Abstract
Introduction
Cetuximab-based chemotherapy showed a statistically significantly higher response rate compared with chemotherapy such as FOLFOX. Therefore, FOLFOX plus cetuximab is suspected to be the best regimen to alleviate tumor-related symptoms with a high response rate.
Case Report
Here we present the results of 8 consecutive patients with metastatic colorectal cancer with poor performance status and/or severe complications who were treated with first-line FOLFOX with cetuximab. Six of 8 patients achieved an apparent clinical benefit, including radiological response and symptoms improvement. Two patients with BRAF mutation could achieve neither clinical benefit nor radiological response.
Conclusion
Although an optimal line of therapy with cetuximab is unclear yet with bevacizumab in mind, we propose that patients who need a tumor response to alleviate their symptoms due to advanced disease might be candidates for first-line cetuximab-based therapy as shown in our cases. Additionally, patients with BRAF mutant tumors might be important candidates for novel targeted therapy in the future to improve their poor prognosis.
Key Words: Colorectal cancer, Poor performance status, FOLFOX, Cetuximab
Introduction
Cetuximab, a recombinant, human-mouse chimeric monoclonal IgG1 antibody that specifically targets the epidermal growth factor receptor (EGFR) has been shown to significantly improve the prognosis of metastatic colorectal cancer (MCRC) compared with best supportive care alone in the third-line setting [1].
Recently, cetuximab was approved as a treatment for MCRC in Japan. Although an optimal line of therapy with cetuximab is unclear yet with bevacizumab in mind [2], a cetuximab-based regimen showed a statistically significantly higher response rate compared with chemotherapy alone [3, 4], in contrast with bevacizumab, which did not show an improvement in objective response rate when given in combination with first-line oxaliplatin with fluoropyrimidine [5]. Therefore, we propose that patients who need a tumor response to alleviate their symptoms due to advanced disease might be candidates for first-line cetuximab-based therapy. To support this strategy, here we present the results of 8 consecutive patients with MCRC with poor performance status and/or severe complications who were treated with first-line FOLFOX with cetuximab.
Case Report
Between October 2009 and April 2010, 8 patients received FOLFOX pus cetuximab as first-line chemotherapy for MCRC (table 1). Eastern Cooperative Oncology Group (ECOG) performance status was 1 in 1 patient, 2 in 6 patients, and 3 in 1 patient. Six patients had symptomatic peritoneal disseminations with ascites. One patient had a complication of icterus due to severe liver metastases. One patient had rectal cancer with anorectal pain due to bone invasion. The primary tumor was present in 6 patients. KRAS status was evaluated using cycleave method and was wild type in all patients. All patients were treated with the approved dosage and schedule of cetuximab: initially 400 mg/m2 followed by weekly infusions of 250 mg/m2. The dose of FOLFOX was adjusted individually based on performance status or organ dysfunction. The median number of cetuximab administrations was 12 (range 3–25). Six of 8 patients achieved an apparent clinical benefit, as shown in fig. 1, fig. 2 and table 1. In contrast, 2 patients could achieve neither clinical benefit nor radiological response; these 2 patients were found to have the BRAF V600 mutation. Grade 3 skin toxicity was observed in 1 patient. Although no treatment-related deaths were observed, 1 patient with icterus experienced infection without neutropenia. The median survival of all patients was 5.2 months (range 2.5–14+ months).
Table 1.
Disease characteristics and outcomes of each patient
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | ||
| Age, gender | 35, F | 60, F | 40, M | 48, F | 68, M | 48, F | 71, M | 74, M | |
| ECOG PS | 3 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | |
| Metastatic sites | Liver, ovary, peritoneum, ascites | Liver, pleural effusion | Peritoneum, ascites | lymph node | Liver, peritoneum ascites | Liver, ovary, peritoneum, ascites | Liver, peritoneum, ascites | Liver, peritoneum, ascites | |
| Complication | Abdominal distension | Icterus (T-bil 5.9 mg/dl) | Abdominal pain | Rectal pain | Ileus | Abdominal distension | Ileus | Abdominal distension | |
| KRAS status* | Wild | Wild | Wild | Wild | Wild | Wild | Wild | Wild | |
| BMF status** | Wild | Wild | Wild | Wild | Mutant | Wild | Wild | Mutant | |
| Cetuximab administration (times) | 19 | 7 | 25 | 8 | 4 | 12 | 6 | 3 | |
| Clinical improvement | Yes | Yes | Yes | Yes | No | Yes | Yes | No | |
| PS 1, followed by colorectomy | PS 1, T-bil 1.3 mg/dl | PS 1, ascites disappeared | Improved pain, surgery (R0) | – | PS1, ascites disappeared, surgery | PS1, improved intestinal obstruction | – | ||
| Skin toxicity (grade) | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 0 | |
| Survival (months) | 14.3+ | 4.2 | 10.4 | 10.3+ | 2.5 | 10 | 5.2 | 3.5 | |
F = Female; M = male; ECOG PS = Eastern Cooperative Oncology Group performance status; T-bil = total bilirubin.
KKAS codon 12, 13, 61
BKAFV600.
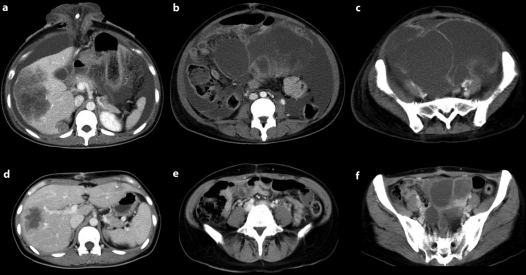
Fig. 1.
a, b CT scans of case 1 before treatment. Multiple liver metastases, massive ascites and ovarian metastases are seen. c, d CT scans of case 1 after 4 cycles of FOLFOX plus cetuximab. The multiple liver metastases and ovarian metastases were reduced in size, and the ascites had almost disappeared. This patient underwent resection of the primary tumor and closure of the colostomy.
Fig. 2.
a, b CT scans of case 3 before treatment. Massive ascites and peritoneum dissemination are seen. This case was complicated with abdominal pain and the patient was unable to eat. c, d CT scans of case 3 after 4 cycles of FOLFOX plus cetuximab. The ascites had almost disappeared. The performance status was improved from 2 to 1 with sufficient oral intake.
Discussion
Although cetuximab was studied initially in pretreated patients, in contrast with bevacizumab [5], the CRYSTAL trial showed a survival benefit of first-line cetuximab, especially in patients with KRAS wild-type tumors [3]. In another study that used cetuximab with an oxaliplatin-based regimen, the combination showed a higher response rate compared with chemotherapy alone [4]. Disappointingly, combination chemotherapy of bevacizumab and cetuximab or panitumumab failed to show an improvement in efficacy [6, 7]. According to these results, the most recent NCCN guideline for the treatment of colon cancer recommended several first-line regimens: bevacizumab or cetuximab/panitumumab (KRAS wild type only) combined with irinotecan or oxaliplatin with fluoropyrimidines [2]. Since cetuximab or panitumumab is also effective even in patients with previous chemotherapy [1, 8], an optimal line of therapy with cetuximab or panitumumab is unclear yet with bevacizumab in mind [2]. As noted previously, since a cetuximab-based regimen showed a statistically significantly higher response rate compared with chemotherapy alone [3, 4], we supposed that patients who need a tumor response to alleviate their symptoms might be candidates for first-line cetuximab-based therapy, although the results of an ongoing phase III study comparing bevacizumab and cetuximab as first-line chemotherapy are eagerly awaited.
For patients with poor general status or severe complications, fluoropyrimidine monotherapy with or without bevacizumab is recommended according to NCCN guidelines [2] and generally selected in clinical practice. However, we suspect that FOLFOX plus cetuximab might become an attractive regimen for these patients according to our results. Although FOLFOX and FOLFIRI (irinotecan with fluoropyrimidine) is considered to be similarly effective as first-line chemotherapy for MCRC, FOLFOX may be preferable in patients with severe abdominal symptoms or liver dysfunction as shown in our cases since toxicity of irinotecan is frequent in these complicated cases.
Our results are provocative for the following reasons: first, the regimen of cetuximab plus FOLFOX might be feasible, as it had activity in patients with MCRC with poor performance status or severe symptoms. In consideration of the risk-benefit balance, further investigations will be needed to clarify the distinct indication for either the intensive chemotherapy such as FOLFOX plus cetuximab or weak chemotherapy like fluoropyrimidine monotherapy for these patients. Second, BRAF might be an important negative marker for response to chemotherapy or a strong negative prognostic factor, as already reported [9, 10, 11, 12]. Although the evaluation of BRAF status prior to treatment with an EGFR antibody is still controversial [2], patients with BRAF mutant tumors might be important candidates for novel targeted therapy in the future to improve their poor prognosis.
References
- 1.Jonker DJ, O'Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, Berry SR, Krahn M, Price T, Simes RJ, Tebbutt NC, van Hazel G, Wierzbicki R, Langer C, Moore MJ. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–2048. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 2.NCCN Clinical Practice Guidelines in Oncology: Colon Cancer. NCCN® Practice Guidelines in Oncology, version 2.2010.
- 3.Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 4.Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C, Loos AH, Zubel A, Koralewski P. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663–671. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 5.Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzén F, Cassidy J. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 6.Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG, Erdkamp FL, Vos AH, van Groeningen CJ, Sinnige HA, Richel DJ, Voest EE, Dijkstra JR, Vink-Börger ME, Antonini NF, Mol L, van Krieken JH, Dalesio O, Punt CJ. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563–572. doi: 10.1056/NEJMoa0808268. [DOI] [PubMed] [Google Scholar]
- 7.Hecht JR, Mitchell E, Chidiac T, Scroggin C, Hagenstad C, Spigel D, Marshall J, Cohn A, McCollum D, Stella P, Deeter R, Shahin S, Amado RG. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27:672–680. doi: 10.1200/JCO.2008.19.8135. [DOI] [PubMed] [Google Scholar]
- 8.Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, Canon JL, Van Laethem JL, Maurel J, Richardson G, Wolf M, Amado RG. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 9.Di Nicolantonio F, Martini M, Molinari F, Sartore-Bianchi A, Arena S, Saletti P, De Dosso S, Mazzucchelli L, Frattini M, Siena S, Bardelli A. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705–5712. doi: 10.1200/JCO.2008.18.0786. [DOI] [PubMed] [Google Scholar]
- 10.Laurent-Puig P, Cayre A, Manceau G, Buc E, Bachet JB, Lecomte T, Rougier P, Lievre A, Landi B, Boige V, Ducreux M, Ychou M, Bibeau F, Bouché O, Reid J, Stone S, Penault-Llorca F. Analysis of, PTEN, BRAF, and EGFR status in determining benefit from cetuximab therapy in wild-type KRAS metastatic colon cancer. J Clin Oncol. 2009;27:5924–5930. doi: 10.1200/JCO.2008.21.6796. [DOI] [PubMed] [Google Scholar]
- 11.Tol J, Nagtegaal ID, Punt CJ. BRAF mutation in metastatic colorectal cancer. N Engl J Med. 2009;361:98–99. doi: 10.1056/NEJMc0904160. [DOI] [PubMed] [Google Scholar]
- 12.Kohne C, Rougier P, Stroh C: Cetuximab with chemotherapy (CT) as first-line treatment for metastatic colorectal cancer (mCRC): a meta-analysis of the CRYSTAL and OPUS studies according to KRAS and BRAF mutation status. ASCO 2010 Gastrointestinal Cancers Symposium, Abstract 406.