Abstract
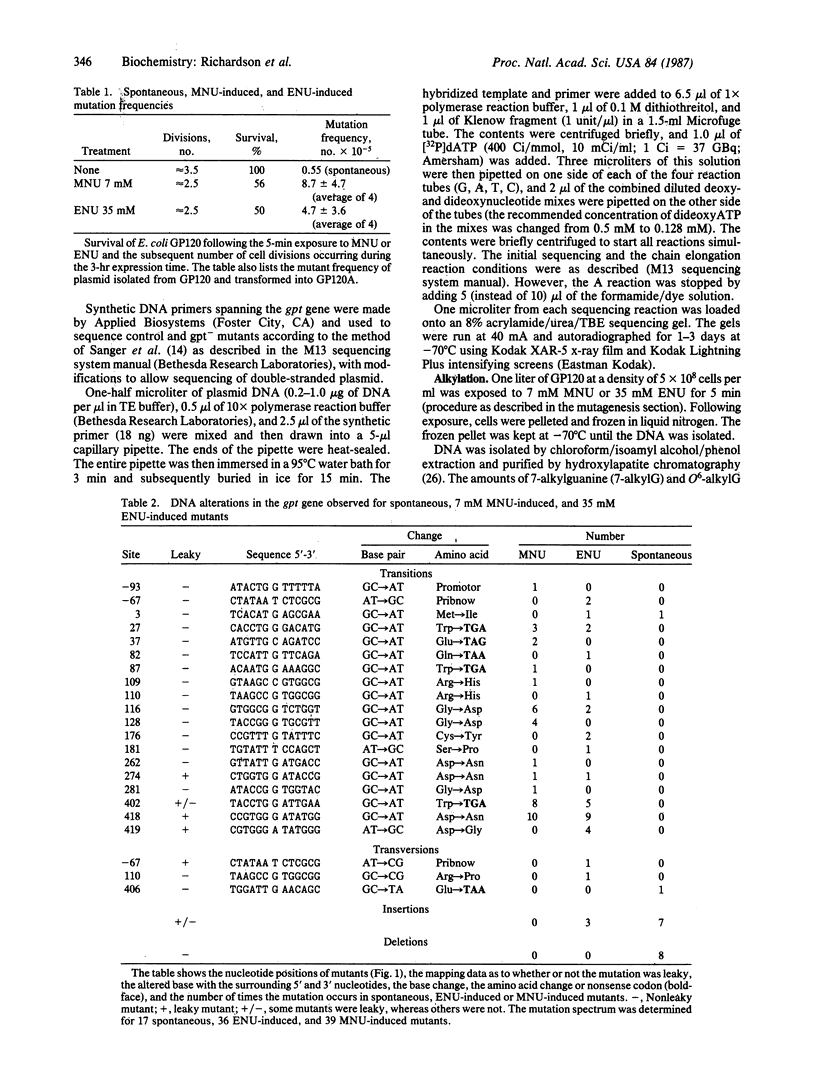
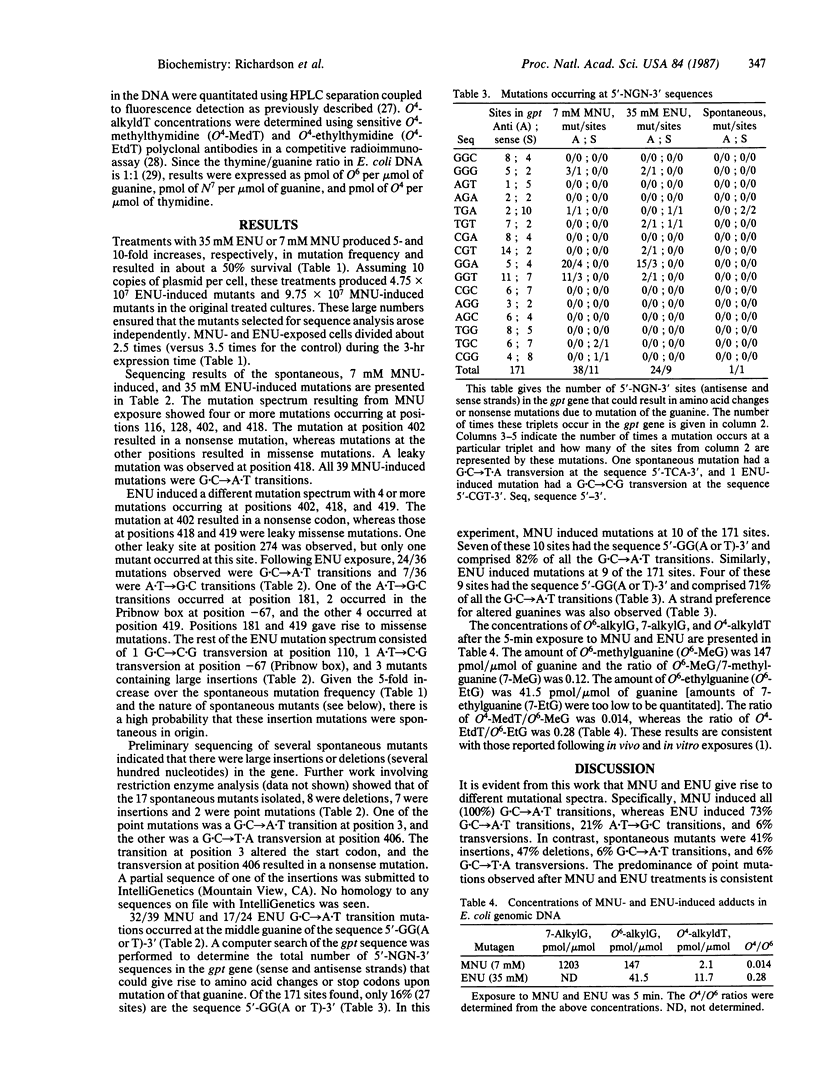
Dideoxy chain-termination DNA sequencing was used to determine the specific DNA base changes induced after in vivo exposure of Escherichia coli to N-methyl-N-nitrosourea (MNU) and N-ethyl-N-nitrosourea (ENU) using the xanthine guanine phosphoribosyltransferase (gpt) gene as the genetic target. The resultant mutation spectra were compared with the levels of O6-alkylguanine and O4-alkylthymidine in genomic DNA immediately after exposure. All (39/39) of the MNU-induced mutations were G X C----A X T transitions. In contrast, 24/33 point mutations isolated following ENU treatment were G X C----A X T transitions, 7/33 were A X T----G X C transitions, 1/33 was a G X C----C X G transversion, and 1/33 was an A X T----C X G transversion. Three large insertions, probably of spontaneous origin, were also isolated. O4-alkylthymidine/O6-alkylguanine ratios were 0.014 for MNU and 0.28 for ENU. These data suggest that the difference in the mutation spectrum of MNU versus ENU may be attributed, in part, to the different ratio of O6-alkylguanine versus O4-alkylthymidine produced in the DNA. Of the G X C----A X T transitions, 82% of the MNU- and 71% of the ENU-induced mutations occurred at the middle guanine of the sequence 5'-GG(A or T)-3'.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashman C. R., Jagadeeswaran P., Davidson R. L. Efficient recovery and sequencing of mutant genes from mammalian chromosomal DNA. Proc Natl Acad Sci U S A. 1986 May;83(10):3356–3360. doi: 10.1073/pnas.83.10.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedell M. A., Lewis J. G., Billings K. C., Swenberg J. A. Cell specificity in hepatocarcinogenesis: preferential accumulation of O6-methylguanine in target cell DNA during continuous exposure to rats to 1,2-dimethylhydrazine. Cancer Res. 1982 Aug;42(8):3079–3083. [PubMed] [Google Scholar]
- Beland F. A., Dooley K. L., Casciano D. A. Rapid isolation of carcinogen-bound DNA and RNA by hydroxyapatite chromatography. J Chromatogr. 1979 Jun 1;174(1):177–186. doi: 10.1016/s0021-9673(00)87048-x. [DOI] [PubMed] [Google Scholar]
- Beranek D. T., Heflich R. H., Kodell R. L., Morris S. M., Casciano D. A. Correlation between specific DNA-methylation products and mutation induction at the HGPRT locus in Chinese hamster ovary cells. Mutat Res. 1983 Jun-Jul;110(1):171–180. doi: 10.1016/0027-5107(83)90026-x. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiteux S., Laval F. Repair of O6-methylguanine, by mammalian cell extracts, in alkylated DNA and poly(dG-m5dC).(poly dG-m5dC) in B and Z forms. Carcinogenesis. 1985 May;6(5):805–807. doi: 10.1093/carcin/6.5.805. [DOI] [PubMed] [Google Scholar]
- Briscoe W. T., Cotter L. E. DNA sequence has an effect on the extent and kinds of alkylation of DNA by a potent carcinogen. Chem Biol Interact. 1985 Dec 31;56(2-3):321–331. doi: 10.1016/0009-2797(85)90014-6. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H., Farabaugh P. J., Gilbert W. Molecular basis of base substitution hotspots in Escherichia coli. Nature. 1978 Aug 24;274(5673):775–780. doi: 10.1038/274775a0. [DOI] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H. Genetic studies of the lac repressor. IV. Mutagenic specificity in the lacI gene of Escherichia coli. J Mol Biol. 1977 Dec 15;117(3):577–606. doi: 10.1016/0022-2836(77)90059-6. [DOI] [PubMed] [Google Scholar]
- Flavell R. A., Sabo D. L., Bandle E. F., Weissmann C. Site-directed mutagenesis: generation of an extracistronic mutation in bacteriophage Q beta RNA. J Mol Biol. 1974 Oct 25;89(2):255–272. doi: 10.1016/0022-2836(74)90517-8. [DOI] [PubMed] [Google Scholar]
- Garner R. C., Pickering C., Martin C. N. Mutagenicity of methyl-, ethyl-, propyl- and butylnitrosourea towards Escherichia coli WP2 strains with varying DNA repair capabilities. Chem Biol Interact. 1979 Jul;26(2):197–205. doi: 10.1016/0009-2797(79)90023-1. [DOI] [PubMed] [Google Scholar]
- Heflich R. H., Beranek D. T., Kodell R. L., Morris S. M. Induction of mutations and sister-chromatid exchanges in Chinese hamster ovary cells by ethylating agents. Mutat Res. 1982 Nov;106(1):147–161. doi: 10.1016/0027-5107(82)90198-1. [DOI] [PubMed] [Google Scholar]
- Hu Y. C., Guttenplan J. B. Evidence for a major premutagenic ethyldeoxythymidine-DNA adduct in an in vivo system: N-nitroso-N-ethylurea-treated Salmonella typhimurium. Carcinogenesis. 1985 Oct;6(10):1513–1516. doi: 10.1093/carcin/6.10.1513. [DOI] [PubMed] [Google Scholar]
- Jensen D. E. Reaction of DNA with alkylating agents. Differential alkylation of poly[dA-dT[ by methylnitrosourea and ethylnitrosourea. Biochemistry. 1978 Nov 28;17(24):5108–5113. doi: 10.1021/bi00617a006. [DOI] [PubMed] [Google Scholar]
- Loechler E. L., Green C. L., Essigmann J. M. In vivo mutagenesis by O6-methylguanine built into a unique site in a viral genome. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6271–6275. doi: 10.1073/pnas.81.20.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Meselson M., Stahl F. W., Vinograd J. EQUILIBRIUM SEDIMENTATION OF MACROMOLECULES IN DENSITY GRADIENTS. Proc Natl Acad Sci U S A. 1957 Jul 15;43(7):581–588. doi: 10.1073/pnas.43.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. H., Low K. B. Specificity of mutagenesis resulting from the induction of the SOS system in the absence of mutagenic treatment. Cell. 1984 Jun;37(2):675–682. doi: 10.1016/0092-8674(84)90400-8. [DOI] [PubMed] [Google Scholar]
- Miller J. H. Mutational specificity in bacteria. Annu Rev Genet. 1983;17:215–238. doi: 10.1146/annurev.ge.17.120183.001243. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Expression of a bacterial gene in mammalian cells. Science. 1980 Sep 19;209(4463):1422–1427. doi: 10.1126/science.6251549. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Factors governing the expression of a bacterial gene in mammalian cells. Mol Cell Biol. 1981 May;1(5):449–459. doi: 10.1128/mcb.1.5.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold R. F., Warren W., Medcalf A. S., Amos J. Mutagenicity of carcinogenic methylating agents is associated with a specific DNA modification. Nature. 1980 Feb 7;283(5747):596–599. doi: 10.1038/283596a0. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Scicchitano D., Dolan M. E. Comparison of the rates of repair of O6-alkylguanines in DNA by rat liver and bacterial O6-alkylguanine-DNA alkyltransferase. Cancer Res. 1984 Sep;44(9):3806–3811. [PubMed] [Google Scholar]
- Richardson F. C., Dyroff M. C., Boucheron J. A., Swenberg J. A. Differential repair of O4-alkylthymidine following exposure to methylating and ethylating hepatocarcinogens. Carcinogenesis. 1985 Apr;6(4):625–629. doi: 10.1093/carcin/6.4.625. [DOI] [PubMed] [Google Scholar]
- Richardson K. K., Fostel J., Skopek T. R. Nucleotide sequence of the xanthine guanine phosphoribosyl transferase gene of E. coli. Nucleic Acids Res. 1983 Dec 20;11(24):8809–8816. doi: 10.1093/nar/11.24.8809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffhill R., Hall J. A. The incorporation of O6-methyldeoxyguanosine monophosphate and O4-methyldeoxythymidine monophosphate into polynucleotide templates leads to errors during subsequent in vitro DNA synthesis. Chem Biol Interact. 1985 Dec 31;56(2-3):363–370. doi: 10.1016/0009-2797(85)90017-1. [DOI] [PubMed] [Google Scholar]
- Saffhill R., Margison G. P., O'Connor P. J. Mechanisms of carcinogenesis induced by alkylating agents. Biochim Biophys Acta. 1985 Dec 17;823(2):111–145. doi: 10.1016/0304-419x(85)90009-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortle D., Nathans D. Local mutagenesis: a method for generating viral mutants with base substitutions in preselected regions of the viral genome. Proc Natl Acad Sci U S A. 1978 May;75(5):2170–2174. doi: 10.1073/pnas.75.5.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B., Chavez F., Spengler S. J. O4-Methyl-, O4-ethyl-, and O4-isopropylthymidine 5'-triphosphates as analogues of thymidine 5'-triphosphate: kinetics of incorporation by Escherichia coli DNA polymerase I. Biochemistry. 1986 Mar 25;25(6):1201–1205. doi: 10.1021/bi00354a001. [DOI] [PubMed] [Google Scholar]
- Singer B., Spengler S. J., Fraenkel-Conrat H., Kuśmierek J. T. O4-Methyl, -ethyl, or -isopropyl substituents on thymidine in poly(dA-dT) all lead to transitions upon replication. Proc Natl Acad Sci U S A. 1986 Jan;83(1):28–32. doi: 10.1073/pnas.83.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B., Sági J., Kuśmierek J. T. Escherichia coli polymerase I can use O2-methyldeoxythymidine or O4-methyldeoxythymidine in place of deoxythymidine in primed poly(dA-dT).poly(dA-dT) synthesis. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4884–4888. doi: 10.1073/pnas.80.16.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skopek T. R., Hutchinson F. DNA base sequence changes induced by bromouracil mutagenesis of lambda phage. J Mol Biol. 1982 Jul 25;159(1):19–33. doi: 10.1016/0022-2836(82)90029-8. [DOI] [PubMed] [Google Scholar]
- Skopek T. R., Wood R. D., Hutchinson F. Sequence specificity of mutagenesis in the cI gene of bacteriophage lambda. Environ Health Perspect. 1985 Oct;62:157–161. doi: 10.1289/ehp.8562157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa K., Nakadate M., Watabe T., Ishidate M., Jr, Kondo S. Differential mutagenicities of 6 N-nitroso-N-alkylurea derivatives in Escherichia coli strains with different DNA-repair capacities. Mutat Res. 1980 Dec;79(4):319–325. doi: 10.1016/0165-1218(80)90155-x. [DOI] [PubMed] [Google Scholar]
- Zarbl H., Sukumar S., Arthur A. V., Martin-Zanca D., Barbacid M. Direct mutagenesis of Ha-ras-1 oncogenes by N-nitroso-N-methylurea during initiation of mammary carcinogenesis in rats. 1985 May 30-Jun 5Nature. 315(6018):382–385. doi: 10.1038/315382a0. [DOI] [PubMed] [Google Scholar]


