Abstract
Purpose
To report a case of bilateral acute angle-closure glaucoma after oral administration of cabergoline for the treatment of galactorrhea.
Methods
A diagnosis of secondary drug-induced angle-closure glaucoma was made in a patient with elevated intraocular pressure (IOP) and myopic refractive shift, which was confirmed by ultrasound biomicroscopy (UBM) of the ciliary body and anterior segment, sonography, and optical coherence tomography. The treatment included the discontinuation of the culprit drug and the administration of topical anti-glaucoma drops. The treatment course was followed with serial measurements of the IOP and refraction, and with performing UBM.
Results
Five hours after he received a single 0.5-mg oral cabergoline tablet, the patient suffered from acute secondary angle-closure glaucoma and myopic refractive error. UBM demonstrated both effusion of the ciliary body and an anterior rotation of the iris-ciliary body. IOP was reduced 8 h after cessation of the causative agent and administration of anti-glaucoma drops. Refractive errors returned to normal levels after 8 days.
Conclusion
Secondary acute angle-closure glaucoma has been reported to occur after the administration of some drugs. In this report, an attempt has been made to describe this adverse reaction after oral cabergoline intake.
Key Words: Bilateral angle-closure glaucoma, Cabergoline, Galactorrhea
Introduction
Drug-induced glaucoma is a form of secondary glaucoma. Several types of drugs, such as adrenergic agonists, cholinergics, anti-cholinergics, sulfa-based drugs (e.g. topiramate), selective serotonin reuptake inhibitors, tricyclic and tetracyclic anti-depressants, anticoagulants, and antihistamines, have been reported to induce secondary acute angle-closure glaucoma [1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12].
Cabergoline (brand names Dostinex and Cabaser), an ergot derivative, is a potent dopamine D2 receptor agonist. The drug is prescribed in Parkinson's disease, prolactin-producing pituitary gland tumors, ablactation and dysfunctions associated with hyperprolactinemia. It stimulates dopamine receptors in lactophilic hypothalamic cells to suppress prolactin secretion in the pituitary gland [13, 14].
To the best of our knowledge, no cabergoline-induced glaucoma report has been published in the literature yet.
Case Report
One year after weaning her child, a 22-year-old female developed galactorrhea. She was prescribed 0.5-mg oral cabergoline tablets by her gynecologist (one tablet per week); however, she did not receive any other medication.
Five hours after ingestion of the first single dose of cabergoline, the patient was referred to our hospital suffering from bilateral painful red eyes, blurred vision, headache, nausea, and vomiting. Initially, the symptoms occured in her left eye, but, consequently, they also developed in her right eye. The patient had no past medical and familial history of glaucoma, other ophthalmologic diseases or refractive errors.
Ocular examination revealed an uncorrected visual acuity of 20/200 in the right and 20/400 in the left eye, normal external ocular motion in both eyes, 5-mm pupils in both eyes that were nonreactive to light, a conjunctival hyperemia and a perilimbal injection. In addition, the examination showed a microcystic corneal edema, a shallow anterior chamber, a closed angle in gonioscopy as well as a clear lens. The fundus demonstrated normal disc and vessels, a normal foveal light reflex as well as some perifoveal retinal wrinkling secondary to choroidal thickening. The intraocular pressure (IOP), measured using Goldmann applanation tonometry, was 40 mm Hg in the right and 42 mm Hg in the left eye. Refractive errors were −7.75 dpt sph and −1.00 × 94° cyl in the right eye, and −9.00 dpt sph and −0.50 × 56° cyl in the left eye.
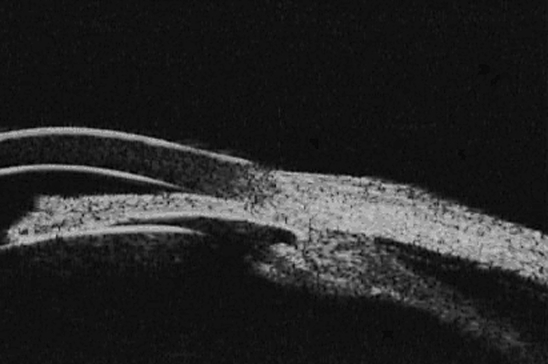
Ultrsonagraphy with a 10-MHz probe revealed choroidal thickening in both eyes. Ultrasound biomicroscopy with a 40-MHz probe showed ciliochoroidal thickening, effusion underneath the ciliary body, narrowing of the angle and anterior rotation of the iris-ciliary body in both eyes (fig. 1). Measured axial lengths were 23.22 and 23.24 mm in the right and left eye, respectively. The anterior chamber depth was 1.33 mm in the right and 1.34 in left eye. Results of a posterior pole optical coherence tomography were normal in both eyes.
Fig. 1.
Choroidal effusion and ciliochoroidal anterior displacement in UBM.
Treatment of the patient was immediately started with the discontinuation of cabergoline as the suspicious causal drug. The elevated IOP was treated with topical timolol maleate 0.5% every 12 h, brimonidine tartrate 0.1% every 8 h, and latanoprost 0.005% every 24 h.
Glaucoma was controlled 8 h after medical treatment, IOP was reduced to 25 mm Hg in both eyes, conjunctival hyperemia was reduced, and ocular pain was alleviated; however, the patient still had a myopic shift in refraction. Finally, by 8 days after cessation of cabergoline, the refraction returned to normal levels.
Discussion
Cabergoline is absorbed from the gastrointestinal tract within 0.5 to 4 h, with an average elimination half-life of 80 h. Seventy-nine percent of the patients taking this drug report at least one side effect. These side effects are chiefly mild or moderate, including nausea, constipation, dry mouth, gastric irritation, dyspepsia, sleep disturbances, vertigo, depression, dyskinesia, hallucinations, systemic hypotension, peripheral edema, arrhythmia, and angina pectoris [15, 16, 17, 18, 19, 20]. We were not able to find any previous report of glaucoma symptoms and signs in the literature.
Acute drug-induced bilateral glaucoma is a relatively uncommon but serious adverse reaction which, if not recognized in a timely manner, may result in severe morbidity and even permanent visual damage. Its treatment differs from that of primary acute angle-closure glaucoma, i.e. it is necessary to discontinue the drug for controlling glaucoma [1, 2, 3, 4, 5].
Footnotes
This is an Open Access article licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 License (www.karger.com/OA-license), applicable to the online version of the article only. Distribution for non-commercial purposes only.
References
- 1.Tripathi RC, Tripathi BJ, Haggerty C. Drug-induced glaucomas: mechanism and management. Drug Saf. 2003;26:749–767. doi: 10.2165/00002018-200326110-00002. [DOI] [PubMed] [Google Scholar]
- 2.Mohammed ZS, Simi ZU, Tariq SM, Ali KR. Bilateral acute angle closure glaucoma in a 50 year old female after oral administration of flavoxate. Br J Clin Pharmacol. 2008;66:726–7. doi: 10.1111/j.1365-2125.2008.03254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cole KL, Wang EE, Aronwald RM: Bilateral acute angle-closure glaucoma in a migraine patient receiving topiramate: a case report. J Emerg Med, Epub ahead of print. [DOI] [PubMed]
- 4.Cruciani F, Lorenzatti M, Nazzarro V, Abdolrahimzadeh S. Bilateral acute angle closure glaucoma and myopia induced by topiramate. Clin Ter. 2009;160:215–216. [PubMed] [Google Scholar]
- 5.Sung VC, Corridan PG. Acute-angle closure glaucoma as a side-effect of oxybutynin. Br J Urol. 1998;81:634–635. doi: 10.1046/j.1464-410x.1998.00403.x. [DOI] [PubMed] [Google Scholar]
- 6.Chapple CR, Parkhouse H, Gardener C, Milroy EJ. Double-blind, placebo-controlled, cross-over study of flavoxate in the treatment of idiopathic detrusor instability. Br J Urol. 1990;66:491–494. doi: 10.1111/j.1464-410x.1990.tb14994.x. [DOI] [PubMed] [Google Scholar]
- 7.Dahm TL, Ostri P, Kristensen JK, Walter S, Frimodt-Møller C, Rasmussen RB, Nøhr M, Alexander N. Flavoxate treatment of micturition disorders accompanying benign prostatic hypertrophy: a double-blind placebo-controlled multicenter investigation. Urol Int. 1995;55:205–208. doi: 10.1159/000282787. [DOI] [PubMed] [Google Scholar]
- 8.Roxburgh C, Cook J, Dublin N. Anticholinergic drugs versus other medications for overactive bladder syndrome in adults. Cochrane Database Syst Rev. 2007;4 doi: 10.1002/14651858.CD003190.pub4. CD003190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato K, Yoshida K, Suzuki K, Murase T, Gotoh M. Managing patients with an overactive bladder and glaucoma: a questionnaire survey of Japanese urologists on the use of anticholinergics. BJU Int. 2005;95:98–101. doi: 10.1111/j.1464-410X.2004.05259.x. [DOI] [PubMed] [Google Scholar]
- 10.Guier CP. Elevated intraocular pressure and myopic shift linked to topiramate use. Optom Vis Sci. 2007;84:1070–1073. doi: 10.1097/OPX.0b013e31815b9e38. [DOI] [PubMed] [Google Scholar]
- 11.Craig JE, Ong TJ, Louis DL, Wells JM. Mechanism of topiramate-induced acute-onset myopia and angle closure glaucoma. Am J Ophthalmol. 2004;137:193–195. doi: 10.1016/s0002-9394(03)00774-8. [DOI] [PubMed] [Google Scholar]
- 12.Banta JT, Hoffman K, Budenz DL, Ceballos E, Greenfield DS. Presumed topiramate-induced bilateral acute angle-closure glaucoma. Am J Ophthalmol. 2001;132:112–114. doi: 10.1016/s0002-9394(01)01013-3. [DOI] [PubMed] [Google Scholar]
- 13.Guay DR. Clinical pharmacokinetics of drugs used to treat urge incontinence. Clin Pharmacokinet. 2003;42:1243–1285. doi: 10.2165/00003088-200342140-00004. [DOI] [PubMed] [Google Scholar]
- 14.Sharma G, Mishra AK, Mishra P, Misra A. Intranasal cabergoline: pharmacokinetic and pharmacodynamic studies. AAPS PharmSciTech. 2009;10:1321–1330. doi: 10.1208/s12249-009-9329-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vallette S, Serri K, Serri O. Cabergoline therapy for prolactinomas: is valvular heart disease a real safety concern? Expert Rev Cardiovasc Ther. 2010;8:49–54. doi: 10.1586/erc.09.167. [DOI] [PubMed] [Google Scholar]
- 16.Filopanti M, Lania AG, Spada A. Pharmacogenetics of D2 dopamine receptor gene in prolactin-secreting pituitary adenomas. Expert Opin Drug Metab Toxicol. 2010;6:43–53. doi: 10.1517/17425250903352501. [DOI] [PubMed] [Google Scholar]
- 17.Uzawa A, Mori M, Kojima S, Mitsuma S, Sekiguchi Y, Kanesaka T, Kuwabara S. Dopamine agonist-induced antecollis in Parkinson's disease. Mov Disord. 2009;24:2408–2411. doi: 10.1002/mds.22779. [DOI] [PubMed] [Google Scholar]
- 18.Jallad RS, Bronstein MD. Optimizing medical therapy of acromegaly: beneficial effects of cabergoline in patients uncontrolled with long-acting release octreotide. Neuroendocrinology. 2009;90:82–92. doi: 10.1159/000218323. [DOI] [PubMed] [Google Scholar]
- 19.Martin NM, Tan T, Meeran K. Dopamine agonists and hyperprolactinaemia. BMJ. 2009;338:b381. doi: 10.1136/bmj.b381. [DOI] [PubMed] [Google Scholar]
- 20.Bogazzi F, Manetti L, Raffaelli V, Lombardi M, Rossi G, Martino E. Cabergoline therapy and the risk of cardiac valve regurgitation in patients with hyperprolactinemia: a meta-analysis from clinical studies. J Endocrinol Invest. 2008;31:1119–1123. doi: 10.1007/BF03345662. [DOI] [PubMed] [Google Scholar]