Abstract
A series of modafinil (1) analogues were synthesized wherein (1) para-halo-substitutents were added to the aryl rings, (2) the sulfoxide function was removed, and (3) the primary amide group was replaced with secondary and tertiary amides and amines to investigate the effects of these chemical modifications on dopamine transporter, serotonin transporter, and norepinephrine transporter binding. In addition, the locomotor-stimulant effects in mice of (±)-modafinil (1), its R- and S-enantiomers, and its para-chloro sulfinylacetamide analogue (5c) were compared to those of cocaine.
Keywords: SAR, monoamine transporters, modafinil analogues
Modafinil {(±)1, 2-[(diphenylmethyl)sulfinyl]acetamide} is used clinically as a wake-promoting agent for the treatment of narcolepsy and other sleep disorders.1 Modafinil has been described as a psychostimulant but does not appear to be amphetamine-like in either pharmacological profile or mechanism of action2 and, as such, has piqued interest for the treatment of cognitive dysfunction in disorders such as attention deficit hyperactivity disorder.1,2 Recently, modafinil has also attracted attention for the treatment of cocaine3,4 and methamphetamine dependence.5 In addition, the emerging emphasis on cognitive impairment in neuropsychiatric disorders, including addiction, has stimulated investigations into the potential pro-cognitive effects of modafinil.6,7
The mechanisms of action by which modafinil produces its wake-promoting and psychostimulant effects appear to be complex and have not been clearly delineated. Several studies suggest that modafinil modulates the activity of hypocretin, histamine, α-adrenergic, γ-aminobutyric acid (GABA), and/or glutamate receptors.1,8 Moreover, modafinil has been shown to bind the dopamine transporter (DAT) and block dopamine reuptake both in vitro and in vivo, although with low affinity as compared to cocaine.9−11 Recently, studies in human subjects, using positron emission tomography (PET),12 show modafinil binding to the DAT, leading to speculation that modafinil may have abuse potential. However, results of animal studies have been equivocal13−16 with at least one study of human stimulant abusers reporting cocaine-like effects of modafinil,17 whereas most studies indicate a low liability for abuse.18
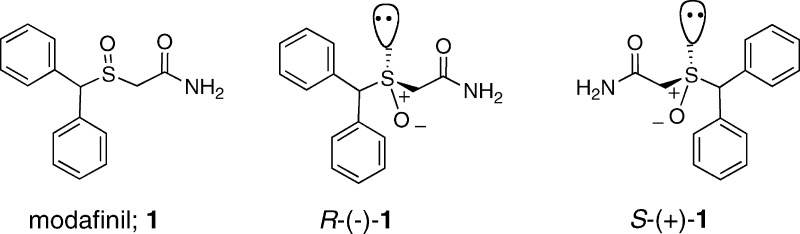
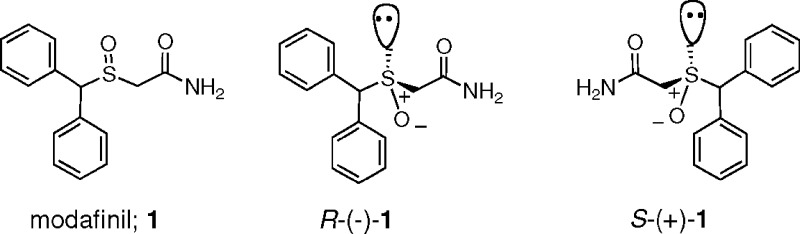
Modafinil is structurally dissimilar to stimulant drugs, such as methamphetamine, and contains an asymmetric sulfoxide group (Figure 1). It was originally prescribed clinically as the racemate (Provigil), as both isomers were presumed to contribute to its pharmacological effects.19 However, more recent studies suggest that R-(−)-modafinil is the more metabolically stable and longer-acting enantiomer [Armodafinil; R-(−)-1].20−22 Comparative pharmacological studies with modafinil, its enantiomers, and structural analogues have not appeared in the literature nor have detailed structure−activity relationship (SAR) studies at any of the suggested pharmacological targets. Therefore, in the present study, we synthesized the R- and S-enantiomers of modafinil and several sets of structural analogues and compared their binding affinities at the monoamine transporters: DAT, serotonin (SERT), and norepinephrine (NET). We first synthesized para-halo-substituted analogues, as the F- and Cl-substituted benztropine [3α-(diphenylmethoxy)tropane] analogues, which also have a biphenyl structural motif, show higher affinity at the DAT than the unsubstituted parent compound.23 In addition, several F and Cl analogues of modafinil have been reported to be “stimulating”, although no binding data were reported.24,25 Furthermore, the optimal S-oxidation state for monoamine transporter binding had not been described, although replacement of this function with a carbonyl group has been reported.24 Finally, modification of the terminal amide through substitution and/or reduction to the amine is reported herein. In addition to the synthesis and in vitro binding profiles of the resulting novel compounds, we report for the first time comparative behavioral effects of (±)-, R-(−)-, and S-(+)-modafinil to cocaine.
Figure 1.
Racemic modafinil and its enantiomers.
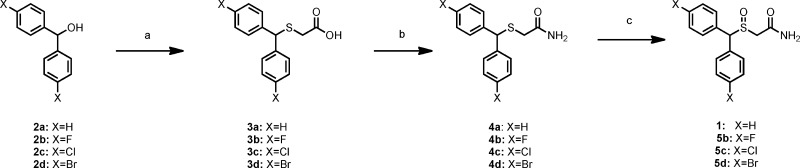
R-(−)- and S-(+)-modafinil enantiomers were synthesized according to the literature procedure,26 with minor modifications described in the Experimental Section in the Supporting Information. Synthesis of novel para-halo-substituted sulfinylacetamide 5b−d was achieved as depicted in Scheme 1. Dihalophenylmethanols 2b−d were coupled with thioglycolic acid in trifluoroacetic acid followed by esterification of the resulting carboxylic acids 3b−d. The esters were then subjected to aminolysis to obtain the thioacetamides 4b−d in 62−92% yield. Oxidation of the thioether was achieved using hydrogen peroxide (30%) in an acetic acid−methanol solution to give sulfinylacetamides 5b−d in 66−76% yield.
Scheme 1. Synthesis of Modafinil Analogues.
Reagents and conditions: (a) Thioglycolic acid (1 equiv), TFA, room temperature, overnight. (b) i) CH3I, K2CO3, acetone, reflux, 4 h; (ii) NH4OH, NH4Cl, MeOH, 50 °C, 72 h. (c) H2O2 (30%), AcOH:MeOH (1:3), 40 °C, 24 h.
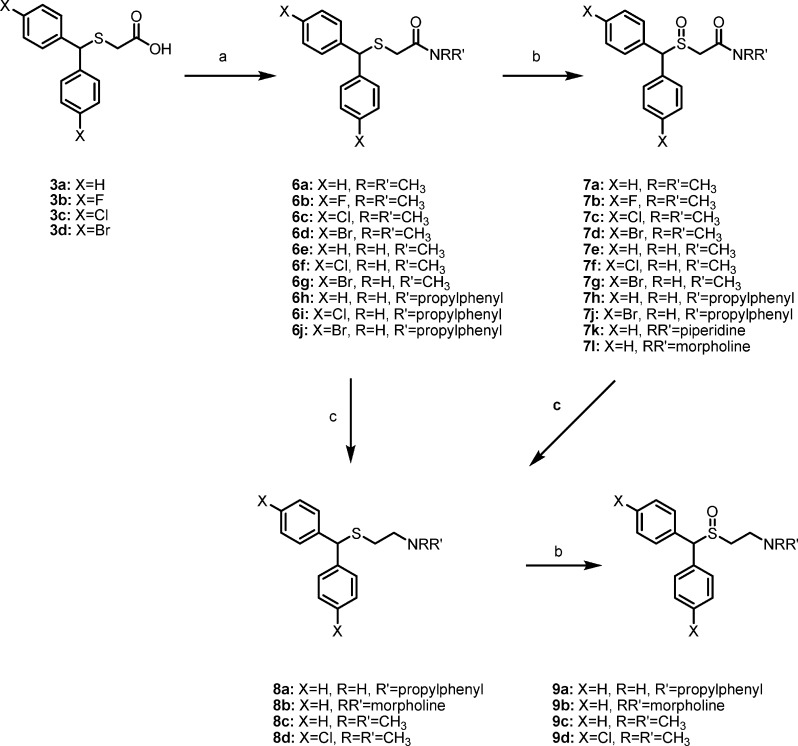
The N-substituted sulfinylacetamides 7a−l were obtained by (1) amidation of carboxylic acids 3a−d using the respective amines and CDI to obtain amides 6a−j followed by (2) oxidation of the thioether moiety to give the desired 7a−l as shown in Scheme 2. N-Substituted sulfinylethanamines 9a−d were obtained by the reduction of 6h, 7l, 7a, and 6c, respectively, using alane in 64−89% yield to give 8a−d, followed by oxidation of the thioether moiety as described in Scheme 1 in 60−76% yield.
Scheme 2. Synthesis of Modafinil Analogues.
Reagents and conditions: (a) (i) CDI, THF, room temperature, 2 h; (ii) HNRR′, THF, room temperature, overnight. (b) H2O2 (30%), AcOH:MeOH (1:3), 40 °C, 24 h. (c) LiAlH4, H2SO4, THF.
In this study, we synthesized a series of (±)-modafinil analogues wherein (1) para-halo-substitutents were added to the aryl rings, (2) the sulfoxide function was removed, and (3) the primary amide group was replaced with secondary and tertiary amides and amines according to synthetic strategies outlined in Schemes 1 and 2. The amino analogues were also designed to improve water solubility, through the formation of salts, as the parent compound is poorly water-soluble. All final compounds were evaluated for binding at the DAT, NET, and SERT in rat brain membranes, using methods previously described.27 These results can be found in Table 1.
Table 1. Binding Data for Modafinil Analoguesa.
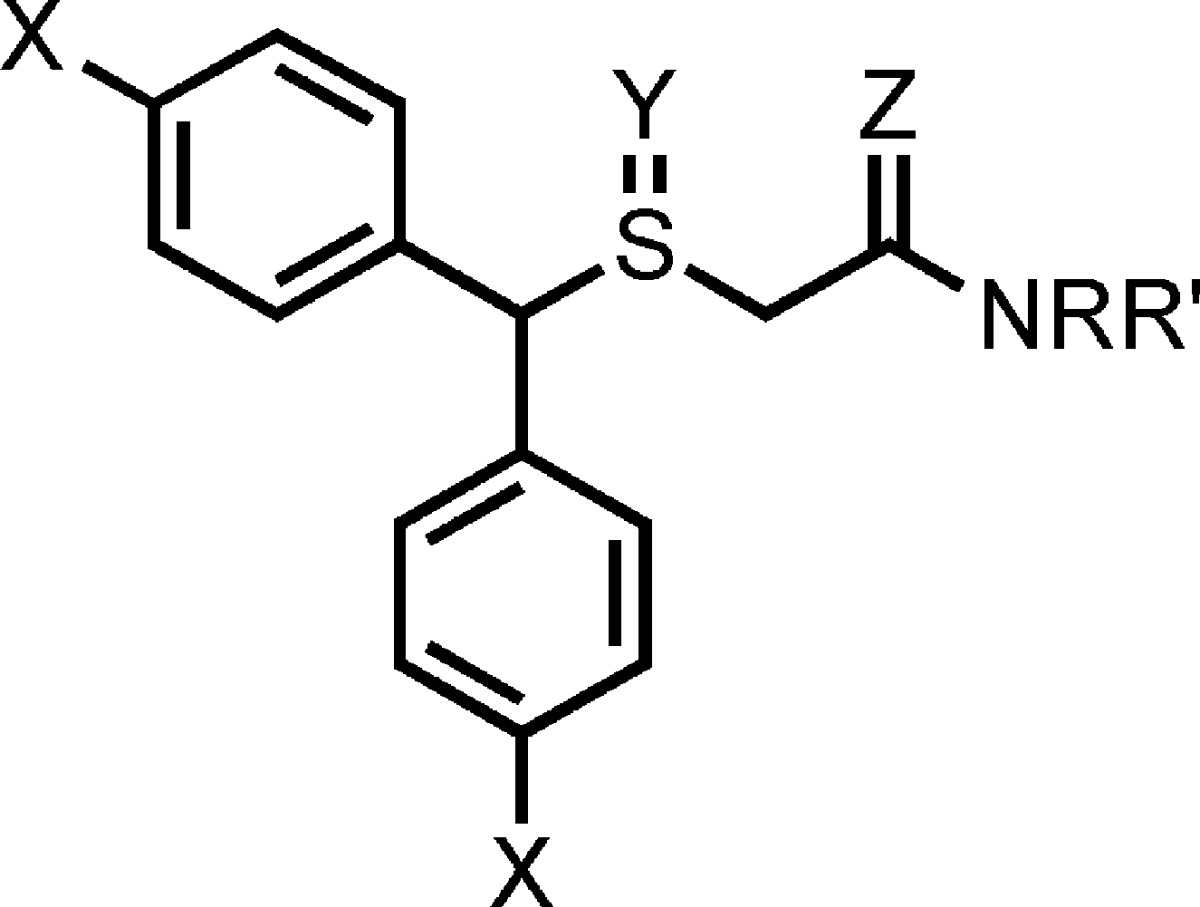
|
Ki (nM) ± SEM |
||||
|---|---|---|---|---|
| compound | substitution X, Y, Z, R, R′ | DAT | SERT | NET |
| (±)-1 | H, O, O, H, H | 2520 ± 204 | ND | ND |
| (+)-1 | H, O, O, H, H | 7640 ± 395 | ND | ND |
| (−)-1 | H, O, O, H, H | 3260 ± 195 | ND | ND |
| 4b | F, −, O, H, H | 1570 ± 68.2 | ND | ND |
| 4c | Cl, −, O, H, H | 2230 ± 166 | 12700 ± 520 | 52100 ± 5510 |
| 4d | Br, −, O, H, H | 1930 ± 95.2 | 2200 ± 278 | 77700 ± 6610 |
| 5b | F, O, O, H, H | 2190 ± 139 | ND | ND |
| 5c | Cl, O, O, H, H | 919 ± 52.8 | 39000 ± 2410 | ND |
| 5d | Br, O, O, H, H | 600 ± 47.3 | 10600 ± 1110 | ND |
| 6a | H, −, O, Me, Me | 16500 ± 2360 | ND | ND |
| 6b | F, −, O, Me, Me | 9510 ± 960 | 25900 ± 1040 | ND |
| 6c | Cl, −, O, Me, Me | 4510 ± 332 | 5980 ± 197 | 42500 ± 7950 |
| 6d | Br, −, O, Me, Me | 2450 ± 374 | 3210 ± 442 | 19200 ± 2760 |
| 7a | H, O, O, Me, Me | ND | ND | ND |
| 7b | F, O, O, Me, Me | ND | 16200 ± 760 | ND |
| 7c | Cl, O, O, Me, Me | 34600 ± 3600 | 22300 ± 1890 | ND |
| 7d | Br, O, O, Me, Me | 21300 ± 2930 | 14200 ± 1740 | ND |
| 7f | Cl, O, O, Me, H | 2440 ± 323 | ND | ND |
| 7g | Br, O, O, Me, H | 1650 ± 124 | 33200 ± 4380 | ND |
| 7h | H, O, O, H, prPh | 2660 ± 122 | ND | ND |
| 7k | H, O, O, −(CH2)4− | ND | ND | ND |
| 9a | H, O, H, H, prPh | 194 ± 16.8 | 1000 ± 120 | 2350 ± 267 |
| 9b | H, O, H, morph | ND | ND | ND |
| 9c | H, O, H, Me, Me | ND | 45800 ± 6740 | ND |
| 9d | Cl, O, H, Me, Me | 2890 ± 344 | 406 ± 18.7 | 36200 ± 3590 |
| cocaine | 71.8 ± 4.6b | 286 ± 38c | 3300 ± 170c | |
Only modest enantioselectivity was observed for the R-(−)- and S-(+)-modafinil, at the DAT, with the R-(−)-enantiomer having slightly higher affinity than the S. All analogues were racemic mixtures, and none showed comparable binding affinities to cocaine (Ki = 71.8 nM) for the DAT, although several showed higher affinity than the parent compound. The S=O motif appears to be optimal for DAT binding, except when the terminal amide is substituted, for example, N(CH3)2, although reducing the sulfoxide did not decrease binding affinities appreciably. Interestingly, with the exception of 4b−d, DAT binding affinity typically increased in each series with halogen substitution at the para-position of both rings in the order: H ≤ F < Cl < Br, in contrast to the comparably substituted analogues in the benztropine series, which also bind to the DAT. In that series, the order of affinities is Br < H < Cl < F.29
Tertiary amides among modafinil analogues were typically less well-tolerated at the DAT, while the amines showed higher binding affinities than the amide analogues, especially the N-propylphenyl analogue (9a), which was the most potent DAT analogue in the series. Most of compounds were less or inactive at SERT and NET, except the single SERT-selective compound, 9d.
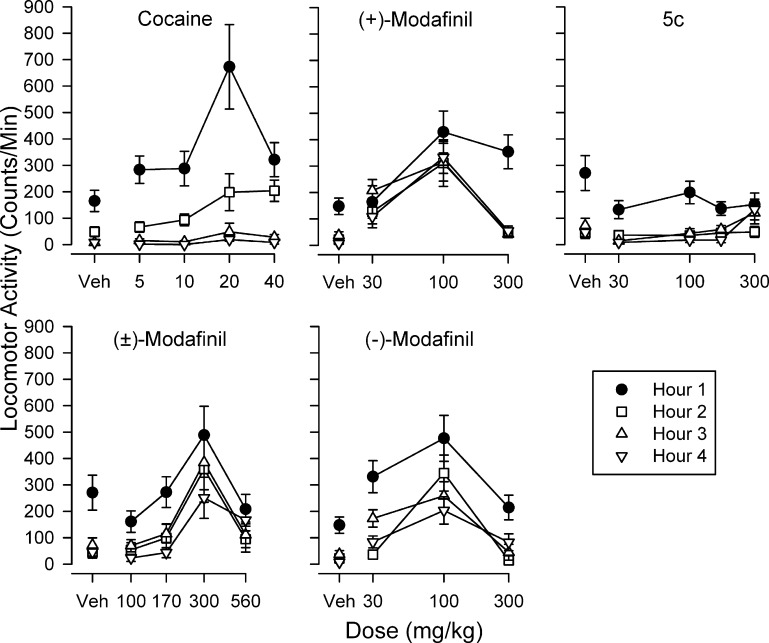
Several of the compounds were evaluated for locomotor stimulant activity, using methods previously described.30 Each of the drugs studied increased locomotor activity in mice at some time after their injection (Figure 2). The maximal effects of cocaine were greatest among the drugs and were substantially diminished 1 h after injection and absent thereafter. (±)-Modafinil and the R-(−)- and S-(+)-enantiomers also increased locomotor activity, although less so than cocaine. In addition, the decreases with time after injection were less pronounced and evident up to 4 h after injection. In contrast, the para-chlorosulfinylacetamide analogue 5c had no effects immediately after injection or in the second hour after injection. However, in the third and fourth hours, a modest stimulant effect was evident at the highest dose studied.
Figure 2.
Dose-dependent effects of (±)-1, its enantiomers R-1 and S-1, and 5c on locomotor activity in mice. Ordinates: horizontal locomotor activity counts after drug administration in counts per min. Abscissae: dose of drug in mg/kg, log scale. Each point represents the average effect determined in six mice. The data are from the 30 min period at the start of each of 4 h after drug administration. Note that neither (±)-modafinil, its enantiomers, or the analogue produced a maximal stimulation of activity that was equivalent to that of cocaine and that compound 5c only had effects in the third and fourth hour after its administration at the highest dose tested.
In summary, a series of modafinil analogues have been synthesized and evaluated for binding at DAT, NET, and SERT. SARs suggest binding interactions at the DAT that appear to contrast to the benztropine analogues, which also have a biphenyl structural motif. Studies of locomotor activity in mice suggest behavioral stimulant effects, although the effectiveness of the drugs studied was less than that of cocaine but greater than that of many benztropine analogues (see ref (30) for comparison). The results of the present studies warrant further investigation of these and other modafinil analogues in additional animal models of psychostimulant abuse.
Supporting Information Available
Experimental section. This material is available free of charge via the Internet at http://pubs.acs.org.
This work was funded by the NIDA-IRP. O.M.O. was supported by a NIH Postdoctoral Intramural Research Training Award (IRTA) Fellowship. M.S. was supported by the NIH Summer Internship Program.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Ballon J. S.; Feifel D. A systematic review of modafinil: Potential clinical uses and mechanisms of action. J. Clin. Psychiatry 2006, 67, 554–566. [DOI] [PubMed] [Google Scholar]
- Minzenberg M. J.; Carter C. S. Modafinil: A review of neurochemical actions and effects on cognition. Neuropsychopharmacology 2008, 33, 1477–1502. [DOI] [PubMed] [Google Scholar]
- Dackis C. A.; Kampman K. M.; Lynch K. G.; Pettinati H. M.; O'Brien C. P. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology 2005, 30, 205–211. [DOI] [PubMed] [Google Scholar]
- Martinez-Raga J.; Knecht C.; Cepeda S. Modafinil: a useful medication for cocaine addiction? Review of the evidence from neuropharmacological, experimental and clinical studies. Curr. Drug Abuse Rev. 2008, 1, 213–221. [DOI] [PubMed] [Google Scholar]
- Shearer J.; Darke S.; Rodgers C.; Slade T.; van Beek I.; Lewis J.; Brady D.; McKetin R.; Mattick R. P.; Wodak A. A double-blind, placebo-controlled trial of modafinil (200 mg/day) for methamphetamine dependence. Addiction 2009, 104, 224–233. [DOI] [PubMed] [Google Scholar]
- Turner D. C.; Robbins T. W.; Clark L.; Aron A. R.; Dowson J.; Sahakian B. J. Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacology 2003, 165, 260–269. [DOI] [PubMed] [Google Scholar]
- Rasetti R.; Mattay V. S.; Stankevich B.; Skjei K.; Blasi G.; Sambataro F.; Arrillaga-Romany I. C.; Goldberg T. E.; Callicott J. H.; Apud J. A.; Weinberger D. R. Modulatory Effects of Modafinil on Neural Circuits Regulating Emotion and Cognition. Neuropsychopharmacology 2010, 35, 2101–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro L.; Antonelli T.; Tanganelli S.; O’Connor W. T.; Perez de la Mora M.; Mendez-Franco J.; Rambert F. A.; Fuxe K. The vigilance promoting drug modafinil increases extracellular glutamate levels in the medial preoptic area and the posterior hypothalamus of the conscious rat: prevention by local GABAA receptor blockade. Neuropsychopharmacology 1999, 20, 346–356. [DOI] [PubMed] [Google Scholar]
- Madras B. K.; Xie Z.; Lin Z.; Jassen A.; Panas H.; Lynch L.; Johnson R.; Livni E.; Spencer T. J.; Bonab A. A.; Miller G. M.; Fischman A. J. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J. Pharmacol. Exp. Ther. 2006, 319, 561–569. [DOI] [PubMed] [Google Scholar]
- Mignot E.; Nishino S.; Guilleminault C.; Dement W. C. Modafinil binds to the dopamine uptake carrier site with low affinity. Sleep 1994, 17, 436–437. [DOI] [PubMed] [Google Scholar]
- Zolkowska D.; Jain R.; Rothman R. B.; Partilla J. S.; Roth B. L.; Setola V.; Prisinzano T. E.; Baumann M. H. Evidence for the involvement of dopamine transporters in behavioral stimulant effects of modafinil. J. Pharmacol. Exp. Ther. 2009, 329, 738–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N. D.; Fowler J. S.; Logan J.; Alexoff D.; Zhu W.; Telang F.; Wang G. J.; Jayne M.; Hooker J. M.; Wong C.; Hubbard B.; Carter P.; Warner D.; King P.; Shea C.; Xu Y.; Muench L.; Apelskog-Torres K. Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. J. Am. Med. Assoc. 2009, 301, 1148–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen M. L.; Kessler E.; Murnane K. S.; McClung J. C.; Tufik S.; Howell L. L. Dopamine transporter-related effects of modafinil in rhesus monkeys. Psychopharmacology 2010, 210, 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopheide M. M.; Morgan R. E.; Rodvelt K. R.; Schachtman T. R.; Miller D. K. Modafinil evokes striatal [(3)H]dopamine release and alters the subjective properties of stimulants. Eur. J. Pharmacol. 2007, 568, 112–123. [DOI] [PubMed] [Google Scholar]
- Gold L. H.; Balster R. L. Evaluation of the cocaine-like discriminative stimulus effects and reinforcing effects of modafinil. Psychopharmacology 1996, 126, 286–292. [DOI] [PubMed] [Google Scholar]
- Reichel C. M.; See R. E. Modafinil effects on reinstatement of methamphetamine seeking in a rat model of relapse. Psychopharmacology 2010, 210, 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinski D. R. An evaluation of the abuse potential of modafinil using methylphenidate as a reference. J. Psychopharmacol. 2000, 14, 53–60. [DOI] [PubMed] [Google Scholar]
- Vosburg S. K.; Hart C. L.; Haney M.; Rubin E.; Foltin R. W. Modafinil does not serve as a reinforcer in cocaine abusers. Drug Alcohol Depend. 2010, 106, 233–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan J. L.; Malcolm R. J.; Markowitz J. S.; DeVane C. L. Chiral analysis of d- and l-modafinil in human serum: Application to human pharmacokinetic studies. Ther. Drug Monit. 2003, 25, 197–202. [DOI] [PubMed] [Google Scholar]
- Dinges D. F.; Arora S.; Darwish M.; Niebler G. E. Pharmacodynamic effects on alertness of single doses of armodafinil in healthy subjects during a nocturnal period of acute sleep loss. Curr. Med. Res. Opin. 2006, 22, 159–167. [DOI] [PubMed] [Google Scholar]
- Robertson P. Jr.; Hellriegel E. T. Clinical pharmacokinetic profile of modafinil. Clin. Pharmacokinet. 2003, 42, 123–137. [DOI] [PubMed] [Google Scholar]
- Garnock-Jones K. P.; Dhillon S.; Scott L. J. Armodafinil. CNS Drugs 2009, 23, 793–803. [DOI] [PubMed] [Google Scholar]
- Newman A. H.; Allen A. C.; Izenwasser S.; Katz J. L. Novel 3 alpha-(diphenylmethoxy)tropane analogs: Potent dopamine uptake inhibitors without cocaine-like behavioral profiles. J. Med. Chem. 1994, 37, 2258–2261. [DOI] [PubMed] [Google Scholar]
- De Risi C.; Ferraro L.; Pollini G. P.; Tanganelli S.; Valente F.; Veronese A. C. Efficient synthesis and biological evaluation of two modafinil analogues. Bioorg. Med. Chem. 2008, 16, 9904–9910. [DOI] [PubMed] [Google Scholar]
- Lafon L.Benzylsulfinylacetamide derivatives and their therapeutic use. European Patent 0097071A1, 1983.
- Prisinzano T.; Podobinski J.; Tidgewell K.; Luo M.; Swenson D. Synthesis and determination of the absolute configuration of the enantiomers of modafinil. Tetrahedron: Asymmetry 2004, 15, 1053–1058. [Google Scholar]
- Zou M. F.; Cao J.; Kopajtic T.; Desai R. I.; Katz J. L.; Newman A. H. Structure-activity relationship studies on a novel series of (S)-2β-substituted 3alpha-[bis(4-fluoro- or 4-chlorophenyl)methoxy]tropane analogues for in vivo investigation. J. Med. Chem. 2006, 49, 6391–6399. [DOI] [PubMed] [Google Scholar]
- Kulkarni S. S.; Grundt P.; Kopajtic T.; Katz J. L.; Newman A. H. Structure-activity relationships at monoamine transporters for a series of N-substituted 3alpha-(bis[4-fluorophenyl]methoxy)tropanes: Comparative molecular field analysis, synthesis, and pharmacological evaluation. J. Med. Chem. 2004, 47, 3388–3398. [DOI] [PubMed] [Google Scholar]
- Newman A. H.; Kline R. H.; Allen A. C.; Izenwasser S.; George C.; Katz J. L. Novel 4′-substituted and 4′,4′′-disubstituted 3 α-(diphenylmethoxy)tropane analogs as potent and selective dopamine uptake inhibitors. J. Med. Chem. 1995, 38, 3933–3940. [DOI] [PubMed] [Google Scholar]
- Katz J. L.; Izenwasser S.; Kline R. H.; Allen A. C.; Newman A. H. Novel 3α-diphenyl-methoxy-tropane analogs: Selective dopamine uptake inhibitors with behavioral effects distinct from those of cocaine. J. Pharmacol. Exp. Ther. 1999, 288, 302–315. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.