Abstract
BACKGROUND
Data regarding health outcomes among living kidney donors are lacking, especially among nonwhite persons.
METHODS
We linked identifiers from the Organ Procurement and Transplantation Network (OPTN) with administrative data of a private U.S. health insurer and performed a retrospective study of 4650 persons who had been living kidney donors from October 1987 through July 2007 and who had post-donation nephrectomy benefits with this insurer at some point from 2000 through 2007. We ascertained post-nephrectomy medical diagnoses and conditions requiring medical treatment from billing claims. Cox regression analyses with left and right censoring to account for observed periods of insurance benefits were used to estimate absolute prevalence and prevalence ratios for diagnoses after nephrectomy. We then compared prevalence patterns with those in the 2005–2006 National Health and Nutrition Examination Survey (NHANES) for the general population.
RESULTS
Among the donors, 76.3% were white, 13.1% black, 8.2% Hispanic, and 2.4% another race or ethnic group. The median time from donation to the end of insurance benefits was 7.7 years. After kidney donation, black donors, as compared with white donors, had an increased risk of hypertension (adjusted hazard ratio, 1.52; 95% confidence interval [CI], 1.23 to 1.88), diabetes mellitus requiring drug therapy (adjusted hazard ratio, 2.31; 95% CI, 1.33 to 3.98), and chronic kidney disease (adjusted hazard ratio, 2.32; 95% CI, 1.48 to 3.62); findings were similar for Hispanic donors. The absolute prevalence of diabetes among all donors did not exceed that in the general population, but the prevalence of hypertension exceeded NHANES estimates in some subgroups. End-stage renal disease was identified in less than 1% of donors but was more common among black donors than among white donors.
CONCLUSIONS
As in the general U.S. population, racial disparities in medical conditions occur among living kidney donors. Increased attention to health outcomes among demographically diverse kidney donors is needed. (Funded by the National Institute of Diabetes and Digestive and Kidney Diseases and others.)
Living kidney transplantation is considered to offer patients with end-stage renal disease the best opportunity for dialysis-free survival.1 In 2006, approximately 27,000 transplantations from registered living kidney donors were performed worldwide,2 and living donors supplied nearly 40% of kidney transplants in the United States.3 Most evidence concerning the safety of living kidney donation for donors derives from single-center studies with limited statistical power and few nonwhite donors.4 In a recent study, investigators at the University of Minnesota achieved high ascertainment of long-term patient and renal survival and reported no adverse effects of living kidney donation on life span or risk of end-stage renal disease, as compared with survey data from the general U.S. population.5 Notably, in the Minnesota cohort, 98.8% of the patients were white.
Linkage of records from the Organ Procurement and Transplantation Network (OPTN) (as supplied by the United Network for Organ Sharing) with the Social Security Administration’s Death Master File recently indicated that although surgical and long-term mortality were higher among black donors than among white donors, the long-term rate of death did not exceed that of corresponding control subjects in the National Health and Nutrition Evaluation Survey (NHANES).6 Although racial disparities in the burden and consequences of diabetes mellitus, hypertension, and chronic kidney disease in the general population have been extensively documented,7–10 few data exist concerning long-term medical outcomes among nonwhite kidney donors.
Currently, the OPTN collects data on living donors for only 2 years of follow-up,11 and incomplete reporting and donor loss to follow-up are common,12 owing in part to compliance barriers, such as cost and inconvenience.13 Thus, additional methods for capturing health outcomes among racially diverse living kidney donors are needed. To determine longer-term postdonation medical outcomes independent of a donor’s interaction with the transplantation center, we linked administrative data from a private insurance provider with OPTN-supplied identifiers for living donors. Using these data, we identified postdonation diagnoses of hypertension, diabetes mellitus, chronic kidney disease, and cardiovascular disease; investigated variation in the risk of postdonation medical diagnoses, according to sociodemographic traits; and estimated the prevalence of these diagnoses in demographic subgroups. We also compared relative and absolute prevalence estimates with those in recent NHANES data.
METHODS
DATA SOURCES AND PARTICIPANT SELECTION
We assembled our study data by linking OPTN records for living kidney donors with administrative data from a national private U.S. health insurer. OPTN data include information on all donors and transplant recipients in the United States, as submitted by OPTN members.14 The Health Resources and Services Administration (HRSA) provides oversight on the activities of the OPTN. After approval by the institutional review board at Saint Louis University, we linked beneficiary identifier numbers from the insurer’s electronic databases, using names and birthdates, with unique OPTN identifiers for living kidney donors. Analyses were performed with the use of limited data sets in compliance with the provisions of the Health Insurance Portability and Accountability Act with all direct identifiers removed.
Study participants were eligible if they had an OPTN record of having served as a living kidney donor from October 1987 through July 2007 and were eligible for benefits under the participating insurer after donor nephrectomy at some point during the period from May 2000 through December 2007, the period of available claims data. All participants were simultaneously enrolled in medical and pharmacy benefits with this company exclusively during the study window. U.S. Census data were incorporated according to residential ZIP Code at the time of donor nephrectomy.
OUTCOME MEASURES
We ascertained medical diagnoses of hypertension, diabetes mellitus, chronic kidney disease, and cardiovascular disease among living kidney donors, using billing claims with corresponding diagnosis codes as listed in the International Classification of Diseases, Ninth Revision, Clinical Modification, similar to algorithms described previously.15–19 We also examined drug-treated hypertension and diabetes (with either insulin or oral agents) in pharmacy claims, using drug-category codes. Stage-specific coding for chronic kidney disease was introduced in October 2005. Therefore, we examined diagnoses of chronic kidney disease of stage 3 to 5 or end-stage renal disease (i.e., chronic kidney disease requiring dialysis) in a prespecified subgroup with insurance eligibility ending June 2006 or later.
BASELINE DEMOGRAPHIC VARIABLES
Demographic data from the OPTN at the time of donor nephrectomy included age, sex, and race or ethnic group, as reported by the donor to the transplantation center. Because the OPTN began collecting information on predonation hypertension in June 2004, we examined baseline hypertension status in a secondary analysis. An index of neighborhood socioeconomic status at the time of nephrectomy was computed from U.S. Census data linked by ZIP Code, according to methods used by the Agency for Healthcare Research and Quality20 (for details, see the Methods section in the Supplementary Appendix, available with the full text of this article at NEJM.org).
COMPARISON DATA FOR THE GENERAL POPULATION
Information about race is not recorded by the insurer and was unavailable for nondonor beneficiaries. Thus, we compared our results with those of population-based survey data from NHANES,21 as has been done in other studies of donor outcomes.5,6 We included participants in the 2005–2006 NHANES survey who were 20 years of age or older. Race or ethnic group in NHANES was self-reported. Hypertension, diabetes, chronic kidney disease, and cardiovascular disease were defined according to the participant’s report of these diagnoses on the basis of encounters with a doctor or other health care professional.
STATISTICAL ANALYSIS
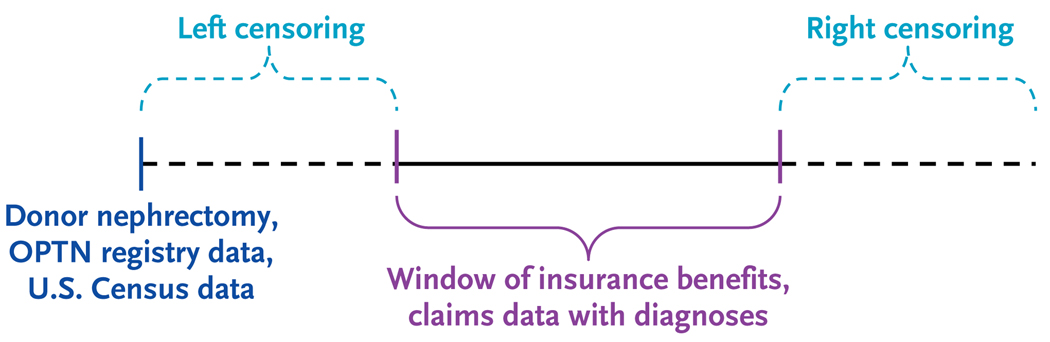
Data sets were merged and analyzed with the use of SAS for Windows software, version 9.2 (SAS Institute). Since windows of insurance benefits varied across the sample, we used Cox regression with left and right censoring to account for observed periods of insurance benefits to model the frequency with 95% confidence intervals and correlates with adjusted hazards ratios of prevalent diagnoses after donor nephrectomy (Fig. 1). The prevalence of diagnoses 5 years after donor nephrectomy in the full cohort and in prespecified subgroups was estimated from outcome-specific Cox models. We estimated correlates of prevalent diagnoses in the general population using SAS Proc Survey logistic software to correct for unequal selection probabilities and response rates in NHANES. The prevalence of medical conditions in subgroups in the general population was estimated by transforming the logistic-regression equation. A P value of less than 0.05 was considered to indicate statistical significance.
Figure 1. Schematic Diagram of Linkage of Study Data Sources.
Identifiers from the Organ Procurement and Transplantation Network (OPTN) were linked to the administrative data of a private U.S. health insurer for 4650 living kidney donors from October 1987 through July 2007. Post-nephrectomy medical diagnoses and conditions requiring medical treatment were ascertained from billing claims. Cox regression analyses with left and right censoring to account for observed periods of insurance benefits were used to estimate absolute prevalence and prevalence ratios for diagnoses after nephrectomy. Prevalence patterns were then compared with those in the 2005–2006 National Health and Nutrition Examination Survey (NHANES) for the general population. U.S. Census data were incorporated according to residential ZIP Code at the time of donor nephrectomy.
RESULTS
DEMOGRAPHIC CHARACTERISTICS OF DONORS
Among 4650 kidney donors in the study cohort, 76.3% were white, 13.1% black, 8.2% Hispanic, and 2.4% another race or ethnic group (Table 1). White donors were significantly older at the time of donation than were nonwhite donors in the study sample and nationally (P<0.001 by analysis of variance). All kidney donors underwent nephrectomy between 1987 and 2007, and the median time from donation to the end of observed insurance eligibility was 7.7 years. The linked donor sample was similar on the basis of race and sex to all living kidney donors in the OPTN and to age-standardized estimates in the general population in NHANES.
Table 1.
Demographic Characteristics of Living Kidney Donors in the Study Sample and in the Organ Procurement and Transplantation Network (OPTN), 1987–2007.*
| Characteristic | Living Donors in the Study Sample (N = 4650) |
Living Donors in OPTN (N = 86,107) |
|---|---|---|
| Male sex (%) | 45.4 | 42.2 |
| Race or ethnic group (%)† | ||
| Non-Hispanic white | 76.3 | 70.9 |
| Non-Hispanic black | 13.1 | 13.1 |
| Hispanic | 8.2 | 11.8 |
| Other | 2.4 | 4.3 |
| Related to recipient (%) | 81.2 | 74.4 |
| Age at donor nephrectomy (yr) | ||
| All donors | 37.2±10.0 | 39.3±10.9 |
| Non-Hispanic white | 38.2±10.0 | 40.7±10.9 |
| Non-Hispanic black | 33.9±9.0 | 35.5±9.9 |
| Hispanic | 34.3±9.6 | 35.9±10.4 |
| Other | 34.8±10.8 | 38.1±11.3 |
| Median time from donation to end of insurance eligibility (yr) | 7.7 | NA |
| Median duration of insurance eligibility (yr) | 2.1 | NA |
Plus–minus values are means ±SD. After adjustment for the sampling technique used in the 2005–2006 National Health and Nutrition Examination Survey (NHANES), 48.1% of respondents represented in the general population were men, 71.7% were non-Hispanic white, 11.5% were non-Hispanic black, 8.0% were Hispanic of Mexican ancestry, and 8.8% were another race or ethnic group. NA denotes not applicable.
Race or ethnic group was self-reported.
FREQUENCY AND VARIATION OF MEDICAL DIAGNOSES
At 5 years after donation, the estimated prevalence of diagnosed hypertension was 17.8% (95% confidence interval [CI], 15.8 to 20.2), and the estimated prevalence of drug-treated hypertension was 13.6% (95% CI, 11.4 to 15.8). Diagnoses of diabetes and cardiovascular disease were identified in 4.0% (95% CI, 2.7 to 5.3) and 3.2% (95% CI, 2.1 to 4.2) of donors, respectively. Chronic kidney disease was indicated as a medical diagnosis in the claims of 5.2% (95% CI, 3.7 to 6.8) of donors by the fifth anniversary of donation.
Older age at donation was associated with an increased risk of postdonation hypertension of 6% per year (Table 2). As compared with white kidney donors, black donors had a relative increase of 52% in the risk of diagnosed hypertension and an increase of 31% in the risk of drug-treated hypertension. The risk of diagnosed hypertension was 36% higher among Hispanic donors than among white donors, although the risk of drug-treated hypertension did not differ significantly between the two groups. Baseline hypertension was reported in 12 of 399 donors (3.0%) from June 2004 through 2007; of these patients, 11 were white, and 1 was Hispanic. Among donors after nephrectomy, reported predonation hypertension was strongly correlated with an increased risk of hypertension (adjusted hazard ratio, 12.2; 95% CI, 5.6 to 26.7) and with drug treatment for hypertension (adjusted hazard ratio, 20.9; 95% CI, 8.8 to 49.3). However, the inclusion of this variable did not have a significant effect on the association between black race or Hispanic ethnic background with hypertension or black race with drug-treated hypertension after donor nephrectomy. In NHANES data, black respondents reported receiving a diagnosis of hypertension more commonly than did white respondents, whereas Hispanic respondents were less likely than white respondents to report diagnosed hypertension.
Table 2.
Adjusted Relative Risk of Hypertension, Diabetes, Chronic Kidney Disease, and Cardiovascular Disease in Living Kidney Donors and in the General Population, According to Demographic Factors.*
| Variable | Hypertension | Diabetes | Chronic Kidney Disease | Cardiovascular Disease | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Living Donors† | NHANES‡ | Living Donors† | NHANES‡ | Living Donors† | NHANES‡ | Living Donors† | NHANES‡ | |||
| Medical Claims | Drug-Treated | Reported | Medical Claims | Drug-Treated | Reported | Medical Claims | Reported | Medical Claims | Reported | |
|
adjusted hazard ratio (95% CI) |
adjusted odds ratio (95% CI) |
adjusted hazard ratio (95% CI) |
adjusted odds ratio (95% CI) |
adjusted hazard ratio (95% CI) |
adjusted odds ratio (95% CI) |
adjusted hazard ratio (95% CI) |
adjusted odds ratio (95% CI) |
|||
| Age (per year) | 1.06 (1.06–1.07)§ | 1.06 (1.05–1.07)§ | 1.06 (1.05–1.07)§ | 1.05 (1.03–1.06)§ | 1.05 (1.03–1.07)§ | 1.05 (1.04–1.06)§ | 1.04 (1.03–1.06)§ | 1.02 (1.01–1.03)§ | 1.09 (1.07–1.19)§ | 1.08 (1.07–1.09)§ |
| Male sex | 1.13 (0.98–1.31) | 1.21 (1.03–1.43)§ | 0.93 (0.82–1.07) | 0.91 (0.68–1.22) | 1.10 (0.73–1.66) | 0.96 (0.71–1.31) | 1.64 (1.16–2.34)§ | 0.59 (0.42–0.84)§ | 2.11 (1.43–3.10)§ | 1.43 (1.10–1.87)§ |
| Race or ethnic group | ||||||||||
| Non-Hispanic white | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Non-Hispanic black | 1.52 (1.23–1.88)§ | 1.31 (1.02–1.68)§ | 1.77 (1.47–2.14)§ | 1.52 (1.00–2.30)¶ | 2.31 (1.33–3.98)§ | 2.74 (2.13–3.51)§ | 2.32 (1.48–3.62)§ | 1.98 (1.34–2.94)§ | 1.15 (0.63–2.11) | 1.44 (1.11–1.88)§ |
| Hispanic | 1.36 (1.04–1.78)§ | 1.03 (0.73–1.46) | 0.65 (0.51–0.83)§ | 1.65 (1.00–2.74)¶ | 2.94 (1.57–5.51)§ | 2.34 (1.76–3.12)§ | 1.90 (1.05–3.43)§ | 1.42 (0.88–2.27) | 0.91 (0.37–2.26) | 1.04 (0.71–1.52) |
| Other | 1.13 (0.68–1.85) | 0.48 (0.20–1.16) | 0.85 (0.66–1.10) | 1.35 (0.50–3.67) | 2.58 (0.80–8.28) | 2.47 (1.52–1.40)§ | 1.74 (0.66–4.76) | 0.95 (0.31–2.96) | 0.49 (0.07–3.54) | 0.86 (0.45–1.67) |
Cardiovascular disease is defined as coronary artery disease, angina, congestive heart failure, heart attack, or stroke. CI denotes confidence interval.
Adjusted hazard ratios for medical diagnoses among donors were calculated by means of multivariate Cox regression with left and right censoring to account for observed periods of insurance benefits.
Adjusted odds ratios for patient-reported diagnoses in the National Health and Nutrition Examination Survey (NHANES) were calculated by means of multivariable logistic regression with correction for unequal selection probabilities and response rates.
P<0.05.
P = 0.05.
The relative frequency of diagnosed diabetes among donors rose 5% for each increase in year of age at the time of donation (Table 2). There were borderline trends toward more frequent diagnoses of diabetes after donation among black and Hispanic donors than among white donors (P = 0.05 for both comparisons). Black and Hispanic donors were more than two times as likely as white donors to have drug-treated diabetes after kidney donation. In NHANES data, reported diabetes was more than twice as common among black and Hispanic respondents as among white respondents.
The relative risk of medically coded chronic kidney disease after donation increased 4% per year of age at the time of donation (Table 2). Black and Hispanic donors were approximately twice as likely to have diagnosed chronic kidney disease after nephrectomy as were white donors. Prespecified subgroup analysis of 2307 donors who had medical benefits after the introduction of stage-specific coding for chronic kidney disease showed that donors had a significant increase in the risk of chronic kidney disease of stage 3 or higher if they were black (adjusted hazard ratio, 3.60; 95% CI, 1.37 to 9.39; P = 0.009) or Hispanic (adjusted hazard ratio, 4.23; 95% CI, 1.52 to 11.75; P = 0.006). Chronic kidney disease requiring dialysis (i.e., end-stage renal disease) was reported in 2 of 271 black donors (0.7%) and 1 of 197 Hispanic donors (0.5%) in this sub-analysis, as compared with no cases among 1786 white donors (P = 0.02 by Fisher’s exact test for the comparison between black and white donors and P = 0.10 for the comparison between Hispanic and white donors). The time from donation to end-stage renal disease ranged from 6.3 to 16.5 years. In NHANES, the relative risk of chronic kidney disease in black respondents was twice that in white respondents.
Although reported cardiovascular disease was significantly more common among black respondents than among white respondents in the general population, we did not detect racial variation in the prevalence of cardiovascular diagnoses among kidney donors.
At 5 years after nephrectomy, the prevalence of diagnosed hypertension varied from 13.9% among white women who were 35 years of age at the time of donation to 47.9% among black men who were 50 years of age at the time of donation (Table 3). At the same time, the prevalence of diagnosed diabetes varied from 3.2% in white men who were 35 years of age at the time of donation to 10.8% in Hispanic women who were 50 years of age at the time of donation. The point estimates for the prevalence of diabetes among black and Hispanic donors were lower than those in the general population, but the estimated prevalence of hypertension among Hispanic donors was higher than that in the general population. There was a trend toward increased point estimates for the prevalence of hypertension, as compared with the general population, among black male and female donors and white male donors who were 50 years of age at the time of donation, but confidence intervals overlapped those of NHANES.
Table 3.
Estimated Prevalence of Hypertension and Diabetes among Living Donors 5 Years after Nephrectomy, as Compared with the General Population, According to Subgroup.
| Age at Evaluation, Sex, and Race or Ethnic Group* |
Hypertension | Diabetes | ||
|---|---|---|---|---|
| Living Donors† | NHANES‡ | Living Donors† | NHANES‡ | |
| percent (95% confidence interval) | ||||
| 40 Yr | ||||
| Female | ||||
| Non-Hispanic white | 13.9 (11.5–16.2) | 16.4 (13.3–19.9) | 3.5 (2.2–4.8) | 3.4 (1.8–6.3) |
| Hispanic | 18.4 (13.4–23.1) | 10.4 (8.5–12.7) | 5.7 (2.6–8.7) | 7.5 (6.0–9.3) |
| Non-Hispanic black | 20.3 (15.8–24.5) | 24.0 (21.8–26.3) | 5.2 (2.7–7.7) | 8.6 (7.2–10.2) |
| Male | ||||
| Non-Hispanic white | 15.6 (12.9–18.1) | 15.5 (12.8–18.5) | 3.2 (2.0–4.4) | 3.3 (1.7–6.0) |
| Hispanic | 20.6 (14.9–25.8) | 9.8 (7.9–12.0) | 5.2 (2.3–8.1) | 7.2 (5.6–9.3) |
| Non-Hispanic black | 22.7 (17.7–27.4) | 24.4 (22.3–26.7) | 4.8 (2.4–7.1) | 8.5 (6.7–10.6) |
| 55 Yr | ||||
| Female | ||||
| Non-Hispanic white | 31.5 (27.1–35.7) | 32.5 (28.4–36.8) | 6.7 (4.4–9.0) | 6.9 (5.5–8.7) |
| Hispanic | 40.2 (30.5–48.6) | 21.6 (18.1–25.6) | 10.8 (4.8–16.4) | 14.5 (11.8–17.7) |
| Non-Hispanic black | 43.7 (35.3–51.1) | 42.8 (40.0–45.8) | 10.0 (5.2–14.6) | 16.5 (14.4–18.9) |
| Male | ||||
| Non-Hispanic white | 34.9 (29.8–39.6) | 31.0 (27.7–36.8) | 6.2 (3.8–8.4) | 6.6 (5.4–8.2) |
| Hispanic | 44.2 (33.3–53.3) | 20.5 (16.9–24.5) | 9.9 (4.2–15.4) | 14.5 (11.8–17.7) |
| Non-Hispanic black | 47.9 (38.5–55.8) | 44.3 (41.3–47.5) | 9.2 (4.5–13.7) | 16.4 (13.3–19.9) |
Values are for living kidney donors who were evaluated 5 years after nephrectomy.
Diagnoses after kidney donation were ascertained from diagnosis codes on billing claims.
Diagnoses in the National Health and Nutrition Examination Survey (NHANES) were defined by respondents’ reports of diagnoses on the basis of clinical encounters.
RACE, SOCIOECONOMIC INDICATORS, AND MEDICAL OUTCOMES AFTER KIDNEY DONATION
Census data were linked for 3385 donors (72.8%) in our study. In this group, the index of socioeconomic indicators was significantly less favorable among black and Hispanic donors than among white donors (Table 4). However, socioeconomic indicators were not associated with any study outcome in bivariate or multivariate analyses. For example, an increased score on the socioeconomic index was not associated with a significant difference in the risk of hypertension (hazard ratio, 1.00; 95% CI, 0.99 to 1.02), diabetes (hazard ratio, 0.99; 95% CI, 0.93 to 1.02), or chronic kidney disease (hazard ratio, 0.97; 95% CI, 0.92 to 1.01).
Table 4.
Variation in Neighborhood Socioeconomic Status Scores among 3385 Living Kidney Donors at the Time of Nephrectomy, According to Race or Ethnic Group.*
| Variable | White | Black | Hispanic |
|---|---|---|---|
| Socioeconomic status index | 49.1±5.3 | 43.9±6.0 | 43.7±7.6 |
| Income score | 24.8±8.5 | 19.6±7.1 | 21.2±8.1 |
| Property-value score | 14.5±10.3 | 11.1±6.0 | 14.5±10.2 |
| Below federal poverty line (%) | 9.1 | 16.4 | 16.4 |
| Unemployed (%) | 9.2 | 15.4 | 15.0 |
| College graduate (%) | 27.5 | 21.2 | 22.5 |
| Education <12th grade (%) | 15.7 | 22.9 | 26.8 |
| Crowded household (%) | 59.9 | 83.3 | 89.2 |
Plus–minus values are means ±SD. Scores on the socioeconomic status index were computed for 3385 of 4650 donors (72.8%) for whom linked Census data were available. Scores for socioeconomic status, income, and property value are standardized to range from 0 to 100, with higher values indicating a higher level. Details about the calculation of these scores are provided in the Supplementary Appendix. P<0.001 for all comparisons of black and Hispanic donors with white donors, except for the comparison for property-value score between Hispanic donors and white donors.
DISCUSSION
Long-term health outcomes have not been well defined among racially diverse living kidney donors. We used administrative insurance data that were collected in the course of actual practice to examine medical diagnoses among living kidney donors, independent of follow-up by the transplantation center. As compared with white donors, black and Hispanic donors had an increased risk of hypertension, drug-treated diabetes, and chronic kidney disease after nephrectomy than did white donors, increases that were not explained by socioeconomic factors. The absolute prevalence of diabetes in donors did not exceed that in the general population, but the prevalence of hypertension was higher than NHANES estimates in some subgroups. End-stage renal disease was reported in less than 1% of donors but was more common among black donors than among white donors. Thus, as in the general U.S. population,7–10 racial disparities in medical conditions appear to occur among kidney donors.
We found that black donors had an increased risk of hypertension, as compared with white donors, similar to racial disparities in the general population. The Amsterdam Forum’s medical guidelines for living kidney donors state that the presence of hypertension at the time of evaluation is a general exclusion to kidney donation, except in patients with hypertension whose condition is defined as “low risk.”22 According to the seminal Mayo Clinic study,23 white race is included among low-risk criteria. Recent data from predominantly white cohorts suggest that there is an increased risk of hypertension among donors, as compared with the general population, possibly due to physiological alterations (including hyperfiltration in the remaining kidney and changes in vascular tone and renin–angiotensin–aldosterone regulation) or heightened follow-up.24,25 Hypertension was recently identified in 41% of 39 black donors who were evaluated at an average of 7 years after nephrectomy at one center.26 In our study, the increased prevalence of hypertension among Hispanic donors, as compared with the general population, may, in part, reflect underreporting of hypertension in this ethnic group, as compared with white respondents, in NHANES. Other studies have reported decreased rates of hypertension among Hispanic persons, as compared with non-Hispanic white persons, on the basis of both self-reporting and measured blood pressure.8,27–30 Nonetheless, in our study, the prevalence of hypertension among Hispanic donors did not exceed that among black donors. We speculate that medical surveillance after kidney donation may mitigate barriers to the recognition of hypertension rather than differentially affect the risk of hypertension among Hispanic donors.
As in the general population, diabetes was more common among black and Hispanic donors, as compared with white donors. Canadian researchers recently found a substantially higher risk of diabetes after kidney donation among aboriginal donors than among white donors, mirroring the disparities in risk in the local population.31 However, in our study, the estimated prevalence of diabetes among black or Hispanic donors did not exceed the prevalence among corresponding subgroups in the general population. A diagnosis of diabetes at evaluation should preclude donation,22 and our data support the finding of a reduction in the absolute prevalence, although not the relative prevalence, of diabetes among black and Hispanic donors, probably as a result of donor-selection practices.
We observed that black and Hispanic donors had approximately twice the risk of chronic kidney disease as white donors. In NHANES, the prevalence of chronic kidney disease was also twice as high among black respondents as among white respondents and tended to be higher among Hispanic respondents than among white respondents. Similarly, the 2008 U.S. Renal Data System registry reported that the national incidence of end-stage renal disease among black persons was 3.7 times that among white persons, and end-stage renal disease among Hispanic persons was 1.5 times that among non-Hispanic white persons.32 Recent queries of registrations of kidney-transplant candidates showed that although 12% of living kidney donors during the period from 1996 through 2007 were black, black donors represented 43% of 148 previous donors who were subsequently listed for kidney transplantation.33,34 Our data also suggest that nonwhite donors have an increased frequency of end-stage renal disease, although the number of such events was low. We did not detect significant race-related differences in cardiovascular diagnoses.
Although we found evidence of socioeconomic disadvantage for nonwhite donors, the donor’s socioeconomic status did not correlate with the studied medical diagnoses. Since all donors had private health insurance during the observation period, it may be that possession of insurance attenuated health disparities that were based on socioeconomic status. In addition, our socioeconomic measure may have lacked precision, since we used neighborhood socioeconomic status as a surrogate for individual status. The exclusion of uninsured donors may have underestimated medical complications in nonwhite donors,35 since a lack of health insurance is more common among nonwhite donors than among white donors.36,37
Our study has inherent limitations, given the available data and sampling approach. Reasons for entry into and exit from the insurance plan are not available, and disenrollment related to events such as health status cannot be identified. Outcome measures that were available in the administrative data differed from those in the NHANES data. Billing claims have been shown to provide sensitive measures of diagnoses of diabetes and cardiovascular disease in other populations15,19 but probably underrepresent the burden of kidney dysfunction, as compared with laboratory-based measures.17
On the basis of claims data that may be left-censored for the absence of insurance benefits within the available data, we were unable to distinguish incident diagnoses definitively. The sub-analysis of the period during which the OPTN collected baseline data on hypertension suggests that some centers have allowed more potential white donors with elevated blood pressure at evaluation to proceed with donation, as compared with those of another race or ethnic group. This finding is consistent with limited data describing white race as a low-risk criterion for hypertension among potential donors.23 Yet despite the apparent exclusion of potential black kidney donors with reported hypertension at evaluation, black donors had an increased rate of hypertension after nephrectomy, as compared with white donors. It is possible that the evaluation and reporting of normal blood pressure from the donor-candidacy evaluation to OPTN vary across centers. The study data also lacked baseline information on body-mass index.
The stringency of living-donor selection has inherent tensions with the goal of increasing the organ supply. Black patients with end-stage renal disease have decreased access to transplantation, including living-donor allografts, as compared with white patients.38,39 As compared with white candidates for kidney transplantation, black candidates are less likely to identify potential living donors, and their potential living donors are less likely to donate for reasons including medical exclusion.40 Despite these exclusions from donation and the demonstrated benefit of selection for kidney donation in reducing the absolute risk of some health complications, such as diabetes, our data show that as in the general population, black kidney donors remain at increased relative risk for hypertension, diabetes, and chronic kidney disease, as compared with white donors. Race and ethnic group should not be used to discourage donor evaluation, but these data may increase awareness of variation in long-term outcomes among living donors and of the need for longer in-depth follow-up of demographically diverse living donors.
Supplementary Material
Acknowledgments
Supported in part by grants (K08DK073036 and P30DK079333) from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
The interpretation and reporting of these data are the responsibility of the authors and do not necessarily reflect the official policy of the OPTN, the Department of Defense, or the National Institutes of Health.
Presented in part at the American Transplant Congress in Boston, June 3, 2009, and the American Society of Nephrology 42nd Annual Renal Week Meeting in San Diego, CA, October 30, 2009.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Terasaki PI, Cecka JM, Gjertson DW, Takemoto S. High survival rates of kidney transplants from spousal and living unrelated donors. N Engl J Med. 1995;333:333–336. doi: 10.1056/NEJM199508103330601. [DOI] [PubMed] [Google Scholar]
- 2.Horvat LD, Shariff SZ, Garg AX. Global trends in the rates of living kidney donation. Kidney Int. 2009;75:1088–1098. doi: 10.1038/ki.2009.20. [DOI] [PubMed] [Google Scholar]
- 3.Organ Procurement and Transplant Network database. [Accessed July 23, 2010]; at http://www.optn.org/data/ [Google Scholar]
- 4.Ommen ES, Winston JA, Murphy B. Medical risks in living kidney donors: absence of proof is not proof of absence. Clin J Am Soc Nephrol. 2006;1:885–895. doi: 10.2215/CJN.00840306. [DOI] [PubMed] [Google Scholar]
- 5.Ibrahim HN, Foley R, Tan L, et al. Long-term consequences of kidney donation. N Engl J Med. 2009;360:459–469. doi: 10.1056/NEJMoa0804883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Segev DL, Muzaale AD, Caffo BS, et al. Perioperative mortality and long-term survival following live kidney donation. JAMA. 2010;303:959–966. doi: 10.1001/jama.2010.237. [DOI] [PubMed] [Google Scholar]
- 7.Carter JS, Pugh JA, Monterrosa A. Non-insulin-dependent diabetes mellitus in minorities in the United States. Ann Intern Med. 1996;125:221–232. doi: 10.7326/0003-4819-125-3-199608010-00011. [DOI] [PubMed] [Google Scholar]
- 8.Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension. 2008;52:818–827. doi: 10.1161/HYPERTENSIONAHA.108.113357. [DOI] [PubMed] [Google Scholar]
- 9.Cowie CC, Port FK, Wolfe RA, Savage PJ, Moll PP, Hawthorne VM. Disparities in incidence of diabetic end-stage renal disease according to race and type of diabetes. N Engl J Med. 1989;321:1074–1079. doi: 10.1056/NEJM198910193211603. [DOI] [PubMed] [Google Scholar]
- 10.Brancati FL, Whittle JC, Whelton PK, Seidler AJ, Klag MJ. The excess incidence of diabetic end-stage renal disease among blacks: a population-based study of potential explanatory factors. JAMA. 1992;268:3079–3084. [PubMed] [Google Scholar]
- 11.Brown RS, Jr, Higgins R, Pruett TL. The evolution and direction of OPTN oversight of live organ donation and transplantation in the United States. Am J Transplant. 2009;9:31–34. doi: 10.1111/j.1600-6143.2008.02433.x. [DOI] [PubMed] [Google Scholar]
- 12.Graham W. Living donor follow-up data: status report from the OPTN. Rockville, MD: Advisory Committee on Organ Transplantation (ACOT); 2008. Nov 13, [Accessed July 23, 2010]. Presentations: at https://www.team-psa.com/DOT/ACOT2008/presentations.asp. [Google Scholar]
- 13.Mandelbrot D, Pavlakis M, Karp SJ, et al. Practices and barriers in long-term living kidney donor follow-up: a survey of U.S. transplant centers. Transplantation. 2009;88:855–860. doi: 10.1097/TP.0b013e3181b6dfb9. [DOI] [PubMed] [Google Scholar]
- 14.Organ Procurement and Transplant Network. [Accessed July 23, 2010];About OPTN data. at http://optn.transplant.hrsa.gov/data/about/
- 15.Hebert PL, Geiss LS, Tierney EF, Engelgau MM, Yawn BP, McBean AM. Identifying persons with diabetes using Medicare claims data. Am J Med Qual. 1999;14:270–277. doi: 10.1177/106286069901400607. [DOI] [PubMed] [Google Scholar]
- 16.Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003;3:178–185. doi: 10.1034/j.1600-6143.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 17.Stevens LA, Fares G, Fleming J, et al. Low rates of testing and diagnostic codes usage in a commercial clinical laboratory: evidence for lack of physician awareness of chronic kidney disease. J Am Soc Nephrol. 2005;16:2439–2448. doi: 10.1681/ASN.2005020192. [DOI] [PubMed] [Google Scholar]
- 18.Methods appendix. [Accessed July 23, 2010];Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; U.S. Renal Data System. USRDS 2008 annual data report. 2008 at http://www.usrds.org/2008/pdf/V2_Appendices_2008.pdf.
- 19.Lentine KL, Schnitzler MA, Abbott KC, Bramesfeld K, Buchanan PM, Brennan DC. Sensitivity of billing claims for cardiovascular disease events among kidney transplant recipients. Clin J Am Soc Nephrol. 2009;4:1213–1221. doi: 10.2215/CJN.00670109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agency for Healthcare Research and Quality. Creating and validating an index of socioeconomic status. [Accessed July 23, 2010]; at http://www.ahrq.gov/qual/medicareindicators/medicareindicators3.htm.
- 21.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey: questionnaires, datasets and related documentation. [Accessed July 23, 2010]; at http://www.cdc.gov/nchs/nhanes/nhanes2005–2006/nhanes05_06.htm.
- 22.Delmonico F. A report of the Amsterdam Forum on the Care of the Live Kidney Donor: data and medical guidelines. Transplantation. 2005;79 Suppl:S53–S66. [PubMed] [Google Scholar]
- 23.Textor SC, Taler SJ, Driscoll N, et al. Blood pressure and renal function after kidney donation from hypertensive living donors. Transplantation. 2004;78:276–282. doi: 10.1097/01.tp.0000128168.97735.b3. [DOI] [PubMed] [Google Scholar]
- 24.Boudville N, Prasad GV, Knoll G, et al. Meta-analysis: risk for hypertension in living kidney donors. Ann Intern Med. 2006;145:185–196. doi: 10.7326/0003-4819-145-3-200608010-00006. [DOI] [PubMed] [Google Scholar]
- 25.Garg AX, Prasad GV, Thiessen-Philbrook HR, et al. Cardiovascular disease and hypertension risk in living kidney donors: an analysis of health administrative data in Ontario, Canada. Transplantation. 2008;86:399–406. doi: 10.1097/TP.0b013e31817ba9e3. [DOI] [PubMed] [Google Scholar]
- 26.Nogueira JM, Weir MR, Jacobs S, et al. A study of renal outcomes in African American living kidney donors. Transplantation. 2009;88:1371–1376. doi: 10.1097/TP.0b013e3181c1e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haffner SM, Mitchell BD, Stern MP, Hazuda HP, Patterson JK. Decreased prevalence of hypertension in Mexican-Americans. Hypertension. 1990;16:225–234. doi: 10.1161/01.hyp.16.3.225. [DOI] [PubMed] [Google Scholar]
- 28.Ramírez EA. Cardiovascular health in Puerto Ricans compared to other population groups in the United States. Ethn Dis. 1991;1:188–199. [PubMed] [Google Scholar]
- 29.Mitchell BD, Stern MP, Haffner SM, Hazuda HP, Patterson JK. Risk factors for cardiovascular mortality in Mexican Americans and non-Hispanic whites: San Antonio Heart Study. Am J Epidemiol. 1990;131:423–433. doi: 10.1093/oxfordjournals.aje.a115517. [DOI] [PubMed] [Google Scholar]
- 30.Pappas G, Gergen PJ, Carroll M. Hypertension prevalence and the status of awareness, treatment, and control in the Hispanic Health and Nutrition Examination Survey (HHANES), 1982-84. Am J Public Health. 1990;80:1431–1436. doi: 10.2105/ajph.80.12.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Storsley LJ, Young A, Rush DN, et al. Long-term medical outcomes among aboriginal living kidney donors. Transplantation. 2010 June 18; doi: 10.1097/TP.0b013e3181e6e79b. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Incidence and prevalence. [Accessed July 23, 2010];Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; U.S. Renal Data System. USRDS 2008 annual data report. 2008 at http://www.usrds.org/adr.htm.
- 33.Gibney EM, King AL, Maluf DG, Garg AX, Parikh CR. Living kidney donors requiring transplantation: focus on African Americans. Transplantation. 2007;84:647–649. doi: 10.1097/01.tp.0000277288.78771.c2. [DOI] [PubMed] [Google Scholar]
- 34.Cherikh WS, Fan PY, Taranto SE, Randall HB, Young CJ. Prior living kidney donors who were subsequently placed on the waiting list: an updated OPTN analysis. Am J Transplant. 2008;8 Suppl:2. 335. abstract. [Google Scholar]
- 35.Ommen ES, Gill JS. The system of health insurance for living organ donors is a disincentive for live donation. Am J Transplant. 2010;10:747–750. doi: 10.1111/j.1600-6143.2009.02994.x. [DOI] [PubMed] [Google Scholar]
- 36.Wainright JL, Cooper M, Bolton DL, Davis CL. The changing landscape of living kidney donors in the US. Am J Transplant. 2009;9 Suppl 2:310–311. abstract. [Google Scholar]
- 37.Herring AA, Woolhandler S, Himmelstein DU. Insurance status of U.S. organ donors and transplant recipients: the uninsured give, but rarely receive. Int J Health Serv. 2008;38:641–652. doi: 10.2190/HS.38.4.d. [DOI] [PubMed] [Google Scholar]
- 38.Young CJ, Gaston RS. Renal transplantation in black Americans. N Engl J Med. 2000;343:1545–1552. doi: 10.1056/NEJM200011233432107. [DOI] [PubMed] [Google Scholar]
- 39.Gore JL, Danovitch GM, Litwin MS, Pham PT, Singer JS. Disparities in the utilization of live donor renal transplantation. Am J Transplant. 2009;9:1124–1133. doi: 10.1111/j.1600-6143.2009.02620.x. [DOI] [PubMed] [Google Scholar]
- 40.Reeves-Daniel A, Adams PL, Daniel K, et al. Impact of race and gender on live kidney donation. Clin Transplant. 2009;23:39–46. doi: 10.1111/j.1399-0012.2008.00898.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.