Abstract
Background
This article constitutes a partial update of the original systematic review evidence by Yengopal et al. from 15 January 2008 (published in the Journal of Oral Science in 2009) with primary focus on research quality in regard to bias risk in trials. Its aim is to update the existing systematic review evidence from the English literature as to whether caries occurrence on pits and fissures of teeth sealed with either GIC or resin is the same.
Methods
In addition to the 12 trials included during the original systematic review, 5 new trials were identified during the database search (up to 26 August 2010) and 2 further trials were included from a hand search and reference check. Of these, 3 trials were excluded and 16 were accepted for data extraction and quality assessment. The quality of accepted trials was assessed, using updated quality criteria, and the risk of bias was investigated in more depth than previously reported. In addition, the focus of quantitative synthesis was shifted to single datasets that were extracted from the accepted trials.
Results
Twenty-six dichotomous and 4 continuous datasets were extracted. Meta-analysis and cumulative meta-analysis were used in combining clinically homogenous datasets. The overall outcome of the computed datasets suggest no difference between the caries-preventive effects of GIC- and resin-based fissure sealants.
Conclusions
This overall outcome is in agreement with the conclusions of the original systematic review. Although the findings of the trials identified in this update may be considered to be less affected by attrition- and publication bias, their risk of selection- and detection-/performance bias is high. Thus, verification of the currently available results requires further high quality randomised control trials.
Introduction
Pits and fissures of posterior teeth are considered to be highly susceptible to the adhesion of micro-organisms and consequently, to caries. Therefore, a significant amount of tooth decay occurs at these sites. Fissure sealants are used to prevent occlusal caries, 71% percent of occlusal decay being preventable after a once-off fissure sealant application [1]. Evidence regarding the efficacy and cost-effectiveness of sealants in reducing occlusal caries in molars has been highlighted [1-5]. The most commonly used sealant material is resin composite [6-8]. Its caries-preventive effect relies on the sealing of pits and fissures through micro-retention, created through tags after enamel acid etching. However, these are easily destroyed by saliva contamination, which reduces micro-retention and, consequently, the caries-preventive effect [9]. Under the generally wet conditions in the oral cavity, Glass Ionomer Cement (GIC) offers an alternative. Owing to its hydrophilic properties, GIC is not as moisture-sensitive as hydrophobic resin [10].
In a previous systematic review Yengopal et al. [11] conducted a meta-analysis in order to quantitatively appraise, for the first time, the evidence regarding the caries-preventive effect of GIC in comparison to that of resin-based fissure sealants. This systematic review with meta-analysis found no evidence that either material was superior to the other in the prevention of dental caries. Therefore, both appeared to be equally suitable for clinical application as fissure sealant materials. These results were based on a systematic search of literature up to 15 January 2008 [11]. It has been suggested that once the search date of a systematic review is older than even 1 year, users should check for more recent trials on the same topic to see whether new evidence has altered the findings of a given systematic review [12]. In addition, the original quality assessment criteria [11] may be questioned on grounds of being ineffective in judging the true internal validity of trials on basis of risk of bias [13,14]. Therefore, the aim of this update is to provide a more in-depth assessment of bias-risk in trials. As the inclusion of non-English language trials in the original systematic review did not decisively influence the overall review results [11] the focus of the in-depth assessment and discussion of bias-risk is limited to English language trials, only.
Thus, the purpose of this article is to update the existing evidence from trials published in English language regarding the review question as to whether caries occurrence on pits and fissures of teeth sealed with either GIC or resin is the same.
Materials and methods
In order to update the existing evidence, the systematic literature search of the English literature was extended beyond the original search date and a further hand search and reference check were done. The quality of accepted trials was assessed, using updated quality criteria (Table 1) [13-16] and the risk of bias was investigated in more depth than previously reported. In addition, the focus of quantitative synthesis was shifted to single datasets (DS) that were extracted from the accepted trials.
Table 1.
Quality assessment criteria of trials
| Selection bias | ||
|---|---|---|
| Score | Criteria | Impact on bias risk |
| Randomisation and concealment | ||
| A | (i) Randomisation: Details of any adequate type of allocation method that generates random sequences with the patient as unit of randomisation are reported.1 | Doubts may still exist whether the trial results are influenced by selection bias but no indication can be found from the trial report to support such doubt. |
| (ii) Concealment: Trial provides evidence2 that concealment was indeed effective and that the random sequence could not have been observed or predicted throughout the duration of the trial. | ||
| B | (i) Randomisation: Details of any adequate type of allocation method that generates random sequences with the patient as unit of randomisation are reported.1 | Despite the implementation of method considered to be able to prevent unmasking of the concealed allocation sequence through direct observation and prediction, there are reasons to expect that the concealed allocation sequence may have been unmasked during the cause of the trial. |
| (ii) Concealment: Trial reports on any adequate method to prevent direct observation3 and prediction4 of the allocation sequence and sequence generation rules. | ||
| C | (i) Randomisation: Details of any adequate type of allocation method that generates random sequences with the patient as unit of randomisation are reported.1 | Despite the implementation of method considered to be able to prevent unmasking of the concealed allocation sequence through direct observation, there are reasons to expect that operators could have predicted the concealed allocation sequence. |
| (ii) Concealment: Trial reports on any adequate method to prevent direct operator observation of allocation sequence and sequence generation rules3. However, the allocation sequence and sequence generation may have been sufficiently predicted. | ||
| D | (i) Randomisation: Details of any adequate type of allocation method that generates random sequences with the patient as unit of randomisation are reported.1 | Despite the theoretical chance for each patient to be allocated to either treatment group, operator knowledge of the allocation sequence may have lead to patient allocation that favoured the outcome of one type of treatment above the other. |
| (ii) Concealment: The trial report does not include information on how the allocation of random sequence was concealed. The allocation could have been directly observed and/or predicted. | ||
| 0 | Trial does not comply with criteria A - D. | No guaranty of equal chance for patients to be allocated to either treatment group, thus allocation may have favoured the outcome of one type of treatment above the other. |
| Baseline data for randomised trials | ||
| A | Baseline data collected before randomisation and reported for both treatment groups. Data shows no significant differences between both groups. | Evidence is given that randomisation has lead to equal groups suggesting little risk of selection bias. |
| B | Baseline data collected before randomisation and reported for both treatment groups. Data shows significant differences between both groups but has been statistically adjusted appropriately. | Differences have been adjusted, thus the influence of possible selection bias appears to be reduced. |
| C | Baseline data collected before randomisation and reported for both treatment groups. Data shows significant differences between both groups without being statistically adjusted. | Reported differences may be due to ineffective randomisation, thus indicate risk of selection bias. |
| 0 | Trial does not comply with criteria A - C. | No evidence is given whether randomisation has indeed lead to equal groups with differences beyond chance, thus differences may exists indicating selection bias. |
| Detection/Performance bias | ||
| Blinding/Masking | ||
| Score | Criteria | Impact on bias risk |
| A | (i) Trial reports on any type of method that is known to prevent patient AND operator AND evaluator to discern whether patients are allocated to the test- or the control group (Blinding/Masking). | Evidence is given that the trial results may not have been influenced by detection/performance bias that may have favored the outcome of one type of treatment above the other. |
| (ii) Trial reports a process with which the effect of Blinding/Masking was evaluated, as well as the results of such evaluation. | ||
| B | (i) Trial reports on any type of method that is known to prevent patient AND operator AND evaluator to discern whether patients are allocated to the test- or the control group (Blinding/Masking). | Doubts may still exist whether the trial results are influenced by detection/performance bias but no indication can be found from the trial report to support such doubt. However, no evaluation of the Blinding/Masking effect has been included in the trial, thus no evidence for lack of bias is given. |
| (ii) Trial report does not give reason for doubt that the patient allocation to either the test- or the control group has been unmasked throughout the duration of the trial. | ||
| C | (i) Trial reports on any type of method that is known to prevent patient AND operator AND evaluator to discern whether patients are allocated to the test- or the control group (Blinding/Masking). | Despite the implementation of method considered to be able to prevent unmasking, there are reasons to expect that operators/patients could have discovered the allocation. |
| (ii) Trial report gives reason for doubt that the patient allocation to either the test- or the control group has been unmasked throughout the duration of the trial. | ||
| 0 | No process reported or implemented able to blind/mask patients AND operators whether patients where allocated to either the test- or the control group (It is insufficient to report that blinding/masking was done without reporting the details of the process). | Knowledge about the patient allocation may have caused patients/operator to act in a way that may have favoured the outcome of one type of treatment above the other, |
| Attrition bias | ||
| Loss - to follow up | ||
| Score | Criteria | Impact on bias risk |
| A | Available case analysis, loss-to-follow up reported per treatment group. Subsequent sensitivity analysis does not indicate a possible risk of bias. | The trial allows extracting evidence that attrition may not have favoured the outcome of one type of treatment above the other. |
| B | Available case analysis, loss-to-follow up reported per treatment group. Subsequent sensitivity analysis indicates a possible risk of bias. | The trial allows assessing the risk that attrition may have favoured the outcome of one type of treatment above the other. |
| 0 | Trial does not report number of included participants per treatment group at baseline or gives any indication that would allow ascertaining the loss-to-follow up rate per treatment group. | The trial carries an unknown risk that attrition may have favoured the outcome of one type of treatment above the other. |
| Run-in phase | ||
| A | No run-in phase reported or discernable during which patients were given the active treatment or the placebo/control. | The trial may not carry the risk of bias due to exclusion of patients who would not respond well to e.g. the active treatment. |
| 0 | Run-in phase reported or discernable during which patients were given the active treatment or the placebo/control. | During a run-in phase only patients were selected for randomisation that have responded/not responded to the active treatment of the placebo/control. This may favour the outcome of one type of treatment above the other as patients who did not respond well to either are excluded. |
| Trial endpoints | ||
| 0 | The trial reports on secondary or surrogate outcomes as endpoints. | Even if the surrogate results would highly correlate with primary (i.e. clinical) outcomes, they cannot serve as valid replacements and need to be regarded for hypothesis development, only. |
| A | The trial reports on primary outcomes as endpoints. | Primary outcomes may provide evidence for hypothesis testing. |
1 Excluded are types of allocation methods that are considered as inadequate: cluster randomisation, fixed block randomisation with block size 2, minimization, alternation, randomisation of teeth, use of date of birth or patient record number, "quasi"-randomisation, split-mouth
2 E.g. by reporting results of the Berger-Exner Test or any other statistical tests that show that covariates of compared groups were similar at baseline
3 E.g. by opening of opaque envelope, obtaining allocation from tables, computer generated or from other sources
4 E.g. central randomisation, sequence allocation by other than operator; excluding varied block randomisation
Literature search, review and quality assessment of trials
The search strategy used in the previous review for English language articles [11] was replicated for this review update using the search terms: "(GIC sealant* OR Glass ionomer cement sealant) AND (caries OR tooth decay)". Only the start and cut-off dates were changed. The English databases, Biomed Central, Cochrane Oral Health Reviews, Cochrane Library, Directory Of Open Access Journals, Expanded Academic ASAP PLUS, Meta Register Of Controlled Trials, PubMed and Science-Direct, were searched for relevant papers published between 15 January 2008 (the search cut-off date of the original systematic review) and 26 August 2010. Criteria for trial inclusion were:
- 2-or multiple arm clinical prospective study designs;
- Comparison of GIC versus Resin fissure sealants;
- Publication in English.
Included trials were excluded after further review if:
- No outcome measure related to caries was reported;
- No computable data, dichotomous or continuous, per treatment group was reported;
- Resin-modified GIC (RMGIC) was used instead of conventional, chemically cured GIC.
Included trials that passed the exclusion criteria test were accepted for further quality assessment and data extraction. Reviewing, data extraction and quality assessment of the accepted trials was undertaken independently by two reviewers (SM and VY). Differences were resolved through discussion and consensus.
Unlike in the original published systematic review [11], quality assessment of accepted trials was undertaken on the basis of availability of evidence indicating successful prevention of selection- and detection/performance bias from the start to end of each trial. The new criteria (Table 1) differed from those previously used in the first review [11]. It has been argued that the inclusion of bias-preventing measures (e.g. randomisation, blinding/masking) into the trial methodology only demonstrates an attempt to reduce bias risk but does not carry proof in itself that such attempt was indeed successful and that it is far more important to judge trial quality according to evidence that indicates to what extent such attempt has succeeded [13]. Against this background the quality criteria were adjusted accordingly. For example; if a trial merely reported that randomisation was conducted; reported only the name of the randomisation method used or included a detailed description of the randomisation process without providing any evidence that randomisation was indeed effective throughout the trial; then this was regarded as inadequate.
Potential attrition- and publication bias was not investigated in the original meta-analysis [11]. In this update, sensitivity analysis was done, using the RevMan Version 4.2 statistical software of The Nordic Cochrane Centre, The Cochrane Collaboration (Copenhagen; 2003), in order to investigate potential attrition bias risk in trials. To investigate publication bias a funnel plot was generated, using the datasets from the included clinical trials. The standard error (SE) of the mean differences was plotted on the Y-axis, and the log of the Relative Risk (RR) on the X-axis, using MIX Version 1.7 meta-analysis software [17]. In addition, Egger's linear regression method [18] was used to calculate an intercept with a 95% Confidence Interval (CI), with statistical significance set at α = 0.05.
We anticipated that many of the identified trials would be of split-mouth design. The split-mouth study design is commonly used in dentistry to test interventions and has the advantage of having an individual serve as both the experiment and control. In this study design, one or more pairs of teeth (e.g. primary molars) form the unit of randomisation. Strictly, these pairs are not independent and should be analysed as "paired data" on a patient basis. However, similar to other systematic reviews where split-mouth trials are included [2] we analysed the pairs of tooth surfaces independently for differences whether they are carious or not. The fact that this would result in slightly narrower confidence intervals was considered in the discussion of the overall results.
Data extraction and analysis
All data concerning primary and secondary outcomes of accepted trials were extracted either as single dichotomous datasets (containing the number of observed effects (n) and the total number of evaluations (N) for both the control and the test groups) or as single continuous datasets (containing the mean value, standard deviation and total number of evaluations for both the control and the test groups). For dichotomous datasets the Relative Risk (RR, 95% CI), and for continuous datasets the Mean Difference (MD, 95% CI), was computed, using the Cochrane RevMan, Version 4.2 software package. Statistical significance was set at α = 0.05.
Meta-analysis, using RevMan Version 4.2 statistical software by The Nordic Cochrane Centre, The Cochrane Collaboration (Copenhagen; 2003), was considered for datasets only if they complied with criteria for clinical homogeneity. Datasets were considered to be clinically homogeneous if they covered the same type of dentition; type of teeth; study length; type of evaluation method; type of GIC (high- or low viscosity) and stated whether the resin-based fissure sealant material contained fluoride. The percentage of total variations across datasets (I2) was used in assessing statistical heterogeneity [19]. Statistical significance for assessing statistical heterogeneity was set at α = 0.10. A fixed effects model was used for meta-analysis under condition of statistical homogeneity of datasets (p > 0.10) and a random-effects model was used for the others. Pooled datasets were assigned a Mantel-Haenszel weight directly proportionate to the sample size.
Cumulative meta-analysis, using MIX Version 1.7 meta-analysis software [17], was performed on datasets of consecutive follow-up periods, in order to investigate whether a trend of the available evidence might be observed in line with increasing time after sealant application. Care was taken to select only clinically homogenous datasets. A random-effects model was used under condition of statistical heterogeneity of datasets (p < 0.10).
Results
Literature search
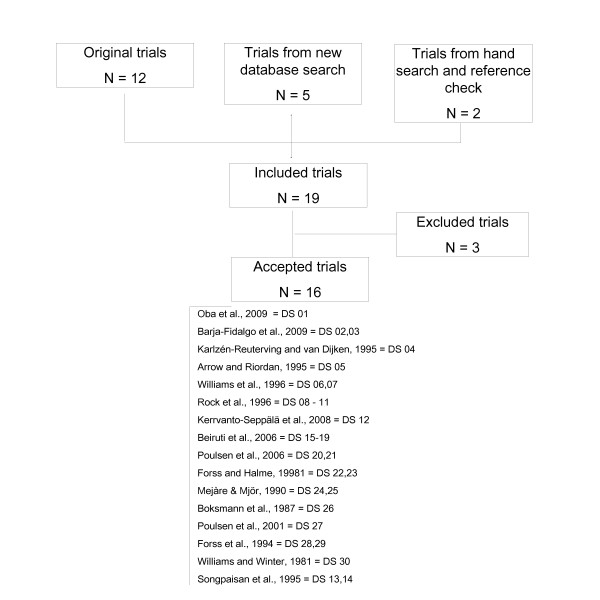
In addition to the 12 trials included during the original systematic review [20-31], five trials [32-36] were identified during the new database search. A further two trials [37,38] from the hand search and reference check were included (Figure 1). Of these 19 trials, 3 were excluded [34-36], owing to lack of reported caries outcome [35] and reporting on RMGIC as a test material [34,36]. Sixteen trials passed the exclusion criteria and were accepted for data extraction and quality assessment [20-33,37,38].
Figure 1.
Flow diagram of trial selection. N = Number of trials; DS = Dataset number.
Dataset extraction and analysis
Twenty-six dichotomous (DS 01-12,15-28,30) and 4 continuous datasets (DS 13,14,23,29) were extracted from the 16 accepted trials. Characteristics of these trials and their datasets are shown in Table 2. The articles by Forss et al. [37] and Forss and Halme [28] reported each of different datasets from the same trial. Three of the accepted 16 trials followed a parallel group design [25,26,33], while all other trials were split-mouth studies.
Table 2.
Details of accepted trials
| Article | DS | Study design | GIC treatment group | Resin treatment group | Outcome measure | Evaluation | Dentition/Teeth/Restoration | Study period | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of material | BSL | N | n | LTF | Type of material | BSL | N | n | LTF | Criteria | Method | ||||||
| Dichotomous datasets | |||||||||||||||||
| Oba et al., 2009 [32] | 01 | SM | Ketac Molar | 91 | 56 | 6 | 35 | Fissurit F | 116 | 81 | 8 | 35 | Caries | Caries present | Visual | First permanent molars | 3 years |
| Barja-Fidalgo et al., 2009 [33] | 02 | PG | Fuji IX | 46 | 46 | 1 | 0 | Delton | 46 | 46 | 2 | 0 | Caries | Cavity that had clearly penetrated the dentin or if a radiolucency in dentin could be seen on the bitewing X- ray | Visual and X-Ray | First permanent molars | 6 months |
| 03 | 46 | 21 | 2 | 25 | 46 | 28 | 7 | 18 | 5 years | ||||||||
| Karlzén-Reuterving and van Dijken, 1995 [20] | 04 | SM | Fuji III | 74 | 72 | 1 | 2 | Delton | 74 | 72 | 3 | 2 | Caries | Caries present | Visual | First permanent molars | 3 years |
| Arrow and Riordan, 1995 [21] | 05 | SM | Ketac Fil | 465 | 412 | 6 | 53 | Delton | 465 | 412 | 31 | 53 | Caries | When a cavity was present | Visual | First permanent molars | Mean 3.64 (SD 0.11) years |
| Williams et al., 1996 [22] | 06 | SM | Fuji III | 430 | 295 | 19 | 135 | Delton | 430 | 295 | 4 | 135 | Caries | Caries present | Visual | First permanent molars | 2 years |
| 07 | 430 | 222 | 22 | 208 | 430 | 222 | 16 | 208 | 4 years | ||||||||
| Rock et al., 1996 [23] | 08 | SM | Baseline | 172 | 160 | 3 | 12 | Fluoro- Shield | 172 | 162 | 0 | 10 | Caries | Caries present | Visual | First permanent molars | 6 months |
| 09 | 172 | 157 | 6 | 15 | 172 | 158 | 1 | 14 | 1 year | ||||||||
| 10 | 172 | 130 | 14 | 42 | 172 | 132 | 2 | 40 | 2 years | ||||||||
| 11 | 172 | 124 | 18 | 48 | 172 | 129 | 3 | 43 | 3 years | ||||||||
| Kerrvanto- Seppälä et al., 2008 [24] | 12 | SM | Fuji III | 1025 | 657 | 27 | 368 | Delton | 1025 | 657 | 7 | 368 | Caries | If dentine caries was detected | Visual | 2nd permanent molars | 3 years |
| Beiruti et al., 2006 | 15 | PG | Fuji IX | 180 | 180 | 0 | 0 | Visio Seal | 180 | 180 | 1 | 0 | Caries | Caries present | Visual | First permanent molars | 1 year |
| [26] | 16 | 180 | 154 | 0 | 26 | 180 | 161 | 6 | 19 | 2 years | |||||||
| 17 | 180 | 154 | 3 | 26 | 180 | 138 | 13 | 42 | 3 years | ||||||||
| 18 | 180 | 143 | 7 | 37 | 180 | 123 | 21 | 57 | 4 years | ||||||||
| 19 | 180 | 80 | 8 | 100 | 180 | 76 | 27 | 104 | 5 years | ||||||||
| Poulsen et al., 2006 [27] | 20 | SM | Fuji III | 364 | 364 | 34 | 0 | Delton | 364 | 364 | 10 | 0 | Radiograp-hically carious | Lesions into dentine | X-Ray | First permanent molars | 2.3 - 3.2 years |
| 21 | 364 | 364 | 23 | 0 | 364 | 364 | 10 | 0 | Clinically carious | Danish municipal dental service criteria | Visual | ||||||
| Forss and Halme, 19981 [28] | 22 | SM | Fuji III | 166 | 97 | 23 | 69 | Delton | 166 | 97 | 16 | 69 | Caries | Caries lesion present/Arrested caries present | Visual | Permanent molars/premolars | 7 years |
| Mejàre and Mjör, 1990 [29] | 24 | SM | Fuji III | 44 | 36 | 0 | 8 | Delton | 117 | 75 | 6 | 42 | Caries | Caries present | Visual | Permanent molars/premolars | 5 years |
| 25 | 44 | 36 | 0 | 8 | Concise | 47 | 18 | 2 | 29 | ||||||||
| Boksmann et al., 1987 [30] | 26 | SM | Fuji III | 125 | 116 | 0 | 9 | Concise | 122 | 115 | 0 | 7 | Caries | Caries present | Visual | Permanent molars | 6 months |
| Poulsen et al., 2001 [31] | 27 | SM | Fuji III | 170 | 116 | 44 | 54 | Delton | 170 | 116 | 13 | 54 | Caries | White, yellow, brown discoloration of the fissure or cavity | Visual | First permanent molars | 3 years |
| Forss et al., 19941 [37] | 28 | SM | Fuji III | 166 | 151 | 7 | 15 | Delton | 166 | 151 | 7 | 15 | Caries | Caries lesion present/Arrested caries present | Visual | Permanent molars/premolars | 2 years |
| Williams and Winter, 1981 [38] | 30 | SM | ASPA | No info | 4862 | 642 | No info | Concise | No info | 4862 | 932 | No info | Caries | Caries present | Visual | First permanent molars | 3.84 years |
| Article | DS | Continuous datasets | |||||||||||||||
| Patient character-istics/potential confounders* | Type of material | N | x | SD | LTF | Type of material | N | x | SD | LTF | Outcome measure | Criteria | Method | Dentition/Teeth/Restoration | Study period | ||
| Songpaisan et al., 1995 [25] | 13 | PG | Fuji III | 128 | 0.48 | 1.03 | 14% | Delton | 133 | 0.05 | 0.57 | 14% | DFS increment | DFS | Visual | Permanent molars | 2 years |
| 14 | 128 | 1.82 | 2.60 | 133 | 0.98 | 1.72 | DMFS increment | DMFS | |||||||||
| Forss and Halme, 19981 [28] | 23 | SM | Fuji III | 97 | 0.13 | 0.40 | 69 | Delton | 97 | 0.13 | 0.37 | 69 | Caries increment on approximal tooth surfaces adjacent to materials | Caries lesion present/Arrested caries present | Visual | Permanent molars/premolars | 7 years |
| Forss et al., 19941 [37] | 29 | SM | Fuji III | 151 | 0.09 | 0.34 | 15 | Delton | 151 | 0.13 | 0.41 | 15 | 2 years | ||||
| Article | Place of trial | Age of patients | Patient characteristics/inclusion/exclusion criteria | Fluoride exposure from other sources | |||||||||||||
| Oba et al., 2009 [32] | Study conducted in a boarding school in the city of Kırıkkale/Turkey | 7-11 years | Children received instructions on good oral health care and were individually shown how to clean their teeth prior to the start of the treatment; inclusion criteria were: (1) sound pits and fissures in fully erupted first molars; and (2) pits and fissures diagnosed with an early enamel lesion; exclusion criteria were: (1) partly erupted first molar; (2) an obvious cavity in the occlusal surface; and (3) the presence of a restoration or a sealant (or part of it) in the pit and fissure system; | Resin-based fissure sealant material containing fluoride | |||||||||||||
| Barja-Fidalgo et al., 2009 [33] | Study carried out in the Department of Paediatric Dentistry, Rio de Janeiro State University, Rio de Janeiro/Brazil; | mean age 6.8 years (±0.98 SD) | With at least 1 permanent first molar erupted and 2 or more primary molars decayed, filled, or extracted due to caries; most from low socio-economic background with high caries-risk; the participants were also given oral hygiene instructions and dietary counselling; those who had dental care needs were referred to the paediatric dental clinic for appropriate restorative and surgical treatment; participants reported brushing their teeth daily, 11% reported using dental floss regularly, and 67% had a dental check-up once a year first molars that presented a sound occlusal surface or occlusal caries at the D1 level (non-cavitated enamel lesion) entered the study; low patient compliance and high saliva contamination during treatment reported. | - | |||||||||||||
| Karlzén-Reuterving and van Dijken, 1995 [20] | Children from Umea/Sweden | mean age 7 years, 1 month | Teeth without clinical evidence of caries were sealed | - | |||||||||||||
| Arrow and Riordan, 1995 [21] | Children from Perth/Australia | mean age 7 (SD 0.72) years | With sound, unsealed, homologous 1st permanent molars | - | |||||||||||||
| Williams et al., 1996 [22] | Children from Suffolk/UK | 6-8 years | Recently erupted visually caries free 1st permanent molars; resealed teeth were excluded | Fluoride concentration of drinking water 0.1 - 0.5 mgF/l | |||||||||||||
| Rock et al., 1996 [23] | Children from Tamworth, Staffordshire/UK | 7-8 years | Caries free fully erupted 1st permanent molars; children had evidence of caries in primary teeth | The resin-based sealant FluroShield contains fluoride; fluoride concentration of drinking water 0.13 ppm | |||||||||||||
| Kerrvanto- Seppälä et al., 2008 [24] | Children from Varkaus/Finland | 12-16 years | 2nd permanent molars considered to be at risk of caries were sealed; teeth with lost resin sealant were resealed and not excluded from the study. | - | |||||||||||||
| Beiruti et al., 2006 [26] | Children from Damascus/Syria | mean age 7.8 years | No cavities in primary teeth; inclusion criteria: sound pits and fissures in fully erupted 1st permanent molars, pits and fissures with early enamel lesion and/or small dentinal lesion; exclusion criteria: partly erupted tooth, obvious occlusal cavity, presence of restoration or sealant in pits and fissures. | - | |||||||||||||
| Poulsen et al., 2006 [27] | Children from Vaerlose/Denmark | 8-13 years | Mean DMFS was between 0.5 and 0.7 for 12-year-old children and between 1.4 and 1.8 for 13-year-old children; sound surfaces, and surfaces with initial or arrested caries (white or brown fissures) were sealed, if the dentist's clinical assessment indicated a caries risk; only children with both clinical and radiographic data were included in the present study | Fluoride content of the drinking water 0.25 ppm; all children commonly use fluoridated toothpaste | |||||||||||||
| Forss and Halme, 2006 [28] | Children from Raisio/Finland | 5-14 years | Contra-lateral pair of newly erupted non-sealed permanent molars or premolars | - | |||||||||||||
| Mejàre and Mjör, 1990 [29] | - | mean age 9.2 (5.7 - 15.0) years | - | - | |||||||||||||
| Boksmann et al., 1987 [30] | - | 6-18 years | Patients had not received topical fluoride treatment for at least 3 months prior. | Fluoride content of the drinking water 1 ppm or more | |||||||||||||
| Poulsen et al., 2001 [31] | Children from Damascus/Syria | 7 years | Only children with at least one pair of caries free permanent 1st molars or with incipient lesions; average DMFT 0.6 -0.7. | - | |||||||||||||
| Forss et al., 1994 [37] | Children from Raisio/Finland | 5-14 years | Contra-lateral pair of newly erupted non-sealed permanent molars or premolars | - | |||||||||||||
| Williams and Winter, 1981 [38] | - | 6-8 and 11-13 years | - | - | |||||||||||||
| Songpaisan et al., 1995 [25] | Children from Bangkok/Thailand | 12-13 years | From very low to medium socio-economic background; children with at least 3 sound permanent molars (erupted sufficiently) | Included in a fluoride mouth rinse programme (0.2% NaF) every 2 weeks; fluoride concentration of drinking water 0.1 - 0.2 ppm | |||||||||||||
DS = Dataset number; BSL = Number of teeth at baseline; N = Number of teeth evaluated; n = Number of teeth with caries, LTF = Loss-to-follow-up; x = Mean; SD = Standard deviation; PG = Parallel group design; SM = Split-mouth design.
1 Different datasets reported from same trial
2 Number of pits reported, instead of the number of sealed teeth
* Potential confounders = Reported fluoride exposure; high-sugary diet; poor oral hygiene; high past caries experience.
Sixteen of the 30 datasets showed no difference between the two materials after periods lasting from 6 months to 7 years (Table 2 and 3). Twelve dichotomous (DS 05, 10,11,17-21,27,30) and two continuous datasets (DS 13,14) showed statistically significant (p < 0.05) different results. Of these, seven dichotomous datasets (DS 06,10-12,20,21,27) extracted from five trials [22-24,27,31] were in favour of resin-based fissure sealants after 2 to 3 years. Five dichotomous datasets (DS 05,17-19,30) extracted from three trials [21,26,38] were in favour of GIC-based fissure sealants after 3 to 5 years. Two continuous datasets (DS 13,14) from one trial [25] reporting on secondary outcomes, such as the DFS and DMFS increment, were also in favour of resin-based fissure sealants after 2 years.
Table 3.
Results of individual datasets
| Article | DS | Dichotomous datasets | ||
|---|---|---|---|---|
| RR | 95% CI | p-value | ||
| Oba et al., 2009 [32] | 01 | 1.08 | 0.40 - 2.96 | 0.87 |
| Barja-Fidalgo et al., 2009 [33] | 02 | 0.50 | 0.05 - 5.32 | 0.57 |
| 03 | 0.38 | 0.09 - 1.65 | 0.20 | |
| Karlzén-Reuterving and van Dijken, 1995 [20] | 04 | 0.33 | 0.04 - 3.13 | 0.34 |
| Arrow and Riordan, 1995 [21] | 05 | 0.19 | 0.08 - 0.46 | 0.0002* |
| Williams et al., 1996 [22] | 06 | 4.75 | 1.64 - 13.79 | 0.004** |
| 07 | 1.38 | 0.74 - 2.55 | 0.31 | |
| Rock et al., 1996 [23] | 08 | 7.09 | 0.37 - 136.11 | 0.19 |
| 09 | 6.04 | 0.74 - 49.58 | 0.09 | |
| 10 | 7.11 | 1.65 - 30.66 | 0.009** | |
| 11 | 6.24 | 1.89 - 20.66 | 0.003** | |
| Kerrvanto- Seppälä et al., 2008 [24] | 12 | 3.86 | 1.69 - 8.79 | 0.001** |
| Beiruti et al., 2006 [26] | 15 | 0.33 | 0.01 - 8.13 | 0.50 |
| 16 | 0.08 | 0.00 - 1.42 | 0.08 | |
| 17 | 0.21 | 0.06 - 0.71 | 0.01* | |
| 18 | 0.29 | 0.13 - 0.65 | 0.003* | |
| 19 | 0.28 | 0.14 - 0.58 | 0.0006* | |
| Poulsen et al., 2006 [27] | 20 | 3.40 | 1.71 - 6.78 | 0.0005** |
| 21 | 2.30 | 1.11 - 4.76 | 0.02** | |
| Forss and Halme, 19981 [28] | 22 | 1.44 | 0.81 - 2.55 | 0.21 |
| Mejàre and Mjör, 1990 [29] | 24 | 0.16 | 0.01 - 2.73 | 0.20 |
| 25 | 0.10 | 0.01 - 2.03 | 0.14 | |
| Boksmann et al., 1987 [30] | 26 | Not estimable | ||
| Poulsen et al., 2001 [31] | 27 | 3.38 | 1.93 - 5.94 | <0.0001** |
| Forss et al., 19941 [37] | 28 | Not estimable | ||
| Williams and Winter, 1981 [38] | 30 | 0.69 | 0.51 - 0.92 | 0.01* |
| Article | DS | Continuous datasets | ||
| MD | 95% CI | p-value | ||
| Songpaisan et al., 1995 [25] | 13 | 0.43 | 0.23, 0.63 | <0.0001** |
| 14 | 0.84 | 0.30, 1.38 | 0.002** | |
| Forss and Halme, 19981 [28] | 23 | 0.00 | -0.11, 0.11 | 1.00 |
| Forss et al., 19941 [37] | 29 | -0.04 | -0.12, 0.04 | 0.36 |
DS = Dataset number; RR = Relative risk; MD = Mean difference; CI = Confidence interval; Not estimable = data from both treatment groups are essentially the same: p = 1.00.
* Statistically significant difference, in favour of GIC
** Statistically significant difference, in favour of Resin
1 Different datasets reported from same trial
Three of the datasets with statistically significant results (DS 17-19) were derived from one trial including high-viscosity GIC [26].
Meta-analysis
Of the 30 datasets, two groups of datasets, DS 04,21,27 after 3 years and DS 05,07 after 4 years, were considered to have each met the criteria for clinical homogeneity and were combined in two meta-analyses. The results were generated in the form of two forest plots (Figure 2 and 3). These datasets included first permanent molars sealed either with low-viscosity GIC or with resin material lacking fluoride and were evaluated by visual, clinical examination.
Figure 2.
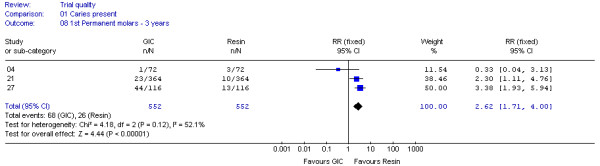
Forrest plot of meta-analysis results concerning caries on sealed first permanent molars after 3 years. Study or sub-category = Dataset number; GIC = Glass-ionomer cement; RR = Relative Risk; CI = Confidence Interval; n = Number of teeth with caries; N = Total number of evaluated teeth.
Figure 3.
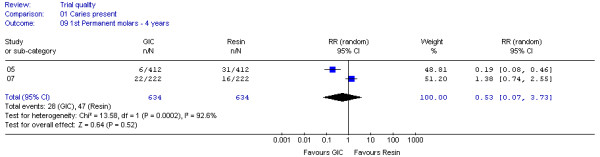
Forrest plot of meta-analysis results concerning caries on sealed first permanent molars after 4 years. Study or sub-category = Dataset number; GIC = Glass-ionomer cement; RR = Relative Risk; CI = Confidence Interval; n = Number of teeth with caries; N = Total number of evaluated teeth.
Figure 2 shows a pooled relative risk of 2.62 (95% CI: 1.71 - 4.00; p < 0.00001), suggesting a 2-3 times higher chance of caries for teeth sealed with low-viscosity GIC than for those filled with resin, after 3 years. Additional analysis established a low risk of statistical heterogeneity (I2 = 56.7%, p = 0.13). For that reason a fixed-effects model was used for this meta-analysis.
Figure 3 shows a pooled relative risk of 0.53 (95% CI: 0.07 - 3.73; p = 0.52), suggesting no difference between the chance of caries development in teeth sealed with low-viscosity GIC or resin after 4 years. Additional analysis established a high risk of statistical heterogeneity (I2 = 92.8%, p = 0.0002). For that reason a random-effects model was used for this meta-analysis. The significant statistical heterogeneity may be related to the inconsistency in the size of the treatment effects as the trials from which both datasets were extracted were similar in type of materials, sample size, outcome measure, evaluation criteria and method, as well as type of dentition, teeth, age of patients and study period (Table 2). Further investigation, by using the available trial information, did not result in any other possible explanation.
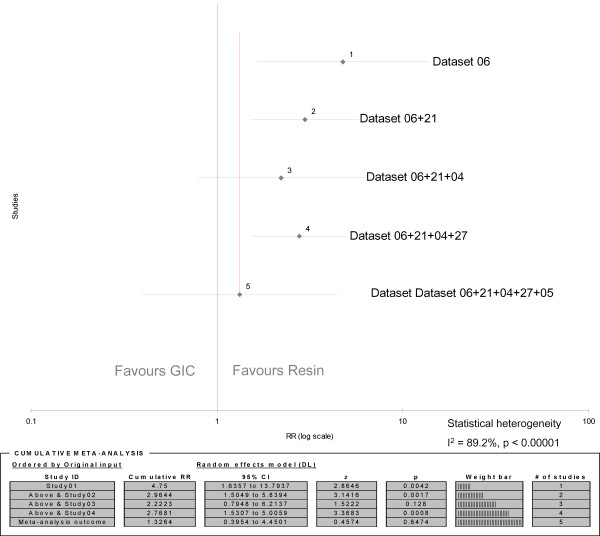
Cumulative meta-analysis
In order to investigate whether a possible trend may be assumed in the comparison of GIC and resin, the chronological results from 5 datasets, concerning sealed teeth after 2 years (DS 06), 2-3 years (DS 21), 3 years (DS 04,27) and 3.64 years (DS 05) [20-22,27,31], were included in a cumulative meta-analysis and its results were generated in the form of a forest plot (Figure 4). As with both meta-analyses (Figure 2 and 3), all datasets included first permanent molars sealed either with low-viscosity GIC or resin material without fluoride and were evaluated by visual, clinical examination. They were thus considered clinically homogenous in all aspects except their follow-up periods. However, a statistical heterogeneity was established (I2 = 89.2%; p < 0.00001) that may be attributed to the differences in the length of follow-up periods, so a random-effects model was used. The cumulative Relative Risk indicates no statistical significant difference between the two materials after 5 years (RR 1.33; 95% CI: 0.39 - 4.45; p = 0.65). A shift of the cumulative relative risks towards the value of 1.00, as well as a shift of the cumulative 95% confidence intervals below a relative risk of 1.00 from the period after 2 to 3.64 years can be observed (Figure 4).
Figure 4.
Forrest plot of cumulative meta-analysis results concerning caries on sealed first permanent molars. RR = Relative Risk
Quality assessment of trials and risk of bias
Selection-, Detection-/Performance bias risk
The results of the quality assessment regarding selection- and detection/performance bias are shown in Table 4. None of the accepted trials reported sufficient details of any randomisation process that had indeed given each patient the same chance to be allocated to either the GIC or the resin group and to ensure that direct observation and prediction of the allocation sequences was successfully prevented. Only three trials [25,26,33] had reported baseline data collected before randomisation and reported for both treatment groups, statistically compared this data between groups and found the difference statistically not significant (p > 0.05). No accepted trial reported on successful blinding/masking of patients, operators and trial evaluators.
Table 4.
Results of quality assessment of accepted trials
| Article | DS | Selection bias | Detection/Performance bias | Attrition bias | Trial outcome | ||
|---|---|---|---|---|---|---|---|
| Randomization | Baseline data | Blinding/Masking | Loss-to-follow up | Run-in phase | |||
| Oba et al., 2009 [32] | 01 | 0 | 0 | 0 | A | A | A |
| Barja-Fidalgo et al., 2009 [33] | 02 | 0 | A | 0 | A | A | A |
| 03 | 0 | A | 0 | A | A | A | |
| Karlzén-Reuterving and van Dijken, 1995 [20] | 04 | 0 | 0 | 0 | A | A | A |
| Arrow and Riordan, 1995 [21] | 05 | 0 | 0 | 0 | A | A | A |
| Williams et al., 1996 [22] | 06 | 0 | 0 | 0 | B | A | A |
| 07 | 0 | 0 | 0 | A | A | A | |
| Rock et al., 1996 [23] | 08 | 0 | 0 | 0 | A | A | A |
| 09 | 0 | 0 | 0 | A | A | A | |
| 10 | 0 | 0 | 0 | B | A | A | |
| 11 | 0 | 0 | 0 | A | A | A | |
| Kerrvanto-Seppälä et al., 2008 [24] | 12 | 0 | 0 | 0 | B | A | A |
| Beiruti et al., 2006 [26] | 15 | 0 | A | 0 | A | A | A |
| 16 | 0 | A | 0 | A | A | A | |
| 17 | 0 | A | 0 | B | A | A | |
| 18 | 0 | A | 0 | B | A | A | |
| 19 | 0 | A | 0 | A | A | A | |
| Poulsen et al., 2006 [27] | 20 | 0 | 0 | 0 | A | A | A |
| 21 | 0 | 0 | 0 | A | A | A | |
| Forss and Halme, 19981 [28] | 22 | 0 | 0 | 0 | A | A | A |
| Mejàre and Mjör, 1990 [29] | 24 | 0 | 0 | 0 | B | A | A |
| 25 | 0 | 0 | 0 | B | A | A | |
| Boksmann et al., 1987 [30] | 26 | 0 | 0 | 0 | A | A | A |
| Poulsen et al., 2001 [31] | 27 | 0 | 0 | 0 | A | A | A |
| Forss et al., 19941 [37] | 28 | 0 | 0 | 0 | A | A | A |
| Williams and Winter, 1981 [38] | 30 | 0 | 0 | 0 | 0 | A | A |
| Songpaisan et al., 1995 [25] | 13 | 0 | A | 0 | n.e. | A | 0 |
| 14 | 0 | A | 0 | n.e. | A | 0 | |
| Forss and Halme, 19981 [28] | 23 | 0 | 0 | 0 | n.e. | A | 0 |
| Forss et al., 19941 [37] | 29 | 0 | 0 | 0 | n.e. | A | 0 |
DS = Dataset number, n.e. = Not evaluated.
1 Different datasets reported from same trial
Attrition bias risk
Sensitivity analysis was used in computing all datasets, under the assumption that either:
(i) All pits and fissures of sealed teeth lost to follow-up developed caries;
(ii) None of the sealed teeth lost to follow-up developed caries.
The results of either situation did not change the conclusions for the majority of the datasets. However, a possible risk of attrition bias was identified for the results of four datasets (DS 06,12,24,25) extracted from three trials [22,24,29]. Under the assumption that all pits and fissures of sealed teeth lost to follow-up developed caries, the results of two datasets (DS 06,12) would not be statistically significantly in favour of resin: DS 06 - RR 1.11 (95% CI: 0.92 - 1.33; p = 0.28); DS 12 - RR 1.05 (95% CI: 0.94 - 1.18; p = 0.36) and the results of two datasets (DS 24,25) would be statistically significantly in favour of GIC: DS 24 - RR 0.44 (95% CI: 0.23 - 0.86; p = 0.02); DS 25 - RR 0.28 (95% CI: 0.14 - 0.53; p = 0.0001).
In line with the potential influence of attrition bias on datasets, the meta-analysis results (Figure 2 and 3) were not affected.
Under the assumption that all pits and fissures of sealed teeth lost to follow-up developed caries, the results of the cumulative meta-analysis (Figure 4) would only change towards a further shift of the cumulative relative risks towards the value of 1.00, and in a narrowing of the cumulative 95% confidence interval after 3.64 years (RR 1.14 - 95% CI: 0.081 - 1.60; p = 0.46).
In addition to the risk of bias due to loss-to-follow up, no trial indicated that a run-in phase was implemented before randomisation (Table 4).
Publication bias risk
Publication bias was investigated, using one funnel plot (Figure 5). The funnel plot concerning data for caries progression showed an even distribution that did not suggest publication bias. Egger's linear regression method for the same datasets showed an intercept of 0.05 (95% CI: -1.74, 1.85; p = 0.95). The regression result was not statistically significant.
Figure 5.
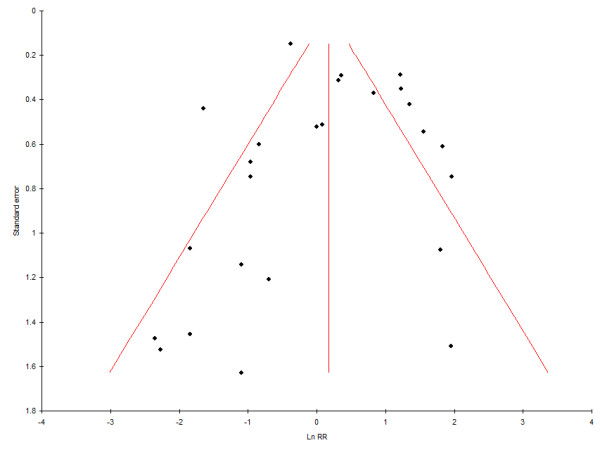
Funnel plot of dataset results (test for publication bias). RR = Relative Risk
Unlike the original systematic review, non-English language articles were not included in this partial update and this needs to be addressed in a future update, including not only those in Portuguese and Spanish but in other languages too.
Discussion
The aim of this article was to update the existing evidence from trials published in English language regarding the review question as to whether caries occurrence on pits and fissures of teeth sealed with either GIC or resin is the same. The article constitutes a partial update of the original systematic review evidence with primary focus on research quality in regard to bias risk in trials.
During the new systematic literature search seven more trials [32-38] could be included for review. The reason for this was that five new trials had been published since the cut-off date of the original search; a more thorough hand search and reference check of the literature had been done, and broader inclusion criteria were used. Of the seven new included trials, three were excluded [34-36] as they did not comply with the stated exclusion criteria.
In comparison to the original published systematic review [11], this update presents an improvement in the output of its systematic search of English trials. However, other aspects in the methodology of this review update might still have contributed to limitations in its results: (i) not all relevant publications were listed in the selected databases; (ii) The chosen search terms may not have been broad enough; (iii) not all relevant publications could be found through a hand search and reference check. In addition and as this update is limited to English language trials, only, a future update of the non-English evidence of the original systematic review is required. Although, the inclusion of non-English language trials in the original systematic review did not decisively influence the overall review results [11] an update of the non-English evidence of the original systematic review would confirm (i) whether the new findings from non-English trials substantially differ from the English language trials and (ii) whether results from any new non-English language trials would influence the overall review results.
Selection-, Detection-/Performance bias risk
Of the accepted 16 trials, only 3 trials followed a parallel group design [25,26,33] and all other were split-mouth studies. Mejáre et al. have cautioned against the split-mouth design as "randomised", as the common practice of including subjects with at least one pair of caries-free molars results in exclusion of caries-active subjects [39]. An obvious selection bias is thus created, as not all subjects will have the same chance to participate. Mejáre et al. have rightfully suggested that the split-mouth trial design should therefore be regarded as "quasi-randomised". Thus, reviews where inclusion criteria include only randomised-control trials should, in theory, exclude trials that use the split-mouth study design. Following the example of other systematic reviews [2], the data from split-mouth trials was analysed as independent data. This will have caused narrower confidence intervals and thus may have favoured the reported outcomes of one type of treatment above the other. However, the so caused differences in confidence intervals may be considered to only be slight [2] and its correction would not have affected the general impact of selection bias on the relative risk (RR, 95% CI) per dataset (Table 3) due to lack of adequate randomisation [39-41]. In addition, wider confidence intervals would have provided only stronger indication of no difference between both types of treatment and would not have changed the overall conclusion from the current analysis results.
All of the accepted trials appear to be limited by risk of selection- and detection-/performance bias. Bias or systematic error may affect studies, causing either an over- or under-estimation of the treatment effect of an investigated clinical procedure. Overestimation has been observed to be the most common [40]. Kjaergard et al. reported a treatment effect overestimation of 48% caused by lack of random sequence allocation [41] and Egger et al. reported a treatment effect overestimation of 54% and 53% due to lack of allocation concealment and lack of evaluator blinding, respectively [42].
It has been emphasized that selection bias can only be successfully prevented if the allocation sequence remains truly random and free from potential interference throughout the trial [13,14]. Thus, it is important that trials should include an effective process for concealing the random allocation sequence and that by the end of each trial this process has indeed prevented direct observation and prediction of the random sequence allocation [13,14]. Quality assessment in terms of the internal validity of trials should therefore be a measure of the result of random sequence allocation and allocation concealment, and not only of its reported attempt. All trials accepted in this systematic review failed to report not only on evidence of successful sequence allocation and allocation concealment results, but also on necessary details about how sequence allocation and allocation concealment were attempted (Table 4). None of the trials, therefore, provide any guarantee that each patient had an equal chance of being allocated to either treatment group and thus, their allocation may have favoured the outcome of one type of treatment above the other. One measure for testing whether random sequence allocation has not been successful is testing whether covariates differ between treatment groups at baseline [13]. Only three articles had included such a test and reported on its outcome [25,26,33]. The statistically non-significant results (p > 0.05) suggest a successful random allocation (Table 4). However, doubt remains regarding potential bias risk, as other non-balanced covariates may exist, that were not tested for and/or not reported.
From the onset, in all trials successful blinding or masking appeared not to have been possible, owing to the obvious differences in clinical appearance between GIC and resin sealants. For that reason the allocation to either treatment group was visible to patients, operators and evaluators. However, the difficulties of successful blinding still carry the danger of detection-/performance bias, which may thus have affected the trials' results. Potential knowledge of superiority claims prior to the trial may have led patients to change their oral hygiene habits, operators to place restorations more carefully or evaluators to apply evaluation criteria more subjectively. This in turn may have favoured the outcome of one type of treatment over the other.
Attrition bias risk
Sensitivity analysis may be used in establishing whether missing data could have affected trial outcomes by assuming that the numbers of restoration lost to evaluation were either failures or successes [43]. Comparison of the analysis results with reported trial outcomes indicates whether different conclusions should be drawn. Sensitivity analysis was conducted for all datasets. The analysis results differed from reported outcomes of four datasets (DS 06,12,24,25) extracted from three trials [22,24,29]. According to the analysis results, more datasets (DS 24,25) would have been in favour of GIC and fewer in favour of resin if all sealed teeth that were lost to follow-up were assumed to have developed caries in their pits and fissures. How high the caries rate in the teeth lost to evaluation really was remains unknown. Owing to uncertainty regarding the real caries prevalence within those lost to follow-up, the results of the sensitivity analysis cannot serve as evidence that GIC would perform better than resin. However, the validity of the four datasets (DS 06,12,24,25) can be questioned on grounds of attrition bias and thus, their results need to be regarded with caution.
None of these datasets were included into the meta-analyses (Figure 2 and 3) and thus do not affect their results. However, one dataset (DS 06) was also included in the cumulative meta-analysis (Figure 4). A re-computation of the data including all loss-to-follow-up (under assumption of caries) did not change the initially observed trend. Rather, the trend was reinforced, as the change from a cumulative RR after 3.65 years of 1.33 to 1.14 would suggest.
A run-in phase is considered to be a stage during a trial where all patients receive, for example, the test treatment and only those patients that respond well to the treatment are later used for random allocation in either the control or the test group [14]. Such practice would effectively exclude patients from the randomisation process and potentially favour one of the groups. No such run-in phase was indicated in any of the accepted trials (Table 4).
Publication bias risk
Publication bias was investigated by generating a funnel plot (Figure 5). Publication bias is present when the results of published research differ from those of all the studies that have been done [44]. Funnel plots are scatter graphs showing the size of studies on the Y-axis (large studies on top; small studies at the bottom) and the effect size, observed in these studies, on the X-axis. The effect sizes of larger studies have the tendency to cluster near the mean. Small studies have effect sizes that are dispersed across a wider range. Results of both types of studies, plotted on a scatter graph, give the shape of an inverted, in absence of publication bias, symmetrical funnel [45]. Publication bias affects a funnel plot in the form of a concentration of studies to one side only (asymmetry). Such asymmetry is created when particular smaller studies are published only when they show a larger than average effect. However, if the number of studies (n) is less than ten, any asymmetry may be due to chance and not to publication bias [46]. For that reason the decision was made to plot results of the 26 extracted dichotomous datasets as units of investigation. These are not all independent from the published trials and this forms a departure from the common application of funnel plots in investigating for publication bias. Despite this departure, the use of datasets (instead of published trials) will also indicate potential publication bias when only datasets that show a larger than average effect are published and other datasets are not. In our update, the funnel plot concerning dichotomous data on caries progression showed a symmetrical spread of dataset results (Figure 5). As the visual judgement of funnel plots is subjective, we calculated intercepts (95% CI), using Eggers regression [18]. The calculated non-significant intercept confirmed the observations from the funnel plot. Both suggest that any potential impact of publication bias may be low in regard to this topic.
Data extraction and analysis results
The extended scope of this update did not change the overall results of the original published systematic review [11]. However, it has to be noted that these results are limited by risk of selection- and detection-/performance bias. As the true extent of such bias impact remains unknown within the reviewed trials, the results need to be regarded with caution.
Five of the accepted studies reported on fluoride exposure of subjects from drinking water [22,23,25,27,30], a fluoride-rinsing programme [25] and toothpaste [27]. Two trials compared GIC fissure sealants with resin materials that reportedly contained fluoride [23,32]. In addition, most of the trials followed a split-mouth design, providing the possibility of fluoride release from the GIC fissure sealants into the oral cavity. This may have provided a caries-preventive effect for the resin-sealed teeth. It can be assumed that this may have increased caries resistance and thus confounded a potential caries-preventive effect of GIC, as suggested by Hara et al. [47]. Notwithstanding any possible confounder impact, the overall results suggest that resin-based materials may be more caries-preventive after 2-3 years of sealant placement (Figure 2). Such benefit does not translate beyond the duration of 3 years as Figure 3, as well as the majority of datasets without statistically significant outcomes (Table 3), suggests. Other statistically significant outcomes in favour of resin from datasets (DS 06,12), not included in the meta-analysis, may be invalid because of attrition bias and datasets of non-significant outcomes (DS 24,25) could very well have shown statistically significant outcomes in favour of GIC if all studied teeth could have been evaluated at the end of the trials. All these observations suggest that weakness exists in any claims of resin superiority over GIC and further, rather suggest that the caries-preventive effects of both types of material are equal.
These considerations should be regarded as hypothetical, owing to the high risk of selection/detection/performance bias in all accepted trials. Plausibility for this conclusion is provided by the results of the included cumulative meta-analysis (Figure 4). Cumulative meta-analysis is used to provide insights into how much the efficacy of treatments, often reported as mean results with 95% confidence intervals, change over time as evidence accumulates [48]. It is the result of conducting a new meta-analysis each time a new set of evidence emerges [49]. A cumulative meta-analysis not only allows evaluation of any additional contributions made by individual studies to the cumulatively combined results of preceding studies [50] but also allows observation of a trend in the direction of evidence over time. The pooling of datasets of different study periods may be questioned on the grounds that their heterogeneity (due to differences in the length of observation periods) could render the result of a meta-analysis meaningless. On the other hand, it can be assumed that datasets covering longer study periods insert a larger weight into the cumulative results and thus generate a potential trend concerning treatment effects observed after increasing periods of time. The length of an observation period is related to the trial outcome as with its length also the length of, e.g. exposure to cariogenic factors increases. Thus the weight of trial outcomes after a longer observation period would be larger. Such period effect may be investigated through cumulative meta-analysis because it allows for the pooling of outcomes from (otherwise clinically homogeneous) trials after continuously increasing time periods. Should the period effect be stronger for one of the compared treatment types than the other, a trend in the direction of the cumulated point estimates and confidence intervals can be shown by use of a forest plot. Such cumulative results may not provide proof in support of a particular hypothesis due to prevailing statistical heterogeneity. However, the results may assist in providing plausibility for hypothesis development. The forest plot in Figure 4 shows a shift of the cumulative results towards the value of RR 1.00, thus indicating that 'no difference' between low-viscosity GIC and resin may be a more plausible hypothesis with increasing time after sealant application than the hypothesis that 'resin is superior to GIC'. Such hypothesis requires thorough testing through further high quality randomised control trials.
The results of two datasets (DS 17,19) from trials in favour of high-viscosity GIC after 3 and 5 years [26] could not be repeated in later trials [32,33]. However, the results of the latter two trials may not be comparable with the former, as Beiruti et al. did not report on annual results that were independent from those of each previous year in this trial [26].
Recommendations for further research
Systematic reviews have been reported to provide the highest form of clinical evidence [51]. However, the internal validity of such evidence can only be as good as the internal validity of the trials reviewed. Although the trials accepted in this update may be considered to be less affected by attrition- and publication bias, their risk of selection- and detection-/performance bias is high. Overestimation due to bias may have affected particularly those datasets that indicated a statistically significant difference between both material groups (Table 3) by increasing (or decreasing) the value of point estimates and by artificially narrowing their confidence intervals. However, the precise effect of bias on all results remains unknown. Thus the results need to be regarded with caution and require verification. For that reason, further high quality randomised control trials (RCT) are needed. Such RCTs should adopt a parallel group design and include randomisation and allocation concealment methods that can effectively prevent direct observation and prediction of the allocation sequence. For this purpose, the maximum randomisation method has been suggested [13]. Covariates of both treatment groups should be tested as to whether they differ at baseline (after randomisation). Recently, inclusion of the Berger-Exner test has been suggested, to enable authors of trials to investigate whether selection bias has been introduced into their studies [13,14]. Where bias risk has been found, it may be adjusted statistically [13]. Both outcomes should be included in the final trial report. In order to ensure that the lack of blinding may not have led to favouring one treatment over the other, trials should use and report on procedures and tests employed that may limit or at least monitor potential bias risk. In addition to these recommendations, future trials should base their reporting on the CONSORT statement [52].
Conclusion
In conclusion, the current evidence to this topic may be considered to be less affected by attrition- and publication bias. However, its risk of selection, detection and performance bias is high. Consequently, further high quality randomised control trials are needed in order to answer the question whether caries occurrence on pits and fissures of teeth sealed with either GIC or resin is the same, more conclusively.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
Both authors contributed equally to the systematic literature search, review, data extraction and to the writing of the manuscript. SM conducted the data analysis.
Contributor Information
Steffen Mickenautsch, Email: neem@global.co.za.
Veerasamy Yengopal, Email: Veerasamy.Yengopal@wits.ac.za.
Acknowledgements
The authors wish to thank Prof Paul Fatti from the School of Statistics & Actuarial Science, University of the Witwatersrand, Johannesburg, South Africa for his valuable advice during the writing of this paper.
References
- Locker D, Jokovic A, Kay EJ. Prevention. Part 8: The use of pit and fissure sealants in preventing caries in the permanent dentition of children. Br Dent J. 2003;195:375–378. doi: 10.1038/sj.bdj.4810556. [DOI] [PubMed] [Google Scholar]
- Ahovuo-Saloranta A, Hiiri A, Nordblad A, Mäkelä M, Worthington HV. Pit and fissure sealants for preventing dental decay in the permanent teeth of children and adolescents. Cochrane Database Syst Rev. 2008;4:CD001830. doi: 10.1002/14651858.CD001830.pub3. [DOI] [PubMed] [Google Scholar]
- Wendt LK, Koch G, Birkhed D. Long- term evaluation of a fissure-sealing programme in Public Dental Service clinics in Sweden. Swedish Dent J. 2001;25:61–65. [PubMed] [Google Scholar]
- Quiñonez RB, Downs SM, Shugars D, Christensen J, Vann WF Jr. Assessing cost-effectiveness of sealant placement in children. J Public Health Dent. 2005;65:82–89. doi: 10.1111/j.1752-7325.2005.tb02791.x. [DOI] [PubMed] [Google Scholar]
- Kitchens DH. The Economics of pit and fissure sealants in preventive dentistry: a review. J Contemp Dent Pract. 2005;6:95–103. [PubMed] [Google Scholar]
- Adair SM. The role of sealants in caries prevention programs. J Calif Dent Assoc. 2003;31:221–227. [PubMed] [Google Scholar]
- Feigal RJ. The use of pit and fissure sealants. Pediatr Dent. 2002;24:415–422. [PubMed] [Google Scholar]
- Simonsen RJ. Pit and fissure sealant: review of the literature. Pediatr Dent. 2002;24:393–414. [PubMed] [Google Scholar]
- Bishara SE, Oonsombat C, Ajlouni R, Denehy G. The effect of saliva contamination on shear bond strength of orthodontic brackets when using a self-etch primer. Angle Orthod. 2002;72:554–557. doi: 10.1043/0003-3219(2002)072<0554:TEOSCO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Smith DC. Development of glass-ionomer cement systems. Biomaterials. 1998;19:467–478. doi: 10.1016/S0142-9612(97)00126-9. [DOI] [PubMed] [Google Scholar]
- Yengopal V, Mickenautsch S, Bezerra AC, Leal SC. Caries-preventive effect of glass ionomer and resin-based fissure sealants on permanent teeth - a meta analysis. J Oral Sci. 2009;51:373–382. doi: 10.2334/josnusd.51.373. [DOI] [PubMed] [Google Scholar]
- Shojania KG, Sampson M, Ansari MT, Ji J, Doucette S, Moher D. How Quickly Do Systematic Reviews Go Out of Date? A Survival Analysis. Ann Intern Med. 2007;147:273–274. doi: 10.7326/0003-4819-147-4-200708210-00179. [DOI] [PubMed] [Google Scholar]
- Berger VW. Selection bias and covariate imbalances in randomised clinical trials. Chichester, UK: John Wiley & Sons, Ltd; 2005. [Google Scholar]
- Berger VW, Alperson SY. A general framework for the evaluation of clinical trial quality. Rev Recent Clin Trials. 2009;4:79–88. doi: 10.2174/157488709788186021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn S, Puffer S, Torgerson DJ, Watson J. Methodological bias in cluster randomised trials. BMC Med Res Methodol. 2005;5:10. doi: 10.1186/1471-2288-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jüni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ. 2001;323:42–46. doi: 10.1136/bmj.323.7303.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bax L, Yu LM, Ikeda N, Tsuruta H, Moons KGM. Development and validation of MIX: comprehensive free software for meta-analysis of causal research data. BMC Medical Research Methodology. 2006;6:50. doi: 10.1186/1471-2288-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SG. Why sources of heterogeneity in meta-analysis should be investigated. BMJ. 1994;309:1351–1355. doi: 10.1136/bmj.309.6965.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlzen-Reuterving G, van Dijken JW. A three-year follow-up of glass ionomer cement and resin fissure sealants. ASDC J Dent Child. 1995;62:108–110. [PubMed] [Google Scholar]
- Arrow P, Riordan PJ. Retention and caries preventive effects of a GIC and a resin-based fissure sealant. Community Dent Oral Epidemiol. 1995;23:282–285. doi: 10.1111/j.1600-0528.1995.tb00249.x. [DOI] [PubMed] [Google Scholar]
- Williams B, Laxton L, Holt RD, Winter GB. Fissure sealants: a 4-year clinical trial comparing an experimental glass polyalkenoate cement with a bis glycidyl methacrylate resin used as fissure sealant. Br Dent J. 1996;180:104–108. doi: 10.1038/sj.bdj.4808989. [DOI] [PubMed] [Google Scholar]
- Rock WP, Foulkes EE, Perry H, Smith AJ. A comparative study of fluoride-releasing composite resin and glass ionomer materials used as fissure sealants. J Dent. 1996;24:275–280. doi: 10.1016/0300-5712(95)00061-5. [DOI] [PubMed] [Google Scholar]
- Kervanto-Seppälä S, Lavonius E, Pietilä I, Pitkäniemi J, Meurman JH, Kerosuo E. Comparing the caries-preventive effect of two fissure sealing modalities in public health care: a single application of glass ionomer and a routine resin-based sealant programme. A randomized split-mouth clinical trial. Int J Paediatr Dent. 2008;18:56–61. doi: 10.1111/j.1365-263X.2007.00855.x. [DOI] [PubMed] [Google Scholar]
- Songpaisan Y, Bratthall D, Phantumvanit P, Somridhivej Y. Effects of glass ionomer cement, resin-based pit and fissure sealant and HF applications on occlusal caries in a developing country field trial. Community Dent Oral Epidemiol. 1995;23:25–29. doi: 10.1111/j.1600-0528.1995.tb00193.x. [DOI] [PubMed] [Google Scholar]
- Beiruti N, Frencken JE, van't Hof MA, Taifour D, van Palenstein Helderman WH. Caries-preventive effect of a one-time application of composite resin and glassionomer sealants after 5 years. Caries Res. 2006;40:52–59. doi: 10.1159/000088907. [DOI] [PubMed] [Google Scholar]
- Poulsen S, Laurberg L, Vaeth M, Jensen U, Haubek D. A field trial of resin-based and glass-ionomer fissure sealants: clinical and radiographic assessment of caries. Community Dent Oral Epidemiol. 2006;34:36–40. doi: 10.1111/j.1600-0528.2006.00248.x. [DOI] [PubMed] [Google Scholar]
- Forss H, Halme E. Retention of a glass ionomer cement and a resin-based fissure sealant and effect on carious outcome after 7 years. Community Dent Oral Epidemiol. 1998;26:21–25. doi: 10.1111/j.1600-0528.1998.tb01919.x. [DOI] [PubMed] [Google Scholar]
- Mejáre I, Mjör I. Glass ionomer and resin-based fissure sealants: a clinical study. Scand J Dent Res. 1990;98:345–350. doi: 10.1111/j.1600-0722.1990.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Boksman L, Gratton DR, McCutcheon E, Plotzke OB. Clinical evaluation of a glass ionomer cement as a fissure sealant. Quintessence Int. 1987;18:707–709. [PubMed] [Google Scholar]
- Poulsen S, Beiruti N, Sadat N. A comparison of retention and the effect on caries of fissure sealing with a glass-ionomer and resin-based sealant. Community Dent Oral Epidemiol. 2001;29:298–301. doi: 10.1034/j.1600-0528.2001.290409.x. [DOI] [PubMed] [Google Scholar]
- Oba AA, Dülgergil T, Sönmez IS, Doğan S. Comparison of caries prevention with glass ionomer and composite resin fissure sealants. J Formos Med Assoc. 2009;108:844–848. doi: 10.1016/S0929-6646(09)60415-0. [DOI] [PubMed] [Google Scholar]
- Barja-Fidalgo F, Maroun S, de Oliveira BH. Effectiveness of a glass ionomer cement used as a pit and fissure sealant in recently erupted permanent first molars. J Dent Child (Chic) 2009;76:34–40. [PubMed] [Google Scholar]
- Amin HE. Clinical and antibacterial effectiveness of three different sealant materials. J Dent Hyg. 2008;82:45. [PubMed] [Google Scholar]
- Subramaniam P, Konde S, Mandanna DK. Retention of a resin-based sealant and a glass ionomer used as a fissure sealant: a comparative clinical study. J Indian Soc Pedod Prev Dent. 2008;26:114–120. doi: 10.4103/0970-4388.43192. [DOI] [PubMed] [Google Scholar]
- Oliveira FS, da Silva SM, Machado MA, Bijella MF, Lima JE, Abdo RC. Resin-modified glass ionomer cement and a resin-based material as occlusal sealants: a longitudinal clinical performance. J Dent Child (Chic) 2008;75:134–143. [PubMed] [Google Scholar]
- Forss H, Saarni UM, Seppä L. Comparison of glass-ionomer and resin-based fissure sealants: a 2-year clinical trial. Community Dent Oral Epidemiol. 1994;22:21–24. doi: 10.1111/j.1600-0528.1994.tb01563.x. [DOI] [PubMed] [Google Scholar]
- Williams B, Winter GB. Fissure sealants. Further results at 4 years. Br Dent J. 1981;150:183–187. doi: 10.1038/sj.bdj.4804568. [DOI] [PubMed] [Google Scholar]
- Mejáre I, Lingström P, Petersson LG, Holm AK, Twetman S, Källestål C, Nordenram G, Lagerlöf F, Söder B, Norlund A, Axelsson S, Dahlgren H. Caries-preventive effect of fissure sealants: a systematic review. Acta Odontol Scand. 2003;61:321–330. doi: 10.1080/00016350310007581. [DOI] [PubMed] [Google Scholar]
- Chalmers TC, Matta RJ, Smith H Jr, Kunzler AM. Evidence favoring the use of anticoagulants in the hospital phase of acute myocardial infarction. N Engl J Med. 1977;297:1091–1096. doi: 10.1056/NEJM197711172972004. [DOI] [PubMed] [Google Scholar]
- Kjaergard LL, Villumsen J, Gluud C. Reported Methodological quality and discrepancies between large and small randomized trials in meta-Analyses. Ann Intern Med. 2001;135:982–989. doi: 10.7326/0003-4819-135-11-200112040-00010. [DOI] [PubMed] [Google Scholar]
- Egger M, Jüni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technol Assess. 2003;7:1–76. [PubMed] [Google Scholar]
- Higgins JPT, Green S. The Cochrane Library. 4. Vol. 82. John Wiley & Sons, Chichester; 2006. Cochrane handbook for systematic reviews of interventions 4.2.6; pp. 113–114. [Google Scholar]
- Rothstein HR, Sutton AJ, Borenstein M. Publication bias in meta-analysis - prevention, assessment and adjustment. John Wiley & Sons, Chichester 1-7; 2005. Publication bias in meta-analysis; pp. 1–7. [Google Scholar]
- Rothstein HR, Sutton AJ, Borenstein M. Publication bias in meta-analysis - prevention, assessment and adjustment. John Wiley & Sons, Chichester; 2005. Software for publication bias; pp. 193–220. [Google Scholar]
- Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. 2006;333:597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara AT, Turssi CP, Ando M, González-Cabezas C, Zero DT, Rodrigues AL Jr, Serra MC, Cury JA. Influence of fluoride-releasing restorative material on root dentine secondary caries in situ. Caries Res. 2006;40:435–439. doi: 10.1159/000094290. [DOI] [PubMed] [Google Scholar]
- Ioannidis J, Lau J. Evolution of treatment effects over time: empirical insight from recursive cumulative meta-analyses. Proc Natl Acad Sci USA. 2001;98:831–836. doi: 10.1073/pnas.021529998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau J, Schmid CH, Chalmers TC. Cumulative meta-analysis of clinical trials builds evidence for exemplary medical care. J Clin Epidemiol. 1995;48:45–57. doi: 10.1016/0895-4356(94)00106-Z. [DOI] [PubMed] [Google Scholar]
- Moles DR, Needleman IG, Niederman R, Lau J. Introduction to cumulative meta-analysis in dentistry: lessons learned from undertaking a cumulative meta-analysis in periodontology. J Dent Res. 2005;84:345–349. doi: 10.1177/154405910508400410. [DOI] [PubMed] [Google Scholar]
- Mickenautsch S. Systematic reviews, systematic error and the acquisition of clinical knowledge. BMC Med Res Methodol. 2010;10:53. doi: 10.1186/1471-2288-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel - group randomised trials. Lancet. 2001;357:1191–1194. doi: 10.1016/S0140-6736(00)04337-3. [DOI] [PubMed] [Google Scholar]