Abstract
The integration of microfabrication technologies with advanced biomaterials has led to the development of powerful tools to control the cellular microenvironment and the microarchitecture of engineered tissue constructs. Here we review this area, with a focus on the work accomplished in our laboratory. In particular, we discuss techniques to develop hydrogel microstructures for controlling cell aggregate formation to regulate stem cell behavior as well as a bottom-up and a top-down microengineering approach to creating biomimic tissue-like structures.
Keywords: microscale, tissue engineering, hydrogels, stem cells
Introduction
At the interface of micro- and nano-fabrication and experimental biology lies enormous potential to address important problems in biology and medicine. This is because biological systems are highly complex and cannot be easily understood without tools that can study such complexity at length scales that are relevant to biological systems. This complexity extends from developmental processes, where a single cell undergoes many rounds of division and proliferation to become a fully developed organism, to the maintenance and regeneration of adult tissues.
Cellular processes are controlled by the genetic factors in each cell, which are not only intrinsically regulated but also controlled by the local cellular microenvironment. Thus, the local chemical, biological, and mechanical environments can provide a coordinate set of regulatory cues to control cell behavior. For example, in the early stages of development, embryonic cells in the inner cell mass communicate with one another through paracrine and autocrine signaling and cell-cell contacts that influences organogenesis.1 Cellular communication and signaling occurs through a number of different mechanisms, including direct cell-to-cell contact, soluble factors and cell-matrix interactions. Soluble factors and signaling molecules provide different cues depending on the identity, concentration and context. Furthermore, mechanical forces imparted by the surrounding extracellular matrix (ECM)can signal cells to specific fate decisions.
The complexity of the interactions between cells and the microenvironment is not only found during development and organogenesis, but is also found in mature organisms. For example, blood cells are continually replenished throughout an organism’s lifetime. It is known that hematopoietic stem cells, which reside in the bone marrow, interact closely with the surrounding endothelial cells, osteoblasts and fibroblasts that regulates their self renewal and differentiation towards different blood cell fates.2, 3
Our research group, along with others, is interested in developing technologies at the interface between micro- and nanoengineering and materials science for engineering controlled microenvironments that can aid in understanding the interactions between cells and their microenvironment. The goal is to overcome a major experimental challenge in experimental biology, which is to recreate the in vivo cell-microenvironment interactions in vitro.4, 5 In a tissue culture Petri dish, cells interact with a two-dimensional (2D) plastic surface that is drastically different from the environment that is found in vivo. A salient example of the deleterious effects due to the disruption of the natural cellular microenvironment is the loss of liver hepatocyte function upon culture outside the body. In the liver, hepatocytes reside in controlled tissue units called liver lobules, which have a high degree of control and complexity.6 Liver lobules are made from organized hepatocytes that are assembled in hexagonal structures that are highly vascularized and are in intimate contact with surrounding endothelial cells. The inability to recreate this complex microarchitecture in vitro may be a key reason that the significant regeneration capacity of the liver and its diverse metabolic functions have not been recreated in vitro.
Our research group aims to develop and use technologies to recreate cell-microenvironment interactions in order to produce in vitro culture conditions that can be used for understanding cell biology or to generate three-dimensional (3D) tissue constructs for cell based therapies. Furthermore, engineered tissues can be used for screening drugs and to investigate the underlying mechanisms of disease.7 These modulations of the cellular microenvironment will be made by using microfabrication to sculpt and assemble advanced materials to control the interactions of cells with the microenvironment.8 For example, through the use of microfluidics it is possible to control the temporal and contextual presentation of soluble factors to induce cellular events9; patterned surfaces can be used to generate spatially controllable co-cultures to control cell-cell interactions10–12. Also cell-laden hydrogels can be created to investigate cell behavior in 3D.13, 14 Finally, it is possible to perturb mechanical properties of materials and local sheer stresses induced by fluids to modify cell responses.15
During the past several decades many tools required to investigate biology at the micro- and nanometer length scales have been developed.8 There is still much work to be accomplished, but great strides have been made in making micro- and nanofabricated systems much more accessible to common laboratory use.16 For example, with rapid prototyping or soft lithography micro- and nano-patterned silicon wafers, or other templates, can be quickly and easily replicated with an elastomeric co-polymer such as polydimethylsiloxane (PDMS).16 PDMS replicas can be used as microfluidic channels, for molding of biomaterials, or as stamps to pattern surfaces. The power of these techniques is that they can be used to control the architecture of materials at length scales much smaller (<100 nm) or much larger (>1 mm) than a typical cell.
In this article, we describe our laboratory’s work in merging biomaterials and advanced fabrication techniques to control the cell microenvironment and engineer tissues with controlled microarchitecture. The common element of the various projects is that they use biomaterials microstructures to engineer cell aggregates, and generate tissue constructs.17,18 Wedescribe two different ways to engineer tissue-like assemblies with controlled microarchitectures by using either a top-down19 or bottom-up approach20. With a bottom-up or “Lego-like” approach, we create small microfabricated tissue units that assemble in to larger tissue-like constructs with controlled microarchitectures. With a top-down engineering approach we sculpt biomaterials into micro- and nanoscale structures that mimic tissue constructs. In each of our approaches we use microfabrication techniques to mold and control the size, shape and microscale features of biocompatible hydrogels. We use photo- and soft lithography as well as micromolding techniques as they are simple methods that can be easily made compatible with a variety of different materials.
Hydrogel microstructures for stem cell bioengineering
Polymers such as poly(ethylene glycol) (PEG) are so hydrophilic that water complexes with their polymer chains to prevent subsequent protein adsorption or cell adhesion. We have been interested in exploiting this phenomenon to create microscale structures with controllable surface properties. Figure 1 shows a number of different microscale hydrogel structures made by micromolding of photo crosslinkable PEG.21 As shown in Figures 1a,b, engineered microwells can be made with exposed underlying glass substrates that can regulate protein adsorption on the surfaces. As cell can adhere to adsorbed protein layers, it is possible to use micropatterning of PEG structures to immobilize cells inside PEG microwells.
Figure 1.
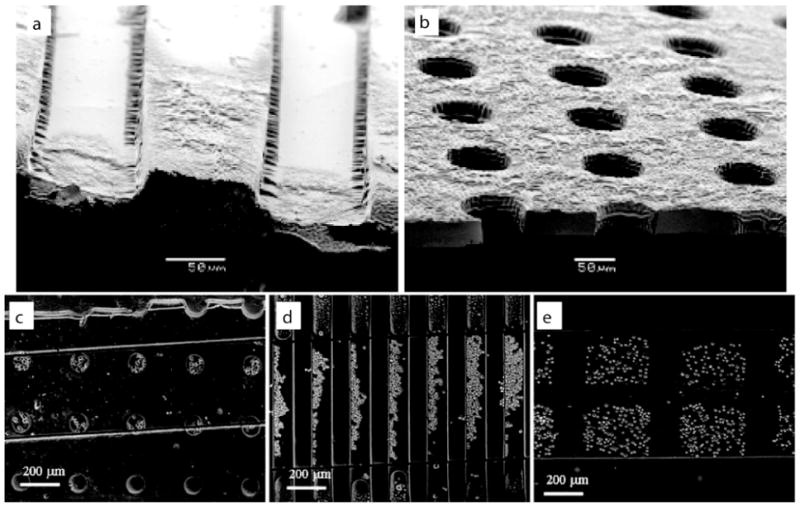
PEG Hydrogel microstructures. Scanning electron micrographs of (a) PEG-bottomed microwells and (b) microwells exposed to the underlying substrate. Cell docking in various microstructures, including (c) round 100 μm diameter microwells, (d) grooves 50 and 75 μm in width (left and right respectively), and (e) 200 μm square microwells. Images reproduced with permission21.
It is important to note that fluid elements around microscale structures behave differently from those in macroscale systems. For example, mathematical modeling of arrays of microwells under fluid flow reveals that as the depth of the microwells increase or the diameter of the wells decrease, the regions in the wells become protected from shear stresses.22 Thus, cells in deeper or smaller diameter microwells are exposed to less shear stress, are not washed away under flow, and can be stably integrated into the device. This system of arrayed microwells made from PEG hydrogel can be used to capture cells and other particles of interest in a rapid and controllable manner.
The flow patterns inside the structures can be also be used to control cell location. For example, we have used mathematical simulations to show that when fluid was flowed on arrays of microwells that were less than 50 μm in diameter, fluid recirculated in the structures, whereas in larger structures fluid penetrated the structures. These results were also confirmed experimentally. Figure 1d shows grooves that are 50 μm and 75 μm in width. In the 75 μm grooves there is no re-circulating flow, or back flow, and cells are somewhat evenly distributed across the width of the grooves. However, in the 50 μm grooves re-circulating flows aligns cells along the the upstream side of the grooves.
Arrayed microwells can be used as a platform to ask questions in stem cell biology and to direct cell fates for therapeutic applications. Specifically, the microwell platform is well suited to control the size of stem cell aggregates.17,23 In these aggregates, embryonic stem (ES) cells begin to initiate differentiation pathways that more closely mimic the developing embryo. In a typical ES cell aggregate experiment, ES cells are plated in a non-adhesive dish, and cells cluster together into aggregates of varying sizes and begin to differentiate. PEG microwells can be used to control this process and form cell aggregates in a simple manner. Cells seeded inside the microwells are in low shear stress regions and remain in the microwells under flow. In this way, we can control the formation and size of cell aggregates, and produce homogeneous populations of microsphere cell aggregates. In Figure 2 we shows examples of controlled cell-cell interactions and the culturing of populations of cell aggregates, embryoid bodies (EBs), with homogeneous size. Figure 2a–d shows EBs of varying sizes cultured in microwells, and Figure 2e–h shows EBs after retrival from the microwells after 10 days of culture.
Figure 2.
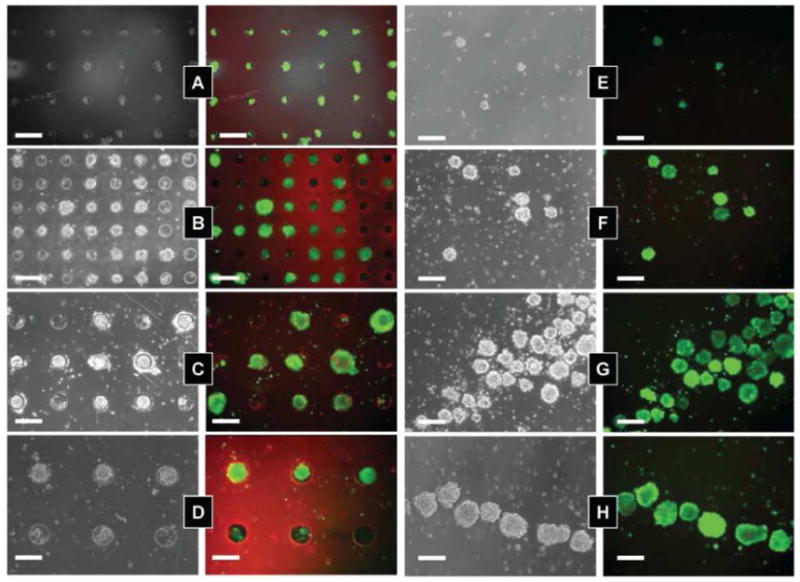
ES cells seeded in microwells of varying size: (A) 40 μm; (B) 75 μm; (C) 100 μm; and (D) 150 μm. Fluorescent images show cell stained with calcein AM (live cells) and ethidium homodimer (dead cells) after 10 days of culturing. (E–H) Harvested cell aggregates after 10 days of culture. Images reproduced with permission.23
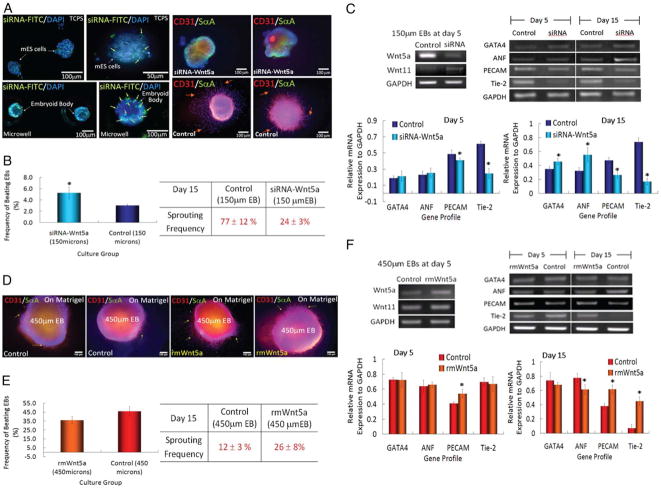
The number of cells that form an EB can be used to direct stem cell differentiation. If one can control the number of cells per EB, then comparisons of small cell number and large cell number EBs are possible. In different sized EBs individual cells will experience a different microenvironment and will be directed toward different fates. Figure 3 shows the outcomes of experiments with EBs of different sizes that were investigated with respect to the size-dependent expression of cardiac and endothelial cell differentiation.24 In this example, we examined the differentiation of EBs of three different sizes by using two different experimental protocols. In both cases ES cells were cultured in microwells of different sizes for 5 days. In one protocol we continued culturing the EBs in microwells for an additional 10 days. Alternatively, we retrieved the EBs and re-plated them on Matrigel for an additional 10 days. In both cases we analyze the outcomes with respect to gene expression and protein markers of cardiac and endothelial cell differentiation. In particular we found that a higher fraction of larger EBs were spontaneously beating, a characteristic of differentiation into cardiac cells. In addition, the cardiac marker sarcomeric alpha actinin (SαA)was highly expressed in the beating cells. Large EBs (450 μm in diameter) cultured in the microwells show strong SαA staining and beat spontaneously. In the smaller EBs (150 μm in diameter) that were cultured throughout the experiment in microwells, we saw minimal expression of SαM and a significant reduction in spontaneous beating. Larger EBs also had more cells with typical cardiac cell morphologies, when re-plated after 5 days of microwell culturing and when culturing in microwells for the course of the experiment.
Figure 3.
Microwell-mediated control of EB size and cardiac and endothelial cell differentiation. (A) Immunocytochemical characterization of Wnt5a-siRNA transfection (green) EBs shows that transfected siRNA is delivered (Left). EBs are analyzed for the presence of endothelial cell marker CD31 (red) and cardiogenic marker sarcomeric-α-actinin (green). (B) Characterization of EB sprouting and beating frequency. (C) Gene expression of endothelial cell and cardiogenic differentiation marker from ES cells cultured in 150 μm diameter microwells. (D) Characterization of ES cell aggregates with the addition of recombinant mouse WNT5a in 450 μm diameter microwells. (Scale bar, 100 μm.) (E and F) Analysis of cardiogenic and endothelial cell differentiation by EB beating, vessel sprouting frequency, and gene expression. (n=3, * indicates P <0.05 compared to controls). Images reproduced with permission.24
Analysis of vascular differentiation shows an opposite response with smaller EBs giving rise to more endothelial-like cells. In these experiments, a higher degree of expression of the endothelial cell marker CD31was observed in smaller EBs compared to larger EBs. Also, CD31+cells formed capillary-like structures. Additionally, smaller EBs that were re-plated on Matrigel started to form sprouts that stained positive for CD31 at a higher frequency and length than those observed in larger EBs, further suggesting that there was more endothelial cell differentiation in smaller EBs than in larger ones.
The microfabricated well arrays can also be used to generate homogeneous cultures for studying the molecular and biochemical mechanisms that regulate the observed size-dependent outcomes. In further analysis of the EBs of different sizes we did not see significant differences in expression of a number of ECM components including fibronectin, collagen IV and laminin-a and -b. We also analyzed the expression of Wnts, a family of signaling molecules that control a number of events during embryonic development. While we did not observe differences in the expression of canonical Wnt family members, beta-catenin or Wnt2, we observed differences in expression of two member of the non-canonical Wnt family molecules. In particular, it was observed that Wnt5a was highly expressed in smaller EBs, whereas Wnt11 was more prominently expressed in larger EBs. The size-dependent Wnt5a expression and the effect on cardiac and vascular differentiation was confirmed by siRNA silencing, and also by exogenous addition of Wnt5a(Figure 3). In small aggregates with Wnt5a silencing endothelial cell differentiation is significantly reduced(Figure 3b,c). Addition of Wnt5a molecules to larger aggregates, which usually promote cardiac over endothelial differentiation, results in increased expression of endothelial markers(Figure 3d,e,f). Additionally, the frequency of endothelial sprouts is increased. Taken together these results show that differential expression of non-canonical Wnt molecules are highly important in EB size-dependant behavior of endothelial and cardiac cell differentiation.
These size-controlled EB experiments demonstrate the utility of microwell-based cell culture in answering interesting questions in stem cell biology; however, there are still many interesting aspects of the microwell culturing that have yet to be explored. For example, we have yet to investigate the effects of size restriction on stem cell proliferation differentiation. Furthermore, the mechanisms that direct stem cell differentiation in these systems are yet to be fully elucidated.
Microwell technologies have many advantages over tradition cell culturing methods, such as the ability to control aggregate size, and the ability to protect cells from external shear stress. It is also possible to control microenvironmental factors including cell-cell and cell-matrix interactions with engineered hydrogel microwells. However, there are some potential shortcomings including, an inability to individually address single microwells, and an inability to dynamically control aggregate size as aggregates expand with cell proliferation. Additionally, aggregate size is controlled by restricting lateral growth, and during long term culturing cell aggregates can proliferate outside of the bounds of the microwell structures. Retrieval of aggregates from the microwell structures has also proven challenging, and great care is needed to retrieve high yields of intact aggregates. With new microscale technologies and microscale hydrogel microstructures we are beginning to address some of these shortcomings.
Bottom-up assembly of microgels for tissue engineering
Natural tissues are made from highly complex tissue microarchitectures. A major challenge in tissue engineering is to recreate tissue constructs with appropriate micro-architectures and functions. To this end, we have explored two different microengineering approaches: 1) the bottom-up assembly of microgels19; and 2) the top-down engineering of microscale biomaterials20. The latter is described in detail in the next section. The former, bottom-up assembly is a bio-inspired approach to the assembly of complex tissue microarchitectures using microgel building blocks.
In nature many tissues are made-up of assemblies of small tissue modules (i.e. repeating functional units). For example, muscles are made from bundles of myofibers; the liver is comprised of lobules; the kidney is made-up of nephrons; and the pancreas contains islets. These functional tissue units are well vascularized and are made of patterned assemblies of the units. In our lab, we are using a bottom-up approach to tissue engineering to mimic this concept. This is done by using microfabrication techniques to engineer microscale tissue units and then assembling the resulting structures to generate tissue-like complexity.
Hydrogels are attractive materials for making microengineered tissue building blocks due to their hydrated nature and biomimetic mechanical properties.25, 26 We have previously utilized a number of methods to engineer cell-laden hydrogels of controlled shapes and sizes. For example, photo crosslinkable hyaluronic acid (HA)-based hydrogels were micromolded into controlled shapes (Figure 4).20 To overcome the potential limitation of micromolding, which include its batch process20, we have used microfluidic systems to create a continuous process to generate controlled shape microgels. In this process the hydrogel precursor solution containing cells was flowed continuously past a light source to produce individual cell-laden microgels (Figure 5).27 A shutter was used to control the exposure of the hydrogel precursor to light and a mask was used to control the microgel shape. In this way, we and our collaborators produced microgel building blocks in a continuous process.
Figure 4.
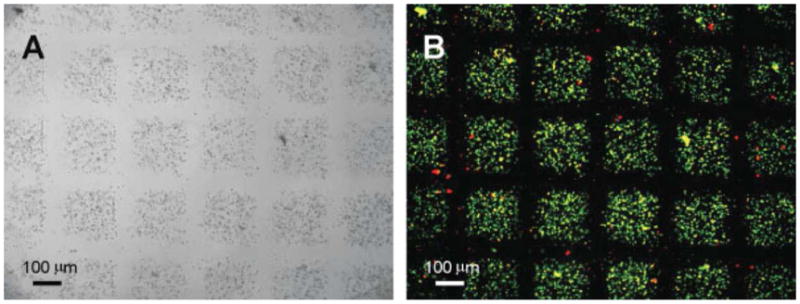
Light (A) and fluorescent (B) micrographs of micromolded cell-laden methacrylated hyaluronic acid (MeHA) hydrogels. The MeHA is crosslinked by exposure to light. The fluorescent micrograph shows cell viability after UV light exposure. Live cells are green (calecinAM) and dead cells are red (ethidium homodimer). Images reproduce with permission.33
Figure 5.
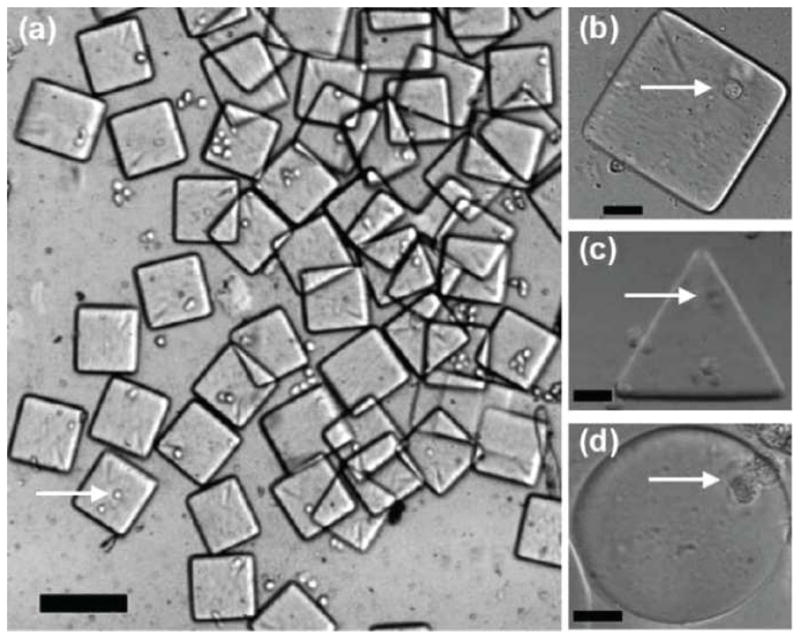
Cell-laden microgels produce by stop-flow lithography. (a) Cell-laden microgel collected at the device outlet reservoir (100 μm scale bar). (b–d) Cell-laden microgels of different shapes (20 μm scale bar). Images reproduced with permission.27
We have also developed methods of inducing the assembly of microgels into tissue-like structures. The self-assembly of microgels differs substantially from molecular self-assembly in that the forces at work in molecular assembly differ from those that drive the assembly of microscale hydrogels. Thus a key aspect that must be considered for microgel assembly is the type of forces that will be used to drive the assembly process.
One of our first ideas was to direct the assembly process by exploiting the tendency of hydrophobic and hydrophilic substances to minimize the interaction with each other. Specifically, we used the difference in chemical properties at a hydrophobic-hydrophilic liquid interface and at an air-liquid interface to drive the assembly process.19, 28 Following on the work of others29, 30, we have used the forces at these interfaces to drive the assembly of microgel building blocks as the energy of the system is minimized.
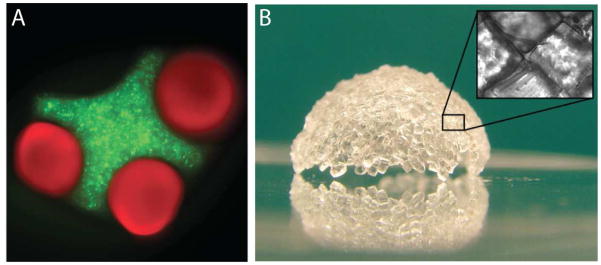
Figure 6 shows two different examples of self-assembled microgel structures. In one case(Figure 6a), two differently shaped microgel building blocks are assembled using an oil-water interface.19 In the simple case of oil in water, the solutions minimize the surface interactions and droplets of water form in oil (or oil droplets in water). With microscale hydrogels in a bulk oil phase a similar process occurs: the hydrogels are driven together as the surface interactions are minimized. Controlling the microgel shape leads to control of the assembly process. With appropriately shaped microgels it is possible to create ‘lock-and-key’ assemblies, where the shape of different microgels are matched to create meso-scale hydrogels with controlled microarchitectures. The examples shown in Figure 6 were made by using an oil-water interface, but we have also been able to create similar structures using an air-liquid interface. We have also been able to use this assembly process to create larger structures. By using two interfaces, oil-water and a wetting substrate we can ‘wrap’ assembled microgels around a wetting substrate to generate 3D structures(Figure 6b).
Figure 6.
Bottom-up assembly of microgels. (a) Cross and cylindrical shaped PEG hydrogels assembled in mineral oil.19(b) A semi-spherical shell assembled from PEG hydrogel microgels.
The examples of bottom-up assembly of microgels demonstrate the potential utility of this technique to create complex tissue-like structures; however, there are some potential disadvantages with the process. For example, it is challenging to create structures that are 10s of cm in length, or that are 100s of mL in volume. Such structures will require strong secondary crosslinking to ensure adequate bonding between microgels, and, if free standing, will require building blocks with mechanical properties sufficient to support the weight of the structure. Additionally, it would be advantageous to develop two-phase liquid systems other than mineral oil and water to drive the assembly process.
Further research is required to overcome these limitations, and we are actively pursuing microscale techniques to advance this bottom-up approach to tissue engineering. We are interested in exploring different aspects of the approach including the ordered and sequential assembly of microgels. We are also interested in exploring new hydrogels for microgel fabrication, and developing composite hydrogels for controlling cell-ECM interactions within each microgel building blocks.
Top-down microscale tissue engineering
Some of the initial studies in the use of microfabrication techniques for tissue engineering were done in attempts to engineer microfluidic networks to recreate vascularized tissue structures in scaffolds made from materials such as silicon, PDMS or degradable synthetic polymers.31, 32 However, while these materials are well suited for microscale fabrication, they are not amenable to cell encapsulation. Recently, we have been creating microfluidic channels with hydrogels made from natural polymers to enable the formation of hydrated vascular networks within which cells can be encapsulated in the bulk phase of the materials.
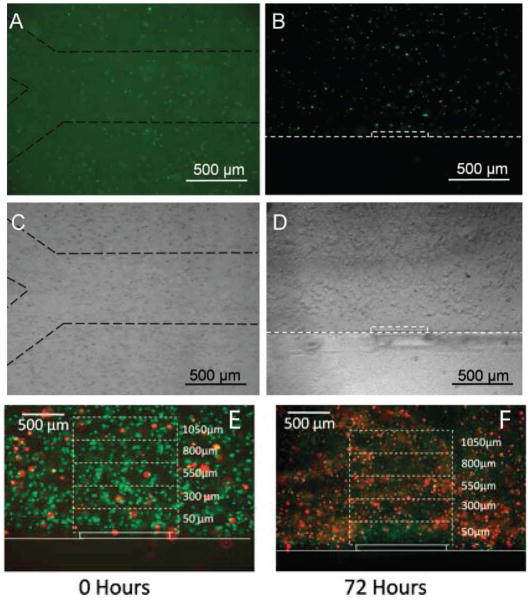
In Figure 7 we demonstrate an example of a micromolding approach to produce cell-laden hydrogel microfluidic channels.33 In this example hepatocytes were embedded in agarose and molded into a microchannel. Perfusion within the hydrogel could be achieved through diffusion from the channels into the hydrogel walls and was confirmed in cross-sections of agarose microfluidic channels after only a few minutes of flow.33 In this way, the microfluidic hydrogel channels have the potential to mimic native vasculature, as oxygen and nutrients from liquid in the channels diffuse into the surrounding hydrogel. This effect can be seen in cell-laden agarose hydrogels with engineered micro-porosity.34 After 3 days of culture a ring of viable cells can be seen on around the channel that corresponds to the diffusion pattern of oxygen and nutrients. With these types of microengineered structures one can begin to create tissue-like constructs with encapsulated cells, and endothelialized channels. Multiple constructs can be stack to create more complex vasculatures and tissue-like constructs.
Figure 7.
Fluorescent and brightfield images of carboxyfluoresceinsuccinimide ester-stained AML-12 cells encapsulated in a microengineering agarose channel. (A, C) top view, and (B, D) are cross-sectional view of the channel. Dashed lines were added to images to aid in visualization at print resolutions.(E,F) Representative live/dead staining of AML-12 hepatocytes immediately after encapsulation and after 72 hours of culturing. Reproduced with permission.33
The size, structure, and complexity of multi-layered constructs are limited by the materials properties of the hydrogels used to create the constructs. While the examples presented here make significant progress in mimicking native vasculature, there are many technological challenges that remain. For example, microscale technologies that can create high densities of microscale channels with complex branching are required. Fabrication of micro-channels that extend-out in 3D, and that have hierarchical structures are also needed. We are actively pursuing new microscale technologies to address these limitations.
Conclusions
We have described three different approaches to merge easily accessible microscale fabrication technologies with hydrogel biomaterials. With hydrogel microstructures we have been able to systematically manipulate stem cell microenvironments and analyze the outcomes of those manipulations. Furthermore, we have developed a bottom-up approach to create cell-laden hydrogels with controlled microarchitecture. We have also developed a top-down approach to microengineer cell-laden hydrogels that mimic native vasculature. These techniques may be of potential benefit for generating tissues and directing stem cell differentiation for regenerative medicine applications as well as for generating in vitro models for drug discovery and pathological studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Armant DR. Blastocysts don’t go it alone. Extrinsic signals fine-tune the intrinsic developmental program of trophoblast cells. Developmental Biology. 2005;280(2):260–280. doi: 10.1016/j.ydbio.2005.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9(6):653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 3.Adams GB, Alley IR, Chung U, Chabner KT, Jeanson NT, Lo Celso C, Marsters ES, Chen M, Weinstein LS, Lin CP, Kronenberg HM, Scadden DT. Haematopoietic stem cells depend on G alpha(s)-mediated signalling to engraft bone marrow. Nature. 2009;459(7243):103-U111. doi: 10.1038/nature07859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23(1):47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 5.Langer R, Vacanti JP. Tissue Engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 6.Ohashi K, Yokoyama T, Yamato M, Kuge H, Kanehiro H, Tsutsumi M, Amanuma T, Iwata H, Yang J, Okano T, Nakajima Y. Engineering functional two- and three-dimensional liver systems in vivo using hepatic tissue sheets. Nat Med. 2007;13(7):880–885. doi: 10.1038/nm1576. [DOI] [PubMed] [Google Scholar]
- 7.Sung JH, Kam C, Shuler ML. A microfluidic device for a pharmacokinetic-pharmacodynamic (PK-PD) model on a chip. Lab on a Chip. 10(4):446–455. doi: 10.1039/b917763a. [DOI] [PubMed] [Google Scholar]
- 8.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci U S A. 2006;103(8):2480–2487. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lii J, Hsu W-J, Parsa H, Das A, Rouse R, Sia SK. Real-Time Microfluidic System for Studying Mammalian Cells in 3D Microenvironments. Anal Chem. 2008;80(10):3640–3647. doi: 10.1021/ac8000034. [DOI] [PubMed] [Google Scholar]
- 10.Fukuda J, Khademhosseini A, Yeh J, Eng G, Cheng JJ, Farokhzad OC, Langer R. Micropatterned cell co-cultures using layer-by-layer deposition of extracellular matrix components. Biomaterials. 2006;27(8):1479–1486. doi: 10.1016/j.biomaterials.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Jinno S, Moeller HC, Chen CL, Rajalingam B, Chung BG, Dokmeci MR, Khademhosseini A. Macrefabricated multilayer parylene-C stencils for the generation of patterned dynamic co-cultures. J Biomed Mater Res Part A. 2008;86A(1):278–288. doi: 10.1002/jbm.a.32030. [DOI] [PubMed] [Google Scholar]
- 12.Khademhosseini A, Ferreira L, Blumling J, Yeh J, Karp JM, Fukuda J, Langer R. Co-culture of human embryonic stem cells with murine embryonic fibroblasts on microwell-patterned substrates. Biomaterials. 2006;27(36):5968–5977. doi: 10.1016/j.biomaterials.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 13.Gil ES, Frankowski DJ, Spontak RJ, Hudson SM. Swelling behavior and morphological evolution of mixed gelatin/silk fibroin hydrogels. Biomacromolecules. 2005;6(6):3079–3087. doi: 10.1021/bm050396c. [DOI] [PubMed] [Google Scholar]
- 14.Doyle AD, Wang FW, Matsumoto K, Yamada KM. One-dimensional topography underlies three-dimensional fibrillar cell migration. J Cell Biol. 2009;184(4):481–490. doi: 10.1083/jcb.200810041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 16.Whitesides GM, Ostuni E, Takayama S, Jiang XY, Ingber DE. Soft lithography in biology and biochemistry. Annual Review of Biomedical Engineering. 2001;3:335–373. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- 17.Moeller HC, Mian MK, Shrivastava S, Chung BG, Khademhosseini A. A microwell array system for stem cell culture. Biomaterials. 2008;29(6):752–763. doi: 10.1016/j.biomaterials.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khademhosseini A, Suh KY, Jon S, Eng G, Yeh J, Chen GJ, Langer R. A soft lithographic approach to fabricate patterned microfluidic channels. Anal Chem. 2004;76(13):3675–3681. doi: 10.1021/ac035415s. [DOI] [PubMed] [Google Scholar]
- 19.Du Y, Lo E, Ali S, Khademhosseini A. Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs. Proceedings of the National Academy of Sciences. 2008;105(28):9522–9527. doi: 10.1073/pnas.0801866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khademhosseini A, Eng G, Yeh J, Fukuda J, Blumling J, Langer R, Burdick JA. Micromolding of photocrosslinkable hyaluronic acid for cell encapsulation and entrapment. J Biomed Mater Res Part A. 2006;79A(3):522–532. doi: 10.1002/jbm.a.30821. [DOI] [PubMed] [Google Scholar]
- 21.Khademhosseini A, Yeh J, Jon S, Eng G, Suh KY, Burdick JA, Langer R. Molded polyethylene glycol microstructures for capturing cells within microfluidic channels. Lab Chip. 2004;4(5):425–430. doi: 10.1039/b404842c. [DOI] [PubMed] [Google Scholar]
- 22.Manbachi A, Shrivastava S, Cioffi M, Chung BG, Moretti M, Demirci U, Yliperttula M, Khademhosseini A. Microcirculation within grooved substrates regulates cell positioning and cell docking inside microfluidic channels. Lab Chip. 2008;8(5):747–754. doi: 10.1039/b718212k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karp JM, Yeh J, Eng G, Fukuda J, Blumling J, Suh KY, Cheng J, Mahdavi A, Borenstein J, Langer R, Khademhosseini A. Controlling size, shape and homogeneity of embryoid bodies using poly(ethylene glycol) microwells. Lab on a Chip. 2007;7(6):786–794. doi: 10.1039/b705085m. [DOI] [PubMed] [Google Scholar]
- 24.Hwang YS, Chung BG, Ortmann D, Hattori N, Moeller HC, Khademhosseini A. Microwell-mediated control of embryoid body size regulates embryonic stem cell fate via differential expression of WNT5a and WNT11. Proc Natl Acad Sci U S A. 2009;106(40):16978–83. doi: 10.1073/pnas.0905550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peppas N, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine. Advanced Materials. 2006;18:1–17. [Google Scholar]
- 26.Brigham MD, Bick A, Lo E, Bendali A, Burdick JA, Khademhosseini A. Mechanically Robust and Bioadhesive Collagen and Photocrosslinkable Hyaluronic Acid Semi-Interpenetrating Networks. Tissue Engineering Part A. 2009;15(7):1645–1653. doi: 10.1089/ten.tea.2008.0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panda P, Ali S, Lo E, Chung BG, Hatton TA, Khademhosseini A, Doyle PS. Stop-flow lithography to generate cell-laden microgel particles. Lab Chip. 2008;8(7):1056–1061. doi: 10.1039/b804234a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du Y, Lo E, Vidula MK, Khabiry M, Khademhosseini A. Method of Bottom-Up Directed Assembly of Cell-Laden Microgels. Cell Mol Bioeng. 2008;1(2–3):157–162. doi: 10.1007/s12195-008-0020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whitesides GM, Boncheva M. Beyond molecules: Self-assembly of mesoscopic and macroscopic components. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(8):4769–4774. doi: 10.1073/pnas.082065899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bowden N, Terfort A, Carbeck J, Whitesides GM. Self-assembly of mesoscale objects into ordered two-dimensional arrays. Science. 1997;276(5310):233–235. doi: 10.1126/science.276.5310.233. [DOI] [PubMed] [Google Scholar]
- 31.Fidkowski C, Kaazempur-Mofrad MR, Borenstein J, Vacanti JP, Langer R, Wang Y. Endothelialized microvasculature based on a biodegradable elastomer. Tissue engineering. 2005;11(1–2):302–9. doi: 10.1089/ten.2005.11.302. [DOI] [PubMed] [Google Scholar]
- 32.Borenstein JT, Terai H, King KR, Weinberg EJ, Kaazempur-Mofrad MR, Vacanti JP. Microfabrication technology for vascularized tissue engineering. Biomedical Microdevices. 2002;4(3):167–175. [Google Scholar]
- 33.Ling Y, Rubin J, Deng Y, Huang C, Demirci U, Karp JM, Khademhosseini A. A cell-laden microfluidic hydrogel. Lab Chip. 2007;7(6):756–762. doi: 10.1039/b615486g. [DOI] [PubMed] [Google Scholar]
- 34.Park JH, Chung BG, Lee WG, Kim J, Brigham MD, Shim J, Lee S, Hwang C, Durmus NG, Demirci U, Khademhosseini A. Microporous cell-laden hydrogels for engineered tissue constructs. Biotechnology and Bioengineering. 9999(999A) doi: 10.1002/bit.22667. n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]