Abstract
Background
Molecular phylogenetic studies on scleractinian corals have shown that most taxa are not reflective of their evolutionary histories. Based principally on gross morphology, traditional taxonomy suffers from the lack of well-defined and homologous characters that can sufficiently describe scleractinian diversity. One of the most challenging clades recovered by recent analyses is 'Bigmessidae', an informal grouping that comprises four conventional coral families, Faviidae, Merulinidae, Pectiniidae and Trachyphylliidae, interspersed among one another with no apparent systematic pattern. There is an urgent need for taxonomic revisions in this clade, but it is vital to first establish phylogenetic relationships within the group. In this study, we reconstruct the evolutionary history of 'Bigmessidae' based on five DNA sequence markers gathered from 76 of the 132 currently recognized species collected from five reef regions in the central Indo-Pacific and the Atlantic.
Results
We present a robust molecular phylogeny of 'Bigmessidae' based on the combined five-gene data, achieving a higher degree of resolution compared to previous analyses. Two Pacific species presumed to be in 'Bigmessidae' are more closely related to outgroup clades, suggesting that other unsampled taxa have unforeseen affinities. As expected, nested within 'Bigmessidae' are four conventional families as listed above, and relationships among them generally corroborate previous molecular analyses. Our more resolved phylogeny supports a close association of Hydnophora (Merulinidae) with Favites + Montastraea (Faviidae), rather than with the rest of Merulinidae, i.e., Merulina and Scapophyllia. Montastraea annularis, the only Atlantic 'Bigmessidae' is sister to Cyphastrea, a grouping that can be reconciled by their septothecal walls, a microstructural feature of the skeleton determined by recent morphological work. Characters at the subcorallite scale appear to be appropriate synapomorphies for other subclades, which cannot be explained using macromorphology. Indeed, wide geographic sampling here has revealed more instances of possible cryptic taxa confused by evolutionary convergence of gross coral morphology.
Conclusions
Numerous examples of cryptic taxa determined in this study support the assertion that diversity estimates of scleractinian corals are erroneous. Fortunately, the recovery of most 'Bigmessidae' genera with only minor degrees of paraphyly offers some hope for impending taxonomic amendments. Subclades are well defined and supported by subcorallite morphological features, providing a robust framework for further systematic work.
Background
For the last two decades, coral systematists have been untangling the complex evolutionary relationships among scleractinian species using DNA sequence data. Seminal molecular phylogenetic work by Romano and Palumbi [1,2] divided the Scleractinia into two major clades, the robust and complex groups, and indicated many problems with traditional taxonomy based on morphology (see also [3]). For instance, Leptastrea was recovered within a Fungiina clade rather than the suborder Faviina, where morphological studies had placed it (e.g., [4,5]). Gradually, using more genetic loci, further evidence was uncovered to show that non-monophyly of coral taxa is widespread in Scleractinia (e.g., [6-11]). This culminated in a comprehensive survey of the entire taxon by Fukami et al. [12], which showed that while Scleractinia is monophyletic, most taxonomic groups within it are not. In fact, a staggering 11 of 16 conventional families are polyphyletic.
Undoubtedly, one of the most challenging clades that have been recovered by recent analyses is a group of robust corals in clade XVII [12]. The disarray within the clade is epitomized by its informal name 'Bigmessidae' [13,14]. This clade contains species from four traditional coral families, Faviidae, Merulinidae, Pectiniidae and Trachyphylliidae, interspersed among one another in the tree based on mitochondrial cytochrome oxidase I (COI) and cytochrome b gene sequences [12]. With the exception of the Montastraea annularis complex, all members of this clade are from the Indo-Pacific. Families with all species included within clade XVII are Trachyphylliidae (monospecific) and Merulinidae, the latter being polyphyletic, while Faviidae and Pectiniidae have representatives present within and outside clade XVII. Although the clade has not been examined in detail, Huang et al. [15] showed that representatives from other families (Merulinidae and Mussidae) are also nested within it, and several genera are not monophyletic (i.e., Echinopora, Favia, Favites, Goniastrea and Montastraea). In addition, Fukami et al. [12] found para- or polyphyly in Leptoria, Oulophyllia and Platygyra for at least one marker.
Clearly, there exists an urgent need for taxonomic revisions in this clade, amidst the ongoing disarray in the Scleractinia. But in order to begin any form of revision for clade XVII, it is first necessary to determine which subclades are problematic, using as complete a morphological and genetic coverage as possible. Up to this point, the largest number of markers used for analysis of this group has been derived from Fukami et al. [12], who used the aforementioned mitochondrial genes, as well as the nuclear β-tubulin and 28S rDNA separately. However, only 33 species represented by 38 terminals were analyzed for clade XVII, and several subclades were not resolved due to their short branches. Resolution was improved in Huang et al. [15], which included 85 terminals from 43 species, but that study used only COI and a noncoding intergenic mitochondrial region (IGR).
In this study, we present data for five molecular markers—two mitochondrial and three nuclear loci—from 76 of the 132 currently recognized species in clade XVII [12]. We also included seven species from other robust corals as outgroups. Corals were sequenced from five reef regions—the central and northern Great Barrier Reef in Australia, Wakayama in Japan, Batangas in the Philippines, Singapore and the Caribbean. We reconstruct the evolutionary history of clade XVII and identify subclade placement of species that have not been studied in a molecular phylogenetic context. As some species were sampled from multiple locations, we also test if these corals were as widespread as previously recorded.
Methods
Specimen collection and DNA extraction
Specimens were collected from coral reefs in five regions—Singapore, Wakayama (Japan), Queensland (Australia), Batangas (The Philippines), and the Caribbean. To ensure consistency in identifications among localities, each coral was sampled by at least two authors, based on morphological features that can be recognized in the field. The identity was later confirmed in the laboratory after examining skeletal traits [5,16-21]. In total, 124 specimens from 83 species in clades XIV-XXI have been included in the present analysis (Table 1; see Additional file 1). We photographed each colony in the field and collected between 10 and 100 cm2 of coral from each colony using a hammer and chisel, with ~2cm2 of tissue preserved in 100% ethanol.
Table 1.
Species and DNA sequences examined in this study.
Unless indicated by roman numerals and/or family names in parentheses, all species belong to clade XVII and Faviidae, respectively. Species placed in a molecular phylogenetic context for the first time are in bold. Specimens with voucher numbers starting with 'G' are from Great Barrier Reef (Australia), 'S' from Singapore, 'J' from Japan, 'P' from the Philippines, and 'A' from the Atlantic. GenBank accession numbers are displayed for each molecular marker.
For each colony from Singapore, Japan and the Caribbean, DNA was extracted from ~2 cm2 of tissue digested in twice their volume of CHAOS solution (not an acronym; 4 M guanidine thiocyanate, 0.1% N-lauroyl sarcosine sodium, 10 mM Tris pH 8, 0.1 M 2-mercaptoethanol) for at least three days at room temperature before DNA extraction using a phenol-chloroform based method with a phenol extraction buffer (100 mM TrisCl pH 8, 10 mM EDTA, 0.1% SDS) [15,22-24]. For specimens from Australia and the Philippines, genomic DNA was extracted from the tissues preserved in ethanol using the Qiagen DNeasy kit, following the manufacturer's instructions.
The rest of the colony was sprayed with a powerful water jet to remove as much tissue as possible before being bleached in 5-10% sodium hypochlorite solution. The skeletons were rinsed in fresh water, dried, and deposited in the Raffles Museum of Biodiversity Research (Singapore), Seto Marine Biological Laboratory (Wakayama, Japan), Museum of Tropical Queensland (Australia), and De La Salle University (Manila, The Philippines) (Table 1).
PCR amplification and sequencing
A total of five molecular markers were amplified for a majority of the samples (Tables 1 and 2). They consist of three nuclear and two mitochondrial loci: (1) 28S rDNA D1 and D2 fragments; (2) histone H3; (3) internal transcribed spacers 1 and 2, including 5.8S rDNA (ITS in short); (4) cytochrome oxidase subunit I (COI); and (5) noncoding intergenic region situated between COI and the formylmethionine transfer RNA gene (IGR in short) [8,23,25-27].
Table 2.
Molecular markers utilized for phylogenetic reconstruction.
| Marker | Primer pairs | Total characters (informative) | Model | Source |
|---|---|---|---|---|
| 28S rDNA | C1': 5'-ACC CGC TGA ATT TAA GCA T-3' D2MAD: 5'-GAC GAT CGA TTT GCA CGT CA-3' |
861 (135) | HKY+Γ | [25] |
| histone H3 | H3F: 5'-ATG GCT CGT ACC AAG CAG ACV GC-3' H3R: 5'-ATA TCC TTR GGC ATR ATR GTG AC-3' |
374 (73) | HKY+Γ | [26] |
| ITS rDNA | A18S: 5'-GATCGAACGGTTTAGTGAGG-3' ITS-4: 5'-TCCTCCGCTTATTGATATGC-3' |
1137 (425) | SYM+Γ | [27] |
| mt COI | MCOIF: 5'-TCTACAAATCATAAAGACATAGG-3' MCOIR: 5'-GAGAAATTATACCAAAACCAGG-3' |
719 (71) | HKY+I | [8] |
| mt IGR | MNC1f: 5'-GAGCTGGGCTTCTTTAGAGTG-3' MNC1r: 5'-GTGAGACTCGAACTCACTTTTC-3' |
1509 (763) | SYM+I | [23] |
The mitochondrial intergenic region (IGR) was too variable to be aligned across the entire clade, so only alignable sequences were included in the analysis. ITS comprises multiple copies in the nuclear genome, but the primers we used have shown high fidelity for a single copy, precluding the need to clone the amplicons [27-33]. Nevertheless, in the unlikely case that paralogs were sequenced, our analyses could be confused by incomplete lineage sorting [7]. We therefore sequenced the ITS locus from at most one representative of each species, unless analyses of the other four markers did not recover its sequences as a clade. In the latter case, sequences may actually belong to separate cryptic species that have been obscured by gross morphological similarities. For COI, not all specimens of each species were necessarily sequenced since intraspecific variation of this gene is limited [15,24].
PCR products were purified with ExoSAP-IT (GE Healthcare, Uppsala, Sweden) and sequencing was performed by Advanced Studies in Genomics, Proteomics and Bioinformatics (ASGPB) at the University of Hawaii at Manoa using the Applied Biosystems BigDye Terminator kit and an ABI 3730XL sequencer. New sequences were deposited in GenBank under accession numbers HQ203246-HQ203689 (Table 1).
Phylogenetic analyses
Sequences were organized into five separate data matrices using Mesquite 2.72 [34], and each aligned with the accurate alignment option (E-INS-i) in MAFFT 6.7 [35-37] under default parameters. Substitution saturation of protein-coding genes was assessed via DAMBE [38,39], where we found histone H3 and COI to be unsaturated at the third codon positions for tree inference. Consequently, we concatenated the five gene matrices into a single partitioned matrix consisting of 4600 characters, 1467 of which were parsimony informative. This was analyzed using maximum parsimony, Bayesian likelihood, and maximum likelihood methods. We also carried out these analyses on a four-gene dataset omitting the ITS partition to determine if the phylogenetic reconstruction was sensitive to the ITS sampling strategy.
Under a maximum parsimony framework, we utilized new search technologies [40,41] in the software TNT 1.1 [42,43]. Tree searches consisted of 50000 random addition sequence replicates under the default sectorial, ratchet, drift and tree fusing parameters. Gaps were treated as missing data and clade stability was inferred using 1000 bootstrap replicates each employing 100 random addition sequences.
For maximum likelihood, neighbor-joining and Bayesian analyses, we determined the most suitable model of molecular evolution for each gene partition and the concatenated matrix using jModelTest 0.1.1 [44,45] to test for a total of 24 models, following the Akaike Information Criterion (AIC). The maximum likelihood tree for each partition and the combined dataset was inferred using RAxML 7.2.3 [46,47] at the Cyberinfrastructure for Phylogenetic Research (CIPRES; http://www.phylo.org), employing the GTRGAMMA model. The proportion of invariable sites and gamma distribution shape parameter for variable sites were estimated during the maximum likelihood analysis. Multiparametric bootstrap analysis was carried out using 1000 bootstrap replicates. Maximum likelihood analysis was also carried out with PhyML 3.0 [45] on the combined data, utilizing the AIC-chosen model (GTR+I+Γ), and generating 1000 bootstrap replicates. The neighbor-joining tree of the combined data was calculated in PAUP*4.0b10 [48] with 1000 bootstrap replicates, employing the evolutionary model selected above.
Bayesian inference was carried out in MrBayes 3.1.2 [49,50], using the resources of the Computational Biology Service Unit from Cornell University, with each partition modeled (Table 2) but unlinked for separate parameter estimations. Four Markov chains of 10 million generations were implemented in twelve runs, saving a tree every 100th generation. MCMC convergence among the runs was monitored using Tracer 1.5 [51], where we ascertained that only four of the twelve runs converged on the shortest trees (only two runs converged for the four-gene analysis; see [52-54]), and the first 40001 trees were to be discarded as burn-in.
Additionally, compensatory base changes because of the secondary structure of the ITS rDNA loci may lead to non-independence and increased homoplasy of characters [55-57]. Hence, analysis of the secondary structure of this region may result in a more rigorous phylogeny [58-61]. Using the ITS2 segment of each ITS sequence, secondary structure was predicted by searching the ITS2 database [62] for the best match template and then modeling its structure based on free energy minimization. The ITS2 sequences and their associated structural information were aligned using 4SALE 1.5 [63,64], and then exported for analysis in ProfDistS 0.9.8 [65-68]. The profile neighbor-joining algorithm was executed with 10000 bootstrap replicates on the RNA structural alignment, using the GTR model and rate matrix 'Q_ITS2.txt' for distance correction. ITS2 could not be amplified from Hydnophora microconos, H. pilosa and Merulina scabricula. Consequently these species were excluded from the analysis.
Results and Discussion
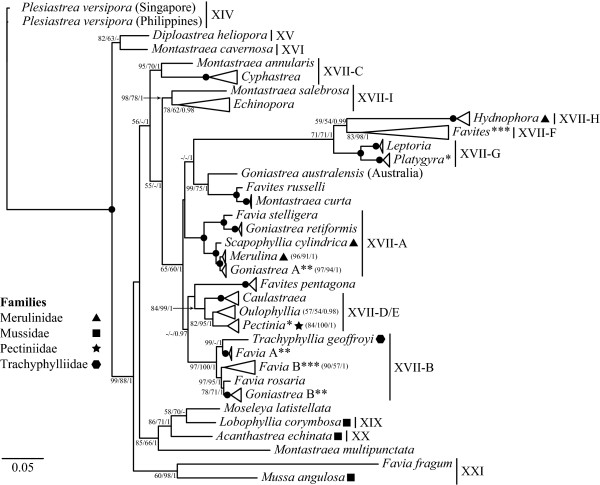
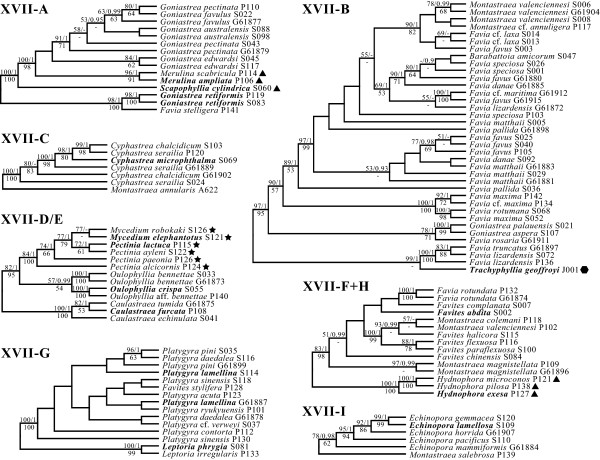
In this study, the evolutionary history of the 'Bigmessidae' corals was robustly reconstructed using five genes. Relations among other clade representatives chosen as outgroups were also inferred. The maximum likelihood reconstructions carried out by RAxML 7.2.3 and PhyML 3.0 had log likelihood values of -36224.67 and -36995.48, respectively. As they were identical when considering nodes with bootstrap values ≥50, we present the RAxML tree that garnered a higher likelihood score (Figures 1 and 2). A total of 182 most parsimonious trees (tree length = 6178) were obtained. No conflicts between tree optimization procedures (including Bayesian inference and the neighbor-joining algorithm) were apparent when considering only the supported nodes (bootstrap ≥50 and posterior probability ≥0.9) (see Additional file 2). Analyses excluding the ITS partition also gave congruent results. Several clades were consistent and well supported among maximum likelihood, parsimony and Bayesian inferences. We named some of these groups within clade XVII from A to I, consistent with the classification in Budd and Stolarski [69]. On the other hand, the neighbor-joining method generated a relatively unresolved tree—subclades A, C, F and I did not achieve bootstrap values of ≥50 (see Additional file 2).
Figure 1.
Maximum likelihood tree of the combined molecular data. Species have been summarized into genera where possible. One asterisk denotes paraphyletic genus, two asterisks polyphyly, and three represents a genus that is both para- and polyphyletic. All taxa from conventional family Faviidae unless otherwise indicated. Clade designations XIV to XXI shown; clade XVII divided into well-supported subclades. Numbers adjacent to branches/taxa are support values (maximum likelihood bootstrap ≥50, maximum parsimony bootstrap ≥50, followed by Bayesian posterior probability ≥0.9). Filled circles indicate well-supported clades (bootstrap values ≥98 and posterior probability of 1).
Figure 2.
Maximum likelihood topologies of each subclade. Numbers above branches are maximum likelihood bootstrap ≥50 and Bayesian posterior probability ≥0.9, while number below denotes maximum parsimony bootstrap ≥50. Family classification follows definitions given for Figure 1. Type species of genera are in bold.
The combined five-gene data yielded the most resolved phylogeny hitherto of clade XVII, with most branches garnering high support values. However, most partitions gave fairly unresolved trees when analyzed individually (see Additional file 3). By examining the support of subclades among trees obtained via different partitions, we found that nuclear markers contributed a greater extent to the final tree topology (Table 3). Histone H3, for instance, supported all higher-level groupings and all subclades except D/E (Figure 1). The 28S and ITS rDNA gene trees had moderate resolution within clade XVII, with only two unresolved subclades each. Surprisingly, the tree based on ITS2 rDNA secondary structure had less resolution than the primary sequence alignment. Indeed, the former has demonstrated potential for resolving intrageneric phylogenies in other anthozoans [70,71], but it is less informative for relationships at higher taxonomic levels [72,73]. Evidently, the COI tree was poorly resolved, with ≥50 bootstrap support for few relationships among major clades and only one subclade. The slow evolution of the mitochondrial COI gene among anthozoans is certainly the reason behind this [24,74,75]. While the intergenic marker (IGR) adjacent to COI on the mitochondrial genome has shown promise for phylogenetic reconstruction among Faviidae and Mussidae [15,23,76], it cannot be unambiguously aligned between the major clades. We urge the development of more nuclear phylogenetic markers that can be reliably applied across diverse scleractinian clades.
Table 3.
Clades supported by maximum likelihood analysis for each partition.
| Clade | Nuclear DNA | mt DNA | 28S rDNA | histone H3 | ITS sequence |
ITS structure |
mt COI | mt IGR |
|---|---|---|---|---|---|---|---|---|
| XV to XXI | √√ | √√ | √√ | √√ | √√ | √√ | √√ | |
| XV+XVI | √√ | X | √√ | √√ | √ | √√ | XX | |
| XVII to XXI | √√ | √√ | √ | √√ | √√ | √ | √√ | |
| XXI | √√ | √√ | √ | √√ | ||||
| XIX+XX1 | √√ | √ | √ | √√ | X | √√ | √ | |
| XVII | √√ | X | √ | √√ | √ | X | X | √√ |
| XVII-A | √√ | X | √√ | √√ | √√ | X | X | X |
| XVII-B | √√ | X | X | √√ | √√ | √√ | X | √ |
| XVII-C | √√ | XX | √√ | √√ | √√ | X | X | |
| XVII-D/E | √√ | XX | X | X | √√ | √ | XX | √√ |
| XVII-F | √√ | X | √√ | √√ | X | √√ | XX | |
| XVII-G | √√ | √√ | √√ | √√ | X | X | √ | √√ |
| XVII-H | √√ | X | √√ | √√ | √√ | √√ | √√ | |
| XVII-I2 | √√ | X | √√ | √√ | √√ | √ | X | X |
1Montastraea multipunctata and Moseleya latistellata are herein considered as part of clade XIX+XX.
2Subclade I is expanded to include Montastraea salebrosa.
'√√': clade present with ≥50 bootstrap support; '√': clade present but not supported (<50 bootstrap); 'XX': contradicted clade with ≥50 bootstrap support; and 'X': contradicted clade not supported. Empty cells indicate insufficient data.
Most relationships among clades XV to XXI obtained in this study corroborate results of Fukami et al. [12] (Figure 1). The only difference occurs in the sister grouping of Diploastrea heliopora (XV) and Montastraea cavernosa (XVI) (supported by all analyses except Bayesian likelihood) that form a grade in Fukami et al. [12]. The monophyly of the clade XVII+XIX+XX (Pacific faviids and mussids) is recovered but not well supported. Montastraea multipunctata and Moseleya latistellata are Pacific faviids, and therefore presumably in clade XVII. But as a result of superficial similarities, they have historically been associated with the Pacific mussids Blastomussa merleti (clade XIV) [77] and Acanthastrea hillae (clade XVIII) [5,18], respectively. Here, we find them to be more closely related to clades XIX and XX instead, revealing a taxonomic situation more challenging than anticipated. Pacific faviids other than Diploastrea heliopora can no longer be restricted to clade XVII, and the possibility exists that yet-to-be sampled taxa provisionally placed in clade XVII—particularly the monotypic genera, Australogyra, Erythrastrea, Boninastrea and Paraclavarina—have unexpected affinities.
Nested within clade XVII are four conventional families—Faviidae, Merulinidae, Pectiniidae and Trachyphylliidae (Figure 1). Two Pectiniidae genera, Pectinia and Mycedium (XVII-E) form the sister clade to Oulophyllia. This is a similar relationship to the results of Fukami et al. [12], although here we also show with reasonable support that Oulophyllia is monophyletic, and Caulastraea is an outgroup rather than nested within Oulophyllia (XVII-D). Merulinidae is represented by Hydnophora, Merulina and Scapophyllia. Hydnophora is more closely related to Favites and Pacific Montastraea spp. than Merulina and Scapophyllia, which form a grade within the clade dominated by Goniastrea. The monospecific Trachyphylliidae is nested within the clade consisting primarily of Favia spp., and is sister to Favia lizardensis and F. truncatus (Figure 2). Work is ongoing to redescribe clade XVII by incorporating the above families and applying a new taxon name since the type species of Faviidae, Favia fragum (Esper, 1797), belongs to clade XXI [12].
The genetic affiliation of Hydnophora and Trachyphyllia with Faviidae has previously been proposed by Fukami et al. [8,12]. However, this is not exclusively a molecular hypothesis. Based on a combination of colony, corallite and subcorallite characters (e.g., polyp budding; wall, septal and columellar structures), Vaughan and Wells, 1943 [78], placed the two taxa within Faviidae. But later, Chevalier, 1975 [79], attempted to distinguish Trachyphyllia from Faviidae based on minor differences in wall and septal structures by elevating it to the rank of family. Correspondingly, Veron, 1985 [17], moved Hydnophora into Merulinidae because of Hydnophora species' macromorphological similarities (i.e., colony growth form and polyp structure) with Merulina ampliata and Scapophyllia cylindrica, which are genetically in the same lineage (subclade A) as several Goniastrea spp. and Favia stelligera (Figures 1 and 2; see also [8,12]).
Montastraea annularis and likely other members of the species complex (M. faveolata and M. franksi) are the only Atlantic species in clade XVII (see also [8,12]). Most significantly here, M. annularis is sister to Cyphastrea, forming clade XVII-C (Figure 1). This placement may seem bizarre in the context of traditional macromorphological characters used to classify scleractinians (e.g., [4,78]). However, recent work at the microstructural scale (centers of rapid accretion and thickening deposits) has suggested that their septothecal walls (formed by fusion of outer margins of septa) may unite the two taxa [69] (see also [80]). These subcorallite features appear to be appropriate synapomorphies for other subclades. For instance, clade XVII-A consists of Merulina, Scapophyllia, Goniastrea A and Favia stelligera (Figure 2). At the corallite level, these corals cannot be reconciled within the same taxon, since Favia stelligera corallites have single centers with separate walls (plocoid), Goniastrea spp. have fused walls (cerioid) and may form valleys (meandroid), while Merulina and Scapophyllia are composed predominantly of elongated valleys (see Additional file 1). On the other hand, they share the apomorphy of having septothecal walls with abortive septa (thin bands between normal septa with their own centers of rapid accretion).
The use of macromorphology for identifying 'Bigmessidae' species is known for being problematic as most of these characters are homoplasious [15,80,81]. The ability to distinguish clades based on microstructural features is encouraging for scleractinian systematics. Micromorphology, at the scale of septal teeth and granules, has also exhibited promise as phylogenetic characters [25,80,82-85]. Interestingly, in light of recent molecular hypotheses, other biological traits, in particular, sexuality and to a lesser extent, breeding mode appear highly conserved and could be further developed as phylogenetic markers [86,87].
Prior to the use of molecular data to build evolutionary trees, it was a great challenge to determine which morphological characters could be useful for classification, given their intraspecific variability [32,88] and phenotypic plasticity [89-94]. Indeed, the general anthozoan body plan is relatively simple, and scleractinians in particular have few discrete morphological characters that are known to be phylogenetically informative at the polyp level [4,95-97]. As a result of the recent disarray in coral systematics, morphological taxonomies of scleractinians have been heavily criticized (e.g., [8,12,98,99]). Molecular characters, which are much more numerous and arguably neutrally evolving, can certainly aid our understanding of evolutionary relationships. However, morphological evidence supporting various molecular clades in the present analysis suggests that morphology at novel scales will play an essential role in the taxonomy of 'Bigmessidae' [80].
Widespread sampling in this study has shown that corals thought to belong to the same species across the central Indo-Pacific are actually from distinct lineages. Consider Goniastrea australensis (Milne Edwards and Haime, 1857), which occurs in two clades (Figures 1 and 2; see also Additional file 1). Since this species was first described from Australia, the Australian specimen that clustered with Favites russelli and Montastraea curta should be considered G. australensis, while the two specimens from Singapore (S088 and S098, subclade A) probably represent new species yet to be described. This is certainly not an isolated case. A similar situation is revealed for Montastraea valenciennesi. Specimens from Australia (G61904) and Singapore (S006 and S008) are in subclade B of mostly Favia spp., while the representative from the Philippines (P102) is in subclade F, a distant clade comprising mainly Favites species. Interestingly, two reproductively isolated morphotypes of M. valenciennesi were recently found to co-occur in Wakayama (Japan), distinguished by the degree of wall fusion among corallites [100]. Chevalier, 1971 [101], upon examination of the holotype, placed the species in Favia on the basis of corallites possessing separate walls and budding intratentacularly (see also [102-108]). This suggests that the name Favia valenciennesi (Milne Edwards and Haime, 1848) could be applied to the Australian and Singaporean specimens in subclade B, while P102 (subclade F) is a new species.
Less extensive issues occur among Goniastrea and Favia species. For instance, G. pectinata (subclade A), collected from three locations, is clearly paraphyletic, with G. australensis and G. favulus nested within them (Figure 2). For Favia (subclade B), of six F. favus specimens collected from three localities, only three of these form a supported clade while the rest are dispersed within clade XVII-B with no apparent biogeographical pattern. The nesting of Barabattoia amicorum among Favia spp. has been consistently recovered in recent molecular phylogenies [12,15], but this affinity was in fact the dominant hypothesis [5,107-109] until Veron, 1986 [18], included the species in its current genus. Conversely, Favia rotundata clusters with Favites spp. rather than its congeners, but it was indeed originally described as Favites rotundata Veron, Pichon and Wijsman-Best, 1977 [5] (see also [109,110]).
The polyphyly of most 'Bigmessidae' genera seems to confer a bleak outlook for revisionary work. However, as we have shown in Figure 1, several genera can be clearly grouped as clades with limited name changes. For instance, subclade F is composed of species from Favites Link, 1807, Montastraea de Blainville, 1830, and Favia Ehrenberg, 1834 (Figure 2). While the remaining Favites spp. (i.e., F. pentagona, F. russelli, and F. stylifera) are not included within this subclade, the type species of this genus is Favites abdita (Ellis and Solander, 1786, type locality 'Probablement les mers des Grandes-Indes', Lamarck, 1816 [111]). The representative of the latter we used falls well within subclade F. Since no other type species were recovered and with Favites Link, 1807, being the oldest valid genus in the subclade, Favites should be expanded to include the other species, while F. pentagona, F. russelli and F. stylifera will have to be subsumed within other genera. Several other multi-species genera in fact appear stable: Caulastraea, Cyphastrea, Echinopora, Hydnophora, Leptoria, Merulina and Oulophyllia. Name changes are certainly not necessary for Favites and Platygyra, since they host their respective type species in the subclades shown in Figure 2.
Conclusions
Numerous instances of cryptic taxa determined in this study support the assertion that coral diversity estimates have been fraught with errors [8]. Traits relating to the gross skeletal morphology of corals are unreliable for species description and identification because of their potential for intraspecific variability [32,88] and environment-induced plasticity [89-94]. Yet, these characters have served as the foundation for scleractinian taxonomy (e.g., [4,5]). Fortunately, using molecular data, the recovery of most genera within the 'Bigmessidae' with only minor degrees of paraphyly spells hope for impending taxonomic amendments. Our results show that most genera only require slight revisions, and most major changes are necessary only at the level of the major clades described in Fukami et al. [12]. Certainly, broad taxonomic sampling within Faviidae has revealed more species with unexpected affinities, such as Moseleya latistellata and Montastraea multipunctata. Clade XVII may consequently have to be redefined to exclude them.
Nevertheless, 'Bigmessidae' subclades are well defined and will no doubt provide a robust framework for taxonomic revisions. The fact that microstructural features support 'Bigmessidae' subclades also offers hope for the morphological approach. Evolutionary relationships among subclades are still provisional due to insufficient statistical support, but they can be clarified with further sampling of nuclear sequences. Eventually, a well-resolved tree of a redescribed clade XVII will be available to reconstruct the morphological evolution of 'Bigmessidae' at various scales.
Authors' contributions
DH obtained the DNA sequences in the laboratory, performed the phylogenetic analyses, and had a major role in writing the manuscript. All authors collected the specimens examined, contributed to and approved the final manuscript.
Supplementary Material
'Bigmessidae' corals. Photographs of most coral specimens sequenced in this study. More photographs are available from the authors.
Maximum likelihood tree topology of the combined molecular data. Numbers above branches are maximum likelihood bootstrap ≥50 and Bayesian posterior probability ≥0.9, while numbers below denote maximum parsimony bootstrap ≥50 and neighbor-joining bootstrap ≥50. Family classification follows definitions given for Figure 1.
Maximum likelihood tree topology of each partition. Numbers adjacent to branches are bootstrap support values ≥50. Definitions for family classification follow Figure 1.
Contributor Information
Danwei Huang, Email: huangdanwei@ucsd.edu.
Wilfredo Y Licuanan, Email: licuananw@dlsu.edu.ph.
Andrew H Baird, Email: andrew.baird@jcu.edu.au.
Hironobu Fukami, Email: hirofukami@cc.miyazaki-u.ac.jp.
Acknowledgements
We thank all who helped with the field collections, including Zeehan Jaafar, Ywee Chieh Tay, Katrina Luzon, Norievill Espana, Eznairah-Jeung Narida and Monica Orquieza. Flavia Nunes kindly provided the Atlantic specimens. We acknowledge Ann Budd for critical discussions on coral morphology; Carmen Ablan-Lagman and Glenn Oyong for lab support at De La Salle University; Rudolf Meier, Loke Ming Chou and Peter Todd for lab support at National University of Singapore; Carden Wallace, Paul Muir and Barbara Done for museum support at Museum of Tropical Queensland; and staff of Orpheus Island Research Station for field support at Orpheus Island. Special thanks go to Gregory Rouse and Nancy Knowlton for valuable advice and support. For comments on this manuscript, we thank Liz Borda, Tito Lotufo, Yun Lei Tan, three anonymous reviewers and the Associate Editor. Collections were made in Australia under Great Barrier Reef Marine Park Authority permit G09/29715.1, and in the Philippines under Department of Agriculture gratuitous permit FBP-0027-09. This study is partly funded by National Geographic Committee for Research and Exploration grant 8449-08.
References
- Romano SL, Palumbi SR. Evolution of scleractinian corals inferred from molecular systematics. Science. 1996;271:640–642. doi: 10.1126/science.271.5249.640. [DOI] [Google Scholar]
- Romano SL, Palumbi SR. Molecular evolution of a portion of the mitochondrial 16S ribosomal gene region in scleractinian corals. J Mol Evol. 1997;45:397–411. doi: 10.1007/PL00006245. [DOI] [PubMed] [Google Scholar]
- Chen CA, Wallace CC, Wolstenholme JK. Analysis of the mitochondrial 12S rRNA gene supports a two-clade hypothesis of the evolutionary history of scleractinian corals. Mol Phylogenet Evol. 2002;23:137–149. doi: 10.1016/S1055-7903(02)00008-8. [DOI] [PubMed] [Google Scholar]
- Wells JW. In: Treatise on Invertebrate Paleontology Part F: Coelenterata. Moore RC, editor. Lawrence KS: University of Kansas Press; 1956. Scleractinia; pp. F328–F444. [Google Scholar]
- Veron JEN, Pichon M, Wijsman-Best M. Scleractinia of Eastern Australia. Part II. Families Faviidae, Trachyphylliidae. Australian Institute of Marine Science Monograph Series. 1977. pp. 1–233.
- Romano SL, Cairns SD. Molecular phylogenetic hypotheses for the evolution of scleractinian corals. Bull Mar Sci. 2000;67:1043–1068. [Google Scholar]
- van Oppen MJH, McDonald BJ, Willis BL, Miller DJ. The evolutionary history of the coral genus Acropora (Scleractinia, Cnidaria) based on a mitochondrial and a nuclear marker: reticulation, incomplete lineage sorting, or morphological convergence? Mol Biol Evol. 2001;18:1315–1329. doi: 10.1093/oxfordjournals.molbev.a003916. [DOI] [PubMed] [Google Scholar]
- Fukami H, Budd AF, Paulay G, Sole-Cava AM, Chen CA, Iwao K, Knowlton N. Conventional taxonomy obscures deep divergence between Pacific and Atlantic corals. Nature. 2004;427:832–835. doi: 10.1038/nature02339. [DOI] [PubMed] [Google Scholar]
- Le Goff-Vitry MC, Rogers AD, Baglow D. A deep-sea slant on the molecular phylogeny of the Scleractinia. Mol Phylogenet Evol. 2004;30:167–177. doi: 10.1016/S1055-7903(03)00162-3. [DOI] [PubMed] [Google Scholar]
- Kerr AM. Molecular and morphological supertree of stony corals (Anthozoa: Scleractinia) using matrix representation parsimony. Biol Rev. 2005;80:543–558. doi: 10.1017/S1464793105006780. [DOI] [PubMed] [Google Scholar]
- Benzoni F, Stefani F, Stolarski J, Pichon M, Mitta G, Galli P. Debating phylogenetic relationships of the scleractinian Psammocora: molecular and morphological evidences. Contrib Zool. 2007;76:35–54. [Google Scholar]
- Fukami H, Chen CA, Budd AF, Collins A, Wallace CC, Chuang YY, Dai CF, Iwao K, Sheppard CRC, Knowlton N. Mitochondrial and nuclear genes suggest that stony corals are monophyletic but most families of stony corals are not (Order Scleractinia, Class Anthozoa, Phylum Cnidaria) PLoS ONE. 2008;3:e3222. doi: 10.1371/journal.pone.0003222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton N, Fukami H, Chen CA, Budd AF. Mitochondrial and nuclear genes suggest that stony corals are monophyletic but most families of stony corals are not [abstract] 11th Int Coral Reef Symp. 2008. p. 251. [DOI] [PMC free article] [PubMed]
- Budd AF. Encyclopedia of Life Synthesis Meeting Report. Smithsonian Institution, National Museum of Natural History; 2009. Systematics and evolution of scleractinian corals. [Google Scholar]
- Huang D, Meier R, Todd PA, Chou LM. More evidence for pervasive paraphyly in scleractinian corals: Systematic study of Southeast Asian Faviidae (Cnidaria; Scleractinia) based on molecular and morphological data. Mol Phylogenet Evol. 2009;50:102–116. doi: 10.1016/j.ympev.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Veron JEN, Pichon M. Scleractinia of Eastern Australia. Part III. Families Agariciidae, Siderastreidae, Fungiidae, Oculinidae, Merulinidae, Mussidae, Pectiniidae, Caryophylliidae, Dendrophylliidae. Australian Institute of Marine Science Monograph Series. 1980. pp. 1–422.
- Veron JEN. New Scleractinia from Australian coral reefs. Rec West Aust Mus. 1985;12:147–183. [Google Scholar]
- Veron JEN. Corals of Australia and the Indo-Pacific. Townsville: Australian Institute of Marine Science; 1986. [Google Scholar]
- Veron JEN. New Scleractinia from Japan and other Indo-West Pacific countries. Galaxea. 1990;9:95–173. [Google Scholar]
- Veron JEN. Corals of the World. Townsville: Australian Institute of Marine Science; 2000. [Google Scholar]
- Veron JEN. New species described in Corals of the World. Australian Institute of Marine Science Monograph Series. 2002. pp. 1–209.
- Sargent TD, Jamrich M, Dawid IB. Cell interactions and the control of gene activity during early development of Xenopus laevis. Dev Biol. 1986;114:238–246. doi: 10.1016/0012-1606(86)90399-4. [DOI] [PubMed] [Google Scholar]
- Fukami H, Budd AF, Levitan DR, Jara J, Kersanach R, Knowlton N. Geographic differences in species boundaries among members of the Montastraea annularis complex based on molecular and morphological markers. Evolution. 2004;58:324–337. [PubMed] [Google Scholar]
- Huang D, Meier R, Todd PA, Chou LM. Slow mitochondrial COI sequence evolution at the base of the metazoan tree and its implications for DNA barcoding. J Mol Evol. 2008;66:167–174. doi: 10.1007/s00239-008-9069-5. [DOI] [PubMed] [Google Scholar]
- Cuif JP, Lecointre G, Perrin C, Tillier A, Tillier S. Patterns of septal biomineralization in Scleractinia compared with their 28S rRNA phylogeny: a dual approach for a new taxonomic framework. Zool Scr. 2003;32:459–473. doi: 10.1046/j.1463-6409.2003.00133.x. [DOI] [Google Scholar]
- Colgan DJ, McLauchlan A, Wilson GDF, Livingston SP, Edgecombe GD, Macaranas J, Cassis G, Gray MR. Histone H3 and U2 snRNA DNA sequences and arthropod molecular evolution. Aust J Zool. 1998;46:419–437. doi: 10.1071/ZO98048. [DOI] [Google Scholar]
- Takabayashi M, Carter DA, Loh WKW, Hoegh-Guldberg O. A coral-specific primer for PCR amplification of the internal transcribed spacer region in ribosomal DNA. Mol Ecol. 1998;7:928–930. [Google Scholar]
- Takabayashi M, Carter DA, Ward S, Hoegh-Guldberg O. Inter- and intra-specific variability in ribosomal DNA sequence in the internal transcribed spacer region of corals. Proc Aust Coral Reef Soc 75th Ann Conf. 1998. pp. 241–248.
- Takabayashi M, Carter DA, Lopez JV, Hoegh-Guldberg O. Genetic variation of the scleractinian coral Stylophora pistillata, from western Pacific reefs. Coral Reefs. 2003;22:17–22. [Google Scholar]
- van Oppen MJH, Worheide G, Takabayashi M. Nuclear markers in evolutionary and population genetic studies of scleractinian corals and sponges. Proc 9th Int Coral Reef Symp. 2000;1:131–138. [Google Scholar]
- Lam KKY, Morton B. Morphological and ITS1, 5.8S, and partial ITS2 ribosomal DNA sequence distinctions between two species Playtygyra (Cnidaria: Scleractinia) from Hong Kong. Mar Biotechnol. 2003;5:555–567. doi: 10.1007/s10126-002-0114-x. [DOI] [PubMed] [Google Scholar]
- Mangubhai S, Souter P, Grahn M. Phenotypic variation in the coral Platygyra daedalea in Kenya: morphometry and genetics. Mar Ecol-Prog Ser. 2007;345:105–115. doi: 10.3354/meps07013. [DOI] [Google Scholar]
- Knittweis L, Kraemer WE, Timm J, Kochzius M. Genetic structure of Heliofungia actiniformis (Scleractinia: Fungiidae) populations in the Indo-Malay Archipelago: implications for live coral trade management efforts. Conserv Genet. 2009;10:241–249. doi: 10.1007/s10592-008-9566-5. [DOI] [Google Scholar]
- Maddison WP, Maddison DR. Mesquite: a modular system for evolutionary analysis. Version 2.72. http://mesquiteproject.org
- Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Asimenos G, Toh H. Multiple alignment of DNA sequences with MAFFT. Bioinformatics for DNA Sequence Analysis. 2009. pp. 39–63. full_text. [DOI] [PubMed]
- Katoh K, Toh H. Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform. 2008;9:286–298. doi: 10.1093/bib/bbn013. [DOI] [PubMed] [Google Scholar]
- Xia X. Data analysis in molecular biology and evolution. Boston: Kluwer Academic Publishers; 2001. [Google Scholar]
- Xia X, Xie Z. DAMBE: Data analysis in molecular biology and evolution. J Hered. 2001;92:371–373. doi: 10.1093/jhered/92.4.371. [DOI] [PubMed] [Google Scholar]
- Goloboff PA. Analyzing large data sets in reasonable times: Solutions for composite optima. Cladistics. 1999;15:415–428. doi: 10.1111/j.1096-0031.1999.tb00278.x. [DOI] [PubMed] [Google Scholar]
- Nixon KC. The Parsimony Ratchet, a new method for rapid parsimony analysis. Cladistics. 1999;15:407–414. doi: 10.1111/j.1096-0031.1999.tb00277.x. [DOI] [PubMed] [Google Scholar]
- Goloboff PA, Farris JS, Nixon KC. Tree Analysis Using New Technology. Version 1.1. http://www.zmuc.dk/public/phylogeny/TNT
- Goloboff PA, Farris JS, Nixon KC. TNT, a free program for phylogenetic analysis. Cladistics. 2008;24:774–786. doi: 10.1111/j.1096-0031.2008.00217.x. [DOI] [Google Scholar]
- Posada D. jModelTest: Phylogenetic model averaging. Mol Biol Evol. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J. A rapid bootstrap algorithm for the RAxML web servers. Syst Biol. 2008;57:758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- Swofford DL. PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4. Sunderland, Massachusetts: Sinauer Associates; 2003. [Google Scholar]
- Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Rambaut A, Drummond AJ. Tracer. Version 1.5. http://beast.bio.ed.ac.uk/Tracer
- Brown JM, Hedtke SM, Lemmon AR, Lemmon EM. When trees grow too long: Investigating the causes of highly inaccurate Bayesian branch-length estimates. Syst Biol. 2010;59:145–161. doi: 10.1093/sysbio/syp081. [DOI] [PubMed] [Google Scholar]
- Marshall DC. Cryptic failure of partitioned Bayesian phylogenetic analyses: Lost in the land of long trees. Syst Biol. 2010;59:108–117. doi: 10.1093/sysbio/syp080. [DOI] [PubMed] [Google Scholar]
- Schwartz RS, Mueller RL. Branch length estimation and divergence dating: estimates of error in Bayesian and maximum likelihood frameworks. BMC Evol Biol. 2010;10:5. doi: 10.1186/1471-2148-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon MT, Hillis DM. Ribosomal RNA secondary structure: Compensatory mutations and implications for phylogenetic analysis. Mol Biol Evol. 1993;10:256–267. doi: 10.1093/oxfordjournals.molbev.a039998. [DOI] [PubMed] [Google Scholar]
- Baldwin BG, Sanderson MJ, Porter JM, Wojciechowski MF, Campbell CS, Donoghue MJ. The ITS region of nuclear ribosomal DNA: A valuable source of evidence on angiosperm phylogeny. Ann Missouri Bot Gard. 1995;82:247–277. doi: 10.2307/2399880. [DOI] [Google Scholar]
- Álvarez I, Wendel JF. Ribosomal ITS sequences and plant phylogenetic inference. Mol Phylogenet Evol. 2003;29:417–434. doi: 10.1016/s1055-7903(03)00208-2. [DOI] [PubMed] [Google Scholar]
- Müller T, Philippi N, Dandekar T, Schultz J, Wolf M. Distinguishing species. RNA. 2007;13:1469–1472. doi: 10.1261/rna.617107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A, Schleicher T, Förster F, Ruderisch B, Dandekar T, Müller T, Wolf M. ITS2 data corroborate a monophyletic chlorophycean DO-group (Sphaeropleales) BMC Evol Biol. 2008;8:218. doi: 10.1186/1471-2148-8-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman AW. Is there a molecular key to the level of "biological species" in eukaryotes? A DNA guide. Mol Phylogenet Evol. 2009;50:197–203. doi: 10.1016/j.ympev.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Schultz J, Wolf M. ITS2 sequence-structure analysis in phylogenetics: A how-to manual for molecular systematics. Mol Phylogenet Evol. 2009;52:520–523. doi: 10.1016/j.ympev.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Koetschan C, Förster F, Keller A, Schleicher T, Ruderisch B, Schwarz R, Müller T, Wolf M, Schultz J. The ITS2 Database III—sequences and structures for phylogeny. Nucleic Acids Res. 2010;38:D275–D279. doi: 10.1093/nar/gkp966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibel PN, Müller T, Dandekar T, Schultz J, Wolf M. 4SALE-A tool for synchronous RNA sequence and secondary structure alignment and editing. BMC Bioinformatics. 2006;7:498. doi: 10.1186/1471-2105-7-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibel PN, Müller T, Dandekar T, Wolf M. Synchronous visual analysis and editing of RNA sequence and secondary structure alignments using 4SALE. BMC Res Notes. 2008;1:91. doi: 10.1186/1756-0500-1-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller T, Rahmann S, Dandekar T, Wolf M. Accurate and robust phylogeny estimation based on profile distances: a study of the Chlorophyceae (Chlorophyta) BMC Evol Biol. 2004;4:20. doi: 10.1186/1471-2148-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich J, Dandekar T, Wolf M, Müller T. ProfDist: a tool for the construction of large phylogenetic trees based on profile distances. Bioinformatics. 2005;21:2108–2109. doi: 10.1093/bioinformatics/bti289. [DOI] [PubMed] [Google Scholar]
- Rahmann S, Müller T, Dandekar T, Wolf M. In: Advanced Data Mining Technologies in Bioinformatics. Hsu HH, editor. Hershey: Idea Group, Inc; 2006. Efficient and robust analysis of large phylogenetic datasets; pp. 104–117. [Google Scholar]
- Wolf M, Ruderisch B, Dandekar T, Schultz J, Müller T. ProfDistS: (profile-) distance based phylogeny on sequence-structure alignments. Bioinformatics. 2008;24:2401–2402. doi: 10.1093/bioinformatics/btn453. [DOI] [PubMed] [Google Scholar]
- Budd AF, Stolarski J. Corallite wall and septal microstructure in scleractinian reef corals: Comparison of molecular clades within the family Faviidae. J Morphol. 2011;272:66–88. doi: 10.1002/jmor.10899. [DOI] [PubMed] [Google Scholar]
- Grajales A, Aguilar C, Sánchez JA. Phylogenetic reconstruction using secondary structures of Internal Transcribed Spacer 2 (ITS2, rDNA): finding the molecular and morphological gap in Caribbean gorgonian corals. BMC Evol Biol. 2007;7:90. doi: 10.1186/1471-2148-7-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez JA, Dorado D. Intragenomic ITS2 variation in Caribbean seafans. Proc 11th Int Coral Reef Symp. 2008. pp. 1383–1387.
- Chen CA, Chang CC, Wei NV, Chen CH, Lein YT, Lin HE, Dai CF, Callace CC. Secondary structure and phylogenetic utility of the ribosomal internal transcribed spacer 2 (ITS2) in scleractinian corals. Zool Stud. 2004;43:759–771. [Google Scholar]
- Wei NV, Wallace CC, Dai CF, Moothien Pillay LR, Chen CA. Analyses of the ribosomal internal transcribed spacers (ITS) and the 5.8S gene indicate that extremely high rDNA heterogeneity is a unique feature in the scleractinian coral genus Acropora (Scleractinia; Acroporidae) Zool Stud. 2006;45:404–418. [Google Scholar]
- Shearer TL, van Oppen MJH, Romano SL, Wörheide G. Slow mitochondrial DNA sequence evolution in the Anthozoa (Cnidaria) Mol Ecol. 2002;11:2475–2487. doi: 10.1046/j.1365-294X.2002.01652.x. [DOI] [PubMed] [Google Scholar]
- Hellberg ME. No variation and low synonymous substitution rates in coral mtDNA despite high nuclear variation. BMC Evol Biol. 2006;6:8. doi: 10.1186/1471-2148-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes F, Fukami H, Vollmer SV, Norris RD, Knowlton N. Re-evaluation of the systematics of the endemic corals of Brazil by molecular data. Coral Reefs. 2008;27:423–432. doi: 10.1007/s00338-007-0349-0. [DOI] [Google Scholar]
- Hodgson G. A new species of Montastrea (Cnidaria, Scleractinia) from the Philippines. Pac Sci. 1985;39:283–290. [Google Scholar]
- Vaughan TW, Wells JW. Revision of the suborders, families, and genera of the Scleractinia. Geol Soc Am Spec Pap. 1943;44:1–345. [Google Scholar]
- Chevalier JP. Les scléractiniaires de la Mélanésie Française (Nouvelle-Calédonie, Iles Chesterfield, Iles Loyauté, Nouvelles Hébrides). Deuxième partie. Expéd Française récifs coralliens Nouvelle Calédonie. 1975;7:1–407. [Google Scholar]
- Budd AF, Stolarski J. Searching for new morphological characters in the systematics of scleractinian reef corals: comparison of septal teeth and granules between Atlantic and Pacific Mussidae. Acta Zool. 2009;90:142–165. doi: 10.1111/j.1463-6395.2008.00345.x. [DOI] [Google Scholar]
- Budd AF, Smith ND. Diversification of a new Atlantic clade of scleractinian reef corals: insights from phylogenetic analysis of morphologic and molecular data. Paleontol Soc Pap. 2005;11:103–128. [Google Scholar]
- Stolarski J, Roniewicz E. Towards a new synthesis of evolutionary relationships and classification of Scleractinia. J Paleontol. 2001;75:1090–1108. doi: 10.1666/0022-3360(2001)075<1090:TANSOE>2.0.CO;2. [DOI] [Google Scholar]
- Stolarski J, Russo A. Microstructural diversity of the stylophyllid (Scleractinia) skeleton. Acta Palaeontol Pol. 2002;47:651–666. [Google Scholar]
- Stolarski J, Vertino A. First Mesozoic record of the scleractinian Madrepora from the Maastrichtian siliceous limestones of Poland. Facies. 2007;53:67–78. doi: 10.1007/s10347-006-0089-6. [DOI] [Google Scholar]
- Zlatarski VN. Need for a more integrative approach to scleractinian taxonomy. Proc 11th Int Coral Reef Symp. 2008. pp. 1406–1410.
- Baird AH, Guest JR, Willis BL. Systematic and biogeographical patterns in the reproductive biology of scleractinian corals. Annu Rev Ecol Evol Syst. 2009;40:551–571. doi: 10.1146/annurev.ecolsys.110308.120220. [DOI] [Google Scholar]
- Kerr AM, Baird AH, Hughes TP. Correlated evolution of sex and reproductive mode in corals (Anthozoa: Scleractinia) Proc R Soc B-Biol Sci. 2011;278:75–81. doi: 10.1098/rspb.2010.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd AF. Longterm patterns of morphological variation within and among species of reef-corals and their relationship to sexual reproduction. Syst Bot. 1990;15:150–165. doi: 10.2307/2419024. [DOI] [Google Scholar]
- Budd AF. Phenotypic plasticity in the reef corals Montastraea annularis (Ellis & Solander) and Siderastrea siderea (Ellis & Solander) J Exp Mar Biol Ecol. 1979;39:25–54. doi: 10.1016/0022-0981(79)90003-0. [DOI] [Google Scholar]
- Budd AF. Large-scale evolutionary patterns in the reef-coral Montastraea: the role of phenotypic plasticity. Proc 6th Int Coral Reef Symp. 1988;3:393–398. [Google Scholar]
- Todd PA, Ladle RJ, Lewin-Koh NJI, Chou LM. Flesh or bone? Quantifying small-scale coral morphology using with-tissue and without-tissue techniques. Mar Biol. 2004;145:323–328. doi: 10.1007/s00227-004-1324-8. [DOI] [Google Scholar]
- Todd PA, Ladle RJ, Lewin-Koh NJI, Chou LM. Genotype × environment interactions in transplanted clones of the massive corals Favia speciosa and Diploastrea heliopora. Mar Ecol-Prog Ser. 2004;271:167–182. doi: 10.3354/meps271167. [DOI] [Google Scholar]
- Todd PA, Sidle RC, Lewin-Koh NJI. An aquarium experiment for identifying the physical factors inducing morphological change in two massive scleractinian corals. J Exp Mar Biol Ecol. 2004;299:97–113. doi: 10.1016/j.jembe.2003.09.005. [DOI] [Google Scholar]
- Todd PA. Morphological plasticity in scleractinian corals. Biol Rev. 2008;83:315–337. doi: 10.1111/j.1469-185X.2008.00045.x. [DOI] [PubMed] [Google Scholar]
- Budd AF, Johnson KG, Potts DC. Recognizing morphospecies in colonial reef corals: I. Landmark-based methods. Paleobiology. 1994;20:484–505. [Google Scholar]
- Wallace CC, Willis BL. Systematics of the coral genus Acropora: implications of new biological findings for species concepts. Annu Rev Ecol Syst. 1994;25:237–262. [Google Scholar]
- Daly M, Brugler MR, Cartwright P, Collins AG, Dawson MN, Fautin DG, France SC, McFadden CS, Opresko DM, Rodriguez E, Romano SL, Stake JL. The phylum Cnidaria: A review of phylogenetic patterns and diversity 300 years after Linnaeus. Zootaxa. 2007;1668:127–182. [Google Scholar]
- Veron JEN, Odorico DM, Chen CA, Miller DJ. Reassessing evolutionary relationships of scleractinian corals. Coral Reefs. 1996;15:1–9. [Google Scholar]
- Knowlton N, Budd AF. In: Evolutionary Patterns: Growth, Form, and Tempo in the Fossil Record. Jackson JBC, Lidgard S, McKinney, FK, editor. Chicago: University of Chicago Press; 2001. Recognizing coral species past and present; pp. 97–119. [Google Scholar]
- Fukami H, Nomura K. Existence of a cryptic species of Montastraea valenciennesi (Milne Edwards and Haime, 1848) in Wakayama, southern Honshu, Japan (in Japanese) J Jpn Coral Reef Soc. 2009;11:25–31. doi: 10.3755/jcrs.11.25. [DOI] [Google Scholar]
- Chevalier JP. Les scléractiniaires de la Mélanésie Française (Nouvelle-Calédonie, Iles Chesterfield, Iles Loyauté, Nouvelles Hébrides). Première partie. Expéd Française récifs coralliens Nouvelle Calédonie. 1971;5:1–307. [Google Scholar]
- Matthai G. A revision of the Recent colonial Astræidæ possessing distinct corallites. Trans Linn Soc Lond. 1914;17:1–140. doi: 10.1111/j.1096-3642.1914.tb00590.x. [DOI] [Google Scholar]
- Matthai G. Report of the madreporarian corals in the collection of the Indian Museum, Calcutta. Mem Indian Mus. 1924;8:1–59. [Google Scholar]
- Crossland C. Madreporaria, Hydrocorallinae, Heliopora and Tubipora. Great Barrier Reef Exped (1928-1929) Sci Rep. 1952;6:85–257. [Google Scholar]
- Wells JW. Recent corals of the Marshall Islands. Geol Surv Prof Pap. 1954;260-I:385–486. [Google Scholar]
- Nemenzo F. Systematic studies on Philippine shallow water scleractinians. II. Suborder Faviida. Nat Appl Sci Bull. 1959;16:73–135. [Google Scholar]
- Wijsman-Best M. Systematics and ecology of New Caledonian Faviinae (Coelenterata - Scleractinia) Contrib Zool. 1972;42:3–90. [Google Scholar]
- Wijsman-Best M. Biological results of the Snellius Expedition. XXV Faviidae collected by the Snellius Expedition. I. The genus Favia. Zool Meded Leiden. 1974;48:249–261. [Google Scholar]
- Scheer G, Pillai CSG. Report on the stony corals from the Red Sea. Zoologica. 1983;131:1–198. [Google Scholar]
- Nemenzo F. Studies on the systematics of scleractinian corals in the Philippines. Proc 4th Int Coral Reef Symp. 1981;1:25–32. [Google Scholar]
- Lamarck JBP. Histoire Naturelle des Animaux sans Vertèbres (Tome Second) Paris: Verdière; 1816. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
'Bigmessidae' corals. Photographs of most coral specimens sequenced in this study. More photographs are available from the authors.
Maximum likelihood tree topology of the combined molecular data. Numbers above branches are maximum likelihood bootstrap ≥50 and Bayesian posterior probability ≥0.9, while numbers below denote maximum parsimony bootstrap ≥50 and neighbor-joining bootstrap ≥50. Family classification follows definitions given for Figure 1.
Maximum likelihood tree topology of each partition. Numbers adjacent to branches are bootstrap support values ≥50. Definitions for family classification follow Figure 1.