi: 10.1117/1.2236301. [DOI] [PubMed] [Google Scholar]
- 84.Huang X, Neretina S, El-Sayed M. Gold Nanorods: From Synthesis and Properties to Biological and Biomedical Applications. Advanced Materials. 2009;21(48):4880–910. doi: 10.1002/adma.200802789. [DOI] [PubMed] [Google Scholar]
- 85.Chamberland D, Agarwal A, Kotov N, et al. Photoacoustic tomography of joints aided by an Etanercept-conjugated gold nanoparticle contrast agentoan ex vivo preliminary rat study. Nanotechnology. 2008;19:095101. doi: 10.1088/0957-4484/19/9/095101. [DOI] [PubMed] [Google Scholar]
- 86.Skrabalak SE, Chen J, Sun Y, et al. Gold nanocages: synthesis, properties, and applications. Acc Chem Res. 2008 Dec;41(12):1587–95. doi: 10.1021/ar800018v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Loo C, Lowery A, Halas N, et al. Immunotargeted Nanoshells for Integrated Cancer Imaging and Therapy. Nano Letters. 2005;5(4):709–11. doi: 10.1021/nl050127s. [DOI] [PubMed] [Google Scholar]
- 88.You J, Zhang G, Li C. Exceptionally high payload of doxorubicin in hollow gold nanospheres for near-infrared light-triggered drug release. ACS Nano. 2010 Feb 23;4(2):1033–41. doi: 10.1021/nn901181c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang X, Skrabalak S, Li Z, et al. Photoacoustic tomography of a rat cerebral cortex in vivo with Au nanocages as an optical contrast agent. Nano Lett. 2007;7(12):3798–802. doi: 10.1021/nl072349r. [DOI] [PubMed] [Google Scholar]
- 90.Cobley CM, Au L, Chen J, et al. Targeting gold nanocages to cancer cells for photothermal destruction and drug delivery. Expert Opin Drug Deliv. 2010 May;7(5):577–87. doi: 10.1517/17425240903571614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Das M, Sanson N, Fava D, et al. Microgels loaded with gold nanorods: photothermally triggered volume transitions under physiological conditions. Langmuir. 2007 Jan 2;23(1):196–201. doi: 10.1021/la061596s. [DOI] [PubMed] [Google Scholar]
- 92.Homan K, Shah J, Gomez S, et al. Silver nanosystems for photoacoustic imaging and image-guided therapy. J Biomed Opt. 2010 Mar-Apr;15(2):021316. doi: 10.1117/1.3365937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu G, Mikhailovsky A, Khant HA, et al. Remotely triggered liposome release by near-infrared light absorption via hollow gold nanoshells. Journal of the American Chemical Society. 2008 Jul 2;130(26):8175–7. doi: 10.1021/ja802656d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mitchem L, Mio C, Snook R. Diffusion of transdermally delivered nitroglycerin through skin mimetics using photoacoustic and attenuated total reflectance spectrometry. Analytica Chimica Acta. 2004;511(2):281–88. [Google Scholar]
- 95.dos Anjos F, Rompe P, Mansanares A, et al. Sunscreen effects in skin analyzed by photoacoustic spectroscopy. J Phys IV France. 2005;125:797–99. [Google Scholar]
- 96.Maswadi S, Glickman R, Barslou N, et al. Investigational detection of pharmacological agents in the eye using photoacoustic spectroscopy. In: Oraevsky AA, Wang LV, editors. Photons Plus Ultrasound: Imaging and Sensing 2007: The Eighth Conference on Biomedical Thermoacoustics, Optoacoustics, and Acousto-optics; 2007; San Jose, CA, USA. 2007. p. 643706. [Google Scholar]
- 97.Su JL, Wang B, Emelianov SY. Photoacoustic imaging of coronary artery stents. Opt Express. 2009 Oct 26;17(22):19894–901. doi: 10.1364/OE.17.019894. [DOI] [PubMed] [Google Scholar]
- 98.Maintz D, Botnar RM, Fischbach R, et al. Coronary magnetic resonance angiography for assessment of the stent lumen: a phantom study. J Cardiovasc Magn Reson. 2002;4(3):359–67. doi: 10.1081/jcmr-120013303. [DOI] [PubMed] [Google Scholar]
- 99.Karlsson B, Ribbing CG. Optical constants and spectral selectivity of stainless steel and its oxides. Journal of Applied Physics. 1982;53(9):6340–46. [Google Scholar]
- 100.Yoon SJ, Mallidi S, Tam JM, et al. Biodegradable plasmonic nanoclusters as contrast agent for photoacoustic imaging. In: Oraevsky AA, Wang LV, editors. Photons Plus Ultrasound: Imaging and Sensing; 2010. San Francisco, CA, USA: 2010. [Google Scholar]